DFT and TD-DFT Study of the Chemical Effect in the SERS Spectra of Piperidine Adsorbed on Silver Colloidal Nanoparticles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Raman Spectroscopy
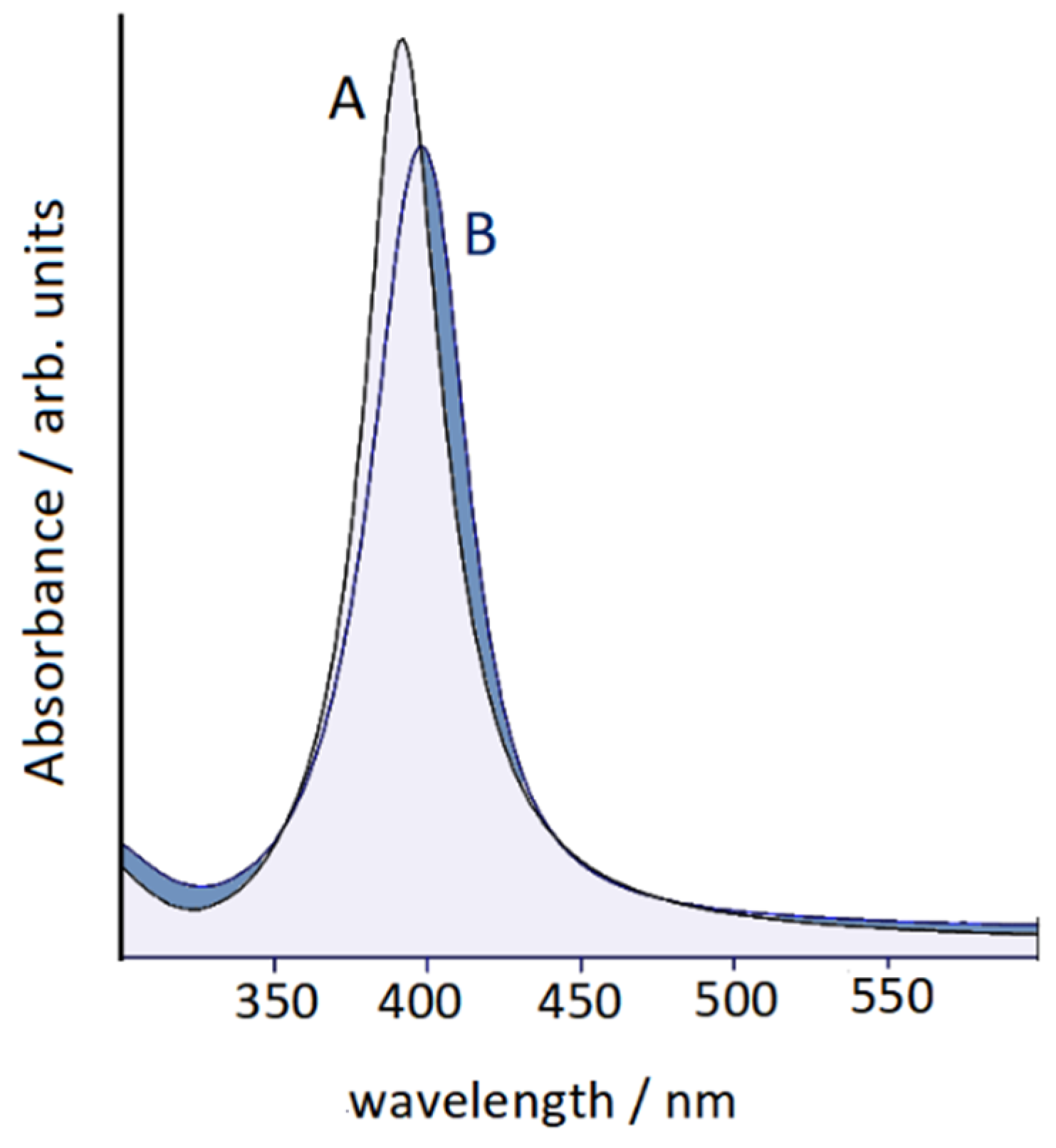
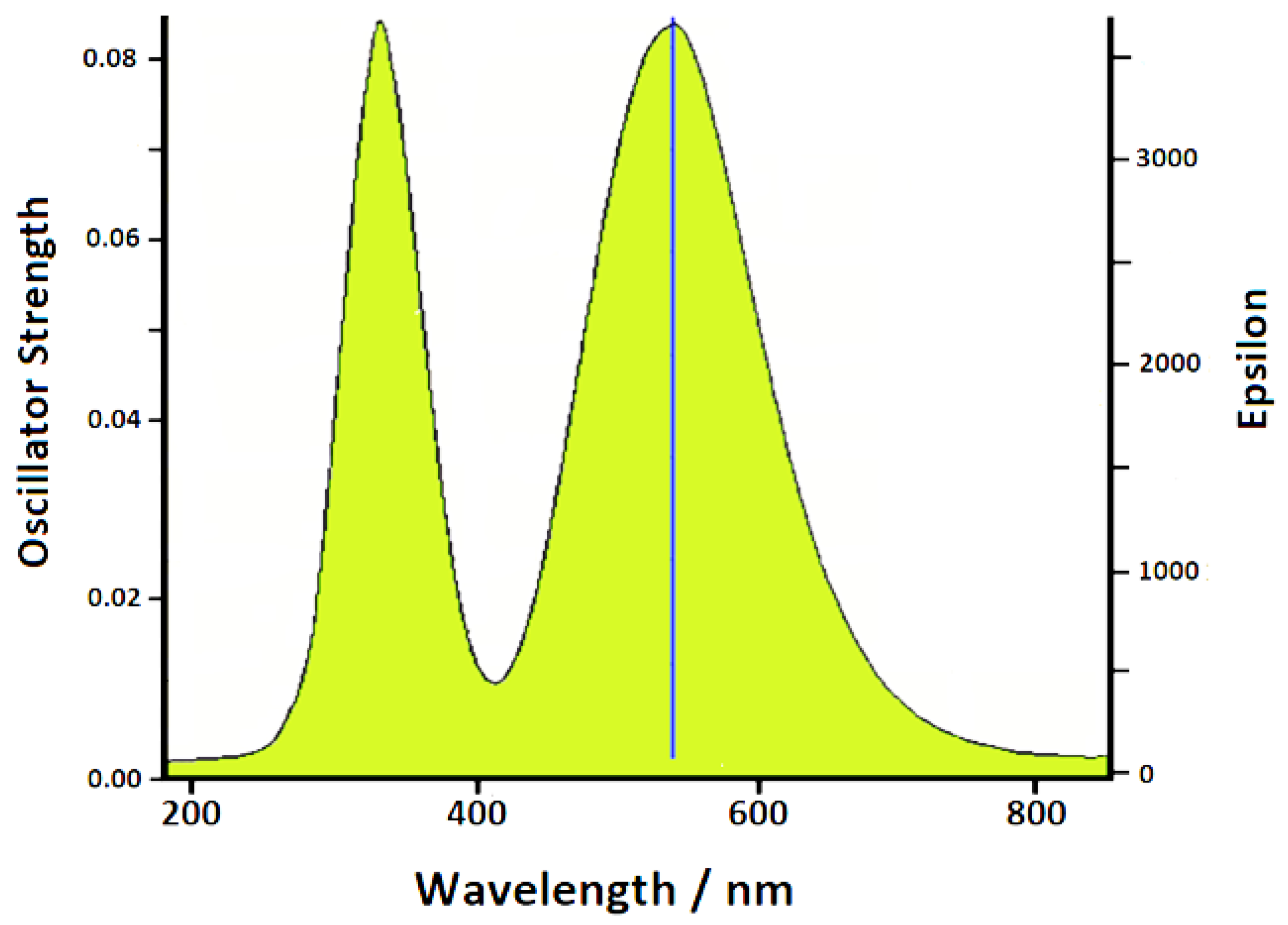
2.3. UV–Vis Extinction Spectroscopy
2.4. Density Functional Theory Calculations
3. Results and Discussion
3.1. Raman/SERS Spectra
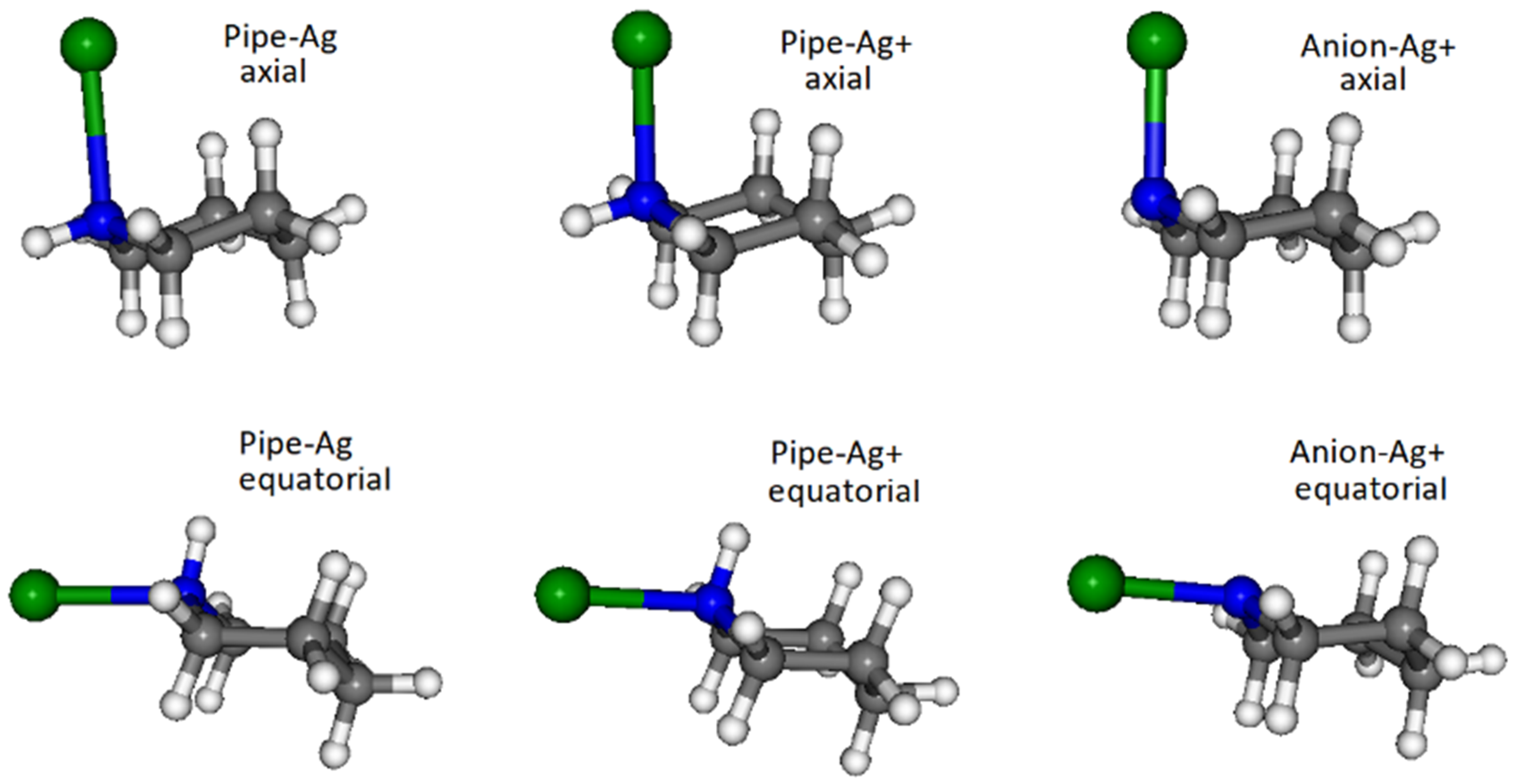
3.2. DFT Calculations
- Molecule linked through the lone pair of nitrogen to a silver atom;
- Molecule linked to a silver ion, because there can be positively charged active sites on the surface of Ag nanoparticles activated by coadsorbed chloride anions;
- Piperidine deprotonated due to a surface reaction with a positive active site, even if the pKa of the molecule in water is 10.45 (at 25°) and the pH of the colloid is around 9; this possible reaction can be schematized as in Figure S2 of the Supplementary Materials.
