Lipid-Based Nanomaterials for Drug Delivery Systems in Breast Cancer Therapy
Abstract
:1. Introduction
2. Conjugation and Targeting Moieties of Liposomes
2.1. Liposomal Nanomaterials
2.2. Liposomes as Drug Carriers
2.3. Active Targeting Liposomes
2.4. Cell Surface Receptor Targeting
2.5. Targeting Transferrin Receptor (TfR)
2.6. Targeting Epidermal Growth Factor Receptor (EGFR)
2.7. Targeting Folic Acid (FA) Receptor
2.8. Carcinoembryonic Antigen-Like Cell Adhesion Molecules (CEACAMs)
2.9. Preparation of Antibody-Conjugated Liposome
2.10. Antibody Conjugation Method
2.10.1. Amine Modification
2.10.2. Carbohydrate Modification
2.10.3. Disulfide Bonds
3. Lipid-Based Drug Delivery Systems and Treatment
3.1. Liposomes
3.2. Exosomes
3.3. Micelles
4. Safety and Efficacy Aspects of Lipid-Based Nanomaterials
4.1. Stealth Liposome Technology
4.2. Non-PEGylated-Based Liposome Technology
4.3. Liposomal-Based Products
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chang, T.-W.; Ko, H.; Huang, W.-S.; Chiu, Y.-C.; Yang, L.-X.; Chia, Z.-C.; Chin, Y.-C.; Chen, Y.-J.; Tsai, Y.-T.; Hsu, C.-W.; et al. Tannic acid-induced interfacial ligand-to-metal charge transfer and the phase transformation of Fe3O4 nanoparticles for the photothermal bacteria destruction. Chem. Eng. J. 2022, 428, 131237. [Google Scholar] [CrossRef]
- Hsu, I.L.; Yeh, F.H.; Chin, Y.-C.; Cheung, C.I.; Chia, Z.C.; Yang, L.-X.; Chen, Y.-J.; Cheng, T.-Y.; Wu, S.-P.; Tsai, P.-J.; et al. Multiplex antibacterial processes and risk in resistant phenotype by high oxidation-state nanoparticles: New killing process and mechanism investigations. Chem. Eng. J. 2021, 409, 128266. [Google Scholar] [CrossRef]
- Yang, Y.-T.; Hsu, I.L.; Cheng, T.-Y.; Wu, W.-J.; Lee, C.-W.; Li, T.-J.; Cheung, C.I.; Chin, Y.-C.; Chen, H.-C.; Chiu, Y.-C.; et al. Off-resonance SERS nanoprobe-targeted screen of biomarkers for antigens recognition of bladder normal and aggressive cancer cells. Anal. Chem. 2019, 91, 8213–8220. [Google Scholar] [CrossRef] [PubMed]
- Mutalik, C.; Okoro, G.; Krisnawati, D.I.; Jazidie, A.; Rahmawati, E.Q.; Rahayu, D.; Hsu, W.-T.; Kuo, T.-R. Copper sulfide with morphology-dependent photodynamic and photothermal antibacterial activities. J. Colloid Interface Sci. 2022, 607, 1825–1835. [Google Scholar] [CrossRef] [PubMed]
- Mutalik, C.; Krisnawati, D.I.; Patil, S.B.; Khafid, M.; Atmojo, D.S.; Santoso, P.; Lu, S.-C.; Wang, D.-Y.; Kuo, T.-R. Phase-dependent MoS2 nanoflowers for light-driven antibacterial application. ACS Sustain. Chem. Eng. 2021, 9, 7904–7912. [Google Scholar] [CrossRef]
- Yougbaré, S.; Chou, H.-L.; Yang, C.-H.; Krisnawati, D.I.; Jazidie, A.; Nuh, M.; Kuo, T.-R. Facet-dependent gold nanocrystals for effective photothermal killing of bacteria. J. Hazard. Mater. 2021, 407, 124617. [Google Scholar] [CrossRef]
- Chang, T.-K.; Cheng, T.-M.; Chu, H.-L.; Tan, S.-H.; Kuo, J.-C.; Hsu, P.-H.; Su, C.-Y.; Chen, H.-M.; Lee, C.-M.; Kuo, T.-R. Metabolic mechanism investigation of antibacterial active cysteine-conjugated gold nanoclusters in Escherichia coli. ACS Sustain. Chem. Eng. 2019, 7, 15479–15486. [Google Scholar] [CrossRef]
- Kuo, T.-R.; Liao, H.-J.; Chen, Y.-T.; Wei, C.-Y.; Chang, C.-C.; Chen, Y.-C.; Chang, Y.-H.; Lin, J.-C.; Lee, Y.-C.; Wen, C.-Y.; et al. Extended visible to near-infrared harvesting of earth-abundant FeS2–TiO2 heterostructures for highly active photocatalytic hydrogen evolution. Green Chem. 2018, 20, 1640–1647. [Google Scholar] [CrossRef]
- Kuo, T.-R.; Chen, W.-T.; Liao, H.-J.; Yang, Y.-H.; Yen, H.-C.; Liao, T.-W.; Wen, C.-Y.; Lee, Y.-C.; Chen, C.-C.; Wang, D.-Y. Improving hydrogen evolution activity of earth-abundant cobalt-doped iron pyrite catalysts by surface modification with phosphide. Small 2017, 13, 1603356. [Google Scholar] [CrossRef]
- Cheng, T.-M.; Chu, H.-L.; Lee, Y.-C.; Wang, D.-Y.; Chang, C.-C.; Chung, K.-L.; Yen, H.-C.; Hsiao, C.-W.; Pan, X.-Y.; Kuo, T.-R.; et al. Quantitative analysis of glucose metabolic cleavage in glucose transporters overexpressed cancer cells by target-specific fluorescent gold nanoclusters. Anal. Chem. 2018, 90, 3974–3980. [Google Scholar] [CrossRef]
- Collaborators, M.W.S. Breast cancer and hormone-replacement therapy in the million women study. Lancet 2003, 362, 419–427. [Google Scholar]
- Berkel, H.; Birdsell, D.C.; Jenkins, H. Breast augmentation: A risk factor for breast cancer? N. Engl. J. Med. 1992, 326, 1649–1653. [Google Scholar] [CrossRef]
- Radice, D.; Redaelli, A. Breast cancer management. Pharmacoeconomics 2003, 21, 383–396. [Google Scholar] [CrossRef] [PubMed]
- O’Shaughnessy, J.; Petrakova, K.; Sonke, G.S.; Conte, P.; Arteaga, C.L.; Cameron, D.A.; Hart, L.L.; Villanueva, C.; Jakobsen, E.; Beck, J.T.; et al. Ribociclib plus letrozole versus letrozole alone in patients with de novo hr+, her2− advanced breast cancer in the randomized monaleesa-2 trial. Breast Cancer Res. Treat. 2018, 168, 127–134. [Google Scholar] [CrossRef] [PubMed]
