Insight into the Effect of Selenization Temperature for Highly Efficient Ni-Doped Cu2ZnSn(S,Se)4 Solar Cells
Abstract
:1. Introduction
2. Experimental
2.1. Preparation of CNZTSSe Thin Films
2.2. Preparation of CNZTSSe Devices
2.3. Characterization
3. Results and Discussion
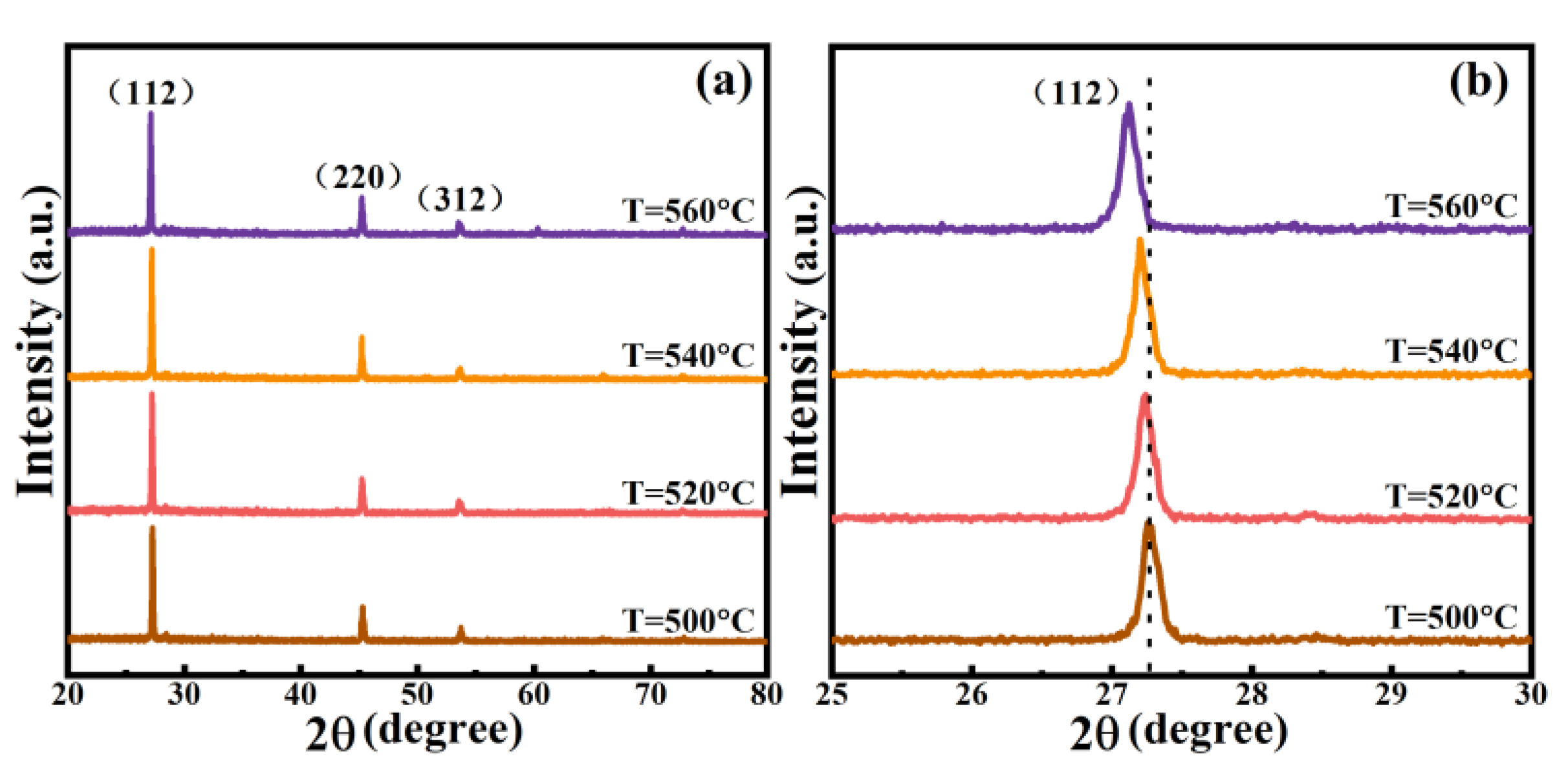
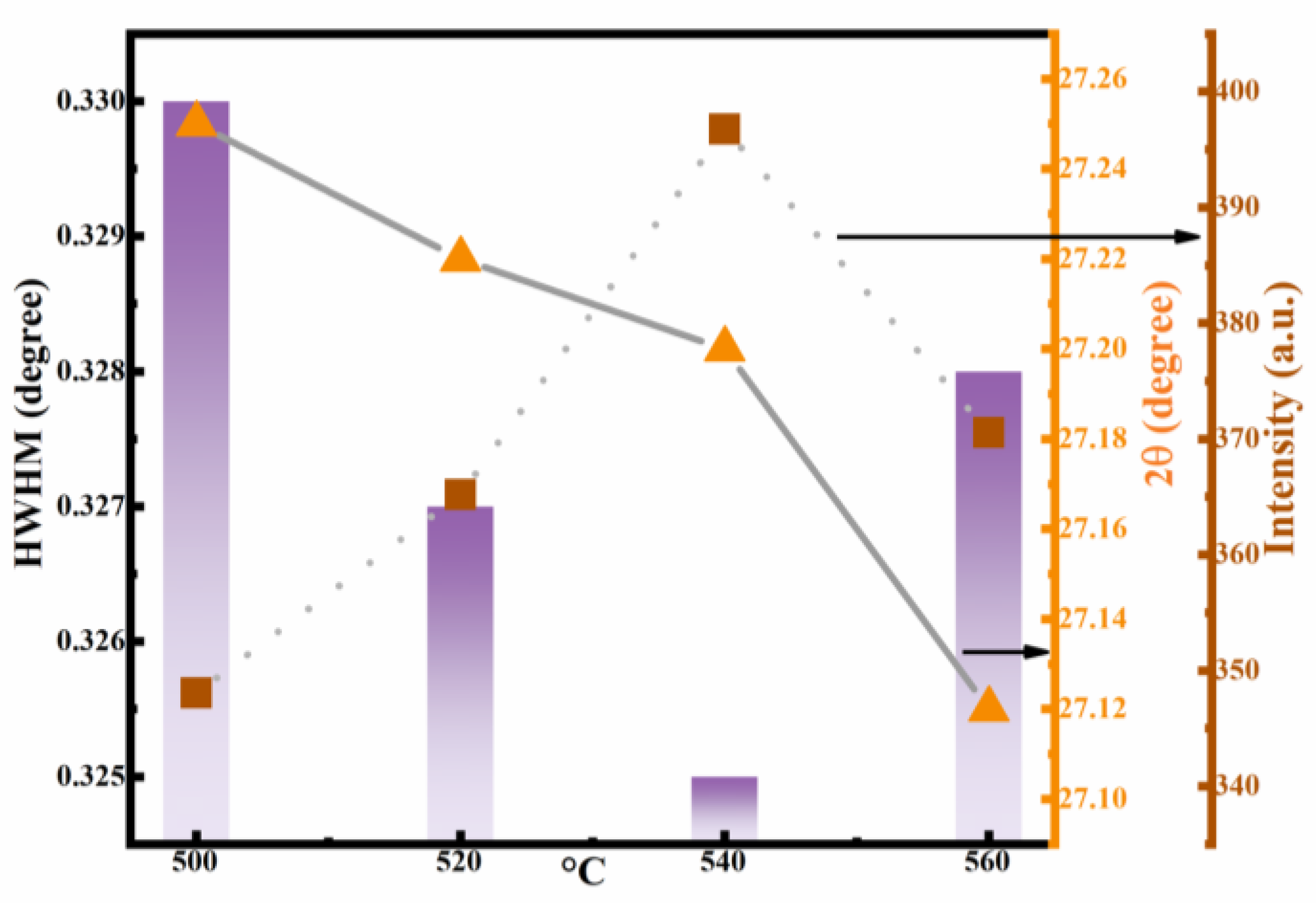
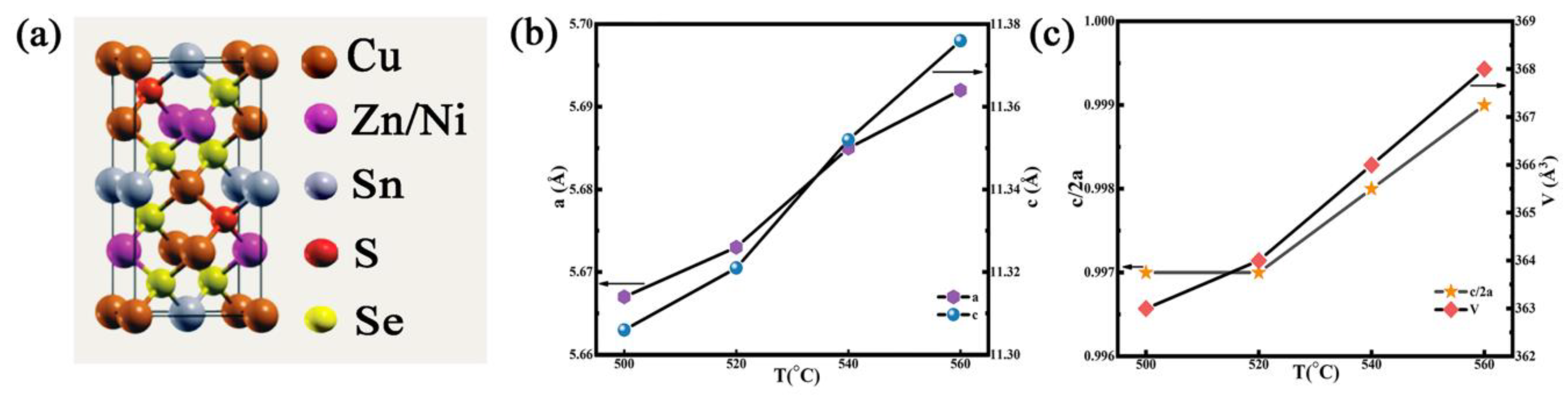
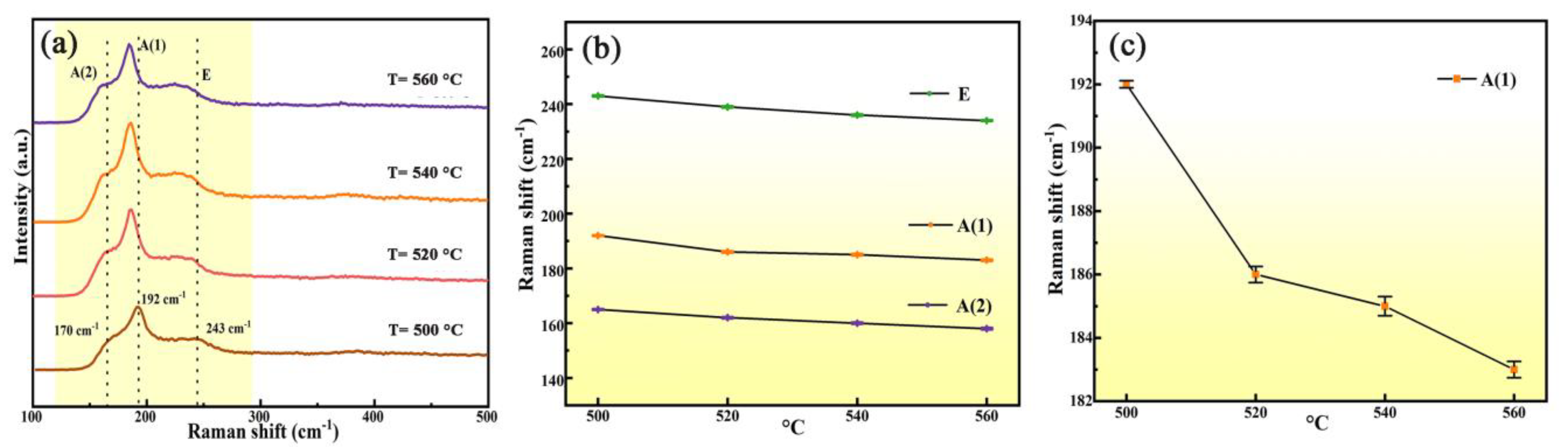
3.1. Structure and Morphology of Thin Films
3.2. Optical and Electrical Performances of Thin Films
3.3. Device Performance
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Yu, Q.; Shi, J.J.; Guo, L.B.; Duan, B.W.; Luo, Y.H.; Wu, H.J.; Li, D.M.; Meng, Q.B. Eliminating multi-layer crystallization of Cu2ZnSn(S,Se)4 absorber by controlling back interface reaction. Nano Energy 2020, 76, 105042. [Google Scholar] [CrossRef]
- Su, Z.H.; Liang, G.X.; Fan, P.; Luo, J.T.; Zheng, Z.H.; Xie, Z.G.; Wang, W.; Chen, S.; Hu, J.G.; Wei, Y.D.; et al. Device Postannealing Enabling over 12% Efficient Solution-Processed Cu2ZnSn(S,Se)4 Solar Cells with Cd(2+) Substitution. Adv. Mater. 2020, 32, 12. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.J.; Son, D.H.; Sung, S.J.; Sim, J.H.; Kim, Y.I.; Park, S.N.; Jeon, D.H.; Kim, J.; Hwang, D.K.; Jeon, C.W.; et al. A band-gap-graded CZTSSe solar cell with 12.3% efficiency. J. Mater. Chem. A 2016, 4, 10151–10158. [Google Scholar] [CrossRef]
- Tiwari, D.; Koehler, T.; Lin, X.; Harniman, R.; Griffiths, I.; Wang, L.; Cherns, D.; Klenk, R.; Fermin, D.J. Cu2ZnSnS4 Thin Films Generated from a Single Solution Based Precursor: The Effect of Na and Sb Doping. Chem. Mater. 2016, 28, 4991–4997. [Google Scholar] [CrossRef]
- Sengar, B.S.; Garg, V.; Kumar, A.; Awasthi, V.; Kumar, S.; Atuchin, V.V.; Mukherjee, S. Band alignment of Cd-free (Zn,Mg)O layer with Cu2 ZnSn(S,Se)4 and its effect on the photovoltaic properties. Opt. Mater. 2018, 84, 748–756. [Google Scholar] [CrossRef]