- (1)
- We considered the Anion-Ag+ equatorial complex, identified as the most likely anchoring geometry, and we performed B3LYP calculations but with a mixed basis set made up of Pople’s 6-311++G(d, p) for the organic anion and LanL2DZ for the metal cation: we did this in order to check if a larger basis set could improve the results presented above.
- (2)
- On the same complex, we also adopted a CAM-B3LYP/LanL2DZ level of theory to check if long-range electronic correlation (better captured by this functional) could improve the agreement with the experiments.In both cases, the latter agreement was actually worse than with the standard B3LYP/LanL2DZ approach used in most of this paper. Indeed, the average difference between computed and experimentally observed frequencies increased, and some intense SERS bands could not be assigned, as shown in Table S2 of the Supplementary Materials.
- (3)
- We performed B3LYP/LanL2DZ calculations on piperidine anion linked to a larger adcluster (Ag5+) in order to better model the silver surface. This model did not show significant improvement in the agreement with SERS spectra, in comparison with calculations with a single Ag+. This result, reported in Table S3 of the Supplementary Materials, is not surprising because the local interaction between molecule and metal active-site is determinant for the SERS profile, as ascertained in different SERS studies of molecules chemisorbed on colloidal silver [7,9,25,37]. In fact, this corroborates the use of simple models to capture most of the chemical interaction relevant for SERS interpretation.