- García-Hermoso, A.; Cavero-Redondo, I.; Ramírez-Vélez, R.; Ruiz, J.R.; Ortega, F.B.; Lee, D.-C.; Martínez-Vizcaíno, V. Muscular strength as a predictor of all-cause mortality in an apparently healthy population: A systematic review and meta-analysis of data from approximately 2 million men and women. Arch. Phys. Med. Rehabil. 2018, 99, 2100–2113.e5. [Google Scholar] [CrossRef]
- Sharma, G.N.; Dave, R.; Sanadya, J.; Sharma, P.; Sharma, K. Various types and management of breast cancer: An overview. J. Adv. Pharm. Technol. Res. 2010, 1, 109. [Google Scholar]
- Park, J.W. Liposome-based drug delivery in breast cancer treatment. Breast Cancer Res. 2002, 4, 95. [Google Scholar] [CrossRef]
- Yougbaré, S.; Mutalik, C.; Okoro, G.; Lin, I.-H.; Krisnawati, D.I.; Jazidie, A.; Nuh, M.; Chang, C.-C.; Kuo, T.-R. Emerging trends in nanomaterials for antibacterial applications. Int. J. Nanomed. 2021, 16, 5831. [Google Scholar] [CrossRef]
- Yougbaré, S.; Mutalik, C.; Krisnawati, D.I.; Kristanto, H.; Jazidie, A.; Nuh, M.; Cheng, T.-M.; Kuo, T.-R. Nanomaterials for the photothermal killing of bacteria. Nanomaterials 2020, 10, 1123. [Google Scholar] [CrossRef]
- Mutalik, C.; Wang, D.-Y.; Krisnawati, D.I.; Jazidie, A.; Yougbare, S.; Kuo, T.-R. Light-activated heterostructured nanomaterials for antibacterial applications. Nanomaterials 2020, 10, 643. [Google Scholar] [CrossRef]
- Kaur, N.; Aditya, R.N.; Singh, A.; Kuo, T.-R. Biomedical applications for gold nanoclusters: Recent developments and future perspectives. Nanoscale Res. Lett. 2018, 13, 302. [Google Scholar] [CrossRef] [PubMed]
- Voinea, M.; Simionescu, M. Designing of ‘intelligent’ liposomes for efficient delivery of drugs. J. Cell. Mol. Med. 2002, 6, 465–474. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bahreyni, A.; Mohamud, Y.; Luo, H. Emerging nanomedicines for effective breast cancer immunotherapy. J. Nanobiotechnol. 2020, 18, 180. [Google Scholar] [CrossRef]
- Lila, A.S.A.; Ishida, T. Liposomal delivery systems: Design optimization and current applications. Biol. Pharm. Bull. 2017, 40, 1–10. [Google Scholar] [CrossRef]
- Malam, Y.; Loizidou, M.; Seifalian, A.M. Liposomes and nanoparticles: Nanosized vehicles for drug delivery in cancer. Trends Pharmacol. Sci. 2009, 30, 592–599. [Google Scholar] [CrossRef] [PubMed]
- Kuo, J.-C.; Tan, S.-H.; Hsiao, Y.-C.; Mutalik, C.; Chen, H.-M.; Yougbaré, S.; Kuo, T.-R. Unveiling the antibacterial mechanism of gold nanoclusters via in situ transmission electron microscopy. ACS Sustain. Chem. Eng. 2022, 10, 464–471. [Google Scholar] [CrossRef]
- Reddy, K.R. Controlled-release, pegylation, liposomal formulations: New mechanisms in the delivery of injectable drugs. Ann. Pharmacother. 2000, 34, 915–923. [Google Scholar] [CrossRef]
- Deshpande, P.P.; Biswas, S.; Torchilin, V.P. Current trends in the use of liposomes for tumor targeting. Nanomedicine 2013, 8, 1509–1528. [Google Scholar] [CrossRef]
- Strebhardt, K.; Ullrich, A. Paul ehrlich’s magic bullet concept: 100 years of progress. Nat. Rev. Cancer 2008, 8, 473–480. [Google Scholar] [CrossRef]
- Huwyler, J.; Drewe, J.; Krähenbühl, S. Tumor targeting using liposomal antineoplastic drugs. Int. J. Nanomed. 2008, 3, 21. [Google Scholar] [CrossRef]
- Rashed, M.H.; Bayraktar, E.; Helal, G.K.; Abd-Ellah, M.F.; Amero, P.; Chavez-Reyes, A.; Rodriguez-Aguayo, C. Exosomes: From garbage bins to promising therapeutic targets. Int. J. Mol. Sci. 2017, 18, 538. [Google Scholar] [CrossRef] [PubMed]
- Pullan, J.E.; Confeld, M.I.; Osborn, J.K.; Kim, J.; Sarkar, K.; Mallik, S. Exosomes as drug carriers for cancer therapy. Mol. Pharm. 2019, 16, 1789–1798. [Google Scholar] [CrossRef] [PubMed]
- Bangham, A.; Standish, M.M.; Watkins, J.C. Diffusion of univalent ions across the lamellae of swollen phospholipids. J. Mol. Biol. 1965, 13, 238–252. [Google Scholar] [CrossRef]
- Kunjachan, S.; Ehling, J.; Storm, G.; Kiessling, F.; Lammers, T. Noninvasive imaging of nanomedicines and nanotheranostics: Principles, progress, and prospects. Chem. Rev. 2015, 115, 10907–10937. [Google Scholar] [CrossRef] [PubMed]
- García-Pinel, B.; Porras-Alcalá, C.; Ortega-Rodríguez, A.; Sarabia, F.; Prados, J.; Melguizo, C.; López-Romero, J.M. Lipid-based nanoparticles: Application and recent advances in cancer treatment. Nanomaterials 2019, 9, 638. [Google Scholar] [CrossRef]
- Lammers, T.; Kiessling, F.; Hennink, W.E.; Storm, G. Drug targeting to tumors: Principles, pitfalls and (pre-) clinical progress. J. Control. Release 2012, 161, 175–187. [Google Scholar] [CrossRef]
- Torchilin, V.P. Passive and active drug targeting: Drug delivery to tumors as an example. In Drug Delivery; Springer: Berlin, Germany, 2010; pp. 3–53. [Google Scholar]
- Torchilin, V. Tumor delivery of macromolecular drugs based on the epr effect. Adv. Drug Deliv. Rev. 2011, 63, 131–135. [Google Scholar] [CrossRef]
