Functionalization with Polyphenols of a Nano-Textured Ti Surface through a High–Amino Acid Medium: A Chemical–Physical and Biological Characterization
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Zeta Potential
2.3. Electron and Fluorescence Microscopies
2.4. UV–Vis Spectroscopy
2.5. Contact Angle Measurements
2.6. Folin–Ciocâlteu Test: Quantification of the Total Phenolic Content
2.7. Biological Experiments
2.7.1. Culture of Osteogenic Cells
2.7.2. Cell Morphology by Epifluorescence Microscopy
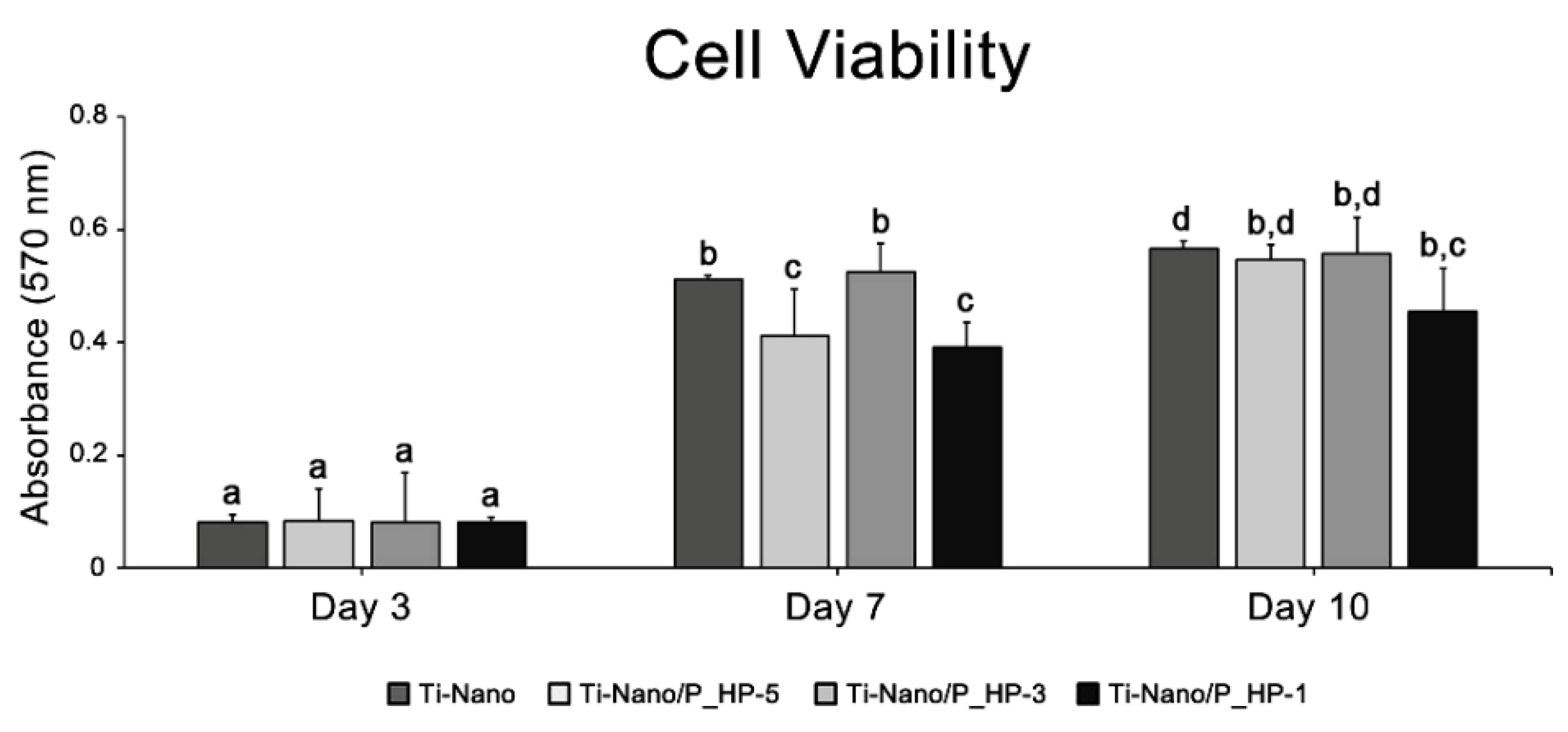
2.7.3. Cell Metabolic Activity/Cell Viability Assay
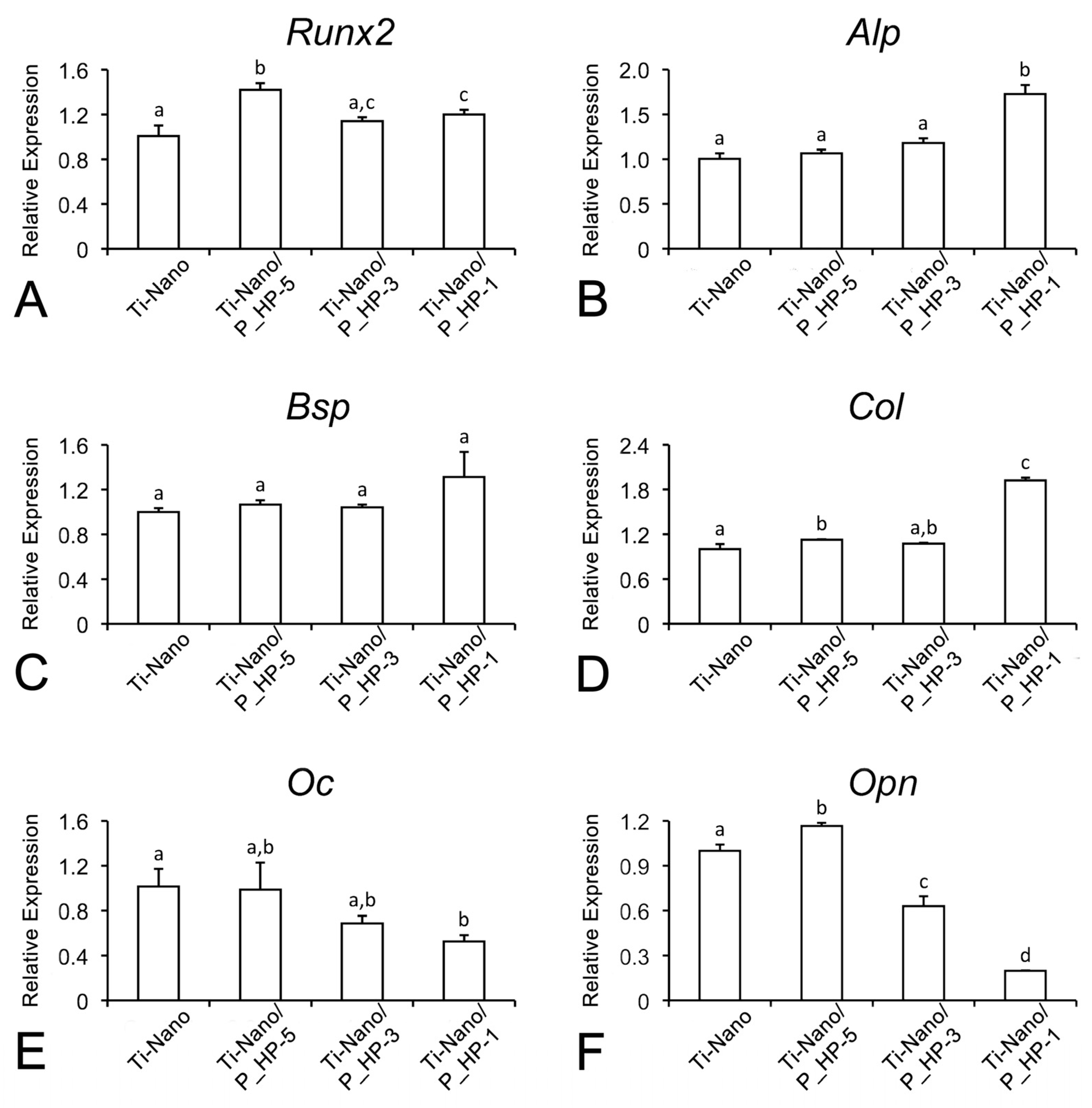
2.7.4. mRNA Expression of Osteogenic Markers by Real-Time Polymerase Chain Reaction (Real-Time PCR)
2.7.5. Mineralized Matrix Formation
2.7.6. Statistical Analysis
3. Results
3.1. Chemical–Physical Characterization
3.1.1. Zeta Potential Titrations
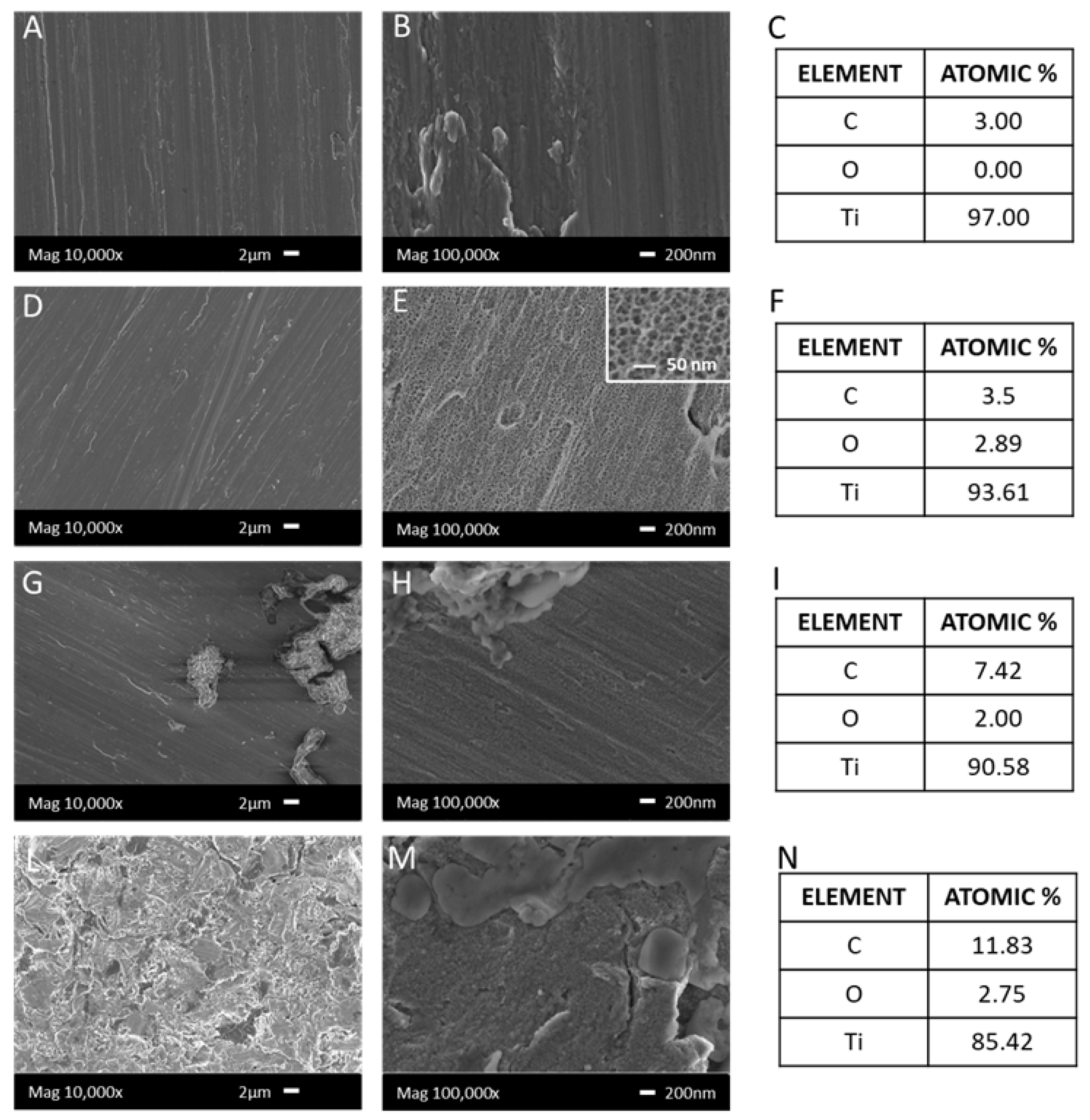
3.1.2. FESEM and EDS Analysis
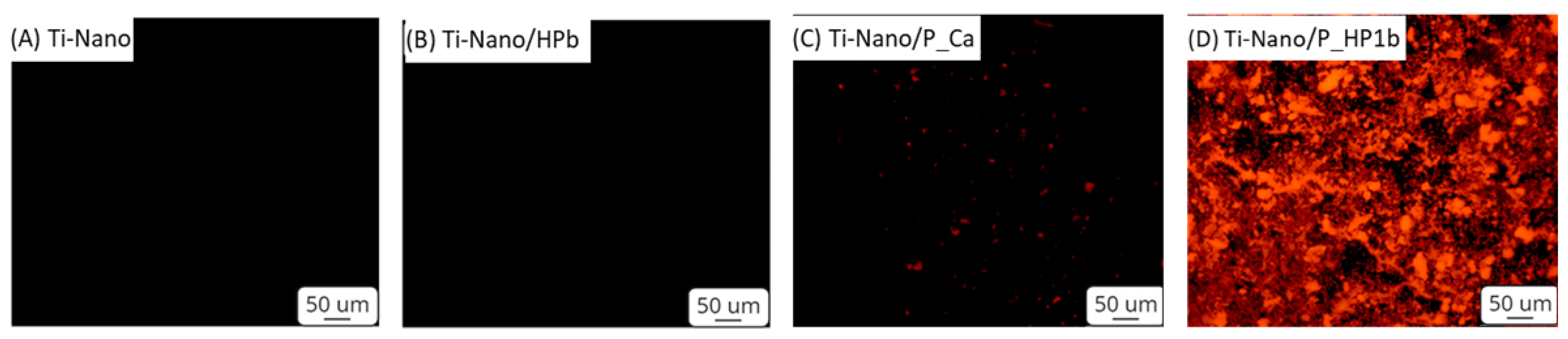
3.1.3. Fluorescence
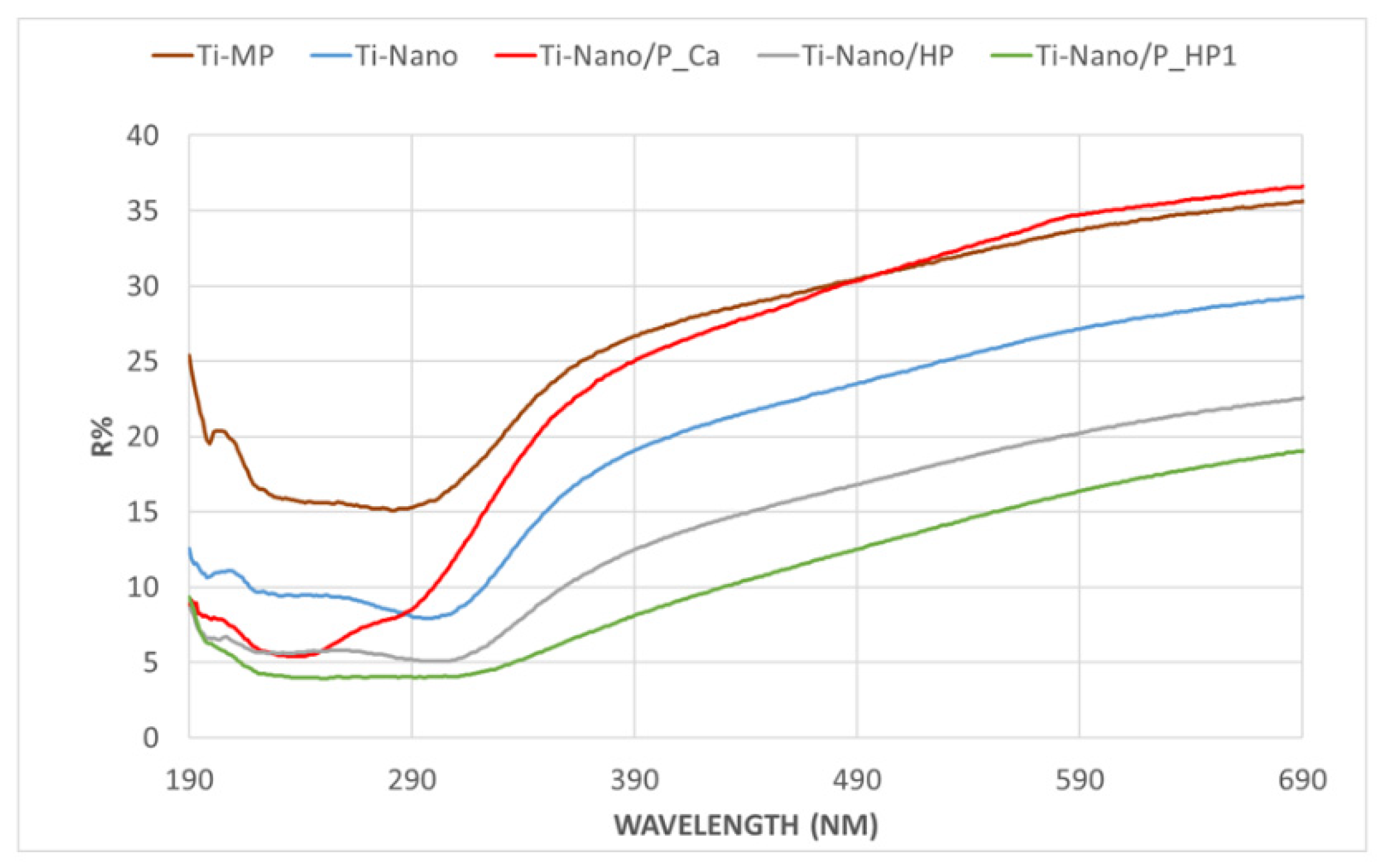
3.1.4. UV–Vis
3.1.5. Contact Angle
3.1.6. Redox Activity and Total Phenolic Content (Folin–Ciocâlteu Test)
3.2. Biological Experiments
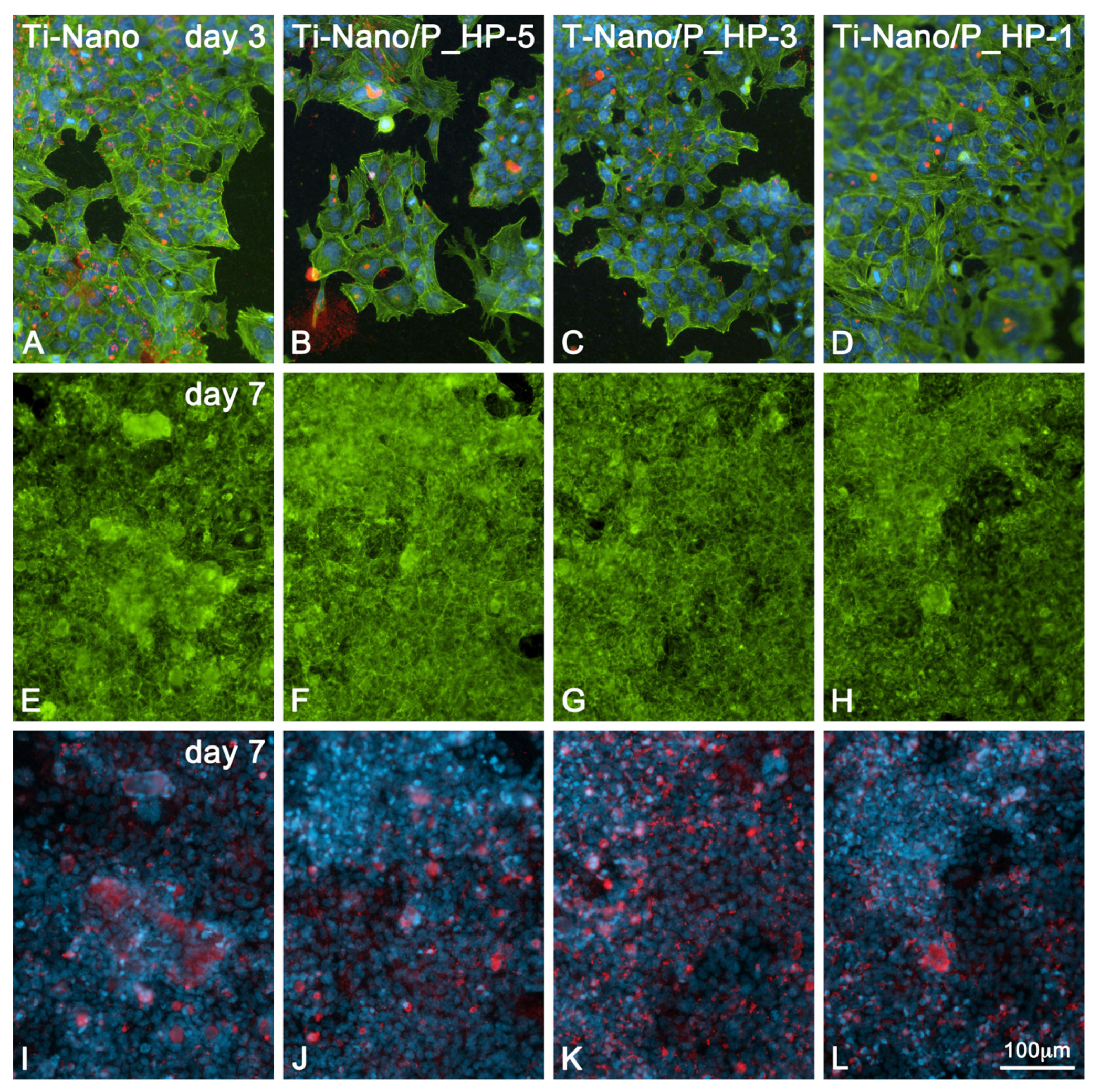
3.2.1. Cell Morphology by Fluorescence Microscopy
3.2.2. Cell Metabolic Activity/Cell Viability Assay
3.2.3. mRNA Expression of Osteogenic Markers by Real-Time PCR
3.2.4. Mineralized Matrix Formation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Souza, J.C.M.; Sordi, M.B.; Kanazawa, M.; Ravindran, S.; Henriques, B.; Silva, F.S.; Aparicio, C.; Cooper, L.F. Nano-scale modification of titanium implant surfaces to enhance osseointegration. Acta Biomater. 2019, 94, 112–131. [Google Scholar] [CrossRef] [PubMed]
- Caneva, M.; Lang, N.P.; Guirado, J.L.C.; Spriano, S.; Iezzi, G.; Botticelli, D. Bone healing at bicortically installed implants with different surface configurations: An experimental study in rabbits. Clin. Oral Implant. Res. 2015, 26, 293–299. [Google Scholar] [CrossRef] [PubMed]
- Barberi, J.; Spriano, S. Titanium and Protein Adsorption: An Overview of Mechanisms and Effects of Surface Features. Materials 2021, 14, 1590. [Google Scholar] [CrossRef]
- Nanci, A.; Wuest, J.D.; Peru, L.; Brunet, P.; Sharma, V.; Zalzal, S.; McKee, M.D. Chemical modification of titanium surfaces for covalent attachment of biological molecules. J. Biomed. Mater. Res. 1998, 40, 324–335. [Google Scholar] [CrossRef]
