Transformation of Amorphous Terbium Metal–Organic Framework on Terbium Oxide TbOx(111) Thin Film on Pt(111) Substrate: Structure of TbxOy Film
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials Used
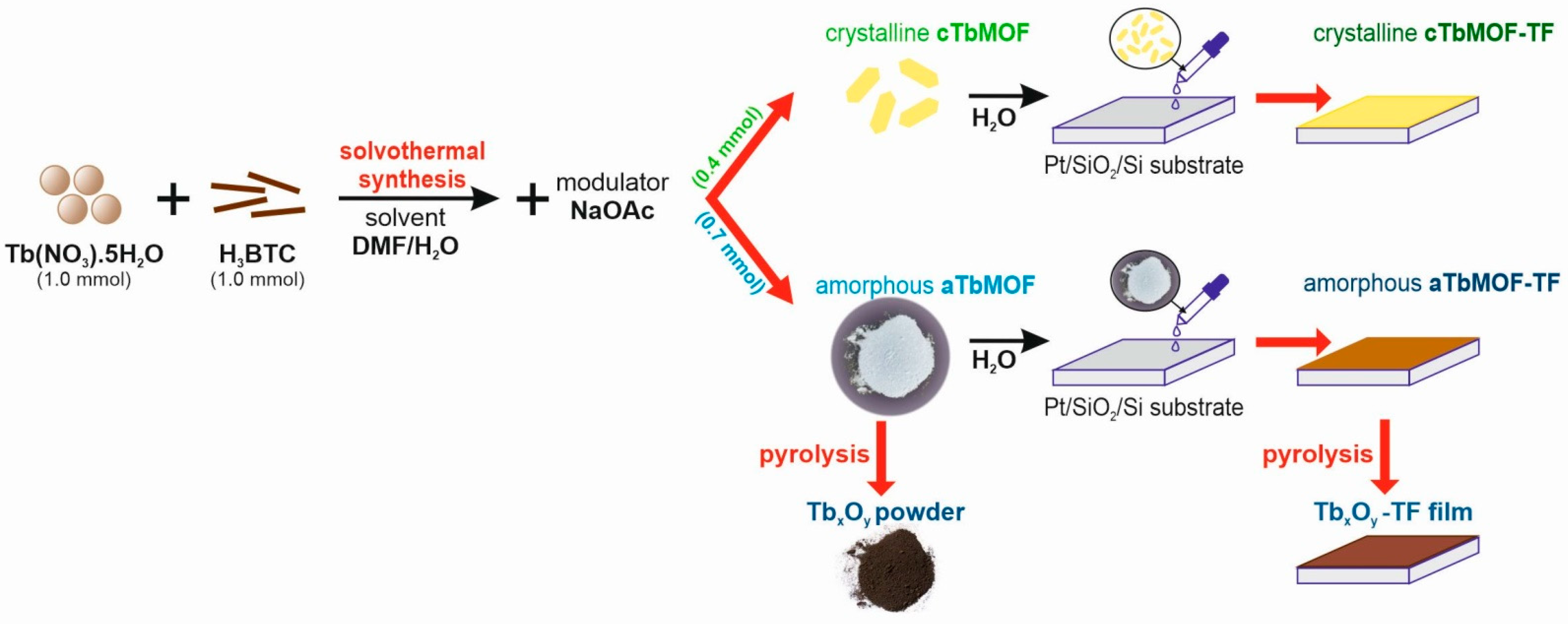
2.2. Preparation of cTbMOF and aTbMOF