- Sengar, B.S.; Garg, V.; Siddharth, G.; Kumar, A.; Pandey, S.K.; Dubey, M.; Atuchin, V.V.; Kumar, S.; Mukherjee, S. Improving the Cu2ZnSn(S,Se)4—Based Photovoltaic Conversion Efficiency by Back-Contact Modification. IEEE Trans. Electron. Devices 2021, 68, 2748–2752. [Google Scholar] [CrossRef]
- Williams, B.A.; Smeaton, M.A.; Trejo, N.D.; Francis, L.F.; Aydil, E.S. Effect of Nanocrystal Size and Carbon on Grain Growth during Annealing of Copper Zinc Tin Sulfide Nanocrystal Coatings. Chem. Mater. 2017, 29, 1676–1683. [Google Scholar] [CrossRef]
- Ki, W.; Hillhouse, H.W. Earth-Abundant Element Photovoltaics Directly from Soluble Precursors with High Yield Using a Non-Toxic Solvent. Adv. Energy Mater. 2011, 1, 732–735. [Google Scholar] [CrossRef]
- Shin, D.; Saparov, B.; Mitzi, D.B. Defect Engineering in Multinary Earth-Abundant Chalcogenide Photovoltaic Materials. Adv. Energy Mater. 2017, 7, 1602366. [Google Scholar] [CrossRef]
- Leitao, J.P.; Santos, N.M.; Fernandes, P.A.; Salome, P.M.P.; da Cunha, A.F.; Gonzalez, J.C.; Ribeiro, G.M.; Matinaga, F.M. Photoluminescence and electrical study of fluctuating potentials in Cu2ZnSnS4-based thin films. Phys. Rev. B 2011, 84, 024120. [Google Scholar] [CrossRef] [Green Version]
- Gokmen, T.; Gunawan, O.; Todorov, T.K.; Mitzi, D.B. Band tailing and efficiency limitation in kesterite solar cells. Appl. Phys. Lett. 2013, 103, 103506. [Google Scholar] [CrossRef]
- Teixeira, J.P.; Sousa, R.A.; Sousa, M.G.; da Cunha, A.F.; Fernandes, P.A.; Salome, P.M.P.; Gonzalez, J.C.; Leitao, J.P. Comparison of fluctuating potentials and donor-acceptor pair transitions in a Cu-poor Cu2ZnSnS4 based solar cell. Appl. Phys. Lett. 2014, 105, 163901. [Google Scholar] [CrossRef]
- Guchhait, A.; Su, Z.; Tay, Y.F.; Shukla, S.; Li, W.J.; Leow, S.W.; Tan, J.M.R.; Lie, S.; Gunawan, O.; Wong, L.H. Enhancement of Open-Circuit Voltage of Solution-Processed Cu2ZnSnS4 Solar Cells with 7.2% Efficiency by Incorporation of Silver. ACS Energy Lett. 2016, 1, 1256–1261. [Google Scholar] [CrossRef]