- De Oliveira, P.T.; Zalzal, S.F.; Beloti, M.M.; Rosa, A.L.; Nanci, A. Enhancement ofin vitro osteogenesis on titanium by chemically produced nanotopography. J. Biomed. Mater. Res. A 2007, 80, 554–564. [Google Scholar] [CrossRef]
- Kokubo, T.; Yamaguchi, S. Simulated body fluid and the novel bioactive materials derived from it. J. Biomed. Mater. Res. Part A 2019, 107, 968–977. [Google Scholar] [CrossRef]
- Riccucci, G.; Cazzola, M.; Ferraris, S.; Gobbo, V.; Guaita, M.; Spriano, S. Surface functionalization of Ti6Al4V with an extract of polyphenols from red grape pomace. Mater. Des. 2021, 206, 109776. [Google Scholar] [CrossRef]
- Riccucci, G.; Cazzola, M.; Ferraris, S.; Gobbo, V.A.; Miola, M.; Bosso, A.; Örlygsson, G.; Ng, C.H.; Verné, E.; Spriano, S. Surface functionalization of bioactive glasses and hydroxyapatite with polyphenols from organic red grape pomace. J. Am. Ceram. Soc. 2021, 105, 1697–1710. [Google Scholar] [CrossRef]
- Mpoyi, E.N.; Cantini, M.; Reynolds, P.M.; Gadegaard, N.; Dalby, M.; Salmerón-Sánchez, M. Protein Adsorption as a Key Mediator in the Nanotopographical Control of Cell Behavior. ACS Nano 2016, 10, 6638–6647. [Google Scholar] [CrossRef]
- Scalbert, A.; Johnson, I.; Saltmarsh, M. Polyphenols: Antioxidants and beyond. Am. J. Clin. Nutr. 2005, 81, 215S–217S. [Google Scholar] [CrossRef] [PubMed]
- Torre, E.; Iviglia, G.; Cassinelli, C.; Morra, M.; Russo, N. Polyphenols from grape pomace induce osteogenic differentiation in mesenchymal stem cells. Int. J. Mol. Med. 2020, 45, 1721–1734. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gómez-Florit, M.; Monjo, M.; Ramis, J.M. Identification of Quercitrin as a Potential Therapeutic Agent for Periodontal Applications. J. Periodontol. 2014, 85, 966–974. [Google Scholar] [CrossRef] [PubMed]
- Byun, M.R.; Sung, M.K.; Kim, A.R.; Lee, C.H.; Jang, E.J.; Jeong, M.G.; Noh, M.; Hwang, E.S.; Hong, J.-H. (−)-Epicatechin Gallate (ECG) Stimulates Osteoblast Differentiation via Runt-related Transcription Factor 2 (RUNX2) and Transcriptional Coactivator with PDZ-binding Motif (TAZ)-mediated Transcriptional Activation. J. Biol. Chem. 2014, 289, 9926–9935. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prakash, M.; Basavaraj, B.V.; Murthy, K.C. Biological functions of epicatechin: Plant cell to human cell health. J. Funct. Foods 2018, 52, 14–24. [Google Scholar] [CrossRef]
- Luxbacher, T. The Zeta Guide, Editor: Anton Paar GmbH. 2014. Available online: https://austria-forum.org/web-books/zeta00en2014iicm (accessed on 10 July 2022).
