Albumin Stabilized Fe@C Core–Shell Nanoparticles as Candidates for Magnetic Hyperthermia Therapy
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Fe@C Structure
3.2. MHT Performance
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sappino, C.; Primitivo, L.; De Angelis, M.; Domenici, M.O.; Mastrodonato, A.; Romdan, I.B.; Tatangelo, C.; Suber, L.; Pilloni, L.; Ricelli, A.; et al. Functionalized Magnetic Nanoparticles as Catalysts for Enantioselective Henry Reaction. ACS Omega 2019, 4, 21809–21817. [Google Scholar] [CrossRef] [PubMed]
- Avasthi, A.; Caro, C.; Pozo-Torres, E.; Leal, M.P.; García-Martín, M.L. Magnetic Nanoparticles as MRI Contrast Agents; Springer International Publishing: Berlin, Germany, 2020; Volume 378, ISBN 0123456789. [Google Scholar]
- McBain, S.C.; Yiu, H.H.P.; Dobson, J. Magnetic Nanoparticles for Gene and Drug Delivery. Int. J. Nanomed. 2008, 3, 169–180. [Google Scholar] [CrossRef] [Green Version]
- Zahn, D.; Landers, J.; Buchwald, J.; Diegel, M.; Salamon, S.; Müller, R.; Köhler, M.; Ecke, G.; Wende, H.; Dutz, S. Ferrimagnetic Large Single Domain Iron Oxide Nanoparticles for Hyperthermia Applications. Nanomaterials 2022, 12, 343. [Google Scholar] [CrossRef] [PubMed]
- Yagawa, Y.; Tanigawa, K.; Kobayashi, Y.; Yamamoto, M. Cancer Immunity and Therapy Using Hyperthermia with Immunotherapy, Radiotherapy, Chemotherapy, and Surgery. J. Cancer Metastasis Treat. 2017, 3, 218. [Google Scholar] [CrossRef]
- Kowalik, P.; Mikulski, J.; Borodziuk, A.; Duda, M.; Kamińska, I.; Zajdel, K.; Rybusinski, J.; Szczytko, J.; Wojciechowski, T.; Sobczak, K.; et al. Yttrium-Doped Iron Oxide Nanoparticles for Magnetic Hyperthermia Applications. J. Phys. Chem. C 2020, 124, 6871–6883. [Google Scholar] [CrossRef]
- Rajan, A.; Sahu, N.K. Review on Magnetic Nanoparticle-Mediated Hyperthermia for Cancer Therapy. J. Nanoparticle Res. 2020, 22, 319. [Google Scholar] [CrossRef]
- Pimentel, B.; Caraballo-Vivas, R.J.; Checca, N.R.; Zverev, V.I.; Salakhova, R.T.; Makarova, L.A.; Pyatakov, A.P.; Perov, N.S.; Tishin, A.M.; Shtil, A.A.; et al. Threshold Heating Temperature for Magnetic Hyperthermia: Controlling the Heat Exchange with the Blocking Temperature of Magnetic Nanoparticles. J. Solid State Chem. 2018, 260, 34–38. [Google Scholar] [CrossRef]