- Zeng, F.C.; Sui, Y.R.; Ma, M.L.; Zhao, N.; Wang, T.Y.; Yang, L.L.; Wang, Z.W.; Wang, F.Y.; Yao, B. Strengthening the Properties of Earth-Abundant Cu2ZnSn(S,Se)4 Photovoltaic Materials via Cation Incorporation with Ni. ACS Appl. Energy Mater. 2022, 5, 1271–1281. [Google Scholar] [CrossRef]
- Li, X.L.; Hou, Z.F.; Gao, S.S.; Zeng, Y.; Ao, J.P.; Zhou, Z.Q.; Da, B.; Liu, W.; Sun, Y.; Zhang, Y. Efficient Optimization of the Performance of Mn2+-Doped Kesterite Solar Cell: Machine Learning Aided Synthesis of High Efficient Cu2(Mn,Zn)Sn(S,Se)(4) Solar Cells. Sol. RRL 2018, 2, 1800198. [Google Scholar] [CrossRef]
- Guo, H.L.; Wang, G.; Meng, R.T.; Sun, Y.L.; Wang, S.Y.; Zhang, S.L.; Wu, J.Y.; Wu, L.; Liang, G.X.; Li, H.; et al. An efficient Li+-doping strategy to optimize the band alignment of a Cu2ZnSn(S,Se)(4)/CdS interface by a Se&LiF co-selenization process. Adv. Energy Mater. 2015, 5, 1500682. [Google Scholar]
- Su, Z.H.; Tan, J.M.R.; Li, X.L.; Zeng, X.; Batabyal, S.K.; Wong, L.H. Cation Substitution of Solution-Processed Cu2ZnSnS4 Thin Film Solar Cell with over 9% Efficiency. J. Mater. Chem. A 2020, 8, 22065–22074. [Google Scholar]
- Kim, K.; Kim, D.; Ahn, K.J.; Jeong, C. Effects of Sulfurization Temperature on Cu(In, Ga)S2 Thin Film Solar Cell Performance by Rapid Thermal Process. J. Nanosci. Nanotechnol. 2016, 16, 4856–4859. [Google Scholar] [CrossRef]
- Chen, G.L.; Wang, W.H.; Zhang, B.Y.; Chen, S.Y.; Huang, Z.G.; Zhuang, B. Influence of selenization atmosphere on the Cu2ZnSn(S,Se)(4) thin films and its correlation to the performance of solar cells. Mater. Res. Bull. 2017, 94, 164–169. [Google Scholar] [CrossRef]
- Chen, G.; Li, J.; Wu, M.; Liu, J.; Lai, F.; Zhu, C. Effect of post sulfurization temperature on the microstructure of Cu2ZnSn(S,Se)(4) thin film. Mater. Lett. 2015, 159, 32–34. [Google Scholar] [CrossRef]
- Kamoun, N.; Bouzouita, H.; Rezig, B. Fabrication and characterization of Cu2ZnSnS4 thin films deposited by spray pyrolysis technique. Thin Solid Film. 2007, 515, 5949–5952. [Google Scholar] [CrossRef]