- Lavid, N.; Schwartz, A.; Yarden, O.; Tel-Or, E. The involvement of polyphenols and peroxidase activities in heavy-metal accumulation by epidermal glands of the waterlily (Nymphaeaceae). Planta 2001, 212, 323–331. [Google Scholar] [CrossRef] [PubMed]
- Talamond, P.; Verdeil, J.-L.; Conéjéro, G. Secondary Metabolite Localization by Autofluorescence in Living Plant Cells. Molecules 2015, 20, 5024–5037. [Google Scholar] [CrossRef] [Green Version]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Fleige, S.; Pfaffl, M.W. RNA integrity and the effect on the real-time qRT-PCR performance. Mol. Asp. Med. 2006, 27, 126–139. [Google Scholar] [CrossRef]
- Schroeder, A.; Mueller, O.; Stocker, S.; Salowsky, R.; Leiber, M.; Gassmann, M.; Lightfoot, S.; Menzel, W.; Granzow, M.; Ragg, T. The RIN: An RNA integrity number for assigning integrity values to RNA measurements. BMC Mol. Biol. 2006, 7, 3. [Google Scholar] [CrossRef] [Green Version]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Gregory, C.A.; Gunn, W.G.; Peister, A.; Prockop, D.J. An Alizarin red-based assay of mineralization by adherent cells in culture: Comparison with cetylpyridinium chloride extraction. Anal. Biochem. 2004, 329, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Kai, G. A Review of Dietary Polyphenol-Plasma Protein Interactions: Characterization, Influence on the Bioactivity, and Structure-Affinity Relationship. Crit. Rev. Food Sci. Nutr. 2012, 52, 85–101. [Google Scholar] [CrossRef] [PubMed]
- Airado-Rodríguez, D.; Durán-Merás, I.; Galeano-Díaz, T.; Wold, J.P. Front-face fluorescence spectroscopy: A new tool for control in the wine industry. J. Food Compos. Anal. 2011, 24, 257–264. [Google Scholar] [CrossRef]
- Praveen, P.; Viruthagiri, G.; Mugundan, S.; Shanmugam, N. Structural, optical and morphological analyses of pristine titanium di-oxide nanoparticles—Synthesized via sol–gel route. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 117, 622–629. [Google Scholar] [CrossRef] [PubMed]
- Santos, J.; Oliveira, M.; Ibáñez, E.; Herrero, M. Phenolic profile evolution of different ready-to-eat baby-leaf vegetables during storage. J. Chromatogr. A 2014, 1327, 118–131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stanford, C.M.; Jacobson, P.A.; Eanes, E.D.; Lembke, L.A.; Midura, R.J. Rapidly Forming Apatitic Mineral in an Osteoblastic Cell Line (UMR 106—01 BSP). J. Biol. Chem. 1995, 270, 9420–9428. [Google Scholar] [CrossRef] [Green Version]
- Maximiano, W.M.A.; Da Silva, E.Z.M.; Santana, A.C.; De Oliveira, P.T.; Jamur, M.C.; Oliver, C. Mast Cell Mediators Inhibit Osteoblastic Differentiation and Extracellular Matrix Mineralization. J. Histochem. Cytochem. 2017, 65, 723–741. [Google Scholar] [CrossRef] [Green Version]