- Torchilin, V.P. Targeted pharmaceutical nanocarriers for cancer therapy and imaging. AAPS J. 2007, 9, E128–E147. [Google Scholar] [CrossRef]
- Wang, M.; Thanou, M. Targeting nanoparticles to cancer. Pharmacol. Res. 2010, 62, 90–99. [Google Scholar] [CrossRef]
- Danhier, F.; Feron, O.; Préat, V. To exploit the tumor microenvironment: Passive and active tumor targeting of nanocarriers for anti-cancer drug delivery. J. Control. Release 2010, 148, 135–146. [Google Scholar] [CrossRef]
- Sawant, R.R.; Torchilin, V.P. Challenges in development of targeted liposomal therapeutics. AAPS J. 2012, 14, 303–315. [Google Scholar] [CrossRef] [PubMed]
- Byrne, J.D.; Betancourt, T.; Brannon-Peppas, L. Active targeting schemes for nanoparticle systems in cancer therapeutics. Adv. Drug Deliv. Rev. 2008, 60, 1615–1626. [Google Scholar] [CrossRef] [PubMed]
- Egusquiaguirre, S.P.; Igartua, M.; Hernández, R.M.; Pedraz, J.L. Nanoparticle delivery systems for cancer therapy: Advances in clinical and preclinical research. Transl. Oncol. 2012, 14, 83–93. [Google Scholar] [CrossRef] [PubMed]
- Talekar, M.; Kendall, J.; Denny, W.; Garg, S. Targeting of nanoparticles in cancer: Drug delivery and diagnostics. Anticancer Drugs 2011, 22, 949–962. [Google Scholar] [CrossRef] [PubMed]
- Liechty, W.B.; Peppas, N.A. Expert opinion: Responsive polymer nanoparticles in cancer therapy. Eur. J. Pharm. Biopharm. 2012, 80, 241–246. [Google Scholar] [CrossRef]
- Allen, T.M. Ligand-targeted therapeutics in anticancer therapy. Nat. Rev. Cancer 2002, 2, 750–763. [Google Scholar] [CrossRef]
- Ying, X.; Wen, H.; Lu, W.-L.; Du, J.; Guo, J.; Tian, W.; Men, Y.; Zhang, Y.; Li, R.-J.; Yang, T.-Y. Dual-targeting daunorubicin liposomes improve the therapeutic efficacy of brain glioma in animals. J. Control. Release 2010, 141, 183–192. [Google Scholar] [CrossRef]
- Yoon, D.J.; Chu, D.S.; Ng, C.W.; Pham, E.A.; Mason, A.B.; Hudson, D.M.; Smith, V.C.; MacGillivray, R.T.; Kamei, D.T. Genetically engineering transferrin to improve its in vitro ability to deliver cytotoxins. J. Control. Release 2009, 133, 178–184. [Google Scholar] [CrossRef]
- Li, X.; Ding, L.; Xu, Y.; Wang, Y.; Ping, Q. Targeted delivery of doxorubicin using stealth liposomes modified with transferrin. Int. J. Pharm. 2009, 373, 116–123. [Google Scholar] [CrossRef]
- Zhai, G.; Wu, J.; Yu, B.; Guo, C.; Yang, X.; Lee, R.J. A transferrin receptor-targeted liposomal formulation for docetaxel. J. Nanosci. Nanotechnol. 2010, 10, 5129–5136. [Google Scholar] [CrossRef]
- Petrilli, R.; Pinheiro, D.P.; de Oliveira, F.D.C.E.; Galvão, G.F.; Marques, L.G.A.; Lopez, R.F.V.; Pessoa, C.; Eloy, J.O. Immunoconjugates for cancer targeting: A review of antibody-drug conjugates and antibody-functionalized nanoparticles. Curr. Med. Chem. 2021, 28, 2485–2520. [Google Scholar] [CrossRef] [PubMed]
- Chiu, G.N.; Edwards, L.A.; Kapanen, A.I.; Malinen, M.M.; Dragowska, W.H.; Warburton, C.; Chikh, G.G.; Fang, K.Y.; Tan, S.; Sy, J. Modulation of cancer cell survival pathways using multivalent liposomal therapeutic antibody constructs. Mol. Cancer Ther. 2007, 6, 844–855. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Low, P.S.; Henne, W.A.; Doorneweerd, D.D. Discovery and development of folic-acid-based receptor targeting for imaging and therapy of cancer and inflammatory diseases. Acc. Chem. Res. 2008, 41, 120–129. [Google Scholar] [CrossRef]
- Kularatne, S.A.; Low, P.S. Targeting of nanoparticles: Folate receptor. In Cancer Nanotechnology; Springer: Berlin, Germany, 2010; pp. 249–265. [Google Scholar]
- Ling, S.S.N.; Yuen, K.H.; Magosso, E.; Barker, S.A. Oral bioavailability enhancement of a hydrophilic drug delivered via folic acid-coupled liposomes in rats. J. Pharm. Pharmacol. 2009, 61, 445–449. [Google Scholar] [CrossRef]
- Xiang, G.; Wu, J.; Lu, Y.; Liu, Z.; Lee, R.J. Synthesis and evaluation of a novel ligand for folate-mediated targeting liposomes. Int. J. Pharm. 2008, 356, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Malhi, S.S.; Budhiraja, A.; Arora, S.; Chaudhari, K.R.; Nepali, K.; Kumar, R.; Sohi, H.; Murthy, R.S. Intracellular delivery of redox cycler-doxorubicin to the mitochondria of cancer cell by folate receptor targeted mitocancerotropic liposomes. Int. J. Pharm. 2012, 432, 63–74. [Google Scholar] [CrossRef] [PubMed]
- Duarte, S.; Faneca, H.; Lima, M.C. Folate-associated lipoplexes mediate efficient gene delivery and potent antitumoral activity in vitro and in vivo. Int. J. Pharm. 2012, 423, 365–377. [Google Scholar] [CrossRef] [PubMed]
- Niu, R.; Zhao, P.; Wang, H.; Yu, M.; Cao, S.; Zhang, F.; Chang, J. Preparation, characterization, and antitumor activity of paclitaxel-loaded folic acid modified and tat peptide conjugated pegylated polymeric liposomes. J. Drug Target. 2011, 19, 373–381. [Google Scholar] [CrossRef] [PubMed]
- Lewis-Wambi, J.S.; Cunliffe, H.E.; Kim, H.R.; Willis, A.L.; Jordan, V.C. Overexpression of ceacam6 promotes migration and invasion of oestrogen-deprived breast cancer cells. Eur. J. Cancer 2008, 44, 1770–1779. [Google Scholar] [CrossRef]