- Sardashti, K.; Haight, R.; Gokmen, T.; Wang, W.; Chang, L.Y.; Mitzi, D.B.; Kummel, A.C. Impact of Nanoscale Elemental Distribution in High-Performance Kesterite Solar Cells. Adv. Energy Mater. 2015, 5, 1402180. [Google Scholar] [CrossRef]
- Jo, E.; Gang, M.G.; Shim, H.; Suryawanshi, M.P.; Ghorpade, U.V.; Kim, J.H. 8% Efficiency Cu2ZnSn(S,Se)(4) (CZTSSe) Thin Film Solar Cells on Flexible and Lightweight Molybdenum Foil Substrates. ACS Appl. Mater. Interfaces 2019, 11, 23118–23124. [Google Scholar] [CrossRef]
- Zhang, Y.B.; Sun, X.D.; Zhang, P.H.; Yuan, X.; Huang, F.Q.; Zhang, W.Q. Structural properties and quasiparticle band structures of Cu-based quaternary semiconductors for photovoltaic applications. J. Appl. Phys. 2012, 111, 063709. [Google Scholar] [CrossRef]
- Fu, J.J.; Fu, J.; Tian, Q.W.; Wang, H.L.; Zhao, F.M.; Kong, J.; Zhao, X.Y.; Wu, S.X. Tuning the Se Content in Cu2ZnSn(S,Se)(4) Absorber to Achieve 9.7% Solar Cell Efficiency from a Thiol/Amine-Based Solution Process. ACS Appl. Energy Mater. 2018, 1, 594–601. [Google Scholar] [CrossRef]
- Kokh, K.A.; Atuchin, V.V.; Adichtchev, S.V.; Gavrilova, T.A.; Bakhadur, A.M.; Klimov, A.O.; Korolkov, I.V.; Kuratieva, N.V.; Mukherjee, S.; Pervukhina, N.V.; et al. Cu2ZnSnS4 crystal growth using a SnCl2 based flux. CrystEngComm 2021, 23, 1025–1032. [Google Scholar] [CrossRef]
- Zeng, F.C.; Sui, Y.R.; Wu, Y.J.; Jiang, D.Y.; Wang, Z.W.; Wang, F.Y.; Yao, B.; Yang, L.L. Structural, optical and electrical properties of indium doped Cu2ZnSn(S, Se)(4) thin films synthesized by the DC and RF reactive magnetron cosputtering. Ceram. Int. 2021, 47, 18376–18384. [Google Scholar] [CrossRef]
- Bakhadur, A.M.; Uralbekov, B.M.; Atuchin, V.V.; Mukherjee, S.; Kokh, K.A. Single-phase CZTSe via isothermal recrystallization in a KI–KCl flux. CrystEngComm 2022, 24, 2291–2296. [Google Scholar] [CrossRef]
- Singh, A.; Geaney, H.; Laffir, F.; Ryan, K.M. Colloidal Synthesis of Wurtzite Cu2ZnSnS4 Nanorods and Their Perpendicular Assembly. J. Am. Chem. Soc. 2012, 134, 2910–2913. [Google Scholar] [CrossRef]
