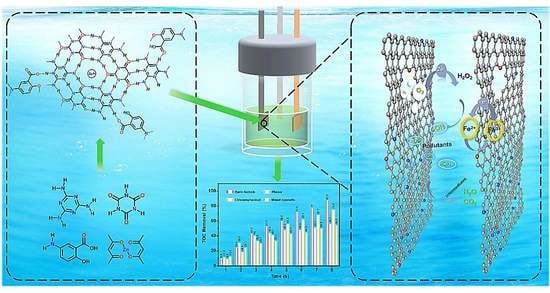
Supramolecular Self-Assembly Strategy towards Fabricating Mesoporous Nitrogen-Rich Carbon for Efficient Electro-Fenton Degradation of Persistent Organic Pollutants
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
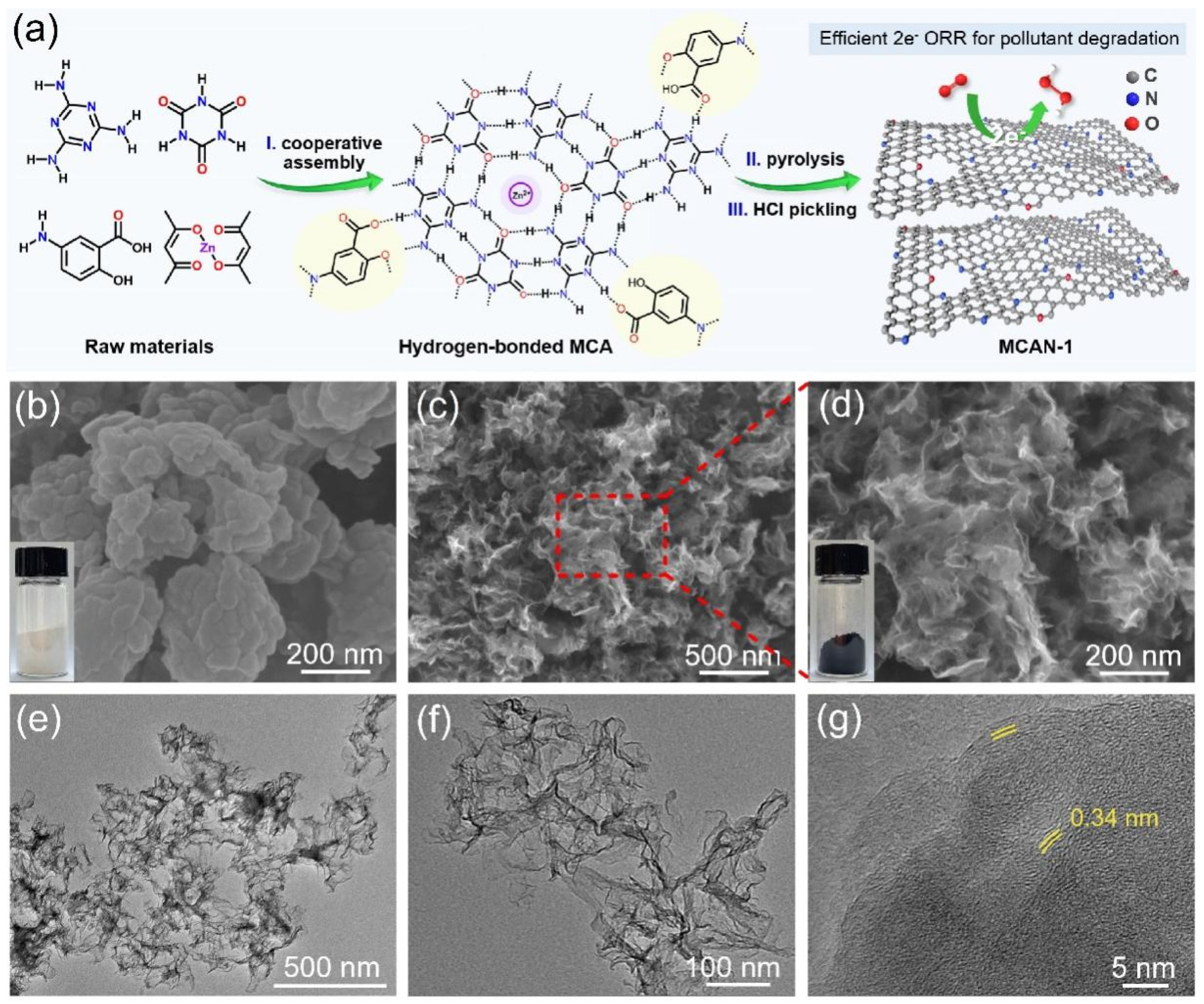
2.2. Preparation of Mesoporous Nitrogen-Rich Carbon Materials
2.3. Characterizations
2.4. Electrochemical Measurements
3. Results and Discussion
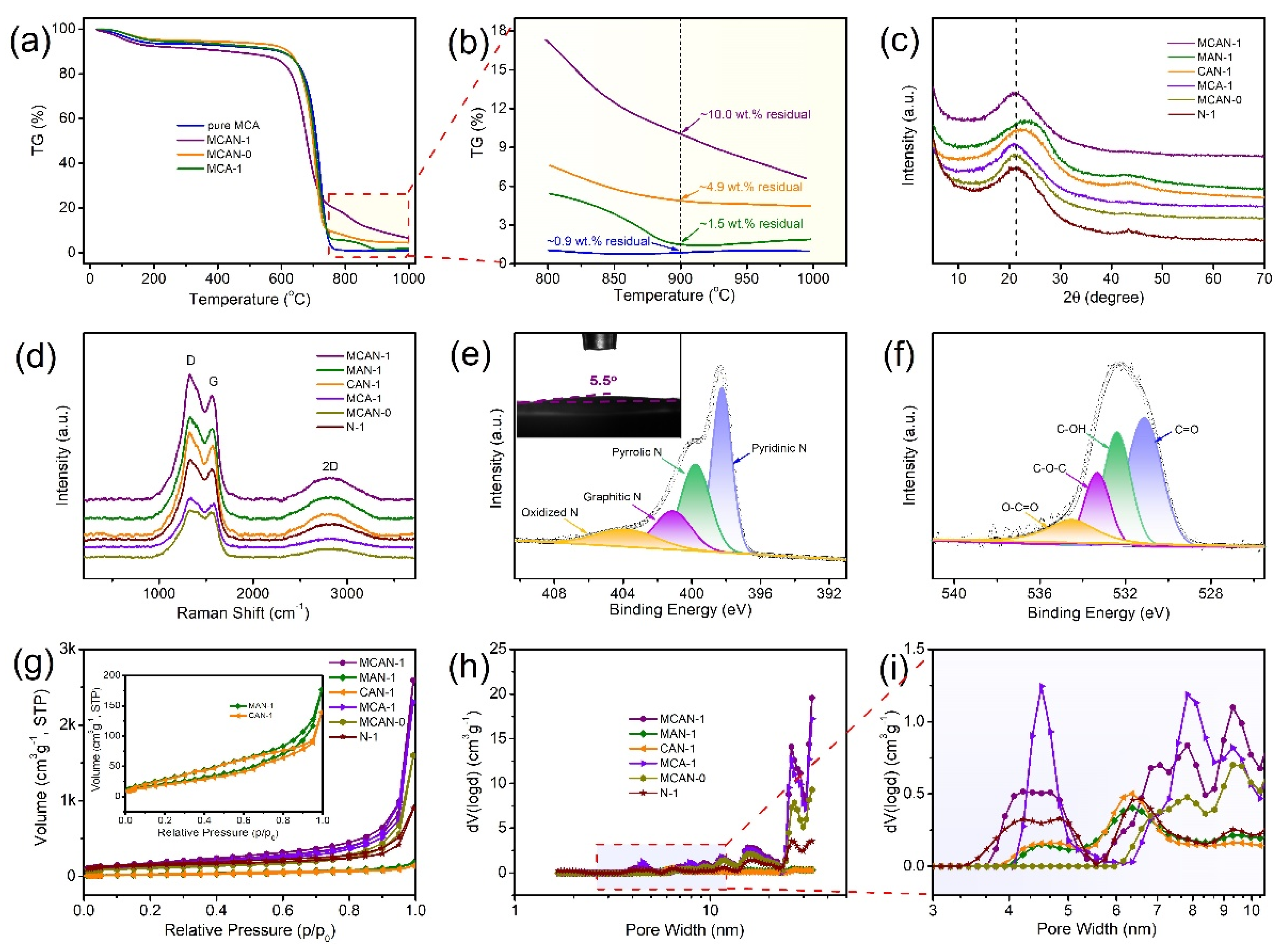
3.1. Catalyst Characterization
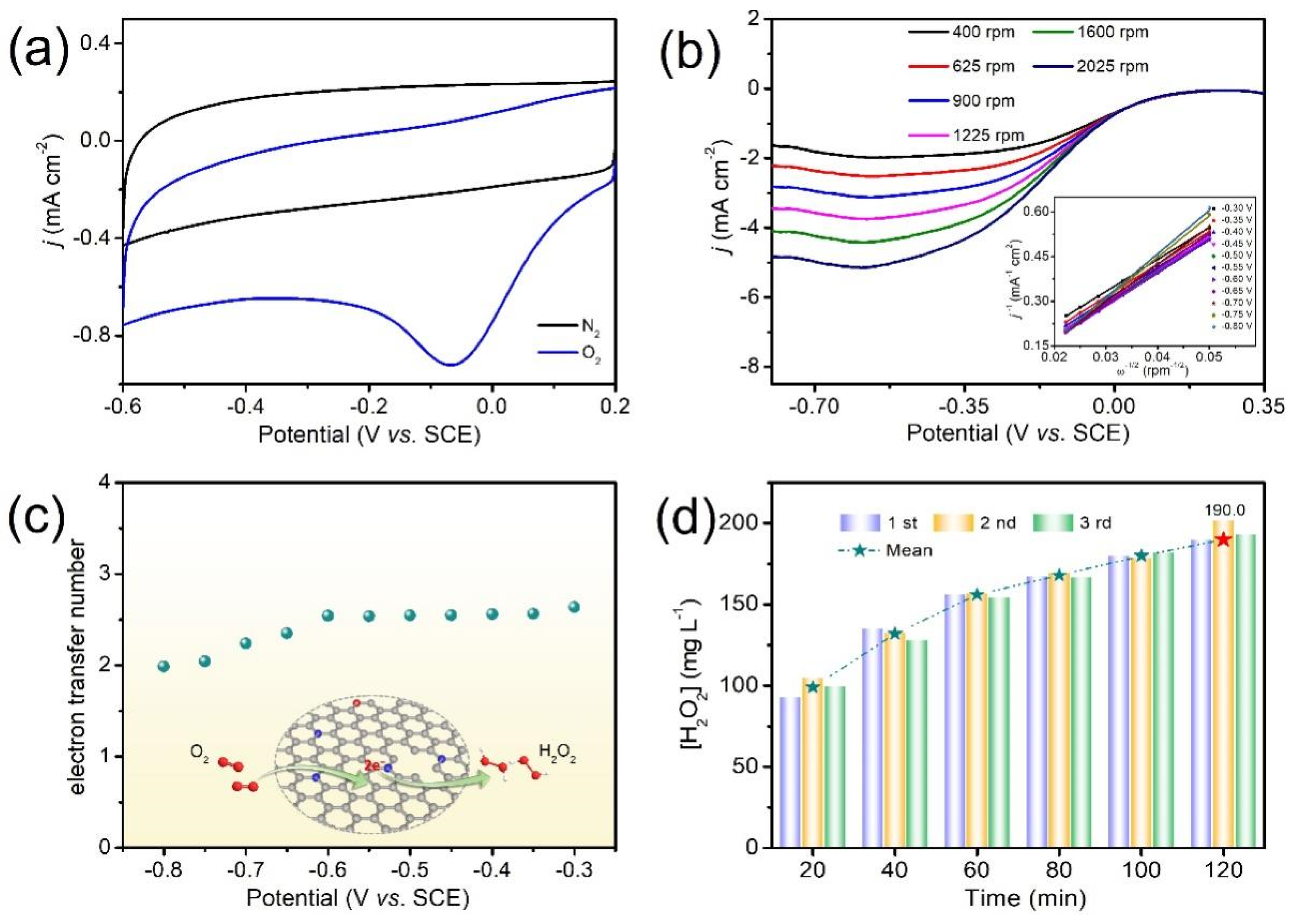
3.2. Electrochemical Measurements
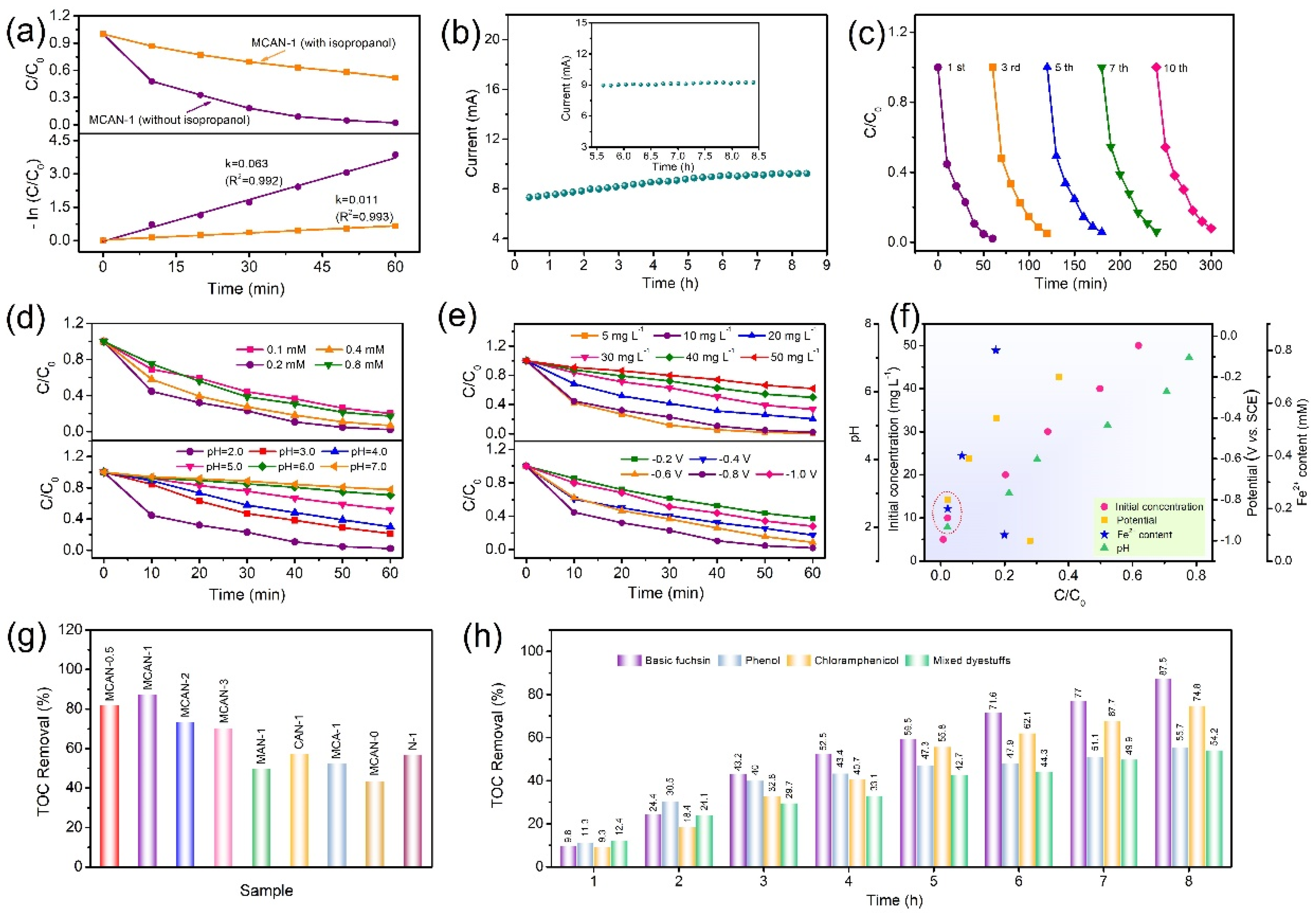
3.3. Degradation of Organic Pollutants
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Tian, Q.; Xiao, F.; Zhao, H.; Fei, X.; Shen, X.; Postole, G.; Zhao, G. Simultaneously accelerating the regeneration of FeII and the selectivity of 2e− oxygen reduction over sulfide iron-based carbon aerogel in electro-Fenton system. Appl. Catal. B Environ. 2020, 272, 119039. [Google Scholar] [CrossRef]
- Huang, B.-C.; Jiang, J.; Wang, W.-K.; Li, W.-W.; Zhang, F.; Jiang, H.; Yu, H.-Q. Electrochemically catalytic degradation of phenol with hydrogen peroxide in situ generated and activated by a municipal sludge-derived catalyst. ACS Sustain. Chem. Eng. 2018, 6, 5540–5546. [Google Scholar] [CrossRef]
- Ko, Y.-J.; Kim, H.-G.; Seid, M.G.; Cho, K.; Choi, J.-W.; Lee, W.-S.; Hong, S.W. Ionic-liquid-derived nitrogen-doped carbon electrocatalyst for peroxide generation and divalent iron regeneration: Its application for removal of aqueous organic compounds. ACS Sustain. Chem. Eng. 2018, 6, 14857–14865. [Google Scholar] [CrossRef]