- Cheng, T.M.; Murad, Y.M.; Chang, C.C.; Yang, M.C.; Baral, T.N.; Cowan, A.; Tseng, S.H.; Wong, A.; Mackenzie, R.; Shieh, D.B.; et al. Single domain antibody against carcinoembryonic antigen-related cell adhesion molecule 6 (ceacam6) inhibits proliferation, migration, invasion and angiogenesis of pancreatic cancer cells. Eur. J. Cancer 2014, 50, 713–721. [Google Scholar] [CrossRef]
- Son, S.M.; Yun, J.; Lee, S.H.; Han, H.S.; Lim, Y.H.; Woo, C.G.; Lee, H.C.; Song, H.G.; Gu, Y.M.; Lee, H.J.; et al. Therapeutic effect of phlip-mediated ceacam6 gene silencing in lung adenocarcinoma. Sci. Rep. 2019, 9, 11607. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Jang, Y.; Park, S.; Jang, H.; Park, E.J.; Kim, H.J.; Kim, H. Development and evaluation of a ceacam6-targeting theranostic nanomedicine for photoacoustic-based diagnosis and chemotherapy of metastatic cancer. Theranostics 2018, 8, 4247–4261. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, J.; Smirnov, A.; Criss, A.K.; Columbus, L. Quantifying carcinoembryonic antigen-like cell adhesion molecule-targeted liposome delivery using imaging flow cytometry. Mol. Pharm. 2019, 16, 2354–2363. [Google Scholar] [CrossRef] [PubMed]
- Wright, S.; Huang, L. Antibody-directed liposomes as drug-delivery vehicles. Adv. Drug Deliv. Rev. 1989, 3, 343–389. [Google Scholar] [CrossRef]
- Endoh, H.; Suzuki, Y.; Hashimoto, Y. Antibody coating of liposomes with 1-ethyl-3-(3-dimethyl-aminopropyl) carbodiimide and the effect on target specificity. J. Immunol. Methods 1981, 44, 79–85. [Google Scholar] [CrossRef]
- Dunnick, J.K.; McDougall, I.R.; Aragon, S.; Goris, M.L.; Kriss, J.P. Vesicle interactions with polyamino acids and antibody: In vitro and in vivo studies. J. Nucl. Med. 1975, 16, 483–487. [Google Scholar]
- Ansell, S.M.; Tardi, P.G.; Buchkowsky, S.S. 3-(2-pyridyldithio) propionic acid hydrazide as a cross-linker in the formation of liposome−antibody conjugates. Bioconjugate Chem. 1996, 7, 490–496. [Google Scholar] [CrossRef]
- Huang, A.; Huang, L.; Kennel, S.J. Monoclonal antibody covalently coupled with fatty acid. A reagent for in vitro liposome targeting. J. Biol. Chem. 1980, 255, 8015–8018. [Google Scholar] [CrossRef]
- Maruyama, K.; Takizawa, T.; Yuda, T.; Kennel, S.J.; Huang, L.; Iwatsuru, M. Targetability of novel immunoliposomes modified with amphipathic poly (ethylene glycol) s conjugated at their distal terminals to monoclonal antibodies. Biochim. Biophys. Acta Biomembr. 1995, 1234, 74–80. [Google Scholar] [CrossRef]
- Barbet, J.; Machy, P.; Leserman, L.D. Monoclonal antibody covalently coupled to liposomes: Specific targeting to cells. J. Supramol. Struct. Cell. Biochem. 1981, 16, 243–258. [Google Scholar] [CrossRef]
- Jones, M.N.; Hudson, M.J. The targeting of immunoliposomes to tumour cells (a431) and the effects of encapsulated methotrexate, Biochim. Biophys. Acta Biomembr. 1993, 1152, 231–242. [Google Scholar] [CrossRef]
- Hansen, C.B.; Kao, G.Y.; Moase, E.H.; Zalipsky, S.; Allen, T.M. Attachment of antibodies to sterically stabilized liposomes: Evaluation, comparison and optimization of coupling procedures. Biochim. Biophys. Acta Biomembr. 1995, 1239, 133–144. [Google Scholar] [CrossRef]
- Carlsson, J.; Drevin, H.; Axén, R. Protein thiolation and reversible protein-protein conjugation. N-succinimidyl 3-(2-pyridyldithio) propionate, a new heterobifunctional reagent. Biochem. J. 1978, 173, 723–737. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Domen, P.L.; Nevens, J.R.; Mallia, A.K.; Hermanson, G.T.; Klenk, D.C. Site-directed immobilization of proteins. J. Chromatogr. A 1990, 510, 293–302. [Google Scholar] [CrossRef]
- Ansell, S.M.; Harasym, T.O.; Tardi, P.G.; Buchkowsky, S.S.; Bally, M.B.; Cullis, P.R. Antibody conjugation methods for active targeting of liposomes. In Drug Targeting; Springer: Berlin, Germany, 2000; pp. 51–68. [Google Scholar]
- Harding, J.A.; Engbers, C.M.; Newman, M.S.; Goldstein, N.I.; Zalipsky, S. Immunogenicity and pharmacokinetic attributes of poly (ethylene glycol)-grafted immunoliposomes. Biochim. Biophys. Acta Biomembr. 1997, 1327, 181–192. [Google Scholar] [CrossRef]
- Chua, M.-M.; Fan, S.-T.; Karush, F. Attachment of immunoglobulin to liposomal membrane via protein carbohydrate. Biochim. Biophys. Acta Gen. Subj. 1984, 800, 291–300. [Google Scholar] [CrossRef]
- Hoffman, A.S. The origins and evolution of “controlled” drug delivery systems. J. Control. Release 2008, 132, 153–163. [Google Scholar] [CrossRef]
- Jain, R.K.; Stylianopoulos, T. Delivering nanomedicine to solid tumors. Nat. Rev. Clin. Oncol. 2010, 7, 653–664. [Google Scholar] [CrossRef]
- Suggitt, M.; Bibby, M.C. 50 years of preclinical anticancer drug screening: Empirical to target-driven approaches. Clin. Cancer Res. 2005, 11, 971–981. [Google Scholar] [CrossRef]