- Qi, Y.F.; Liu, Y.; Kou, D.X.; Zhou, W.H.; Zhou, Z.J.; Tian, Q.W.; Yuan, S.J.; Meng, Y.N.; Wu, S.X. Enhancing Grain Growth for Efficient Solution-Processed (Cu,Ag)(2)ZnSn(S,Se)(4) Solar Cells Based on Acetate Precursor. ACS Appl. Mater. Interfaces 2020, 12, 14213–14223. [Google Scholar] [CrossRef]
- Qi, Y.F.; Zhao, X.Y.; Liu, Y.; Kou, D.X.; Zhou, W.H.; Zhou, Z.J.; Yuan, S.J.; Meng, Y.N.; Wu, S.X. Synergistic effect of Mn on bandgap fluctuations and surface electrical characteristics in Ag-based Cu2ZnSn(S,Se)(4) solar cells. J. Mater. Chem. A 2021, 9, 2292–2300. [Google Scholar] [CrossRef]
- Atuchin, V.V.; Kesler, V.G.; Ursaki, V.V.; Tezlevan, V.E. Electronic structure of HgGa2S4. Solid State Commun. 2006, 138, 250–254. [Google Scholar] [CrossRef]
- Atuchin, V.V.; Kesler, V.G.; Parassyuk, O.V. Electronic structure of AgCd2 GaS4. Surf. Rev. Lett. 2007, 14, 403–409. [Google Scholar] [CrossRef]
- Atuchin, V.V.; Isaenko, L.I.; Kesler, V.G.; Lobanov, S.I. Core level photoelectron spectroscopy of LiGaS2 and Ga–S bonding in complex sulfides. J. Alloy. Compd. 2010, 497, 244–248. [Google Scholar] [CrossRef]
- Liu, W.C.; Guo, B.L.; Wu, X.S.; Zhang, F.M.; Mak, C.L.; Wong, K.H. Facile hydrothermal synthesis of hydrotropic Cu2ZnSnS4 nanocrystal quantum dots: Band-gap engineering and phonon confinement effect. J. Mater. Chem. A 2013, 1, 3182–3186. [Google Scholar] [CrossRef]
- Li, J.J.; Huang, Y.C.; Huang, J.L.; Liang, G.X.; Zhang, Y.X.; Rey, G.; Guo, F.; Su, Z.H.; Zhu, H.B.; Cai, L.L.; et al. Defect Control for 12.5% Efficiency Cu2ZnSnSe4 Kesterite Thin-Film Solar Cells by Engineering of Local Chemical Environment. Adv. Mater. 2020, 32, 2005268. [Google Scholar] [CrossRef]
- Atuchin, V.V.; Golyashov, V.A.; Kokh, K.A.; Korolkov, I.V.; Kozhukhov, A.S.; Kruchinin, V.N.; Makarenko, S.V.; Pokrovsky, L.D.; Prosvirin, I.P.; Romanyuk, K.N.; et al. Formation of Inert Bi2Se3 (0001) Cleaved Surface. Cryst. Growth Des. 2011, 11, 5507–5514. [Google Scholar] [CrossRef]