- Zhu, Y.; Deng, F.; Qiu, S.; Ma, F.; Zheng, Y.; Lian, R. Enhanced electro-Fenton degradation of sulfonamides using the N, S co-doped cathode: Mechanism for H2O2 formation and pollutants decay. J. Hazard. Mater. 2021, 403, 123950. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Qiao, S.; Zhou, J.; Quan, X. A novel porous-carbon-based hollow fiber membrane with electrochemical reduction mediated by in-situ hydroxyl radical generation for fouling control and water treatment. Appl. Catal. B Environ. 2019, 255, 117772. [Google Scholar] [CrossRef]
- Ren, G.; Zhou, M.; Su, P.; Yang, W.; Lu, X.; Zhang, Y. Simultaneous sulfadiazines degradation and disinfection from municipal secondary effluent by a flow-through electro-Fenton process with graphene-modified cathode. J. Hazard. Mater. 2019, 368, 830–839. [Google Scholar] [CrossRef]
- Huang, J.; Lu, Y.; Zhang, H.; Shangguan, L.; Mou, Z.; Sun, J.; Sun, S.; He, J.; Lei, W. Template-free synthesis of mesh-like graphic carbon nitride with optimized electronic band structure for enhanced photocatalytic hydrogen evolution. Chem. Eng. J. 2021, 405, 126685. [Google Scholar] [CrossRef]
- Wu, J.; Chen, J.; Huang, Y.; Feng, K.; Deng, J.; Huang, W.; Wu, Y.; Zhong, J.; Li, Y. Cobalt atoms dispersed on hierarchical carbon nitride support as the cathode electrocatalyst for high-performance lithium-polysulfide batteries. Sci. Bull. 2019, 64, 1875–1880. [Google Scholar] [CrossRef]
- Yang, H.; Liu, Y.; Luo, Y.; Lu, S.; Su, B.; Ma, J. Achieving high activity and selectivity of nitrogen reduction via Fe–N3 coordination on iron single-atom electrocatalysts at ambient conditions. ACS Sustain. Chem. Eng. 2020, 8, 12809–12816. [Google Scholar] [CrossRef]
- Zhao, L.; Sui, X.-L.; Li, J.-Z.; Zhang, J.-J.; Zhang, L.-M.; Huang, G.-S.; Wang, Z.-B. Supramolecular assembly promoted synthesis of three-dimensional nitrogen doped graphene frameworks as efficient electrocatalyst for oxygen reduction reaction and methanol electrooxidation. Appl. Catal. B Environ. 2018, 231, 224–233. [Google Scholar] [CrossRef]