- Oakman, C.; Santarpia, L.; Di Leo, A. Breast cancer assessment tools and optimizing adjuvant therapy. Nat. Rev. Clin. Oncol. 2010, 7, 725–732. [Google Scholar] [CrossRef]
- Akbari, A.; Akbarzadeh, A.; Rafiee Tehrani, M.; Ahangari Cohan, R.; Chiani, M.; Mehrabi, M.R. Development and characterization of nanoliposomal hydroxyurea against bt-474 breast cancer cells. Adv. Pharm. Bull. 2020, 10, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.-J.; Yang, S.-C.; Liu, X.-L.; Gao, Y.; Dong, X.; Lai, X.; Zhu, M.-H.; Feng, H.-Y.; Zhu, X.-D.; Lu, Q.; et al. Nanobowl-supported liposomes improve drug loading and delivery. Nano Lett. 2020, 20, 4177–4187. [Google Scholar] [CrossRef]
- Kim, B.; Shin, J.; Wu, J.; Omstead, D.T.; Kiziltepe, T.; Littlepage, L.E.; Bilgicer, B. Engineering peptide-targeted liposomal nanoparticles optimized for improved selectivity for her2-positive breast cancer cells to achieve enhanced in vivo efficacy. J. Control. Release 2020, 322, 530–541. [Google Scholar] [CrossRef] [PubMed]
- Tokunaga, E.; Kimura, Y.; Oki, E.; Ueda, N.; Futatsugi, M.; Mashino, K.; Yamamoto, M.; Ikebe, M.; Kakeji, Y.; Baba, H.; et al. Akt is frequently activated in her2/neu-positive breast cancers and associated with poor prognosis among hormone-treated patients. Int. J. Cancer 2006, 118, 284–289. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Chen, J.; Wang, L.; Li, Q.; Yang, Y.; Lv, Z.; Bao, H.; Li, Y.; Luan, X.; Li, Y.; et al. Co-delivery of epirubicin and paclitaxel using an estrone-targeted pegylated liposomal nanoparticle for breast cancer. Int. J. Pharm. 2020, 573, 118806. [Google Scholar] [CrossRef] [PubMed]
- Tang, B.; Peng, Y.; Yue, Q.; Pu, Y.; Li, R.; Zhao, Y.; Hai, L.; Guo, L.; Wu, Y. Design, preparation and evaluation of different branched biotin modified liposomes for targeting breast cancer. Eur. J. Med. Chem. 2020, 193, 112204. [Google Scholar] [CrossRef]
- Swami, R.; Kumar, Y.; Chaudhari, D.; Katiyar, S.S.; Kuche, K.; Katare, P.B.; Banerjee, S.K.; Jain, S. pH sensitive liposomes assisted specific and improved breast cancer therapy using co-delivery of sirt1 shrna and docetaxel. Mater. Sci. Eng. C 2021, 120, 111664. [Google Scholar] [CrossRef]
- Huang, M.; Pu, Y.; Peng, Y.; Fu, Q.; Guo, L.; Wu, Y.; Zheng, Y. Biotin and glucose dual-targeting, ligand-modified liposomes promote breast tumor-specific drug delivery. Bioorg. Med. Chem. Lett. 2020, 30, 127151. [Google Scholar] [CrossRef]
- Yang, Y.; Zhao, Z.; Xie, C.; Zhao, Y. Dual-targeting liposome modified by glutamic hexapeptide and folic acid for bone metastatic breast cancer. Chem. Phys. Lipids 2020, 228, 104882. [Google Scholar] [CrossRef]
- Ghandhariyoun, N.; Jaafari, M.R.; Nikoofal-Sahlabadi, S.; Taghdisi, S.M.; Moosavian, S.A. Reducing doxorubicin resistance in breast cancer by liposomal foxm1 aptamer: In vitro and in vivo. Life Sci. 2020, 262, 118520. [Google Scholar] [CrossRef]
- Zhao, Z.; Zhao, Y.; Xie, C.; Chen, C.; Lin, D.; Wang, S.; Lin, D.; Cui, X.; Guo, Z.; Zhou, J. Dual-active targeting liposomes drug delivery system for bone metastatic breast cancer: Synthesis and biological evaluation. Chem. Phys. Lipids 2019, 223, 104785. [Google Scholar] [CrossRef] [PubMed]
- Cai, D.; Gao, W.; He, B.; Dai, W.; Zhang, H.; Wang, X.; Wang, J.; Zhang, X.; Zhang, Q. Hydrophobic penetrating peptide pfvyli-modified stealth liposomes for doxorubicin delivery in breast cancer therapy. Biomaterials 2014, 35, 2283–2294. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liu, D.; Guan, S.; Zhu, W.; Fan, L.; Zhang, Q.; Cai, D. Hyaluronic acid-modified liposomal honokiol nanocarrier: Enhance anti-metastasis and antitumor efficacy against breast cancer. Carbohydr. Polym. 2020, 235, 115981. [Google Scholar] [CrossRef]
- Gallez, A.; Palazzo, C.; Blacher, S.; Tskitishvili, E.; Noël, A.; Foidart, J.-M.; Evrard, B.; Pequeux, C.; Piel, G. Liposomes and drug-in-cyclodextrin-in-liposomes formulations encapsulating 17β-estradiol: An innovative drug delivery system that prevents the activation of the membrane-initiated steroid signaling (miss) of estrogen receptor α. Int. J. Pharm. 2020, 573, 118861. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wu, X.; Yang, H.; Li, L.; Ye, Z.; Rao, Y. A nuclear targeted dox-aptamer loaded liposome delivery platform for the circumvention of drug resistance in breast cancer. Biomed. Pharmacother. 2019, 117, 109072. [Google Scholar] [CrossRef]
- Ağardan, N.B.M.; Değim, Z.; Yılmaz, Ş.; Altıntaş, L.; Topal, T. Tamoxifen/raloxifene loaded liposomes for oral treatment of breast cancer. J. Drug Deliv. Sci. Technol. 2020, 57, 101612. [Google Scholar] [CrossRef]
- Zhu, Y.-X.; Jia, H.-R.; Duan, Q.-Y.; Liu, X.; Yang, J.; Liu, Y.; Wu, F.-G. Photosensitizer-doped and plasma membrane-responsive liposomes for nuclear drug delivery and multidrug resistance reversal. ACS Appl. Mater. Interfaces 2020, 12, 36882–36894. [Google Scholar] [CrossRef]