- Kaur, P.; Aliru, M.L.; Chadha, A.S.; Asea, A.; Krishnan, S. Hyperthermia Using Nanoparticles-Promises and Pitfalls. Int. J. Hyperth. 2016, 32, 76–88. [Google Scholar] [CrossRef]
- Bonvin, D.; Alexander, D.T.L.; Millán, A.; Piñol, R.; Sanz, B.; Goya, G.F.; Martínez, A.; Bastiaansen, J.A.M.; Stuber, M.; Schenk, K.J.; et al. Tuning Properties of Iron Oxide Nanoparticles in Aqueous Synthesis without Ligands to Improve MRI Relaxivity and SAR. Nanomaterials 2017, 7, 225. [Google Scholar] [CrossRef] [Green Version]
- Hadadian, Y.; Ramos, A.P.; Pavan, T.Z. Role of Zinc Substitution in Magnetic Hyperthermia Properties of Magnetite Nanoparticles: Interplay between Intrinsic Properties and Dipolar Interactions. Sci. Rep. 2019, 9, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Manohar, A.; Geleta, D.D.; Krishnamoorthi, C.; Lee, J. Synthesis, Characterization and Magnetic Hyperthermia Properties of Nearly Monodisperse CoFe2O4 Nanoparticles. Ceram. Int. 2020, 46, 28035–28041. [Google Scholar] [CrossRef]
- Vamvakidis, K.; Katsikini, M.; Sakellari, D.; Paloura, E.C.; Kalogirou, O.; Dendrinou-Samara, C. Reducing the Inversion Degree of MnFe2O4 Nanoparticles through Synthesis to Enhance Magnetization: Evaluation of Their 1H NMR Relaxation and Heating Efficiency. Dalt. Trans. 2014, 43, 12754–12765. [Google Scholar] [CrossRef] [PubMed]
- Menelaou, M.; Georgoula, K.; Simeonidis, K.; Dendrinou-Samara, C. Evaluation of Nickel Ferrite Nanoparticles Coated with Oleylamine by NMR Relaxation Measurements and Magnetic Hyperthermia. Dalt. Trans. 2014, 43, 3626–3636. [Google Scholar] [CrossRef] [PubMed]
- Shlapa, Y.; Kulyk, M.; Kalita, V.; Polek, T.; Tovstolytkin, A.; Greneche, J.M.; Solopan, S.; Belous, A. Iron-Doped (La,Sr)MnO3 Manganites as Promising Mediators of Self-Controlled Magnetic Nanohyperthermia. Nanoscale Res. Lett. 2016, 11, 24. [Google Scholar] [CrossRef] [Green Version]
- Aneja, M.; Tovstolytkin, A.; Singh Lotey, G. Superparamagnetic LaSrMnO3 Nanoparticles for Magnetic Nanohyperthermia and Their Biocompatibility. J. Magn. Magn. Mater. 2017, 442, 423–428. [Google Scholar] [CrossRef]