- Smeets, R.; Stadlinger, B.; Schwarz, F.; Beck-Broichsitter, B.; Jung, O.; Precht, C.; Kloss, F.; Gröbe, A.; Heiland, M.; Ebker, T. Impact of Dental Implant Surface Modifications on Osseointegration. BioMed Res. Int. 2016, 2016, 6285620. [Google Scholar] [CrossRef] [Green Version]
- Jemat, A.; Ghazali, M.J.; Razali, M.; Otsuka, Y. Surface Modifications and Their Effects on Titanium Dental Implants. BioMed Res. Int. 2015, 2015, 791725. [Google Scholar] [CrossRef] [Green Version]
- Variola, F.; Brunski, J.B.; Orsini, G.; de Oliveira, P.T.; Wazen, R.; Nanci, A. Nanoscale surface modifications of medically relevant metals: State-of-the art and perspectives. Nanoscale 2010, 3, 335–353. [Google Scholar] [CrossRef] [Green Version]
- Salou, L.; Hoornaert, A.; Louarn, G.; Layrolle, P. Enhanced osseointegration of titanium implants with nanostructured surfaces: An experimental study in rabbits. Acta Biomater. 2015, 11, 494–502. [Google Scholar] [CrossRef] [PubMed]
- Ferraris, S.; Cazzola, M.; Zuardi, L.R.; de Oliveira, P.T. Metal nanoscale systems functionalized with organic compounds. In Woodhead Publishing Series in Biomaterials, Nanostructured Biomaterials for Regenerative Medicine; Guarino, V., Iafisco, M., Spriano, S., Eds.; Woodhead Publishing: Sawston, UK, 2020; pp. 407–436. ISBN 9780081025949. [Google Scholar] [CrossRef]
- Tao, Z.-S.; Zhou, W.-S.; Xu, H.-G.; Yang, M. Parathyroid hormone (1–34) can reverse the negative effect of valproic acid on the osseointegration of titanium rods in ovariectomized rats. J. Orthop. Transl. 2020, 27, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; He, R.; Deng, X.; Shao, Z.; Deganello, D.; Yan, C.; Xia, Z. Three-dimensional biofabrication of an aragonite-enriched self-hardening bone graft substitute and assessment of its osteogenicity in vitro and in vivo. Biomater. Transl. 2020, 1, 69–81. [Google Scholar] [CrossRef] [PubMed]
- Castro-Raucci, L.M.S.; Francischini, M.S.; Teixeira, L.N.; Ferraz, E.P.; Lopes, H.B.; de Oliveira, P.T.; Hassan, M.Q.; Rosa, A.L.; Beloti, M.M. Titanium with Nanotopography Induces Osteoblast Differentiation by Regulating Endogenous Bone Morphogenetic Protein Expression and Signaling Pathway. J. Cell. Biochem. 2015, 117, 1718–1726. [Google Scholar] [CrossRef] [PubMed]