3.3. TD-DFT Calculations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Schlücker, S. Surface Enhanced Raman Spectroscopy: Analytical, Biophysical and Life Science Applications; Wiley-VCH: Weinheim, Germany, 2011. [Google Scholar]
- Prcházka, M. Surface-Enhanced Raman Spectroscopy, Bioanalytical, Biomolecular and Medical Applications; Springer: Basel, Switzerland, 2016. [Google Scholar]
- Maiti, N.; Thomas, S.; Debnath, A.; Kappor, S. Raman and XPS study on the interaction of taurine with silver nanoparticles. RSC Adv. 2016, 6, 56406–56411. [Google Scholar] [CrossRef]
- Dhayagude, A.C.; Maiti, N.; Debnath, A.K.; Joshi, S.S.; Kapoor, S. Metal nanoparticle catalyzed charge rearrangement in selenourea probed by surface-enhanced Raman scattering. RSC Adv. 2016, 6, 17405–17414. [Google Scholar] [CrossRef]
- Muniz-Miranda, M.; Muniz-Miranda, F.; Pedone, A. Raman and DFT study of methimazole chemisorbed on gold colloidal nanoparticles. Phys. Chem. Chem. Phys. 2016, 18, 5974–5980. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, D.B.; Nguyen, T.D.; Kim, S.; Joo, S.W. Raman spectroscopy and quantum-mechanical analysis of tautomeric forms in cytosine and 5-methylcytosine on gold surfaces. Spectrochim. Acta Part A 2017, 174, 183–188. [Google Scholar] [CrossRef]
- Gellini, C.; Deepak, F.L.; Muniz-Miranda, M.; Caporali, S.; Muniz-Miranda, F.; Pedone, A.; Innocenti, C.; Sangregorio, C. Magneto-plasmonic colloidal nanoparticles obtained by laser ablation of nickel and silver targets in water. J. Phys. Chem. C 2017, 121, 3597–3606. [Google Scholar] [CrossRef] [Green Version]
- Muniz-Miranda, M.; Muniz-Miranda, F.; Caporali, S.; Calisi, N.; Pedone, A. SERS, XPS and DFT investigation on palladium surfaces coated with 2,2′-bipyridine monolayers. Appl. Surf. Sci. 2018, 457, 98–103. [Google Scholar] [CrossRef]
- Lopez-Tocón, I.; Valdivia, S.; Soto, J.; Otero, J.C.; Muniz-Miranda, F.; Menziani, M.C.; Muniz-Miranda, M. A DFT Approach to the Surface-Enhanced Raman Scattering of 4-Cyanopyridine Adsorbed on Silver Nanoparticles. Nanomaterials 2019, 9, 1211. [Google Scholar] [CrossRef] [Green Version]