2.3. Preparation of cTbMOF-TF and aTbMOF-TF Thin Films
2.4. Characterization of the Obtained Powders (TbMOF and TbxOy) and Thin Films (TbMOF-TF and TbxOy-TF)
3. Results and Discussion
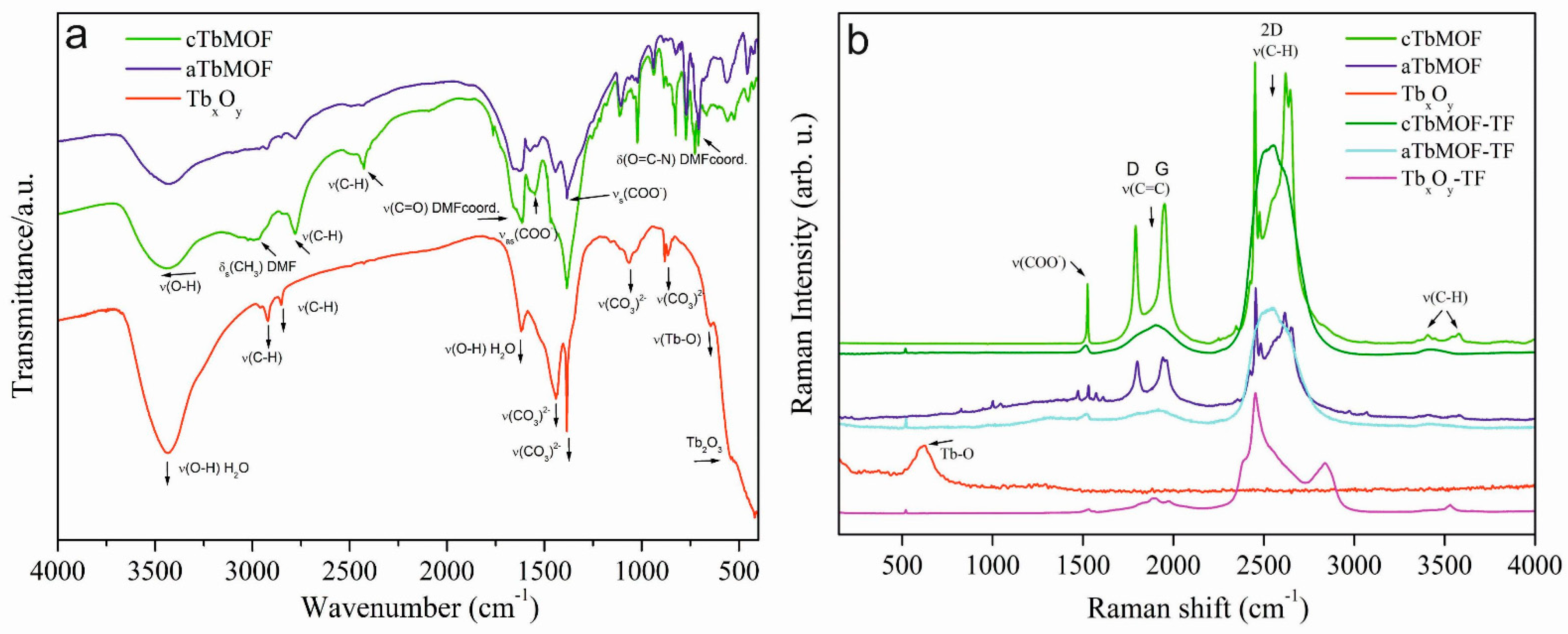
3.1. Structural Characterization of Tb-Based Powders and Thin Films