- Abuna, R.P.F.; Oliveira, F.S.; Lopes, H.B.; Freitas, G.P.; Fernandes, R.R.; Rosa, A.L.; Beloti, M.M. The Wnt/β-catenin signaling pathway is regulated by titanium with nanotopography to induce osteoblast differentiation. Colloids Surf. B Biointerfaces 2019, 184, 110513. [Google Scholar] [CrossRef]
- Bueno, R.D.B.E.L.; Ponce, K.; Dias, A.; Bello, D.G.; Brunski, J.; Nanci, A. Influence of Nanotopography on Early Bone Healing during Controlled Implant Loading. Nanomaterials 2020, 10, 2191. [Google Scholar] [CrossRef]
- Mendonca, G.; Mendonça, D.B.S.; Aragão, F.J.L.; Cooper, L.F. The combination of micron and nanotopography by H2SO4/H2O2 treatment and its effects on osteoblast-specific gene expression of hMSCs. J. Biomed. Mater. Res. A 2010, 94, 169–179. [Google Scholar] [CrossRef]
- Ballo, A.; Agheli, H.; Thomsen, P.; Petronis, S. Nanostructured model implants for in vivo studies: Influence of well-defined nanotopography on de novo bone formation on titanium implants. Int. J. Nanomed. 2011, 6, 3415–3428. [Google Scholar] [CrossRef] [Green Version]
- Yi, Z.; Chen, G.; Chen, X.; Ma, X.; Cui, X.; Sun, Z.; Su, W.; Li, X. Preparation of Strong Antioxidative, Therapeutic Nanoparticles Based on Amino Acid-Induced Ultrafast Assembly of Tea Polyphenols. ACS Appl. Mater. Interfaces 2020, 12, 33550–33563. [Google Scholar] [CrossRef]
- Han, Y.; Lin, Z.; Zhou, J.; Yun, G.; Guo, R.; Richardson, J.J.; Caruso, F. Polyphenol-Mediated Assembly of Proteins for Engineering Functional Materials. Angew. Chem. Int. Ed. 2020, 59, 15618–15625. [Google Scholar] [CrossRef]
- Bello, D.G.; Fouillen, A.; Badia, A.; Nanci, A. A nanoporous titanium surface promotes the maturation of focal adhesions and formation of filopodia with distinctive nanoscale protrusions by osteogenic cells. Acta Biomater. 2017, 60, 339–349. [Google Scholar] [CrossRef] [PubMed]
- Bello, D.G.; Fouillen, A.; Badia, A.; Nanci, A. Nanoporosity Stimulates Cell Spreading and Focal Adhesion Formation in Cells with Mutated Paxillin. ACS Appl. Mater. Interfaces 2020, 12, 14924–14932. [Google Scholar] [CrossRef] [PubMed]
- Dai, P.; Mao, Y.; Sun, X.; Li, X.; Muhammad, I.; Gu, W.; Zhang, D.; Zhou, Y.; Ma, J.; Ni, Z.; et al. Attenuation of Oxidative Stress-Induced Osteoblast Apoptosis by Curcumin is Associated with Preservation of Mitochondrial Functions and Increased Akt-GSK3β Signaling. Cell. Physiol. Biochem. 2017, 41, 661–677. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malaval, L.; Wade-Guéye, N.M.; Boudiffa, M.; Fei, J.; Zirngibl, R.; Chen, F.; Laroche, N.; Roux, J.-P.; Burt-Pichat, B.; Duboeuf, F.; et al. Bone sialoprotein plays a functional role in bone formation and osteoclastogenesis. J. Exp. Med. 2008, 205, 1145–1153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Y.; Huang, Y.; Zhang, L.; Zhang, C. Transcriptional regulation of bone sialoprotein gene expression by Osx. Biochem. Biophys. Res. Commun. 2016, 476, 574–579. [Google Scholar] [CrossRef]