- Yu, W.; Zhang, T.; Zhao, Z. Garland-like intercalated carbon nitride prepared by an oxalic acid-mediated assembly strategy for highly-efficient visible-light-driven photoredox catalysis. Appl. Catal. B Environ. 2020, 278, 119342. [Google Scholar] [CrossRef]
- Guo, Y.; Li, J.; Yuan, Y.; Li, L.; Zhang, M.; Zhou, C.; Lin, Z. A rapid microwave-assisted thermolysis route to highly crystalline carbon nitrides for efficient hydrogen generation. Angew. Chem. Int. Ed. 2016, 55, 14693–14697. [Google Scholar] [CrossRef]
- Chen, J.; Mao, Z.; Zhang, L.; Wang, D.; Xu, R.; Bie, L.; Fahlman, B.D. Nitrogen-deficient graphitic carbon nitride with enhanced performance for lithium ion battery anodes. ACS Nano 2017, 11, 12650–12657. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, Y.; Zhang, L.; Xie, F.; Vasileff, A.; Qiao, S.Z. Graphitic carbon nitride (g-C3N4)-derived N-rich graphene with tuneable interlayer distance as a high-rate anode for sodium-ion batteries. Adv. Mater. 2019, 31, e1901261. [Google Scholar] [CrossRef]
- Ding, Y.; Yu, C.; Chang, J.; Yao, C.; Yu, J.; Guo, W.; Qiu, J. Effective fixation of carbon in g-C3N4 enabled by Mg-induced selective reconstruction. Small 2020, 16, e1907164. [Google Scholar] [CrossRef]
- Li, X.; Guan, B.Y.; Gao, S.; Lou, X.W. A general dual-templating approach to biomass-derived hierarchically porous heteroatom-doped carbon materials for enhanced electrocatalytic oxygen reduction. Energy Environ. Sci. 2019, 12, 648–655. [Google Scholar] [CrossRef]
- Tian, M.; Zhu, Y.; Chen, Y.; Liu, X.; Yang, Y.; Gao, S. Template-assisted self-activation of mesoporous carbon with active nitrogen/oxygen configurations for sustainable triboelectric nanogenerator powered electro-Fenton degradation. Nano Energy 2021, 83, 105825. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, Y.; Chen, Y.; Liu, X.; Li, X.; Gao, S. A treasure map for nonmetallic catalysts: Optimal nitrogen and fluorine distribution of biomass-derived carbon materials for high-performance oxygen reduction catalysts. J. Mater. Chem. A 2021, 9, 18251–18259. [Google Scholar] [CrossRef]
- Tian, M.; Zhu, Y.; Zhang, D.; Wang, M.; Chen, Y.; Yang, Y.; Gao, S. Pyrrolic-nitrogen-rich biomass-derived catalyst for sustainable degradation of organic pollutant via a self-powered electro-Fenton process. Nano Energy 2019, 64, 103940. [Google Scholar] [CrossRef]
- Chen, C.; Zhu, Y.; Tian, M.; Chen, Y.; Yang, Y.; Jiang, K.; Gao, S. Sustainable self-powered electro-Fenton degradation using N, S co-doped porous carbon catalyst fabricated with adsorption-pyrolysis-doping strategy. Nano Energy 2021, 81, 105623. [Google Scholar] [CrossRef]
- Yi, S.; Qin, X.; Liang, C.; Li, J.; Rajagopalan, R.; Zhang, Z.; Song, J.; Tang, Y.; Cheng, F.; Wang, H.; et al. Insights into KMnO4 etched N-rich carbon nanotubes as advanced electrocatalysts for Zn-air batteries. Appl. Catal. B Environ. 2020, 264, 118537. [Google Scholar] [CrossRef]
- Chen, Y.; Zhu, Y.; Tian, M.; Chen, C.; Jia, X.; Gao, S. Sustainable self-powered electro-Fenton degradation of organic pollutants in wastewater using carbon catalyst with controllable pore activated by EDTA-2Na. Nano Energy 2019, 59, 346–353. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, Y.; Liu, Y.; Liu, X.; Gao, S. Self-catalyzed growth of Zn/Co-N-C carbon nanotubes derived from metal-organic frameworks as efficient oxygen reduction catalysts for Zn-air battery. Sci. China Mater. 2022, 65, 653–662. [Google Scholar] [CrossRef]
- Qi, H.; Sun, X.; Sun, Z. Porous graphite felt electrode with catalytic defects for enhanced degradation of pollutants by electro-Fenton process. Chem. Eng. J. 2021, 403, 126270. [Google Scholar] [CrossRef]