- Rego, G.N.d.A.; Mamani, J.B.; Souza, T.K.F.; Nucci, M.P.; Silva, H.R.d.; Gamarra, L.F. Therapeutic Evaluation of Magnetic Hyperthermia Using Fe3O4-Aminosilane-Coated Iron Oxide Nanoparticles in Glioblastoma Animal Model. Einstein (Sao Paulo) 2019, 17, eAO4786. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gawali, S.L.; Shelar, S.B.; Gupta, J.; Barick, K.C.; Hassan, P.A. Immobilization of Protein on Fe3O4 Nanoparticles for Magnetic Hyperthermia Application. Int. J. Biol. Macromol. 2021, 166, 851–860. [Google Scholar] [CrossRef]
- Shaw, S.K.; Kailashiya, J.; Gangwar, A.; Alla, S.K.; Gupta, S.K.; Prajapat, C.L.; Meena, S.S.; Dash, D.; Maiti, P.; Prasad, N.K. γ-Fe2O3 Nanoflowers as Efficient Magnetic Hyperthermia and Photothermal Agent. Appl. Surf. Sci. 2021, 560, 150025. [Google Scholar] [CrossRef]
- El Mendili, Y.; Bardeau, J.F.; Randrianantoandro, N.; Greneche, J.M.; Grasset, F. Structural Behavior of Laser-Irradiated γ-Fe2O3 Nanocrystals Dispersed in Porous Silica Matrix: γ-Fe2O3to α-Fe2O3Phase Transition and Formation of ε-Fe2O3. Sci. Technol. Adv. Mater. 2016, 17, 597–609. [Google Scholar] [CrossRef] [Green Version]
- Billas, I.M.L.; Chatelain, A.; de Heer, W.A. Magnetism from the Atom to the Bulk in Iron, Cobalt, and Nickel Clusters. Science 1994, 265, 1682–1684. [Google Scholar] [CrossRef]
- Mehdaoui, B.; Meffre, A.; Lacroix, L.M.; Carrey, J.; Lachaize, S.; Gougeon, M.; Respaud, M.; Chaudret, B. Large Specific Absorption Rates in the Magnetic Hyperthermia Properties of Metallic Iron Nanocubes. J. Magn. Magn. Mater. 2010, 322, L49–L52. [Google Scholar] [CrossRef] [Green Version]
- Abdullaeva, Z.; Omurzak, E.; Iwamoto, C.; Ihara, H.; Ganapathy, H.S.; Sulaimankulova, S.; Koinuma, M.; Mashimo, T. Pulsed Plasma Synthesis of Iron and Nickel Nanoparticles Coated by Carbon for Medical Applications. Jpn. J. Appl. Phys. 2013, 52, 01AJ01. [Google Scholar] [CrossRef]
- Eremin, A.V.; Gurentsov, E.V.; Musikhin, S.A. Temperature Influence on the Properties of Carbon-Encapsulated Iron Nanoparticles Forming in Pyrolysis of Gaseous Precursors. J. Alloy. Compd. 2017, 727, 711–720. [Google Scholar] [CrossRef]