- Liu, T.M.; Lee, E.H. Transcriptional Regulatory Cascades in Runx2-Dependent Bone Development. Tissue Eng. Part B Rev. 2013, 19, 254–263. [Google Scholar] [CrossRef] [Green Version]
- Addison, W.N.; Masica, D.L.; Gray, J.J.; McKee, M.D. Phosphorylation-Dependent Inhibition of Mineralization by Osteopontin ASARM Peptides is Regulated by PHEX Cleavage. J. Bone Miner. Res. 2009, 25, 695–705. [Google Scholar] [CrossRef]
- Zoch, M.L.; Clemens, T.L.; Riddle, R.C. New insights into the biology of osteocalcin. Bone 2015, 82, 42–49. [Google Scholar] [CrossRef] [Green Version]
- da Cunha, L.R.; Muniz-Junqueira, M.I.; Borges, T.K.D.S. Impact of polyphenols in phagocyte functions. J. Inflamm. Res. 2019, 12, 205–217. [Google Scholar] [CrossRef] [Green Version]
- Niu, Y.; Wang, Z.; Shi, Y.; Dong, L.; Wang, C. Modulating macrophage activities to promote endogenous bone regeneration: Biological mechanisms and engineering approaches. Bioact. Mater. 2020, 6, 244–261. [Google Scholar] [CrossRef]
- Watson, S.; Beydoun, D.; Scott, J.; Amal, R. Preparation of nanosized crystalline TiO2 particles at low temperature for photocatalysis. J. Nanoparticle Res. 2004, 6, 193–207. [Google Scholar] [CrossRef]
- Nakamura, M.; Aoki, T.; Hatanaka, Y.; Korzec, D.; Engemann, J. Comparison of hydrophilic properties of amorphous TiOx films obtained by radio frequency sputtering and plasma-enhanced chemical vapor deposition. J. Mater. Res. 2001, 16, 621–626. [Google Scholar] [CrossRef]
- Davis, K.M.; Tomozawa, M. An infrared spectroscopic study of water-related species in silica glasses. J. Non. Cryst. Solids 1996, 201, 177–198. [Google Scholar] [CrossRef]
- Urlaub, R.; Posset, U.; Thull, R. FT-IR spectroscopic investigations on sol–gel-derived coatings from acid-modified titanium alkoxides. J. Non. Cryst. Solids 2000, 265, 276–284. [Google Scholar] [CrossRef]
- Ferraris, S.; Spriano, S.; Miola, M.; Bertone, E.; Allizond, V.; Cuffini, A.M.; Banche, G. Surface modification of titanium surfaces through a modified oxide layer and embedded silver nanoparticles: Effect of reducing/stabilizing agents on precipitation and properties of the nanoparticles. Surf. Coatings Technol. 2018, 344, 177–189. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, C.; Chen, J.; Li, X. Biocompatible, functional spheres based on oxidative coupling assembly of green tea polyphenols. J. Am. Chem. Soc. 2013, 135, 4179–4182. [Google Scholar] [CrossRef]