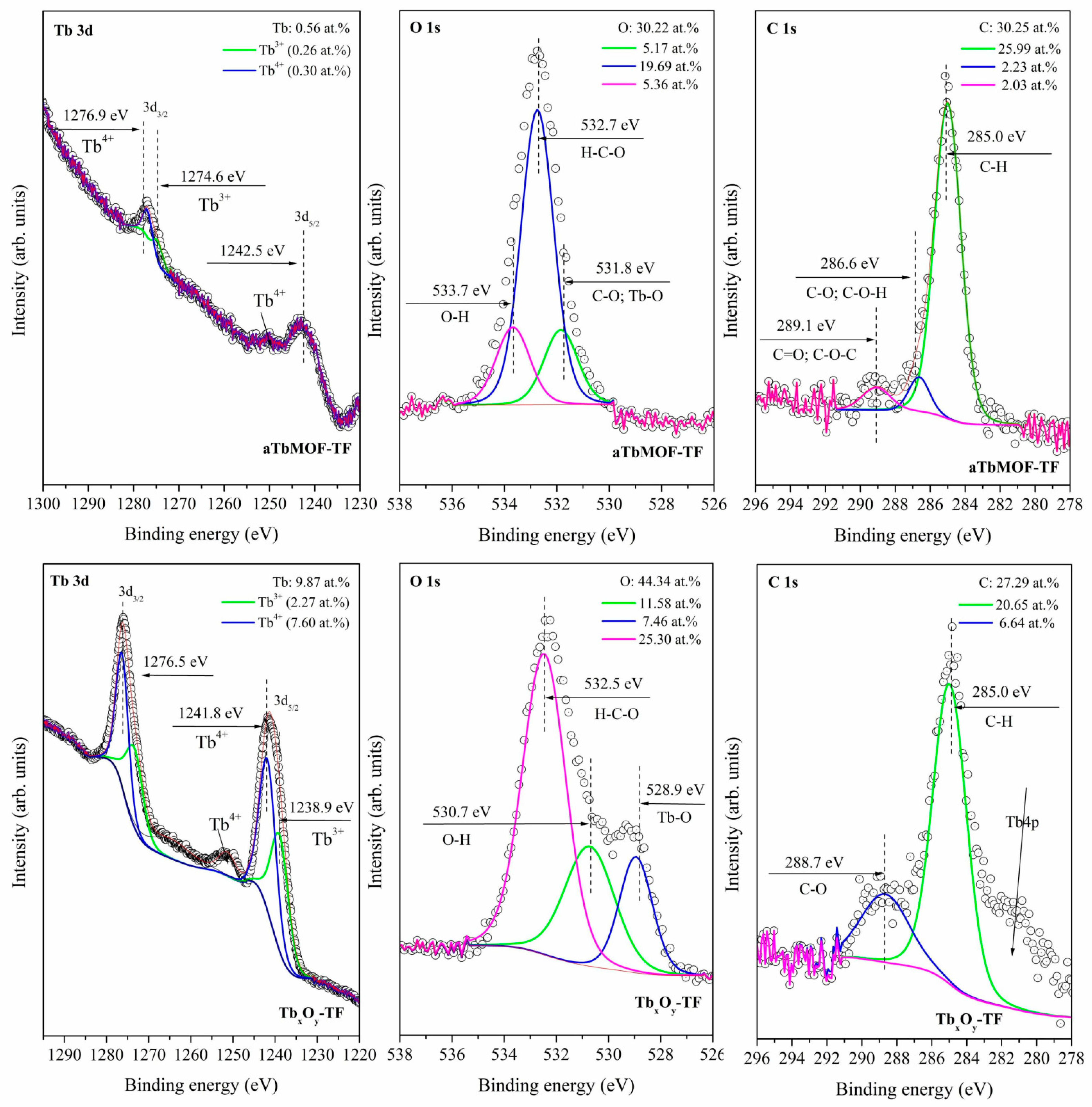
3.2. XPS Characterization of the Surfaces of Powders and Films