- Zhang, Z.; Wen, G. Synthesis and Characterization of Carbon-Encapsulated Magnetite, Martensite and Iron Nanoparticles by High-Energy Ball Milling Method. Mater. Charact. 2020, 167, 110502. [Google Scholar] [CrossRef]
- Yan, Q.; Street, J.; Yu, F. Synthesis of Carbon-Encapsulated Iron Nanoparticles from Wood Derived Sugars by Hydrothermal Carbonization (HTC) and Their Application to Convert Bio-Syngas into Liquid Hydrocarbons. Biomass Bioenergy 2015, 83, 85–95. [Google Scholar] [CrossRef] [Green Version]
- Bystrzejewski, M.; Łabedź, O.; Kaszuwara, W.; Huczko, A.; Lange, H. Controlling the Diameter and Magnetic Properties of Carbon-Encapsulated Iron Nanoparticles Produced by Carbon Arc Discharge. Powder Technol. 2013, 246, 7–15. [Google Scholar] [CrossRef]
- Zhigach, A.N.; Leipunsky, I.O.; Kuskov, M.L.; Berezkina, N.G.; Afanasenkova, E.S.; Safronova, O.A.; Kudrov, B.V.; Lopez, G.W.; Skryleva, E.A. Synthesis of Pure Titanium Carbide and Titanium Carbide/Hydride Core-Shell Nanoparticles via the Flow-Levitation Method, and Their Characterization. J. Alloy. Compd. 2020, 819, 153054. [Google Scholar] [CrossRef]
- Tsurin, V.A.; Yermakov, A.Y.; Uimin, M.A.; Mysik, A.A.; Shchegoleva, N.N.; Gaviko, V.S.; Maikov, V.V. Synthesis, Structure, and Magnetic Properties of Iron and Nickel Nanoparticles Encapsulated into Carbon. Phys. Solid State 2014, 56, 287–301. [Google Scholar] [CrossRef]
- Dadej, A.; Wo, A.; Bilski, P.; Piotrowska-kempisty, H. For Phenylbutazone. Materials 2022, 41, 3–16. [Google Scholar]
- Bartelmess, J.; Frasconi, M.; Balakrishnan, P.B.; Signorelli, A.; Echegoyen, L.; Pellegrino, T.; Giordani, S. Non-Covalent Functionalization of Carbon Nano-Onions with Pyrene-BODIPY Dyads for Biological Imaging. RSC Adv. 2015, 5, 50253–50258. [Google Scholar] [CrossRef]
- Zhao, W.; Song, C.; Pehrsson, P.E. Water-Soluble and Optically PH-Sensitive Single-Walled Carbon Nanotubes from Surface Modification. J. Am. Chem. Soc. 2002, 124, 12418–12419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zaremba, O.; Goldt, A.; Ramirez-Morales, M.; Khabushev, E.M.; Shulga, E.; Eremin, T.; Prikazchikova, T.; Orekhov, A.; Grebenko, A.; Zatsepin, T.S.; et al. Robust Technique for Dispersion of Single-Walled Carbon Nanotubes in Aqueous Solutions with TRNA. Carbon N. Y. 2019, 151, 175–180. [Google Scholar] [CrossRef]