- Yu, H.; Guo, Z.; Wang, S.; Fernando, G.S.N.; Channa, S.; Kazlauciunas, A.; Martin, D.P.; Krasnikov, S.A.; Kulak, A.; Boesch, C. Fabrication of hybrid materials from titanium dioxide and natural phenols for efficient radical scavenging against oxidative stress. ACS Biomater. Sci. Eng. 2019, 5, 2778–2785. [Google Scholar] [CrossRef]
- Huang, X.; Wu, H.; Liao, X.; Shi, B. One-step, size-controlled synthesis of gold nanoparticles at room temperature using plant tannin. Green Chem. 2010, 12, 395–399. [Google Scholar] [CrossRef]
- Bulut, E.; Ozacar, M. Rapid, facile synthesis of silver nanostructure using hydrolyzable tannin. Ind. Eng. Chem. Res. 2009, 48, 5686–5690. [Google Scholar] [CrossRef]
- Socrates, G. Infrared and Raman Characteristic Group Frequencies: Tables and Charts, 3rd ed.; Wiley: Chichester, UK, 2004; ISBN 0470093072. [Google Scholar]
- Pereira, K.K.Y.; Alves, O.C.; Novaes, A.B., Jr.; de Oliveira, F.S.; Yi, J.-H.; Zaniquelli, O.; Brandstetter, C.W.; Scharnweber, D.; Variola, F.; Nanci, A.; et al. Progression of Osteogenic Cell Cultures Grown on Microtopographic Titanium Coated with Calcium Phosphate and Functionalized with a Type I Collagen-Derived Peptide. J. Periodontol. 2013, 84, 1199–1210. [Google Scholar] [CrossRef] [Green Version]
- de Oliveira, P.T.; Andrade de Oliva, M.; Maximiano, W.M.A.; Sebastião, K.E.V.; Crippa, G.E.; Ciancaglini, P.; Beloti, M.M.; Nanci, A.; Rosa, A.L. Effects of a Mixture of Growth Factors and Proteins on the Development of the Osteogenic Phenotype in Human Alveolar Bone Cell Cultures. J. Histochem. Cytochem. 2008, 56, 629–638. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stábile, M.F.; Soubelet, C.G.; Albano, M.P.; Rosa, A.L.; de Castro-Raucci, L.M.S.; de Oliveira, P.T. Effect of 64S bioglass addition on sintering kinetic, flexural strength and osteoblast cell response of yttria-partially stabilized zirconia ceramics. Int. J. Appl. Ceram. 2019, 16, 517–530. [Google Scholar] [CrossRef]


| Surface Treatment | Sample Acronym | ||||
|---|---|---|---|---|---|
| Polishing | Chemical Etching (Nano-Texture) | Adsorption of Polyphenols in an Inorganic Solution | Adsorption of Polyphenols in a High–Amino Acid Medium | Soaking in the High–Amino Acid Medium | |
| X | Ti-MP | ||||
| X | X | Ti-Nano | |||
| X | X | X | Ti-Nano/HP Ti-Nano/HPb (no phenol red) | ||
| X | X | X (1 mg/mL) | Ti-Nano/P_Ca | ||