- Tian, M.; Zhang, D.; Wang, M.; Zhu, Y.; Chen, C.; Chen, Y.; Jiang, T.; Gao, S. Engineering flexible 3D printed triboelectric nanogenerator to self-power electro-Fenton degradation of pollutants. Nano Energy 2020, 74, 104908. [Google Scholar] [CrossRef]
- Han, G.F.; Li, F.; Zou, W.; Karamad, M.; Jeon, J.P.; Kim, S.W.; Kim, S.J.; Bu, Y.; Fu, Z.; Lu, Y.; et al. Building and identifying highly active oxygenated groups in carbon materials for oxygen reduction to H2O2. Nat. Commun. 2020, 11, 2209. [Google Scholar] [CrossRef]
- Peng, L.; Hung, C.T.; Wang, S.; Zhang, X.; Zhu, X.; Zhao, Z.; Wang, C.; Tang, Y.; Li, W.; Zhao, D. Versatile nanoemulsion assembly approach to synthesize functional mesoporous carbon nanospheres with tunable pore sizes and architectures. J. Am. Chem. Soc. 2019, 141, 7073–7080. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Geng, K.; Liu, H.; Wei, X.; Zhang, M.; Wang, P.; Wang, J. Transforming organic-rich amaranthus waste into nitrogen-doped carbon with superior performance of the oxygen reduction reaction. Energy Environ. Sci. 2015, 8, 221–229. [Google Scholar] [CrossRef]
- Chang, Q.; Zhang, P.; Mostaghimi, A.H.B.; Zhao, X.; Denny, S.R.; Lee, J.H.; Gao, H.; Zhang, Y.; Xin, H.L.; Siahrostami, S.; et al. Promoting H2O2 production via 2-electron oxygen reduction by coordinating partially oxidized Pd with defect carbon. Nat. Commun. 2020, 11, 2178. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.-H.; Wang, D.; Lu, X.; Zhang, X.; Xie, Z.; Liu, Y.; Su, B.-J.; Chen, J.-M.; Su, D.-S.; Qi, W.; et al. Highly selective hydrogen peroxide electrosynthesis on carbon: In situ interface engineering with surfactants. Chem 2020, 6, 1443–1458. [Google Scholar] [CrossRef]