- Du, P.; Bléger, D.; Charra, F.; Bouchiat, V.; Kreher, D.; Mathevet, F.; Attias, A.J. A Versatile Strategy towards Non-Covalent Functionalization of Graphene by Surface-Confined Supramolecular Self-Assembly of Janus Tectons. Beilstein J. Nanotechnol. 2015, 6, 632–639. [Google Scholar] [CrossRef] [Green Version]
- Nosrati, H.; Sefidi, N.; Sharafi, A.; Danafar, H.; Kheiri Manjili, H. Bovine Serum Albumin (BSA) Coated Iron Oxide Magnetic Nanoparticles as Biocompatible Carriers for Curcumin-Anticancer Drug. Bioorg. Chem. 2018, 76, 501–509. [Google Scholar] [CrossRef] [PubMed]
- Jurašin, D.D.; Ćurlin, M.; Capjak, I.; Crnković, T.; Lovrić, M.; Babič, M.; Horák, D.; Vrček, I.V.; Gajović, S. Surface Coating Affects Behavior of Metallic Nanoparticles in a Biological Environment. Beilstein J. Nanotechnol. 2016, 7, 246–262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kratz, F. Albumin as a Drug Carrier: Design of Prodrugs, Drug Conjugates and Nanoparticles. J. Control. Release 2008, 132, 171–183. [Google Scholar] [CrossRef]
- Ostroverkhov, P.; Semkina, A.; Naumenko, V.; Plotnikova, E.; Yakubovskaya, R.; Vodopyanov, S.; Abakumov, A.; Majouga, A.; Grin, M.; Chekhonin, V.; et al. HSA—Coated Magnetic Nanoparticles for Mri-Guided Photodynamic Cancer Therapy. Pharmaceutics 2018, 10, 248. [Google Scholar] [CrossRef] [Green Version]
- Chubarov, A.S. Serum Albumin for Magnetic Nanoparticles Coating. Magnetochemistry 2022, 8, 13. [Google Scholar] [CrossRef]
- Zhigach, A.N.; Leipunsky, I.O.; Kuskov, M.L.; Berezkina, N.G.; Afanasenkova, E.S.; Lopez, G.W.; Skryleva, E.A.; Menushenkov, V.P.; Zhigalina, O.M.; Khmelenin, D.N. On the Production of Dispersive Single-Crystal Iron Carbide (Fe3C) Nanoparticulate. Bull. Mater. Sci. 2022, 45, 38. [Google Scholar] [CrossRef]
- Kekalo, K.; Baker, I.; Meyers, R.; Shyong, J. Magnetic Nanoparticles with High Specific Absorption Rate at Low Alternating Magnetic Field. Nano Life 2015, 5, 1550002. [Google Scholar] [CrossRef]
- Zeng, Q.; Baker, I.D.D.S.; Loudis, J.A.; Liao, Y.F.; Hoopes, P.J. Synthesis and Heating Effect of Iron/Iron Oxide Composite and Iron Oxide Nanoparticles. Proc. SPIE 2007, 6440, 149–159. [Google Scholar] [CrossRef] [Green Version]