| X | X | X (1 mg/mL) | Ti-Nano/P_HP1 Ti-Nano/P_HP1b (no phenol red) | ||
| X | X | X (1 × 10−3 mg/mL) | Ti-Nano/P_HP-3 | ||
| X | X | X (1 × 10−5 mg/mL) | Ti-Nano/P_HP-5 | ||
| Gene | Gene Name | Identification |
|---|---|---|
| Runx2 | Runt-related transcription factor 2 (Runx2) | Rn01512298_m1 |
| Alp | Alkaline phosphatase (Alp) | Rn01516028_m1 |
| Ibsp | Integrin binding sialoprotein (Bone sialoprotein, Bsp) | Rn00561414_m1 |
| Spp1 | Secreted phosphoprotein 1 (Osteopontin, Opn) | Rn00681031_m1 |
| Bglap | Bone gamma-carboxyglutamic acid–containing protein (Osteocalcin, Oc) | Rn00566386_g1 |
| Col1a1 | Collagen type 1 alpha 1 (Col) | Rn01523366_m1 |
| Gapdh | Glyceraldeyde-3-phosphaste dehydrogenase (Gapdh) | Rn01775763_g1 |
| Sample | Contact Angle (°) | Standard Deviation |
|---|---|---|
| Ti-MP | 70.0 | 2.5 |
| Ti-Nano | 31.4 | 1.4 |
| Ti-Nano/HP | 10.2 | 6.0 |
| Ti-Nano/P_Ca | 36.9 | 1.6 |
| Ti-Nano/P_HP1 | 40.4 | 3.2 |
| Samples | GAE Units (mg/mL) | Standard Deviation |
|---|---|---|
| Ti-Nano | 0 | - |
| Ti-Nano/HPb | 0 | - |
| Ti-Nano/HP | 0.0011 | - |
| Ti-Nano/P_Ca | 0.0020 | 0.0008 |
| Ti_Nano/P_HP1 | 0.0082 | 0.0025 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scannavino, R.C.P.; Riccucci, G.; Ferraris, S.; Duarte, G.L.C.; de Oliveira, P.T.; Spriano, S. Functionalization with Polyphenols of a Nano-Textured Ti Surface through a High–Amino Acid Medium: A Chemical–Physical and Biological Characterization. Nanomaterials 2022, 12, 2916. https://doi.org/10.3390/nano12172916
Scannavino RCP, Riccucci G, Ferraris S, Duarte GLC, de Oliveira PT, Spriano S. Functionalization with Polyphenols of a Nano-Textured Ti Surface through a High–Amino Acid Medium: A Chemical–Physical and Biological Characterization. Nanomaterials. 2022; 12(17):2916. https://doi.org/10.3390/nano12172916
Chicago/Turabian StyleScannavino, Rafaella C. P., Giacomo Riccucci, Sara Ferraris, Gabriel L. C. Duarte, Paulo T. de Oliveira, and Silvia Spriano. 2022. "Functionalization with Polyphenols of a Nano-Textured Ti Surface through a High–Amino Acid Medium: A Chemical–Physical and Biological Characterization" Nanomaterials 12, no. 17: 2916. https://doi.org/10.3390/nano12172916
APA StyleScannavino, R. C. P., Riccucci, G., Ferraris, S., Duarte, G. L. C., de Oliveira, P. T., & Spriano, S. (2022). Functionalization with Polyphenols of a Nano-Textured Ti Surface through a High–Amino Acid Medium: A Chemical–Physical and Biological Characterization. Nanomaterials, 12(17), 2916. https://doi.org/10.3390/nano12172916