- Carballeira, L.; Pérez-Juste, I. Influence of calculation level and effect of methylation on axial/equatorial equilibria in piperidines. J. Comput. Chem. 1998, 19, 961–976. [Google Scholar] [CrossRef]
- Blackburne, J.D.; Katritzky, A.R.; Takeuchi, Y. Conformation of piperidine and of derivatives with additional ring hetero atoms. Acc. Chem. Res. 1975, 8, 300–306. [Google Scholar] [CrossRef]
- Anet, F.A.L.; Yavari, I. Nitrogen inversion in piperidine. J. Am. Chem. Soc. 1977, 99, 2794–2796. [Google Scholar] [CrossRef]
- Creighton, J.A.; Blatchford, C.G.; Albrecht, M.G. Plasma resonance enhancement of Raman scattering by pyridine adsorbed on silver or gold sol particles of size comparable to the excitation wavelength. J. Chem. Soc. Faraday Trans. II 1979, 75, 790–798. [Google Scholar] [CrossRef]
- Muniz-Miranda, M.; Neto, N.; Sbrana, G. Surface enhanced Raman scattering of pyrazine adsorbed on silver colloidal particles. J. Mol. Struct. 1986, 143, 275–278. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 09; Revision D.01; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar] [CrossRef] [Green Version]
- Becke, A.D. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef] [Green Version]
- Hay, P.J.; Wadt, W.R. Ab initio effective core potentials for molecular calculations. Potentials for the transition metal atoms Sc to Hg. J. Chem. Phys. 1985, 82, 270–283. [Google Scholar]
- Wadt, W.R.; Hay, P.J. Ab initio effective core potentials for molecular calculations. Potentials for main group elements Na to Bi. J. Chem. Phys. 1985, 82, 284–298. [Google Scholar]
- Hay, P.J.; Wadt, W.R. Ab initio effective core potentials for molecular calculations. Potentials for K to Au including the outermost core orbitals. J. Chem. Phys. 1985, 82, 299–310. [Google Scholar]
- Scalmani, G.; Frisch, M.J. Continuous surface charge polarizable continuum models of solvation. I. General formalism. J. Chem. Phys. 2010, 132, 114110. [Google Scholar] [CrossRef]
- Grimme, S.; Ehrlich, S.; Goerigk, L. Effect of the damping function in dispersion corrected density functional theory. J. Comp. Chem. 2011, 32, 1456–1465. [Google Scholar] [CrossRef]