- Iacovita, C.; Stiufiuc, G.F.; Dudric, R.; Vedeanu, N.; Tetean, R.; Stiufiuc, R.I.; Lucaciu, C.M. Saturation of Specific Absorption Rate for Soft and Hard Spinel Ferrite Nanoparticles Synthesized by Polyol Process. Magnetochemistry 2020, 6, 23. [Google Scholar] [CrossRef]
- Kossatz, S.; Grandke, J.; Couleaud, P.; Latorre, A.; Aires, A.; Crosbie Staunton, K.; Ludwig, R.; Dähring, H.; Ettelt, V.; Lazaro-Carrillo, A.; et al. Efficient Treatment of Breast Cancer Xenografts with Multifunctionalized Iron Oxide Nanoparticles Combining Magnetic Hyperthermia and Anti-Cancer Drug Delivery. Breast Cancer Res. 2015, 17, 66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ludwig, R.; Stapf, M.; Dutz, S.; Müller, R.; Teichgräber, U.; Hilger, I. Structural Properties of Magnetic Nanoparticles Determine Their Heating Behavior-an Estimation of the in Vivo Heating Potential. Nanoscale Res. Lett. 2014, 9, 602. [Google Scholar] [CrossRef] [Green Version]
- Cervadoro, A.; Giverso, C.; Pande, R.; Sarangi, S.; Preziosi, L.; Wosik, J.; Brazdeikis, A.; Decuzzi, P. Design Maps for the Hyperthermic Treatment of Tumors with Superparamagnetic Nanoparticles. PLoS ONE 2013, 8, e57332. [Google Scholar] [CrossRef] [Green Version]
- Lanier, O.L.; Korotych, O.I.; Monsalve, A.G.; Wable, D.; Savliwala, S.; Grooms, N.W.F.; Nacea, C.; Tuitt, O.R.; Dobson, J. Evaluation of Magnetic Nanoparticles for Magnetic Fluid Hyperthermia. Int. J. Hyperth. 2019, 36, 687–701. [Google Scholar] [CrossRef]
- Singh, G.; Kumar, P.A.; Lundgren, C.; van Helvoort, T.J.; Methieu, R.; Washlstrom, R.; Glomm, W.R. Tunability in Crystallinity and Magnetic Properties of Core–Shell Fe Nanoparticles. Part. Part. Syst. Char. 2014. 31, 1054–1059. [CrossRef]
- Omelyanchik, A.; Varvaro, G.; Gorshenkov, M.; Medvedev, A.; Bagazeev, A.; Beketov, I.; Rodionova, V. High-Quality α-Fe Nanoparticles Synthesized by the Electric Explosion of Wires. J. Magn. Magn. Mater. 2019, 484, 196–200. [Google Scholar] [CrossRef]
- Hou, D.L.; Nie, X.F.; Luo, H.L. Magnetic Anisotropy and Coercivity of Ultrafine Iron Particles. J. Magn. Magn. Mater. 1998, 188, 169–172. [Google Scholar] [CrossRef]