- Wang, L.; Liang, K.; Deng, L.; Liu, Y.-N. Protein hydrogel networks: A unique approach to heteroatom self-doped hierarchically porous carbon structures as an efficient ORR electrocatalyst in both basic and acidic conditions. Appl. Catal. B Environ. 2019, 246, 89–99. [Google Scholar] [CrossRef]
- Chen, Y.P.; Yang, L.M.; Paul Chen, J.; Zheng, Y.M. Electrospun spongy zero-valent iron as excellent electro-Fenton catalyst for enhanced sulfathiazole removal by a combination of adsorption and electro-catalytic oxidation. J. Hazard. Mater. 2019, 371, 576–585. [Google Scholar] [CrossRef]
- Deng, F.; Olvera-Vargas, H.; Garcia-Rodriguez, O.; Zhu, Y.; Jiang, J.; Qiu, S.; Yang, J. Waste-wood-derived biochar cathode and its application in electro-Fenton for sulfathiazole treatment at alkaline pH with pyrophosphate electrolyte. J. Hazard. Mater. 2019, 377, 249–258. [Google Scholar] [CrossRef]
- Haider, M.R.; Jiang, W.-L.; Han, J.-L.; Sharif, H.M.A.; Ding, Y.-C.; Cheng, H.-Y.; Wang, A.-J. In-situ electrode fabrication from polyaniline derived N-doped carbon nanofibers for metal-free electro-Fenton degradation of organic contaminants. Appl. Catal. B Environ. 2019, 256, 117774. [Google Scholar] [CrossRef]
- Song, X.; Zhang, H.; Bian, Z.; Wang, H. In situ electrogeneration and activation of H2O2 by atomic Fe catalysts for the efficient removal of chloramphenicol. J. Hazard. Mater. 2021, 412, 125162. [Google Scholar] [CrossRef]
- Qin, X.; Zhao, K.; Quan, X.; Cao, P.; Chen, S.; Yu, H. Highly efficient metal-free electro-Fenton degradation of organic contaminants on a bifunctional catalyst. J. Hazard. Mater. 2021, 416, 125859. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, M.; Tian, M.; Zhu, Y.; Wei, X.; Jiang, T.; Gao, S. An innovative electro-Fenton degradation system self-powered by triboelectric nanogenerator using biomass-derived carbon materials as cathode catalyst. Nano Energy 2017, 42, 314–321. [Google Scholar] [CrossRef]