- Yanai, T.; Tew, D.; Handy, N. A new hybrid exchange-correlation functional using the Coulomb-attenuating method (CAM-B3LYP). Chem. Phys. Lett. 2004, 393, 51–57. [Google Scholar] [CrossRef] [Green Version]
- Leopold, N.; Stefancu, A.; Herman, K.; Todor, I.S.; Iancu, S.D.; Moisoiu, V.; Leopold, L.F. The role of adatoms in chloride activated colloidal silver nanoparticles for surface-enhanced Raman scattering enhancement. Beilstein J. Nanotechnol. 2018, 9, 2236–2247. [Google Scholar] [CrossRef]
- Gellini, C.; Muniz-Miranda, F.; Pedone, A.; Muniz-Miranda, M. SERS active Ag-SiO2 nanoparticles obtained by laser ablation of silver in colloidal silica. Beilstein J. Nanotechnol. 2018, 9, 2396–2404. [Google Scholar] [CrossRef] [Green Version]
- Muniz-Miranda, M. SERS investigation on the adsorption and photoreaction of 4-nitroanisole in Ag hydrosols. J. Raman Spectrosc. 2013, 44, 1416–1421. [Google Scholar] [CrossRef]
- Sanchez-Cortes, S.; Garcia-Ramos, J.V.; Morcillo, G.; Tinti, A. Morphological Study of Silver Colloids Employed in Surface Enhanced Raman Spectroscopy: Activation when Exciting in Visible and Near-Infrared Regions. J. Colloid Interface Sci. 1995, 175, 358–368. [Google Scholar] [CrossRef]
- Parkin, A.; Oswald, J.D.H.; Parsons, S. Structures of piperazine, piperidine and morpholine. Acta Crystallogr. Sect. B 2004, 60, 219–227. [Google Scholar] [CrossRef]
- Vedal, D.; Ellestad, O.H.; Klaboe, P. The vibrational spectra of piperidine and morpholine and their N-deuterated analogs. Spectrochim. Acta Part A 1976, 32, 877–890. [Google Scholar] [CrossRef]
- Sbrana, G.; Neto, N.; Muniz-Miranda, M.; Nocentini, M. Surface-enhanced Raman spectra of 2,2′-bipyrimidine adsorbed on silver sol. J. Phys. Chem. 1990, 94, 3706–3710. [Google Scholar] [CrossRef]
- Foresti, M.L.; Funtikov, A.M.; Guidelli, R.; Muniz-Miranda, M. Relation between surface enhanced Raman intensity and surface concentration for 2-amino 5-nitro pyridine adsorbed on roughened polycrystalline silver electrodes. J. Electroanal. Chem. 1994, 367, 223–237. [Google Scholar] [CrossRef]
- Neto, N.; Muniz-Miranda, M.; Sbrana, G. SER Spectra of Bipyrazine Adsorbed on Silver Sols and Silver Electrodes. J. Phys. Chem. 1996, 100, 9911–9917. [Google Scholar] [CrossRef]