- Wood, I.G.; Vočadlo, L.; Knight, K.S.; Dobson, D.P.; Marshall, W.G.; Price, G.D.; Brodholt, J. Thermal Expansion and Crystal Structure of Cementite, Fe3C, between 4 and 600 K Determined by Time-of-Flight Neutron Powder Diffraction. J. Appl. Crystallogr. 2004, 37, 82–90. [Google Scholar] [CrossRef] [Green Version]
- Guskos, N.; Typek, J.; Maryniak, M.; Narkiewicz, U.; Arabczyk, W.; Kucharewicz, I. Temperature Dependence of FMR Spectrum of Fe3C magnetic agglomerates. J. Phys. Conf. Ser. 2005, 10, 151–154. [Google Scholar] [CrossRef]
- Francia, V.; Montizaan, D.; Salvati, A. Interactions at the Cell Membrane and Pathways of Internalization of Nano-Sized Materials for Nanomedicine. Beilstein J. Nanotechnol. 2020, 11, 338–353. [Google Scholar] [CrossRef] [PubMed]
- Fang, R.H.; Kroll, A.V.; Gao, W.; Zhang, L. Cell Membrane Coating Nanotechnology1. Adv. Mater. 2018, 30, 1706759. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.; Yeap, S.P.; Che, H.X.; Low, S.C. Characterization of magnetic nanoparticle by dynamic light scattering. Nanoscale Res Lett. 2013, 8, 381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Freitas, C.; Müller, R.H. Effect of Light and Temperature on Zeta Potential and Physical Stability in Solid Lipid Nanoparticle (SLN®) Dispersions. Int. J. Pharm. 1998, 168, 221–229. [Google Scholar] [CrossRef]
- Conde-Leboran, I.; Baldomir, D.; Martinez-Boubeta, C.; Chubykalo-Fesenko, O.; Morales, M.d.P.; Salas, G.; Cabrera, D.; Camarero, J.; Teran, F.J.; Serantes, D. A Single Picture Explains Diversity of Hyperthermia Response of Magnetic Nanoparticles. J. Phys. Chem. C 2015, 119, 15698–15706. [Google Scholar] [CrossRef]
- Feng, Q.; Liu, Y.; Huang, J.; Chen, K.; Huang, J.; Xiao, K. Uptake, Distribution, Clearance, and Toxicity of Iron Oxide Nanoparticles with Different Sizes and Coatings. Sci. Rep. 2018, 8, 1–14. [Google Scholar]
- El-Boubbou, K.; Lemine, O.M.; Ali, R.; Huwaizi, S.M.; Al-Humaid, S.; AlKushi, A. Evaluating Magnetic and Thermal Effects of Various Polymerylated Magnetic Iron Oxide Nanoparticles for Combined Chemo-Hyperthermia. New J. Chem. 2022, 46, 5489–5504. [Google Scholar] [CrossRef]