- Atuchin, V.V.; Isaenko, L.I.; Lobanov, S.I.; Goloshumova, A.A.; Molokeev, M.S.; Zhang, Z.M.; Zhang, X.Y.; Jiang, X.X.; Lin, Z.S. Anisotropic Thermal Expansion and Electronic Structure of LiInSe2. Molecules 2022, 27, 5078. [Google Scholar] [CrossRef]
- Thompson, M.J.; Blakeney, K.J.; Cady, S.D.; Reichert, M.D.; Del Pilar-Albaladejo, J.; White, S.T.; Vela, J. Cu2ZnSnS4 Nanorods Doped with Tetrahedral, High Spin Transition Metal Ions: Mn2+, Co2+ and Ni2+. Chem. Mater. 2016, 28, 1668–1677. [Google Scholar] [CrossRef]
- Matsumura, Y.; Enomoto, Y.; Sugiyama, M.; Akamatsu, K.; Nawafune, H. Direct metallization of nickel on polymeric template patterns for fabrication of copper circuits on glass substrates. J. Mater. Chem. 2008, 18, 5078–5082. [Google Scholar] [CrossRef]
- Li, Y.F.; Deng, R.; Yao, B.; Xing, G.Z.; Wang, D.D.; Wu, T. Tuning ferromagnetism in MgxZn1-xO thin films by band gap and defect engineering. Appl. Phys. Lett. 2010, 97, 102506. [Google Scholar] [CrossRef]
- Atuchin, V.V.; Isaenko, L.I.; Kesler, V.G.; Lin, Z.S.; Molokeev, M.S.; Yelisseyev, A.P.; Zhurkov, S.A. Exploration on anion ordering, optical properties and electronic structure in K3WO3F3 elpasolite. J. Solid State Chem. 2012, 187, 159–164. [Google Scholar] [CrossRef]
- Mudavakkat, V.H.; Atuchin, V.V.; Kruchinin, V.N.; Kayani, A.; Ramana, C.V. Structure, morphology and optical properties of nanocrystalline yttrium oxide (Y2O3) thin films. Opt. Mater. 2012, 34, 893–900. [Google Scholar] [CrossRef]
- Ji, H.; Huang, Z.; Xia, Z.; Molokeev, M.S.; Jiang, X.; Lin, Z.; Atuchin, V.V. Comparative investigations of the crystal structure and photoluminescence property of eulytite-type Ba3Eu(PO4)3 and Sr3Eu(PO4)3. Dalton Trans. 2015, 44, 7679–7686. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Walsh, A.; Luo, Y.; Yang, J.H.; Gong, X.G.; Wei, S.H. Wurtzite-derived polytypes of kesterite and stannite quaternary chalcogenide semiconductors. Phys. Rev. B 2010, 82, 195203. [Google Scholar] [CrossRef]