- Muniz-Miranda, M.; Sbrana, G. Evidence for Surface Ag+ Complex Formation by an Anion-Induced Effect in the SER Spectra of Phthalazine Adsorbed on Silver Sols. J. Raman Spectrosc. 1996, 27, 105–110. [Google Scholar] [CrossRef]
- Muniz-Miranda, M. Surface Enhanced Raman Scattering and Normal Coordinate Analysis of 1,10-Phenanthroline Adsorbed on Silver Sols. J. Phys. Chem. A 2000, 104, 7803–7810. [Google Scholar] [CrossRef]
- Pergolese, B.; Muniz-Miranda, M.; Bigotto, A. Study of the Adsorption of 1,2,3-Triazole on Silver and Gold Colloidal Nanoparticles by Means of Surface Enhanced Raman Scattering. J. Phys. Chem. B 2004, 108, 5698–5702. [Google Scholar] [CrossRef]
- Muniz-Miranda, M.; Gellini, C.; Pagliai, M.; Innocenti, M.; Salvi, P.R.; Schettino, V. SERS and Computational Studies on MicroRNA Chains Adsorbed on Silver Surfaces. J. Phys. Chem. C 2010, 114, 13730–13735. [Google Scholar] [CrossRef]
- Muniz-Miranda, F.; Pedone, A.; Muniz-Miranda, M. Raman and Computational Study on the Adsorption of Xanthine on Silver Nanocolloids. ACS Omega 2018, 3, 13530–13537. [Google Scholar] [CrossRef]
- Hao, Y.; Fang, Y. Piperidine adsorption on two different silver electrodes: A combined surface enhanced Raman spectroscopy and density functional theory study. J. Nanopart. Res. 2007, 9, 817–824. [Google Scholar] [CrossRef]
- Chang, R.K.; Laube, B.L. Surface-enhanced Raman scattering and nonlinear optics applied to electrochemistry. Crit. Rev. Solid State 1984, 12, 1–73. [Google Scholar] [CrossRef]
- Pagliai, M.; Muniz-Miranda, F.; Schettino, V.; Muniz-Miranda, M. Competitive Solvation and Chemisorption in Silver Colloidal Suspensions. Prog. Colloid Polym. Sci. 2012, 139, 39–44. [Google Scholar]
- Martin, T.; Brehm, M.; Fligg, R.; Vöhringer, P.; Kirchner, B. Computing vibrational spectra from ab initio molecular dynamics. Phys. Chem. Chem. Phys. 2013, 15, 6608–6622. [Google Scholar]
- Muniz-Miranda, F.; Pagliai, M.; Cardini, G.; Schettino, V. Wavelet Transform for Spectroscopic Analysis: Application to Diols in Water. J. Chem. Theory Comput. 2011, 7, 1109–1118. [Google Scholar] [CrossRef]
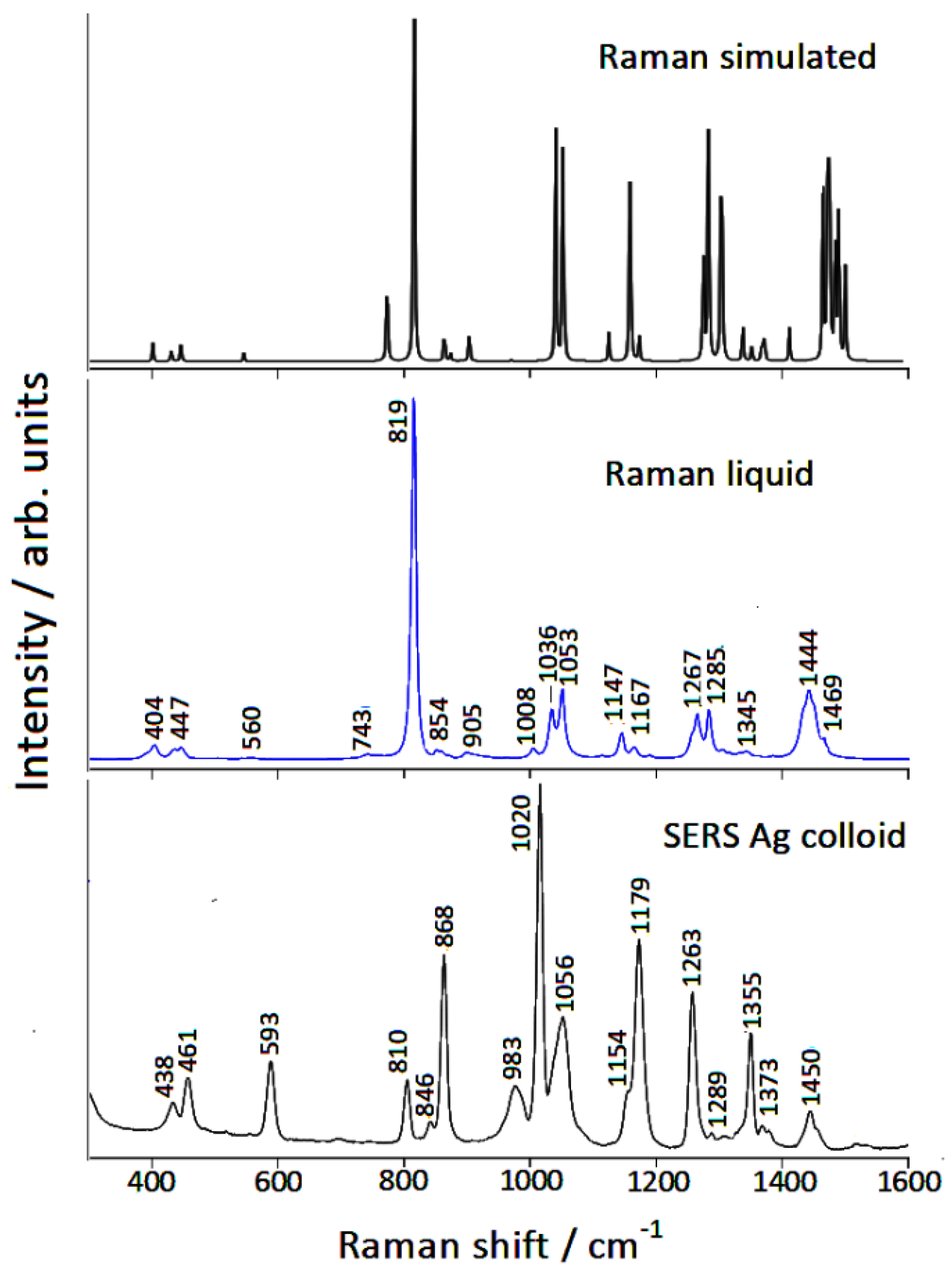