- Belfiore, L.; Saunders, D.N.; Ranson, M.; Vine, K.L. N-alkylisatin-loaded liposomes target the urokinase plasminogen activator system in breast cancer. Pharmaceutics 2020, 12, 641. [Google Scholar] [CrossRef]
- Burande, A.S.; Viswanadh, M.K.; Jha, A.; Mehata, A.K.; Shaik, A.; Agrawal, N.; Poddar, S.; Mahto, S.K.; Muthu, M.S. Egfr targeted paclitaxel and piperine Co-loaded liposomes for the treatment of triple negative breast cancer. AAPS PharmSciTech 2020, 21, 151. [Google Scholar] [CrossRef]
- Rayamajhi, S.; Nguyen, T.D.T.; Marasini, R.; Aryal, S. Macrophage-derived exosome-mimetic hybrid vesicles for tumor targeted drug delivery. Acta Biomater. 2019, 94, 482–494. [Google Scholar] [CrossRef]
- Skog, J.; Würdinger, T.; van Rijn, S.; Meijer, D.H.; Gainche, L.; Curry, W.T.; Carter, B.S.; Krichevsky, A.M.; Breakefield, X.O. Glioblastoma microvesicles transport rna and proteins that promote tumour growth and provide diagnostic biomarkers. Nat. Cell Biol. 2008, 10, 1470–1476. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Wu, Y.; Ding, F.; Yang, J.; Li, J.; Gao, X.; Zhang, C.; Feng, J. Engineering macrophage-derived exosomes for targeted chemotherapy of triple-negative breast cancer. Nanoscale 2020, 12, 10854–10862. [Google Scholar] [CrossRef] [PubMed]
- Bianco, P. “Mesenchymal” stem cells. Annu. Rev. Cell Dev. Biol. 2014, 30, 677–704. [Google Scholar] [CrossRef]
- Hass, R.; Kasper, C.; Böhm, S.; Jacobs, R. Different populations and sources of human mesenchymal stem cells (msc): A comparison of adult and neonatal tissue-derived msc. Cell Commun. Signal. 2011, 9, 12. [Google Scholar] [CrossRef] [PubMed]
- Phinney, D.G. Building a consensus regarding the nature and origin of mesenchymal stem cells. J. Cell. Biochem. 2002, 85, 7–12. [Google Scholar] [CrossRef]
- Pittenger, M.F.; Mackay, A.M.; Beck, S.C.; Jaiswal, R.K.; Douglas, R.; Mosca, J.D.; Moorman, M.A.; Simonetti, D.W.; Craig, S.; Marshak, D.R. Multilineage potential of adult human mesenchymal stem cells. Science 1999, 284, 143–147. [Google Scholar] [CrossRef]
- Melzer, C.; Rehn, V.; Yang, Y.; Bähre, H.; von der Ohe, J.; Hass, R. Taxol-loaded msc-derived exosomes provide a therapeutic vehicle to target metastatic breast cancer and other carcinoma cells. Cancers 2019, 11, 798. [Google Scholar] [CrossRef]
- Yu, M.; Gai, C.; Li, Z.; Ding, D.; Zheng, J.; Zhang, W.; Lv, S.; Li, W. Targeted exosome-encapsulated erastin induced ferroptosis in triple negative breast cancer cells. Cancer Sci. 2019, 110, 3173–3182. [Google Scholar] [CrossRef]
- Wang, K.; Ye, H.; Zhang, X.; Wang, X.; Yang, B.; Luo, C.; Zhao, Z.; Zhao, J.; Lu, Q.; Zhang, H.; et al. An exosome-like programmable-bioactivating paclitaxel prodrug nanoplatform for enhanced breast cancer metastasis inhibition. Biomaterials 2020, 257, 120224. [Google Scholar] [CrossRef]
- Risha, Y.; Minic, Z.; Ghobadloo, S.M.; Berezovski, M.V. The proteomic analysis of breast cell line exosomes reveals disease patterns and potential biomarkers. Sci. Rep. 2020, 10, 1–12. [Google Scholar] [CrossRef]
- Duncan, R. The dawning era of polymer therapeutics. Nat. Rev. Drug Discov. 2003, 2, 347–360. [Google Scholar] [CrossRef] [PubMed]
- Barriga, H.M.G.; Holme, M.N.; Stevens, M.M. Cubosomes: The next generation of smart lipid nanoparticles? Angew. Chem. Int. Ed. 2019, 58, 2958–2978. [Google Scholar] [CrossRef] [PubMed]
- Pramanik, A.; Xu, Z.; Shamsuddin, S.H.; Khaled, Y.S.; Ingram, N.; Maisey, T.; Tomlinson, D.; Coletta, P.L.; Jayne, D.; Hughes, T.A.; et al. Affimer tagged cubosomes: Targeting of carcinoembryonic antigen expressing colorectal cancer cells using in vitro and in vivo models. ACS Appl. Mater. Interfaces 2022, 14, 11078–11091. [Google Scholar] [CrossRef] [PubMed]
- Pramanik, A.; Xu, Z.; Ingram, N.; Coletta, P.L.; Millner, P.A.; Tyler, A.I.I.; Hughes, T.A. Hyaluronic-acid-tagged cubosomes deliver cytotoxics specifically to cd44-positive cancer cells. Mol. Pharm. 2022. [Google Scholar] [CrossRef] [PubMed]
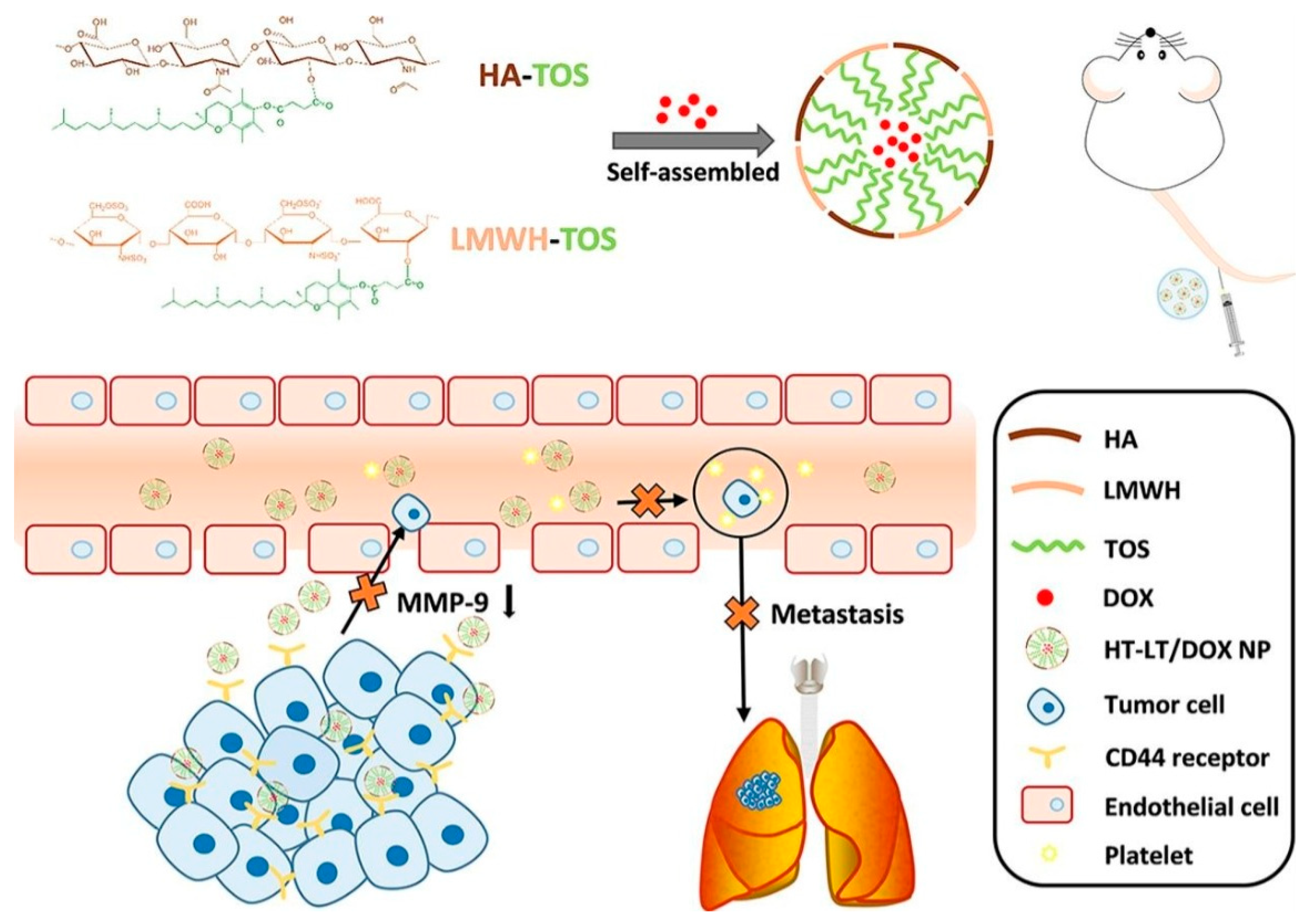
- Chai, Z.; Teng, C.; Yang, L.; Ren, L.; Yuan, Z.; Xu, S.; Cheng, M.; Wang, Y.; Yan, Z.; Qin, C.; et al. Doxorubicin delivered by redox-responsive hyaluronic acid–ibuprofen prodrug micelles for treatment of metastatic breast cancer. Carbohydr. Polym. 2020, 245, 116527. [Google Scholar] [CrossRef]
- Yang, Y.; Long, Y.; Wang, Y.; Ren, K.; Li, M.; Zhang, Z.; Xiang, B.; He, Q. Enhanced anti-tumor and anti-metastasis therapy for triple negative breast cancer by cd44 receptor-targeted hybrid self-delivery micelles. Int. J. Pharm. 2020, 577, 119085. [Google Scholar] [CrossRef]
- Cai, Y.; Xu, Z.; Shuai, Q.; Zhu, F.; Xu, J.; Gao, X.; Sun, X. Tumor-targeting peptide functionalized peg-pla micelles for efficient drug delivery. Biomater. Sci. 2020, 8, 2274–2282. [Google Scholar] [CrossRef]