- Jia, N.; Yang, T.; Shi, S.; Chen, X.; An, Z.; Chen, Y.; Yin, S.; Chen, P. N,F-codoped carbon nanocages: An efficient electrocatalyst for hydrogen peroxide electroproduction in alkaline and acidic solutions. ACS Sustain. Chem. Eng. 2020, 8, 2883–2891. [Google Scholar] [CrossRef]
- Sheng, H.; Janes, A.N.; Ross, R.D.; Kaiman, D.; Huang, J.; Song, B.; Schmidt, J.R.; Jin, S. Stable and selective electrosynthesis of hydrogen peroxide and the electro-Fenton process on CoSe2 polymorph catalysts. Energy Environ. Sci. 2020, 13, 4189–4203. [Google Scholar] [CrossRef]
- Tang, H.; Zhu, Z.; Shang, Q.; Tang, Y.; Zhang, D.; Du, Y.; Liu, M.; Yin, K.; Liu, C. Highly efficient continuous-flow electro-Fenton treatment of antibiotic wastewater using a double-cathode system. ACS Sustain. Chem. Eng. 2021, 9, 1414–1422. [Google Scholar] [CrossRef]
- Fan, W.; Zhang, B.; Wang, X.; Ma, W.; Li, D.; Wang, Z.; Dupuis, M.; Shi, J.; Liao, S.; Li, C. Efficient hydrogen peroxide synthesis by metal-free polyterthiophene via photoelectrocatalytic dioxygen reduction. Energy Environ. Sci. 2020, 13, 238–245. [Google Scholar] [CrossRef]
- Zhai, L.-F.; Sun, Y.-M.; Guo, H.-Y.; Sun, M. Surface modification of graphite support as an effective strategy to enhance the electro-Fenton activity of Fe3O4/graphite composites in situ fabricated from acid mine drainage using an air-cathode fuel cell. ACS Sustain. Chem. Eng. 2019, 7, 8367–8374. [Google Scholar] [CrossRef]
- Li, Z.; Shen, C.; Liu, Y.; Ma, C.; Li, F.; Yang, B.; Huang, M.; Wang, Z.; Dong, L.; Wolfgang, S. Carbon nanotube filter functionalized with iron oxychloride for flow-through electro-Fenton. Appl. Catal. B Environ. 2020, 260, 118204. [Google Scholar] [CrossRef]