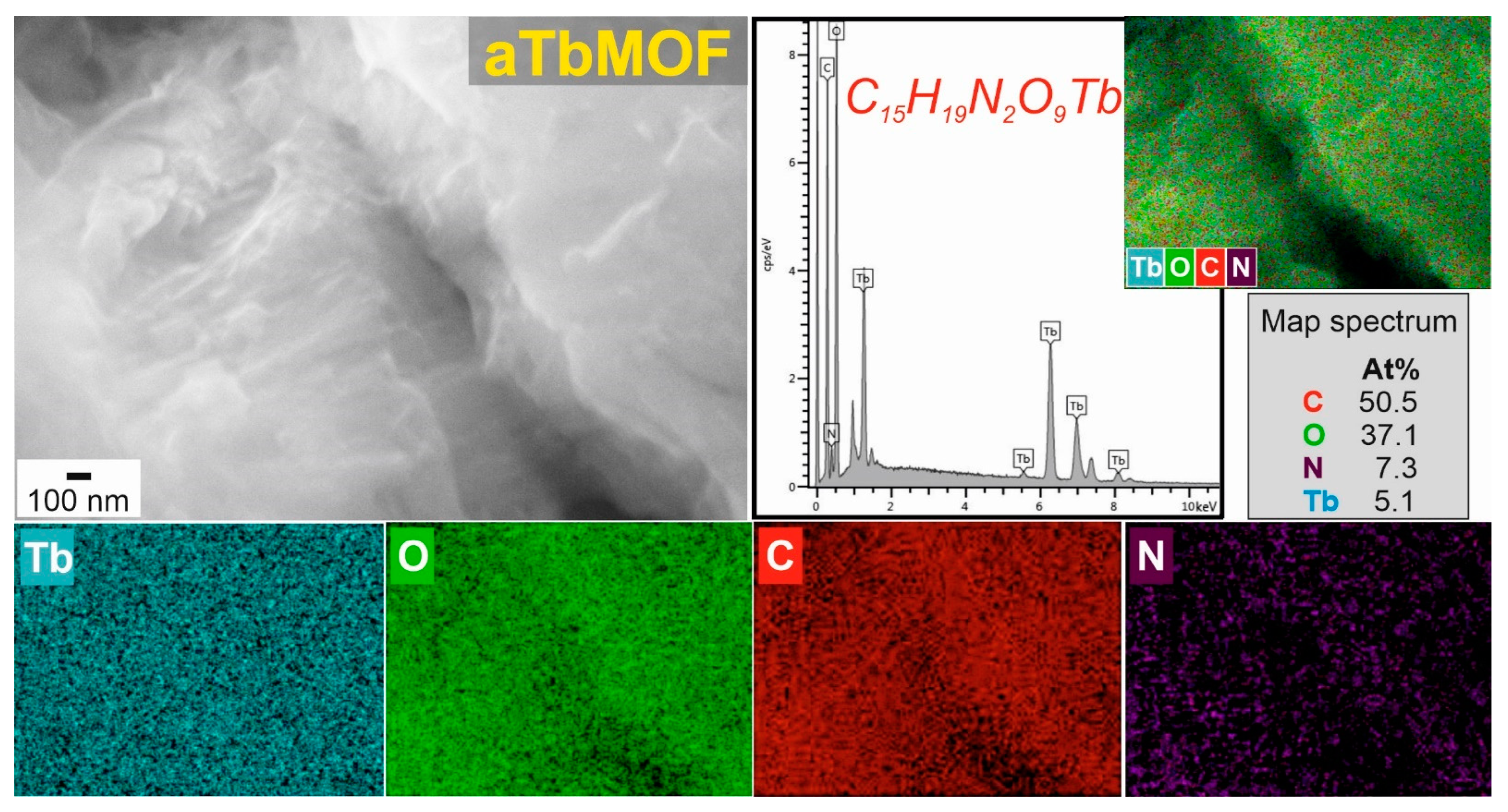
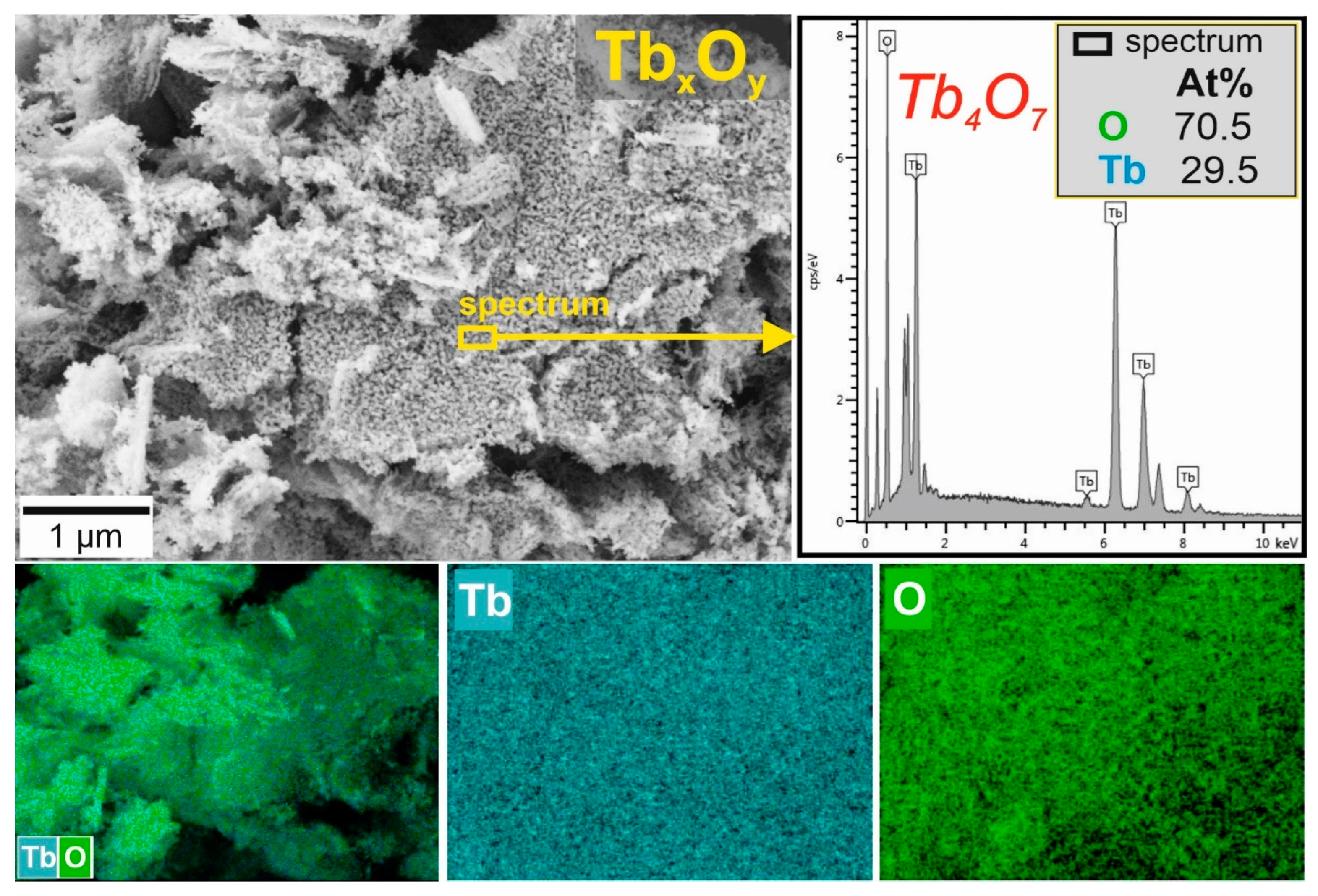
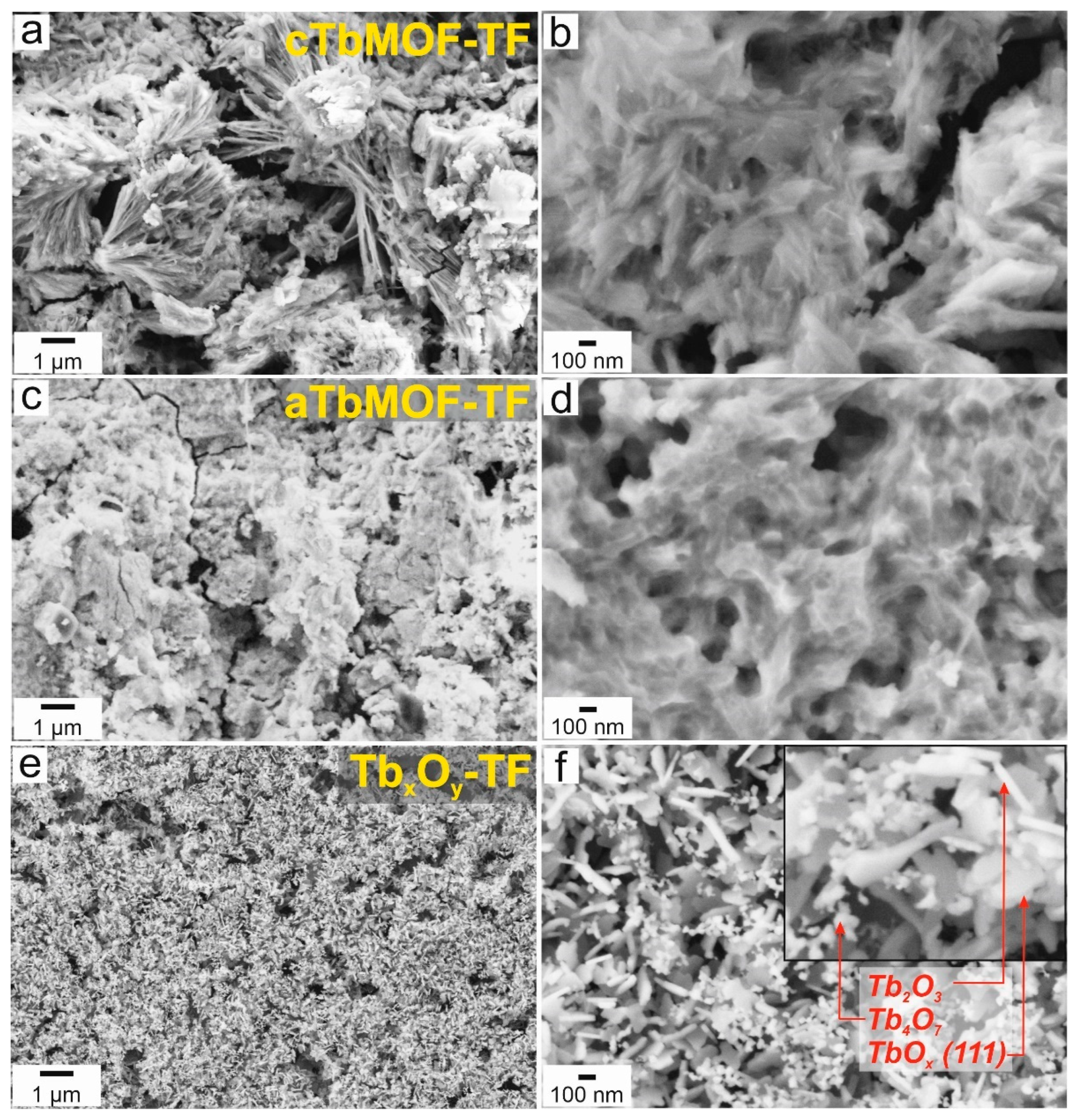
3.3. Morphological Characterization of Powders