- Park, J.; Yoo, H.; Karade, V.; Gour, K.S.; Choi, E.; Kim, M.; Hao, X.J.; Shin, S.J.; Kim, J.; Shim, H.; et al. Investigation of low intensity light performances of kesterite CZTSe, CZTSSe, and CZTS thin film solar cells for indoor applications. J. Mater. Chem. A 2020, 8, 14538–14544. [Google Scholar] [CrossRef]
- He, W.J.; Sui, Y.R.; Zeng, F.C.; Wang, Z.W.; Wang, F.Y.; Yao, B.; Yang, L.L. Enhancing the Performance of Aqueous Solution-Processed Cu2ZnSn(S,Se)(4) Photovoltaic Materials by Mn(2+)Substitution. Nanomaterials 2020, 10, 1250. [Google Scholar] [CrossRef] [PubMed]
- Suryawanshi, M.P.; Agawane, G.L.; Bhosale, S.M.; Shin, S.W.; Patil, P.S.; Kim, J.H.; Moholkar, A.V. CZTS based thin film solar cells: A status review. Mater. Technol. 2013, 28, 98–109. [Google Scholar] [CrossRef]
- Sun, Y.L.; Qiu, P.F.; Yu, W.; Li, J.J.; Guo, H.L.; Wu, L.; Luo, H.; Meng, R.T.; Zhang, Y.; Liu, S.Z. N-Type Surface Design for p-Type CZTSSe Thin Film to Attain High Efficiency. Adv. Mater. 2021, 33, 2104330. [Google Scholar] [CrossRef]
- He, M.R.; Zhang, X.; Huang, J.L.; Li, J.J.; Yan, C.; Kim, J.; Chen, Y.S.; Yang, L.M.; Cairney, J.M.; Zhang, Y.; et al. Efficiency Cu2ZnSn(S,Se)(4) Solar Cells with Shallow Li-Zn Acceptor Defects Enabled by Solution-Based Li Post-Deposition Treatment. Adv. Energy Mater. 2021, 11, 2003783. [Google Scholar] [CrossRef]
- Emrani, A.; Vasekar, P.; Westgate, C.R. Effects of sulfurization temperature on CZTS thin film solar cell performances. Sol. Energy 2013, 98, 335–340. [Google Scholar] [CrossRef]

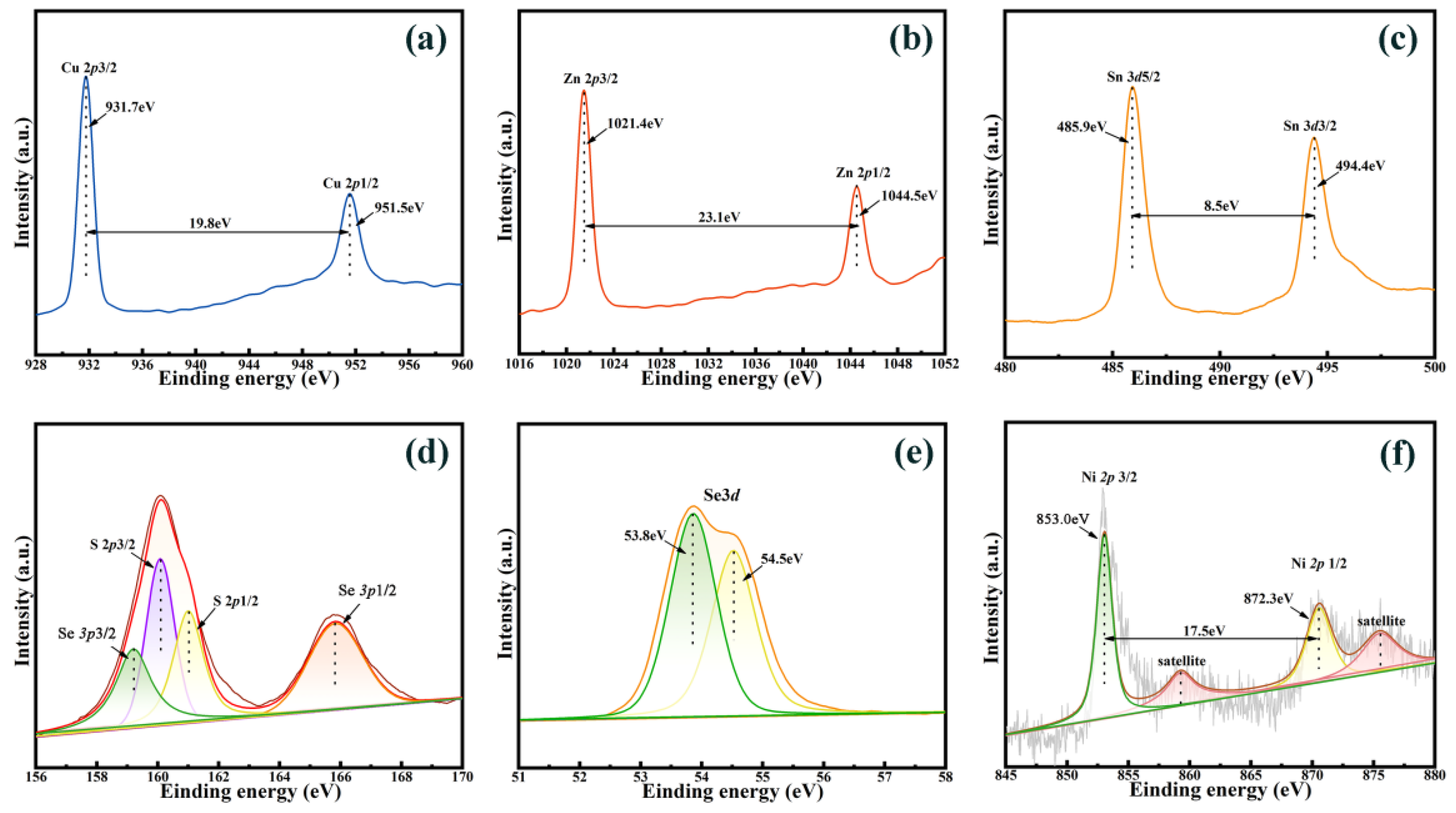

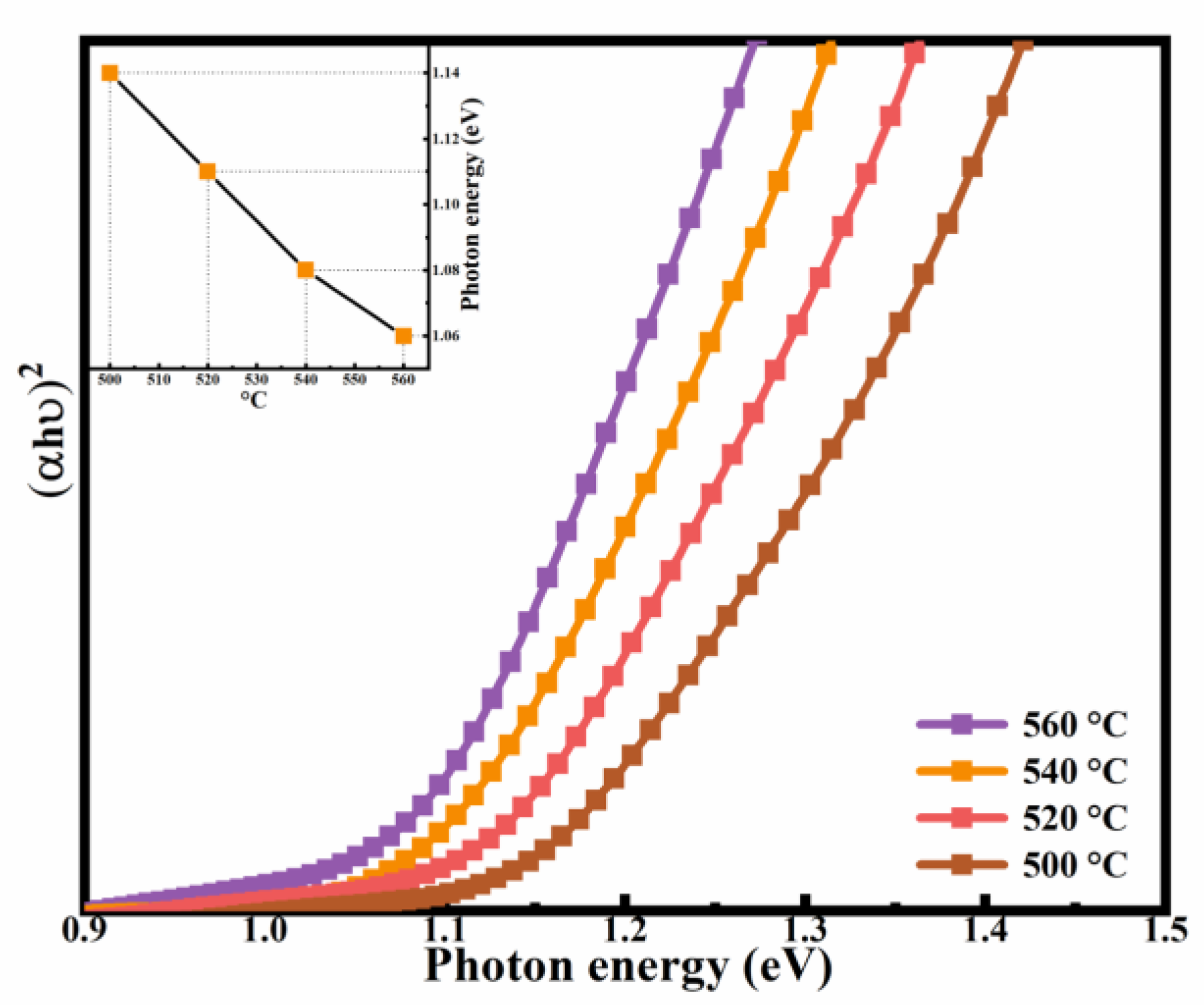
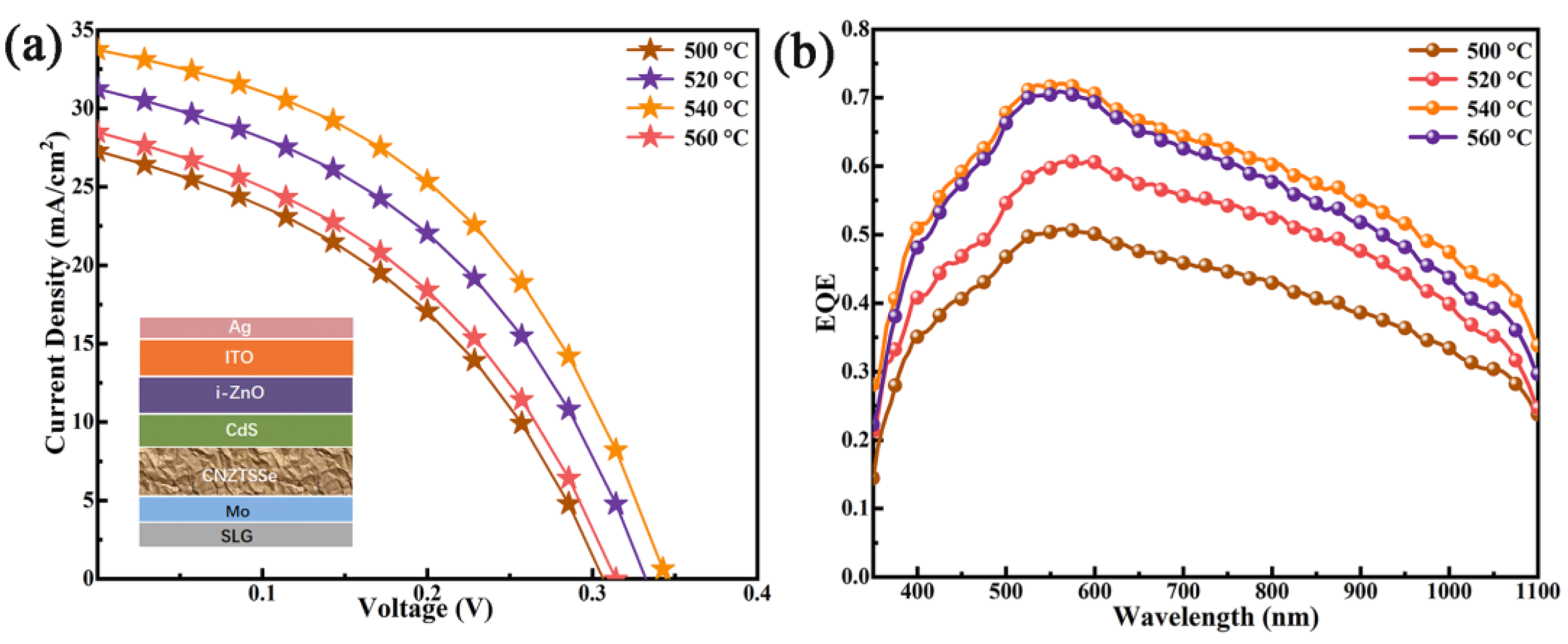

| Sample | Cu (% at) | Ni (% at) | Zn (% at) | Sn (% at) | S (% at) | Se (% at) | Cu/(Zn + Ni + Sn) | (Ni + Zn)/Sn |
|---|---|---|---|---|---|---|---|---|
| 500 | 23.81 | 0.34 | 16.28 | 10.98 | 2.80 | 45.79 | 0.862 | 1.513 |
| 520 | 23.75 | 0.36 | 16.26 | 10.97 | 2.47 | 46.22 | 0.860 | 1.515 |
| 540 | 23.79 | 0.35 | 16.30 | 10.99 | 2.06 | 46.51 | 0.860 | 1.515 |