| Sample | (A)SBET [m2·g−1] | ID/IG | N Content [at.%] | O Content [at.%] | (B)Removal Efficiency [%] | k [min−1] (R2) | (C)TOC Removal [%] |
| MCAN-0.5 | 626 | 1.47 | 15.42 | 8.66 | 95.7 | 0.050 (0.992) | 81.8 |
| MCAN-1 | 595 | 1.52 | 19.62 | 4.39 | 98.0 | 0.063 (0.992) | 87.5 |
| MCAN-2 | 492 | 1.51 | 19.34 | 4.94 | 93.2 | 0.044 (0.990) | 73.3 |
| MCAN-3 | 397 | 1.53 | 18.26 | 5.12 | 90.9 | 0.039 (0.993) | 70.3 |
| MAN-1 | 84 | 1.44 | 15.61 | 6.21 | 84.1 | 0.030 (0.995) | 49.9 |
| CAN-1 | 77 | 1.41 | 13.63 | 7.25 | 78.0 | 0.024 (0.994) | 43.6 |
| MCAN-0 | 374 | 1.38 | 13.42 | 4.03 | 88.3 | 0.034 (0.993) | 52.5 |
| MCA-1 | 494 | 1.43 | 14.39 | 4.24 | 93.6 | 0.043 (0.990) | 57.3 |
| N-1 | 467 | 1.44 | 5.79 | 10.38 | 81.1 | 0.026 (0.990) | 57.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Tian, M.; Liu, X. Supramolecular Self-Assembly Strategy towards Fabricating Mesoporous Nitrogen-Rich Carbon for Efficient Electro-Fenton Degradation of Persistent Organic Pollutants. Nanomaterials 2022, 12, 2821. https://doi.org/10.3390/nano12162821
Chen Y, Tian M, Liu X. Supramolecular Self-Assembly Strategy towards Fabricating Mesoporous Nitrogen-Rich Carbon for Efficient Electro-Fenton Degradation of Persistent Organic Pollutants. Nanomaterials. 2022; 12(16):2821. https://doi.org/10.3390/nano12162821
Chicago/Turabian StyleChen, Ye, Miao Tian, and Xupo Liu. 2022. "Supramolecular Self-Assembly Strategy towards Fabricating Mesoporous Nitrogen-Rich Carbon for Efficient Electro-Fenton Degradation of Persistent Organic Pollutants" Nanomaterials 12, no. 16: 2821. https://doi.org/10.3390/nano12162821
APA StyleChen, Y., Tian, M., & Liu, X. (2022). Supramolecular Self-Assembly Strategy towards Fabricating Mesoporous Nitrogen-Rich Carbon for Efficient Electro-Fenton Degradation of Persistent Organic Pollutants. Nanomaterials, 12(16), 2821. https://doi.org/10.3390/nano12162821