- Andreu, I.; Natividad, E. Accuracy of Available Methods for Quantifying the Heat Power Generation of Nanoparticles for Magnetic Hyperthermia. Int. J. Hyperth. 2013, 29, 739–751. [Google Scholar] [CrossRef] [Green Version]
- Dutz, S.; Hergt, R. Magnetic Particle Hyperthermia-A Promising Tumour Therapy? Nanotechnology 2014, 25, 452001. [Google Scholar] [CrossRef]
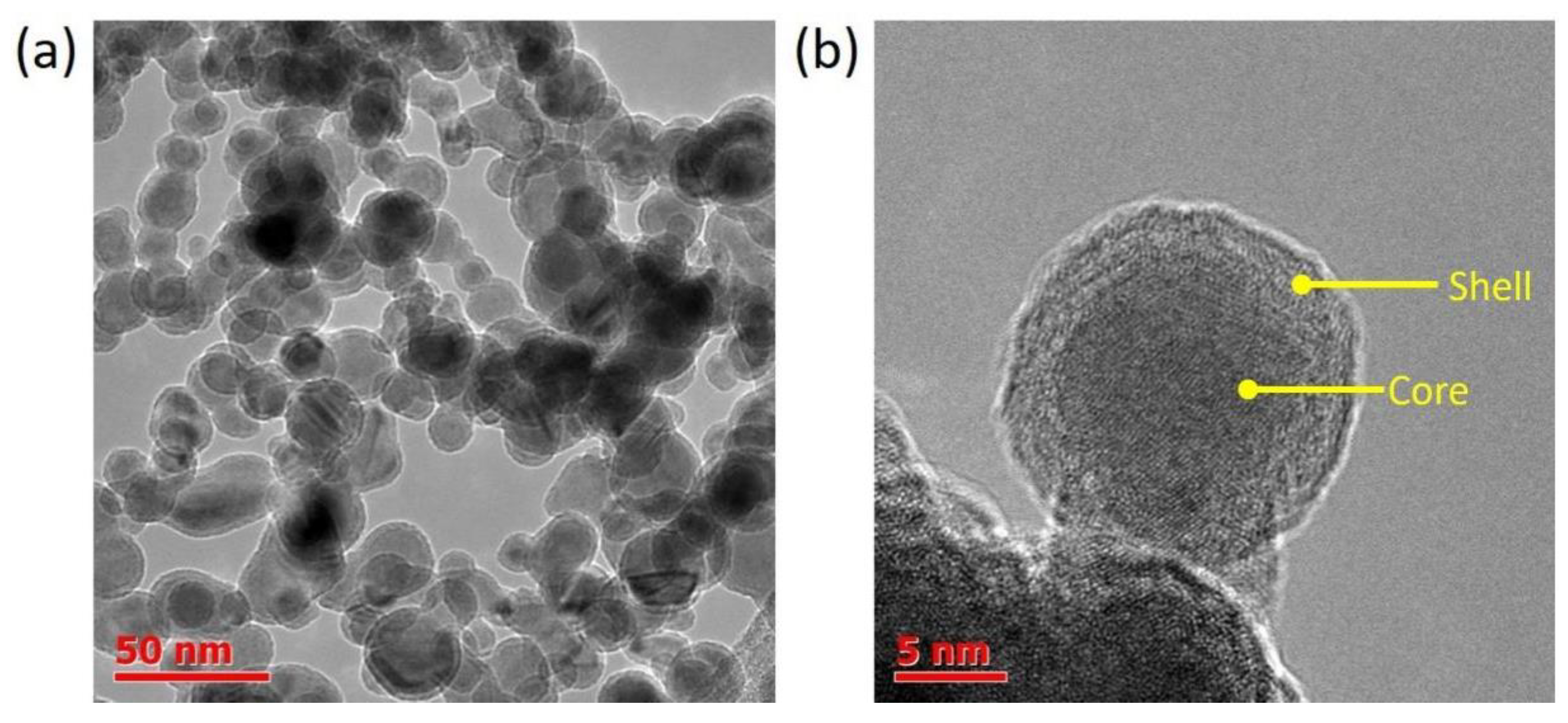
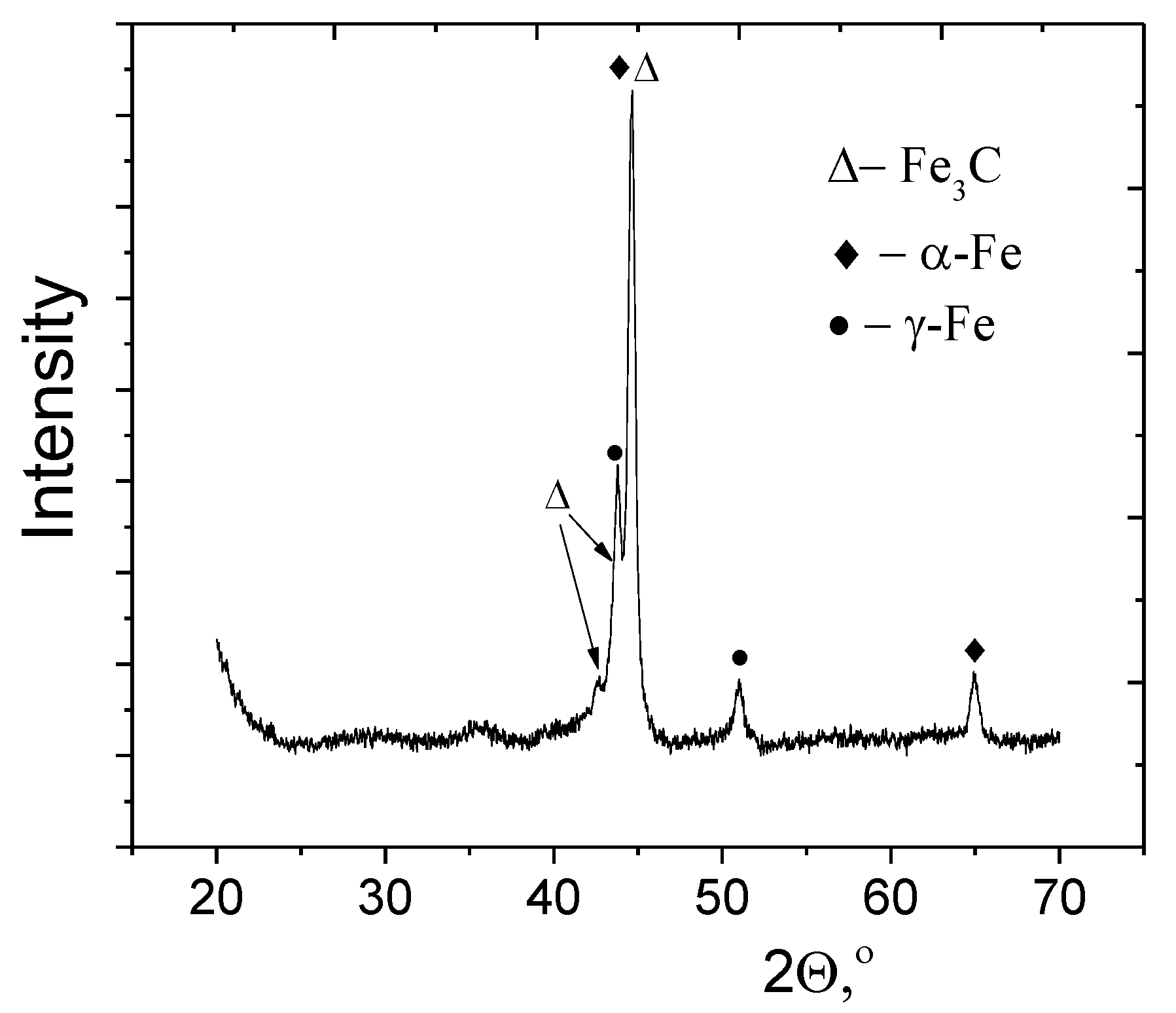
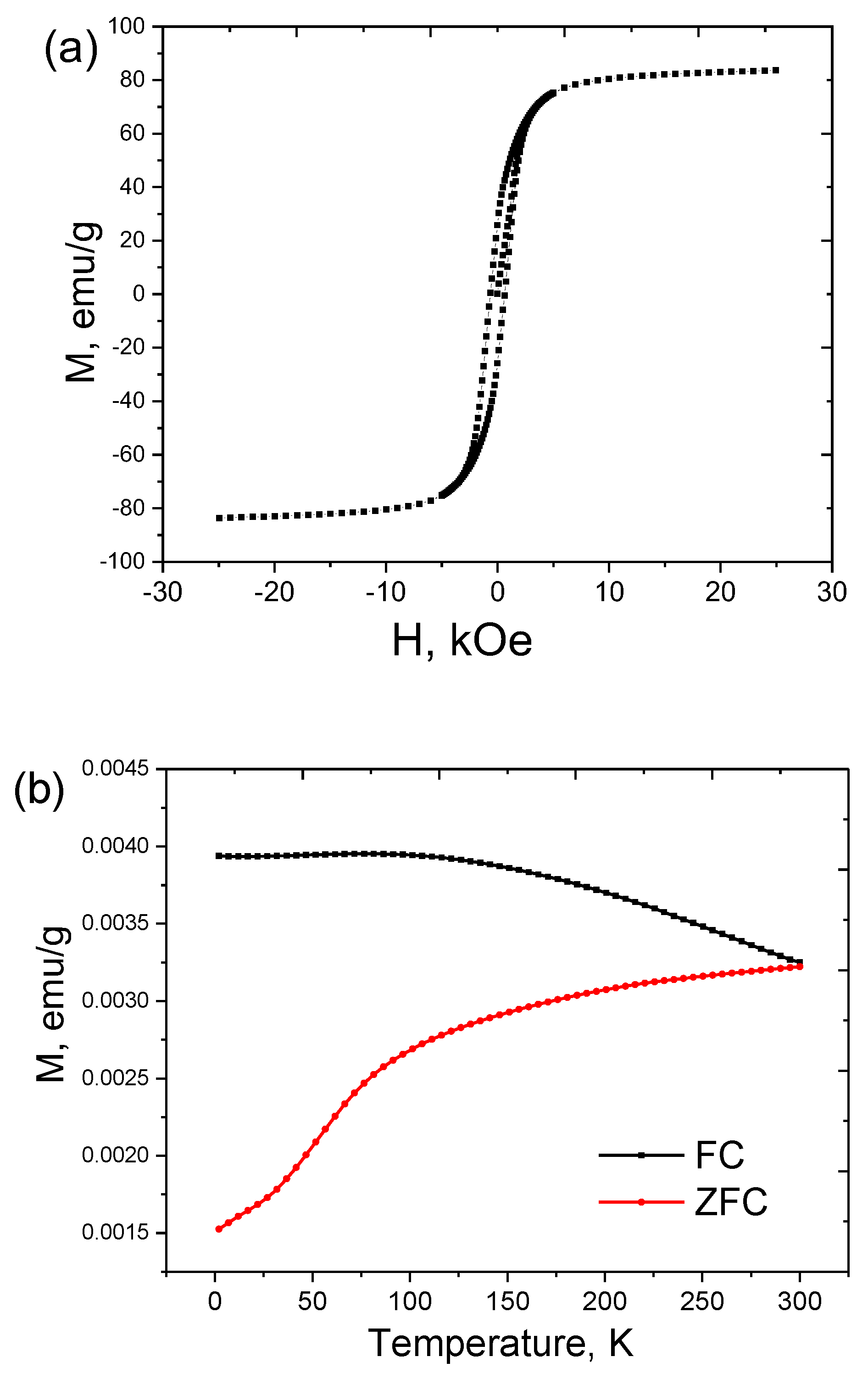
| Fe@C Concentration, mg/mL | Diameter, nm | Zeta Potential, mV |
|---|---|---|
| 1.0 | 17 ± 3 | −32 ± 5 |
| 2.5 | 22 ± 5 | −32 ± 7 |
| 5.0 | 24 ± 7 | −31 ± 7 |
| Concentration, mg/mL | SAR, W/g |
|---|---|
| 1.0 | 437.64 |
| 2.5 | 129.36 |
| 5.0 | 50.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramírez-Morales, M.A.; Goldt, A.E.; Kalachikova, P.M.; Ramirez B., J.A.; Suzuki, M.; Zhigach, A.N.; Ben Salah, A.; Shurygina, L.I.; Shandakov, S.D.; Zatsepin, T.; et al. Albumin Stabilized Fe@C Core–Shell Nanoparticles as Candidates for Magnetic Hyperthermia Therapy. Nanomaterials 2022, 12, 2869. https://doi.org/10.3390/nano12162869
Ramírez-Morales MA, Goldt AE, Kalachikova PM, Ramirez B. JA, Suzuki M, Zhigach AN, Ben Salah A, Shurygina LI, Shandakov SD, Zatsepin T, et al. Albumin Stabilized Fe@C Core–Shell Nanoparticles as Candidates for Magnetic Hyperthermia Therapy. Nanomaterials. 2022; 12(16):2869. https://doi.org/10.3390/nano12162869
Chicago/Turabian StyleRamírez-Morales, Maria Antonieta, Anastasia E. Goldt, Polina M. Kalachikova, Javier A. Ramirez B., Masashi Suzuki, Alexey N. Zhigach, Asma Ben Salah, Liliya I. Shurygina, Sergey D. Shandakov, Timofei Zatsepin, and et al. 2022. "Albumin Stabilized Fe@C Core–Shell Nanoparticles as Candidates for Magnetic Hyperthermia Therapy" Nanomaterials 12, no. 16: 2869. https://doi.org/10.3390/nano12162869
APA StyleRamírez-Morales, M. A., Goldt, A. E., Kalachikova, P. M., Ramirez B., J. A., Suzuki, M., Zhigach, A. N., Ben Salah, A., Shurygina, L. I., Shandakov, S. D., Zatsepin, T., Krasnikov, D. V., Maekawa, T., Nikolaev, E. N., & Nasibulin, A. G. (2022). Albumin Stabilized Fe@C Core–Shell Nanoparticles as Candidates for Magnetic Hyperthermia Therapy. Nanomaterials, 12(16), 2869. https://doi.org/10.3390/nano12162869