| 560 | 23.74 | 0.36 | 16.25 | 10.95 | 1.78 | 46.91 | 0.861 | 1.516 |
| Samples | Resistivity (Ω·cm) | Carrier Concentration (cm−3) | Mobility (cm2V−1s−1) | Type |
|---|---|---|---|---|
| T = 500 °C | 1.65 × 102 | 2.25 × 1016 | 1.27 | p |
| T = 520 °C | 1.63 × 102 | 2.63 × 1016 | 1.43 | p |
| T = 540 °C | 1.52 × 102 | 1.15 × 1017 | 1.15 | p |
| T = 560 °C | 2.70 × 102 | 1.35 × 1016 | 1.71 | p |
| Device | Active Area | VOC (mV) | JSC (mA/cm2) | FF (%) | PCE (%) | Rs (Ω cm2) | Rsh (Ω cm2) |
|---|---|---|---|---|---|---|---|
| CNZTSSe (T = 500 °C) | 0.19 cm2 | 306 | 28.37 | 40.98 | 3.56 | 2.20 | 188 |
| CNZTSSe (T = 520 °C) | 0.19 cm2 | 332 | 31.14 | 42.85 | 4.43 | 2.00 | 211 |
| CNZTSSe (T = 540 °C) | 0.19 cm2 | 344 | 33.63 | 44.56 | 5.16 | 1.59 | 270 |
| CNZTSSe (T = 560 °C) | 0.19 cm2 | 314 | 28.41 | 41.26 | 3.68 | 2.33 | 185 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zeng, F.; Sui, Y.; Ma, M.; Zhao, N.; Wang, T.; Wang, Z.; Yang, L.; Wang, F.; Li, H.; Yao, B. Insight into the Effect of Selenization Temperature for Highly Efficient Ni-Doped Cu2ZnSn(S,Se)4 Solar Cells. Nanomaterials 2022, 12, 2942. https://doi.org/10.3390/nano12172942
Zeng F, Sui Y, Ma M, Zhao N, Wang T, Wang Z, Yang L, Wang F, Li H, Yao B. Insight into the Effect of Selenization Temperature for Highly Efficient Ni-Doped Cu2ZnSn(S,Se)4 Solar Cells. Nanomaterials. 2022; 12(17):2942. https://doi.org/10.3390/nano12172942
Chicago/Turabian StyleZeng, Fancong, Yingrui Sui, Meiling Ma, Na Zhao, Tianyue Wang, Zhanwu Wang, Lili Yang, Fengyou Wang, Huanan Li, and Bin Yao. 2022. "Insight into the Effect of Selenization Temperature for Highly Efficient Ni-Doped Cu2ZnSn(S,Se)4 Solar Cells" Nanomaterials 12, no. 17: 2942. https://doi.org/10.3390/nano12172942
APA StyleZeng, F., Sui, Y., Ma, M., Zhao, N., Wang, T., Wang, Z., Yang, L., Wang, F., Li, H., & Yao, B. (2022). Insight into the Effect of Selenization Temperature for Highly Efficient Ni-Doped Cu2ZnSn(S,Se)4 Solar Cells. Nanomaterials, 12(17), 2942. https://doi.org/10.3390/nano12172942