- Chu, H.-L.; Cheng, T.-M.; Chen, H.-W.; Chou, F.-H.; Chang, Y.-C.; Lin, H.-Y.; Liu, S.-Y.; Liang, Y.-C.; Hsu, M.-H.; Wu, D.-S.; et al. Synthesis of apolipoprotein b lipoparticles to deliver hydrophobic/amphiphilic materials. ACS Appl. Mater. Interfaces 2013, 5, 7509–7516. [Google Scholar] [CrossRef]
- Emami, J.; Kazemi, M.; Hasanzadeh, F.; Minaiyan, M.; Mirian, M.; Lavasanifar, A. Novel pH-triggered biocompatible polymeric micelles based on heparin–α-tocopherol conjugate for intracellular delivery of docetaxel in breast cancer. Pharm. Dev. Technol. 2020, 25, 492–509. [Google Scholar] [CrossRef]
- Xu, C.; Xu, J.; Zheng, Y.; Fang, Q.; Lv, X.; Wang, X.; Tang, R. Active-targeting and acid-sensitive pluronic prodrug micelles for efficiently overcoming mdr in breast cancer. J. Mater. Chem. B 2020, 8, 2726–2737. [Google Scholar] [CrossRef]
- Gener, P.; Montero, S.; Xandri-Monje, H.; Díaz-Riascos, Z.V.; Rafael, D.; Andrade, F.; Martínez-Trucharte, F.; González, P.; Seras-Franzoso, J.; Manzano, A.; et al. Zileuton™ loaded in polymer micelles effectively reduce breast cancer circulating tumor cells and intratumoral cancer stem cells. Nanotechnol. Biol. Med. 2020, 24, 102106. [Google Scholar] [CrossRef] [PubMed]
- Mehnath, S.; Chitra, K.; Karthikeyan, K.; Jeyaraj, M. Localized delivery of active targeting micelles from nanofibers patch for effective breast cancer therapy. Int. J. Pharm. 2020, 584, 119412. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Alakhova, D.Y.; Zhao, X.; Band, V.; Batrakova, E.V.; Kabanov, A.V. Eradication of cancer stem cells in triple negative breast cancer using doxorubicin/pluronic polymeric micelles. Nanotechnol. Biol. Med. 2020, 24, 102124. [Google Scholar] [CrossRef]
- Weissig, V. Liposomes came first: The early history of liposomology. In Liposomes; Springer: Berlin, Germany, 2017; pp. 1–15. [Google Scholar]
- Gu, Z.; Da Silva, C.G.; Van der Maaden, K.; Ossendorp, F.; Cruz, L.J. Liposome-based drug delivery systems in cancer immunotherapy. Pharmaceutics 2020, 12, 1054. [Google Scholar] [CrossRef] [PubMed]
- Ni, C.; Fang, J.; Qian, H.; Xu, Q.; Shen, F. Liposomal doxorubicin-related palmar–plantar erythrodysesthesia (hand–foot syndrome): A case report. J. Int. Med. Res. 2020, 48, 0300060520974854. [Google Scholar] [CrossRef] [PubMed]
- He, K.; Tang, M. Safety of novel liposomal drugs for cancer treatment: Advances and prospects. Chem. Biol. Interact. 2018, 295, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Filipczak, N.; Pan, J.; Yalamarty, S.S.K.; Torchilin, V.P. Recent advancements in liposome technology. Adv. Drug Deliv. Rev. 2020, 156, 4–22. [Google Scholar] [CrossRef]
- Bangale, G.; Rajesh, K.; Shinde, G. Stealth liposomes: A novel approach of targeted drug delivery in cancer therapy. Int. J. Pharm. Sci. Res. 2014, 5, 750–759. [Google Scholar]
- Liu, Y.; Gao, D.; Zhang, X.; Liu, Z.; Dai, K.; Ji, B.; Wang, Q.; Luo, L. Antitumor drug effect of betulinic acid mediated by polyethylene glycol modified liposomes. Mater. Sci. Eng. C 2016, 64, 124–132. [Google Scholar] [CrossRef]
- Veronese, F.M.; Harris, J.M. Introduction and overview of peptide and protein pegylation. Adv. Drug Deliv. Rev. 2002, 54, 453–456. [Google Scholar]
- Bulbake, U.; Doppalapudi, S.; Kommineni, N.; Khan, W. Liposomal formulations in clinical use: An updated review. Pharmaceutics 2017, 9, 12. [Google Scholar] [CrossRef] [PubMed]
- Soares, S.; Sousa, J.; Pais, A.; Vitorino, C. Nanomedicine: Principles, properties, and regulatory issues. Front. Chem. 2018, 6, 360. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.C.; Huang, L. Non-viral vector as vaccine carrier. Adv. Genet. 2005, 54, 315–337. [Google Scholar]
- Alberts, D.S.; Muggia, F.M.; Carmichael, J.; Winer, E.P.; Jahanzeb, M.; Venook, A.P.; Skubitz, K.M.; Rivera, E.; Sparano, J.A.; Dibella, N.J. Seminars in oncology. In Efficacy and Safety of Liposomal Anthracyclines in Phase i/II Clinical Trials; Elsevier: Amsterdam, The Netherlands, 2004; pp. 53–90. [Google Scholar]
- Schwendener, R.A. Liposomes as vaccine delivery systems: A review of the recent advances. Ther. Adv. Vaccines 2014, 2, 159–182. [Google Scholar] [CrossRef] [PubMed]
- Leoncini, E.; Ricciardi, W.; Cadoni, G.; Arzani, D.; Petrelli, L.; Paludetti, G.; Brennan, P.; Luce, D.; Stucker, I.; Matsuo, K. Adult height and head and neck cancer: A pooled analysis within the inhance consortium. Eur. J. Epidemiol. 2014, 29, 35–48. [Google Scholar] [CrossRef] [Green Version]
- Schwendener, R.A.; Ludewig, B.; Cerny, A.; Engler, O. Liposome-based vaccines. In Liposomes; Springer: Berlin, Germany, 2010; pp. 163–175. [Google Scholar]
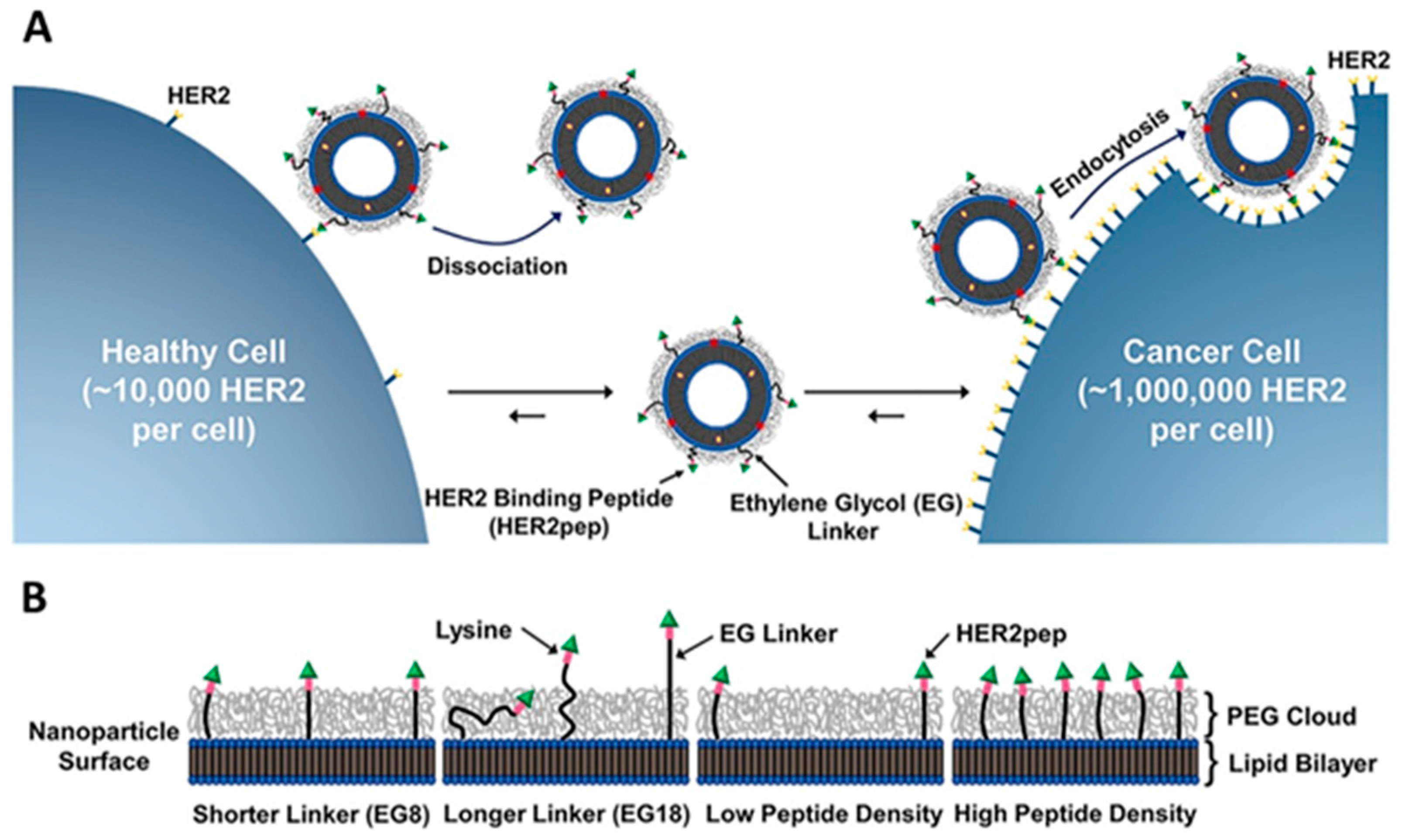
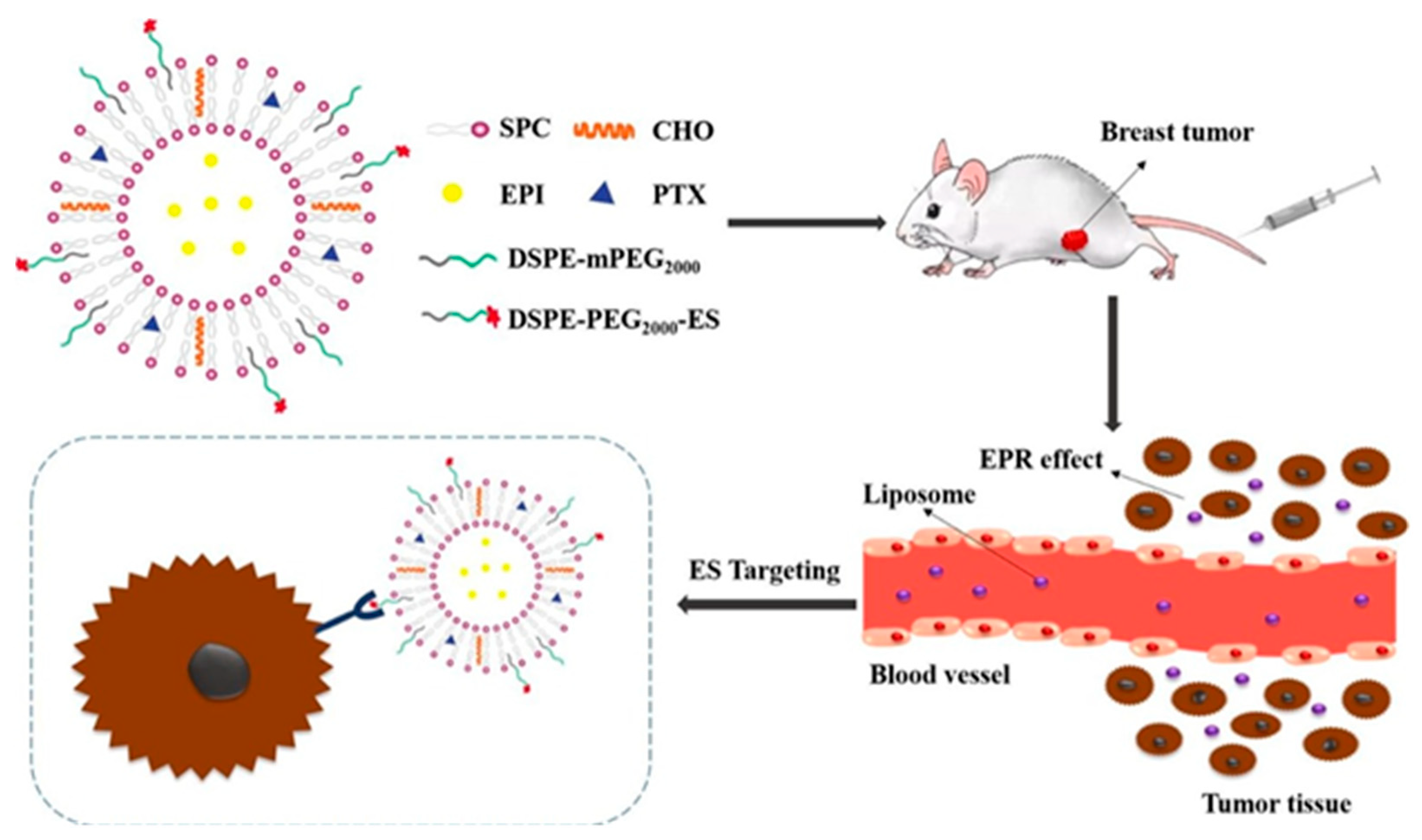
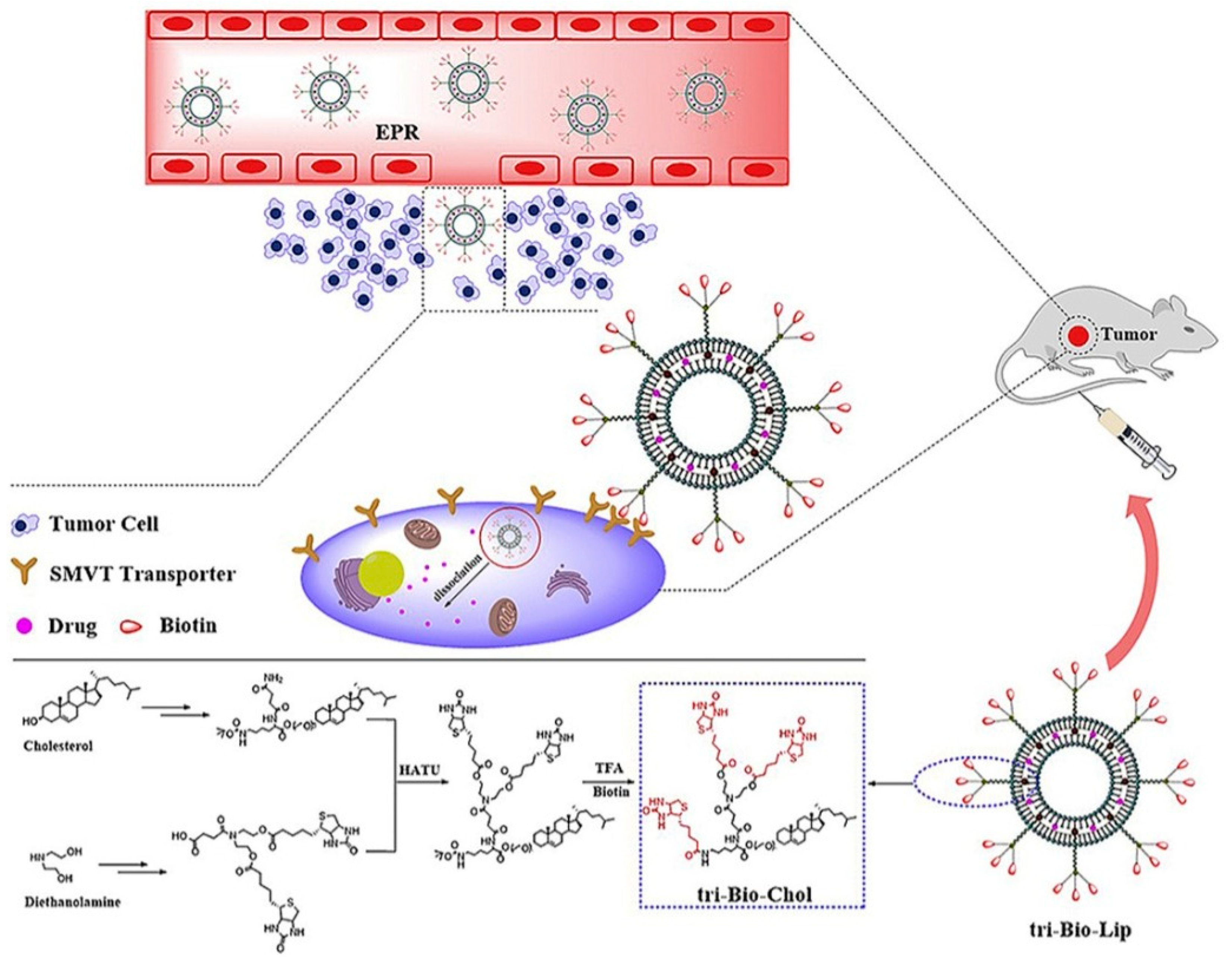
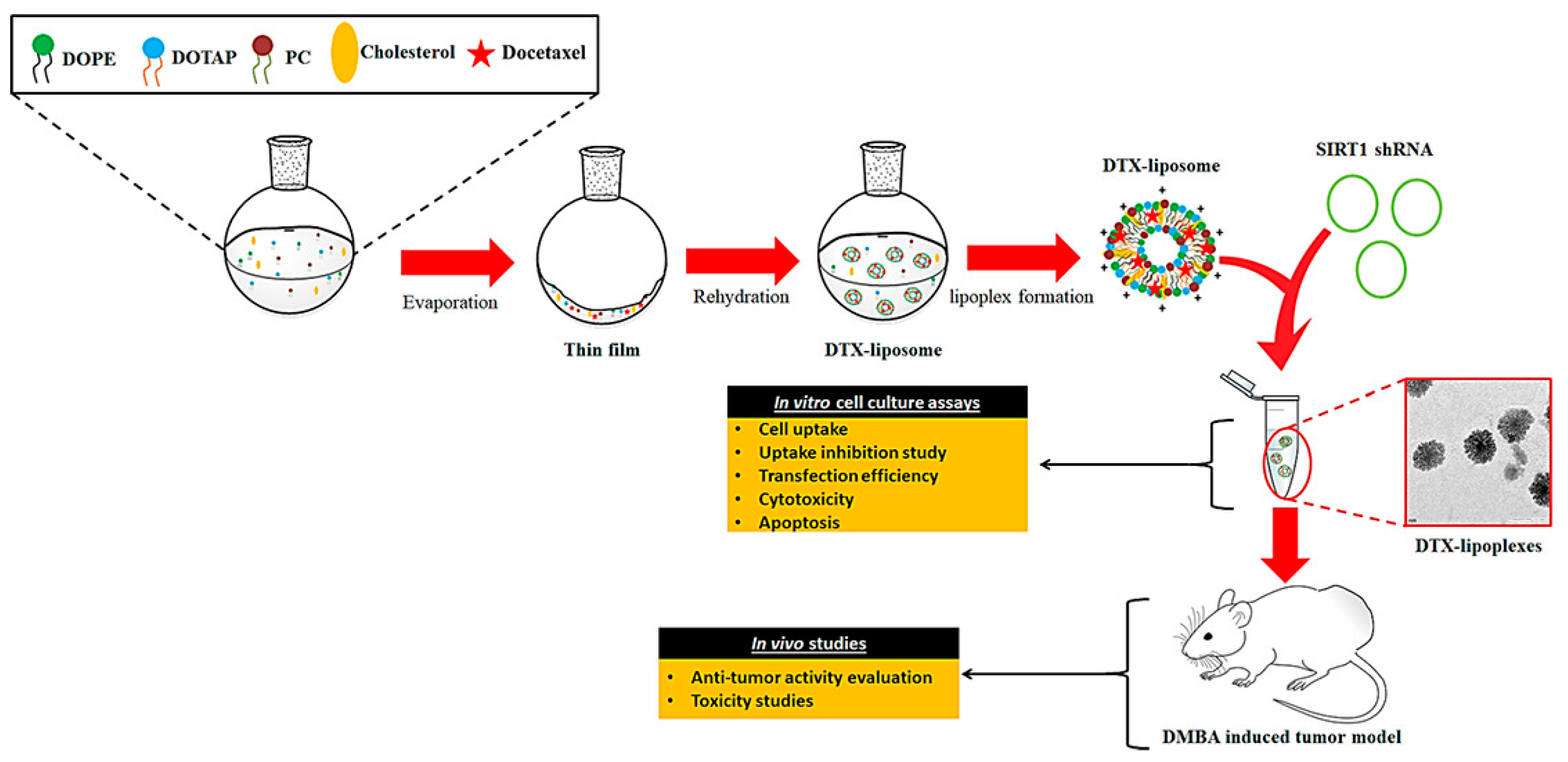

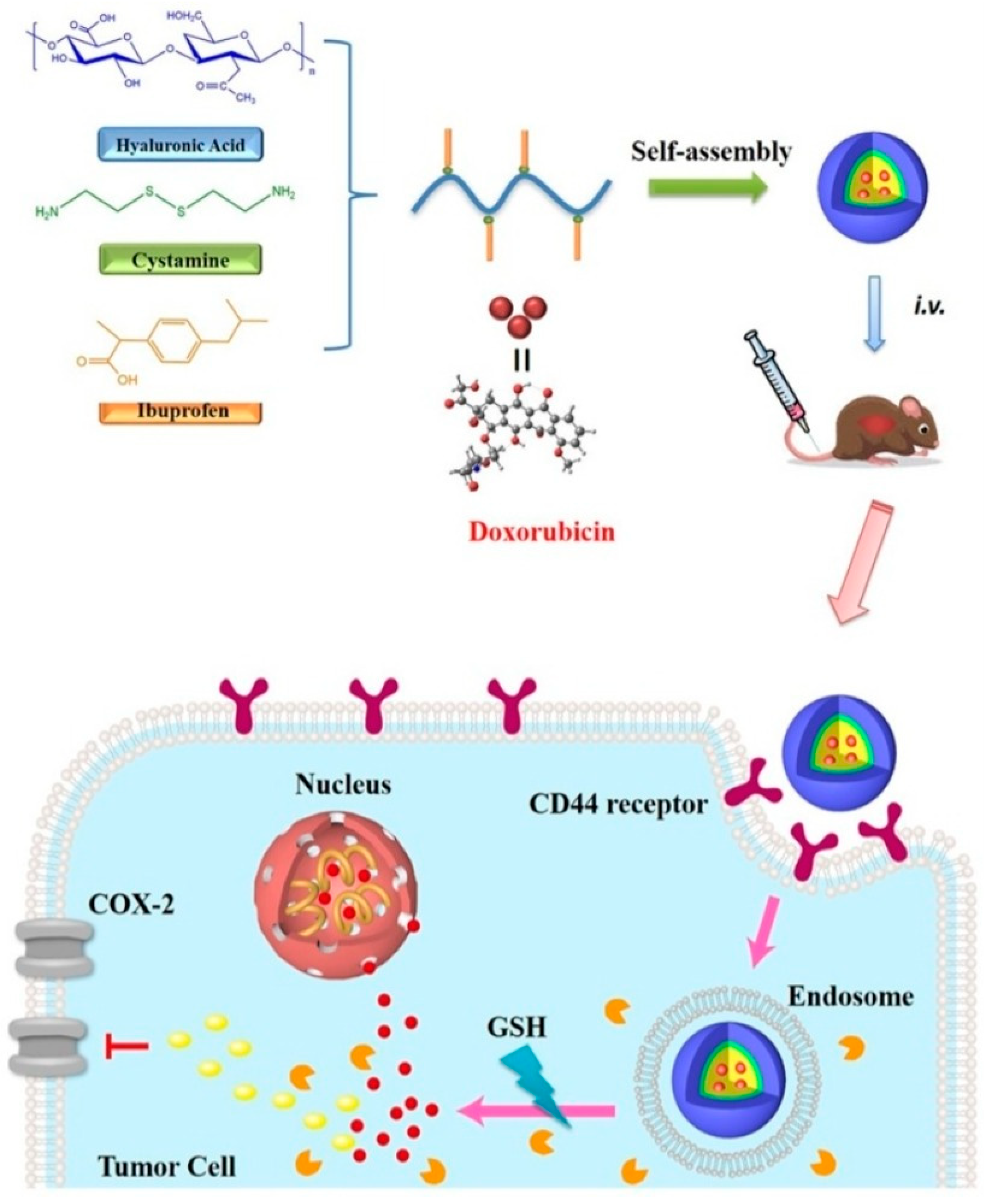
| Vesicular System | Drug/s/API/s | Pathway/Receptor/Targeting Moiety/ Overexpressed Factors/Mode of Action | Progress | Reference |
|---|---|---|---|---|
| Ligand modified liposome | PTX | SMVTs receptors/biotin and glucose targeting complex | in vitro and in vivo | [91] |