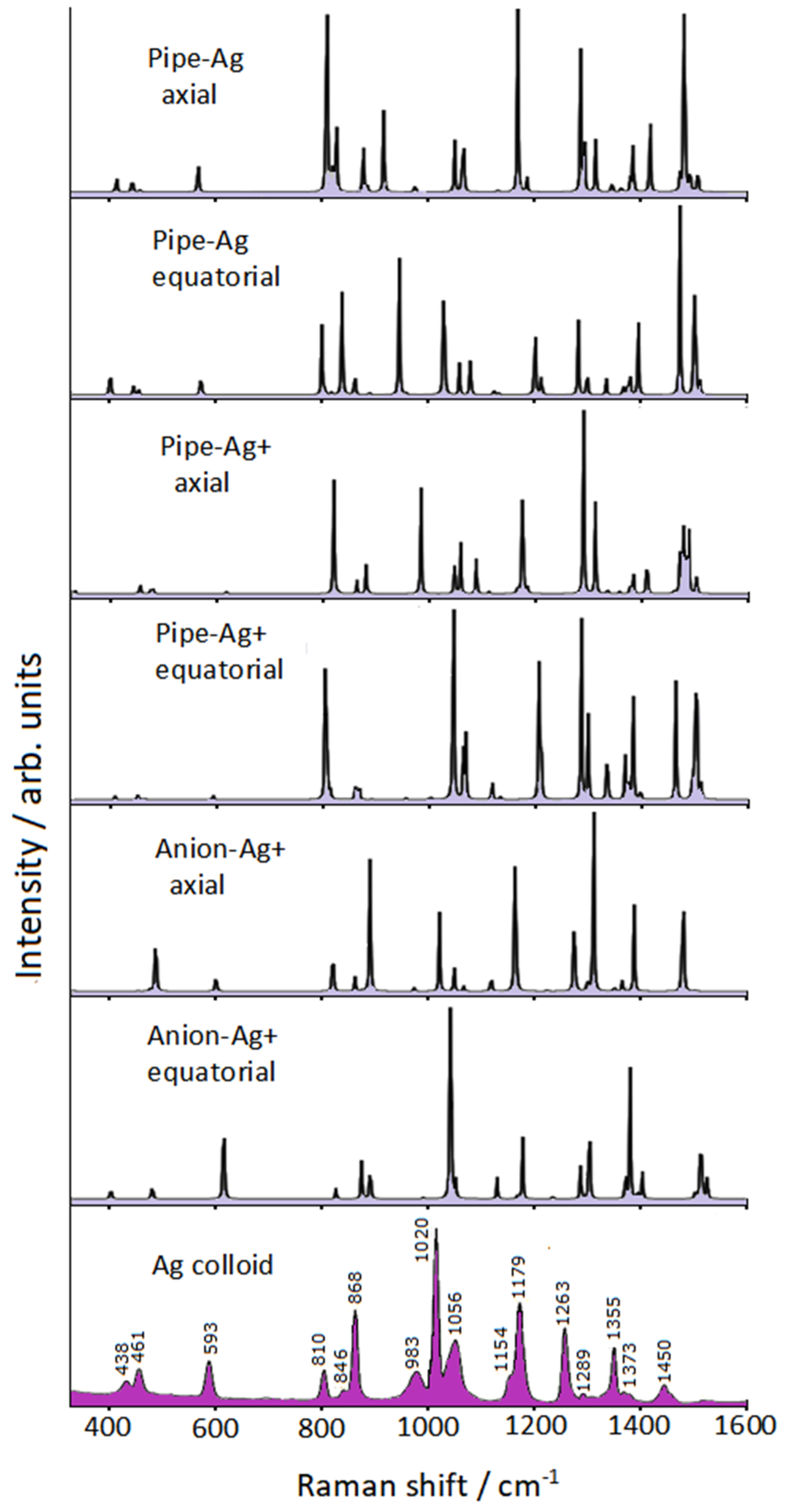
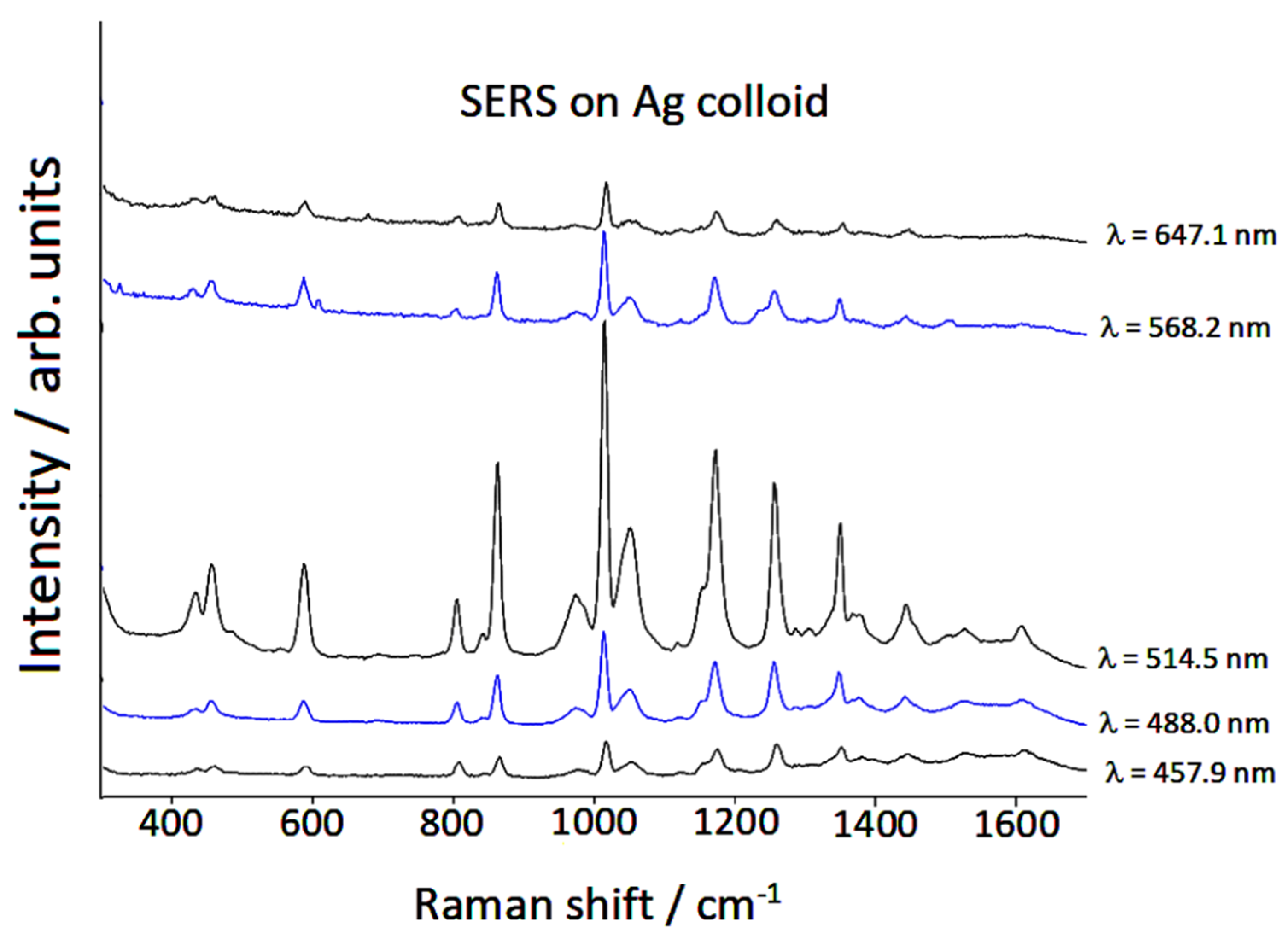

| Infrared Liquid [29] | Raman Liquid | Calculated | SERS Ag Colloid | Calculated # |
|---|---|---|---|---|
| 404 | 404 | 406 | ||
| 432 | 436 | 438 | 452 | |
| 445 | 447 | 452 | 461 | 478 |
| 546 | 560 | 550 | 593 | 611 |
| 743 | 743 | 779 | ||
| 822 | 819 | 822 | 810 | 821 |
| 846 | 868 | |||
| 859 | 854 | 871 | 868 | 884 |
| 906 | 905 | 909 | ||
| 964 | 976 | |||
| 1006 | 1008 | 1048 | 983 | 981 |
| 1035 | 1036 | 1059 | 1020 | 1032 |
| 1052 | 1053 | 1062 | 1056 | 1041 |
| 1115 | 1132 | |||
| 1146 | 1147 | 1165 | 1154 | 1157 |
| 1164 | 1167 | 1166 | 1179 | 1166 |
| 1191 | 1180 | |||
| 1258 | 1280 | |||
| 1266 | 1267 | 1282 | 1263 | 1275 |
| 1285 | 1285 | 1290 | 1289 | 1290 |
| 1318 | 1310 | |||
| 1332 | 1345 | 1345 | 1355 | 1367 |
| 1365 | 1360 | |||
| 1386 | 1380 | 1373 | 1389 | |
| 1436 | 1420 | |||
| 1444 | 1444 | 1471 | ||
| 1452 | 1479 | 1450 | 1494 | |
| 1460 | 1482 | |||
| 1468 | 1469 | 1491 | 1470 | 1498 |
| 1476 | 1497 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muniz-Miranda, F.; Pedone, A.; Menziani, M.C.; Muniz-Miranda, M. DFT and TD-DFT Study of the Chemical Effect in the SERS Spectra of Piperidine Adsorbed on Silver Colloidal Nanoparticles. Nanomaterials 2022, 12, 2907. https://doi.org/10.3390/nano12172907
Muniz-Miranda F, Pedone A, Menziani MC, Muniz-Miranda M. DFT and TD-DFT Study of the Chemical Effect in the SERS Spectra of Piperidine Adsorbed on Silver Colloidal Nanoparticles. Nanomaterials. 2022; 12(17):2907. https://doi.org/10.3390/nano12172907
Chicago/Turabian StyleMuniz-Miranda, Francesco, Alfonso Pedone, Maria Cristina Menziani, and Maurizio Muniz-Miranda. 2022. "DFT and TD-DFT Study of the Chemical Effect in the SERS Spectra of Piperidine Adsorbed on Silver Colloidal Nanoparticles" Nanomaterials 12, no. 17: 2907. https://doi.org/10.3390/nano12172907
APA StyleMuniz-Miranda, F., Pedone, A., Menziani, M. C., & Muniz-Miranda, M. (2022). DFT and TD-DFT Study of the Chemical Effect in the SERS Spectra of Piperidine Adsorbed on Silver Colloidal Nanoparticles. Nanomaterials, 12(17), 2907. https://doi.org/10.3390/nano12172907