| Peptide Based liposome | PTX | Folate receptors/Glutamic hexapeptide-folic acid-targeting complex | in vitro and in vivo | [92] |
| Aptamer Based liposome | DOX | Forkhead box M1 (FOXM1) aptamer | in vitro and in vivo | [93] |
| Peptide Based liposome | PTX | Glutamic oligopeptide- RGD Peptide targeting moiety | in vitro and in vivo | [94] |
| Hydrophobic peptide-based liposome | DOX | Hydrophobic penetrating peptide PFVYL1 targeting moiety | in vitro and in vivo | [95] |
| HA modified Cationic liposome | Honokiol (HNK) | CD44 receptors/HA-liposome-HNK targeting and therapeutic complex | in vitro and in vivo | [96] |
| Drug-in-cyclodextrin in-liposome (DCL) | 17β-Estradiol | Membrane Isolated Steroid Signaling (MISS) pathway/Estrogen α receptors | in vitro and in vivo | [97] |
| Aptamer based liposome | DOX | AS1411 targeting nucleotide/P-glycoprotein (P-gp) overexpression | in vitro and in vivo | [98] |
| Drug based liposome | Tamoxifen and Raloxifene | Estrogen and progesterone receptors | in vitro and in vivo | [99] |
| Drug based liposome | DOX | Protoporphyrin IX nucleus targeting complex | in vitro and in vivo | [100] |
| API based liposome | N-alkylIsatin | SerpinB2 inhibitor/uPA/uPAR receptors and targeting ligand | in vitro and in vivo | [101] |
| Drug and API based liposome | PTX, Cetuximab, and Piperine | EGFR inhibition pathways/EGFR receptors | in vitro | [102] |
| Vesicular System | Drug/s/API/s/RNA/DNA | Pathway/Receptor/Targeting Moiety/ Overexpressed Factors/Mode of Action | Progress | Reference |
|---|---|---|---|---|
| Drug based exosome | Erastin | Folate overexpressed receptors | in vitro | [111] |
| Tumor cells derived exosomes | PTX/ Linoleic acid/Cucurbutacin B | CD44 and CD47 receptors | in vitro and in vivo | [112] |
| MSCs derived exosomes | MicroRNA | LNA-antimiR-142-3p targeting microRNA | in vitro | [113] |
| Vesicular System | Drug/s/API/s | Pathway/Receptor/ Targeting Moiety/ Overexpressed Factors/ Mode of Action | Progress | Reference |
|---|---|---|---|---|
| Drug loaded micelle | PTX | F3 targeting peptide/nucleolin overexpression | in vitro and in vivo | [120] |
| Lipoprotein based micelle | Tetra-O-methyl nordihydroguaiaretic acid (M4N) | LDL receptor, Apolipoprotein B targeting moiety | in vitro and in vivo | [121] |
| Drug loaded micelle | DTX, Coumarin, Taxotere® | Heparin targeting complex and pH based drug delivery system | in vitro and in vivo | [122] |
| Polymeric prodrug micelle | DOX -P 123 (prodrugs group) | Phenylboric acid-modified F127 tumor-targeting copolymer | in vitro and in vivo | [123] |
| Drug loaded micelle | Zileuton™ | ALOX5 pathway inhibitor of cancer stem cells | in vitro and in vivo | [124] |
| Stimuli-responsive nano polymeric micelle | PTX | Stimuli-responsive nano polymeric micelle targeting complex | in vitro and ex vivo | [125] |
| Drug based polymeric micelle | DOX | CD44 and CD24 receptors/ polymeric targeting complex | in vitro and in vivo | [126] |
| Products | Stage | References |
|---|---|---|
| Liposome-stabilized prostate cancer vaccine | Phase I trials | [137] |
| Liposome-lipid A-prostate-specific antigen formulation | Phase II trials | [137] |
| Liposomal anthracyclines (pegylated liposomal doxorubicin, nonpegylated liposomal doxorubicin, and liposomal daunorubicin) | Phase I and phase II clinical trials | [138] |
| CAF01-adjuvant liposomes as vaccine formulation | Phase I trial | [139] |
| Vascular targeting cationic liposomes encapsulating paclitaxel (EndoTAG-1 [ET]) for human HNSCC (head and neck squamous cell carcinoma). | Phase I/II clinical trial | [140] |
| The formulation of liposomal peptides vaccines or plasmid–DNA vaccines | in vivo antigen loading | [141] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rethi, L.; Mutalik, C.; Anurogo, D.; Lu, L.-S.; Chu, H.-Y.; Yougbaré, S.; Kuo, T.-R.; Cheng, T.-M.; Chen, F.-L. Lipid-Based Nanomaterials for Drug Delivery Systems in Breast Cancer Therapy. Nanomaterials 2022, 12, 2948. https://doi.org/10.3390/nano12172948
Rethi L, Mutalik C, Anurogo D, Lu L-S, Chu H-Y, Yougbaré S, Kuo T-R, Cheng T-M, Chen F-L. Lipid-Based Nanomaterials for Drug Delivery Systems in Breast Cancer Therapy. Nanomaterials. 2022; 12(17):2948. https://doi.org/10.3390/nano12172948
Chicago/Turabian StyleRethi, Lekshmi, Chinmaya Mutalik, Dito Anurogo, Long-Sheng Lu, Hsiu-Yi Chu, Sibidou Yougbaré, Tsung-Rong Kuo, Tsai-Mu Cheng, and Fu-Lun Chen. 2022. "Lipid-Based Nanomaterials for Drug Delivery Systems in Breast Cancer Therapy" Nanomaterials 12, no. 17: 2948. https://doi.org/10.3390/nano12172948
APA StyleRethi, L., Mutalik, C., Anurogo, D., Lu, L.-S., Chu, H.-Y., Yougbaré, S., Kuo, T.-R., Cheng, T.-M., & Chen, F.-L. (2022). Lipid-Based Nanomaterials for Drug Delivery Systems in Breast Cancer Therapy. Nanomaterials, 12(17), 2948. https://doi.org/10.3390/nano12172948