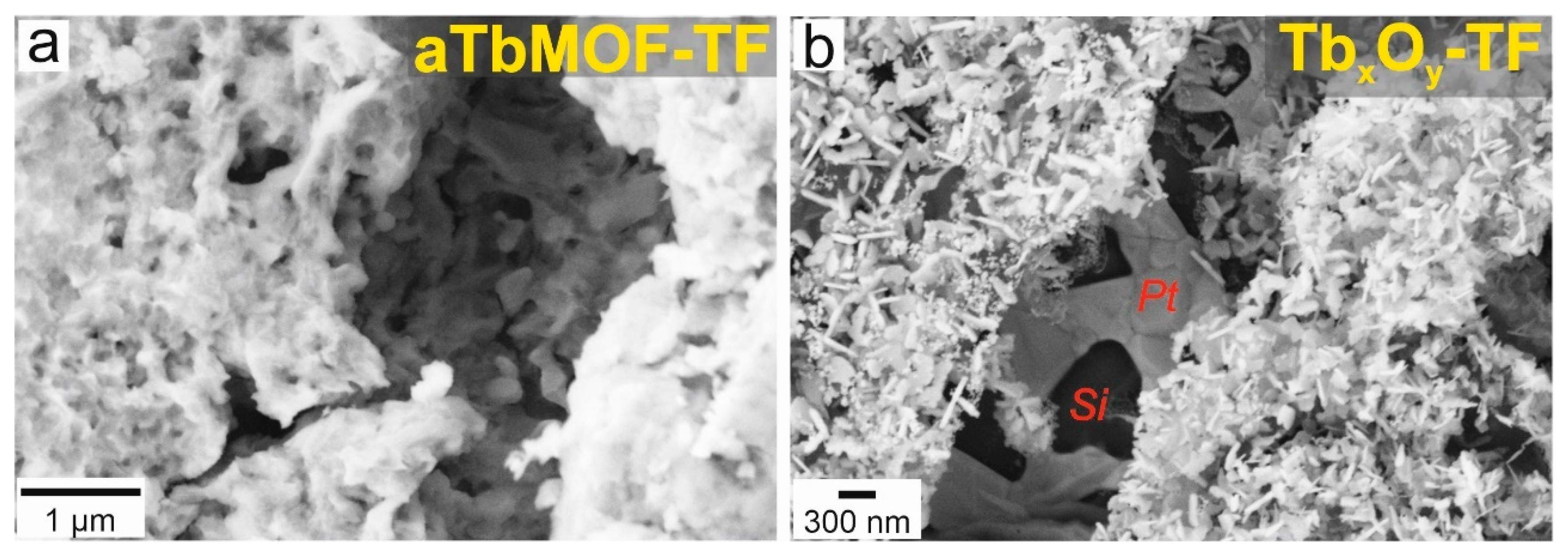
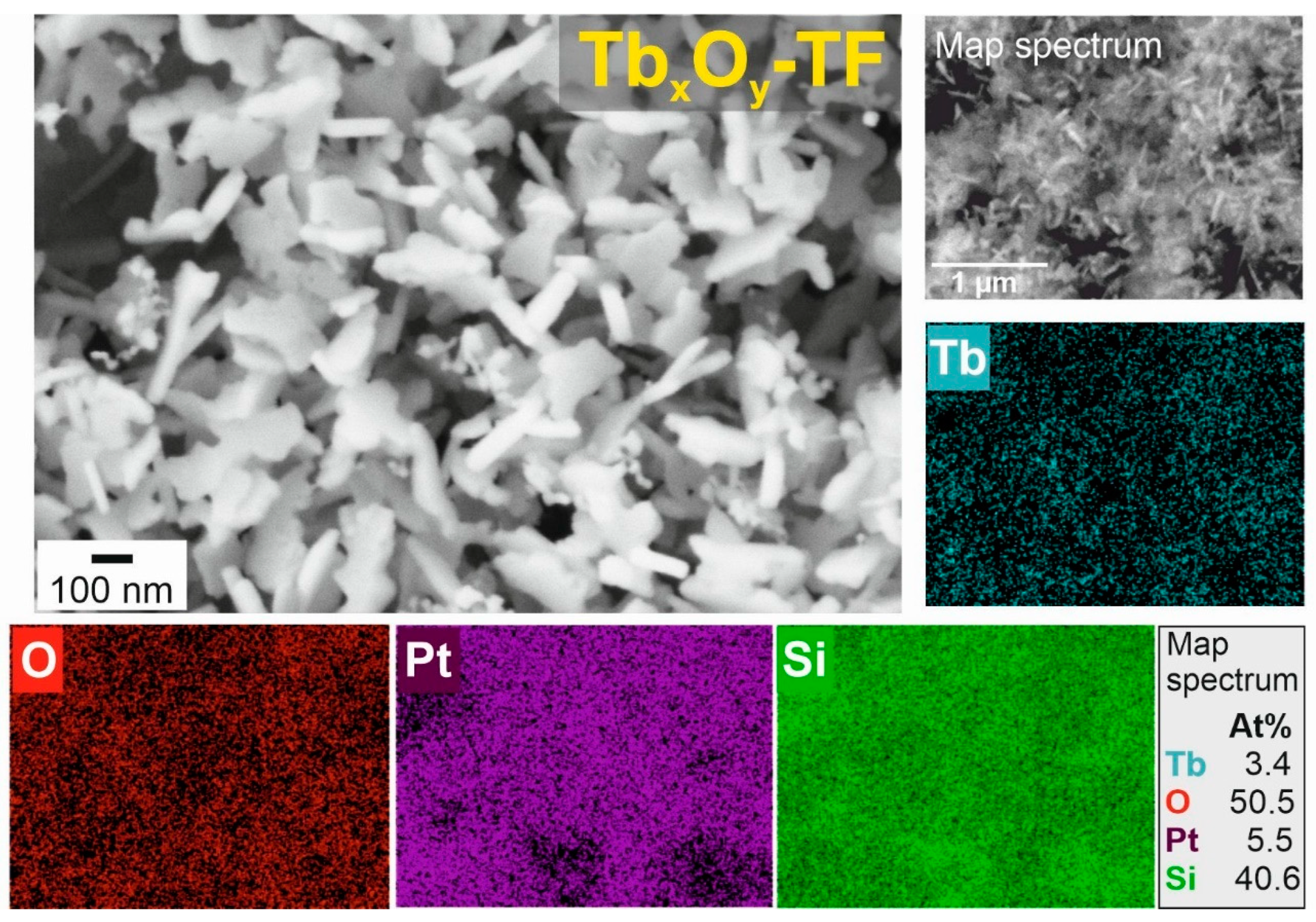
3.4. Morphological Characterization of Films
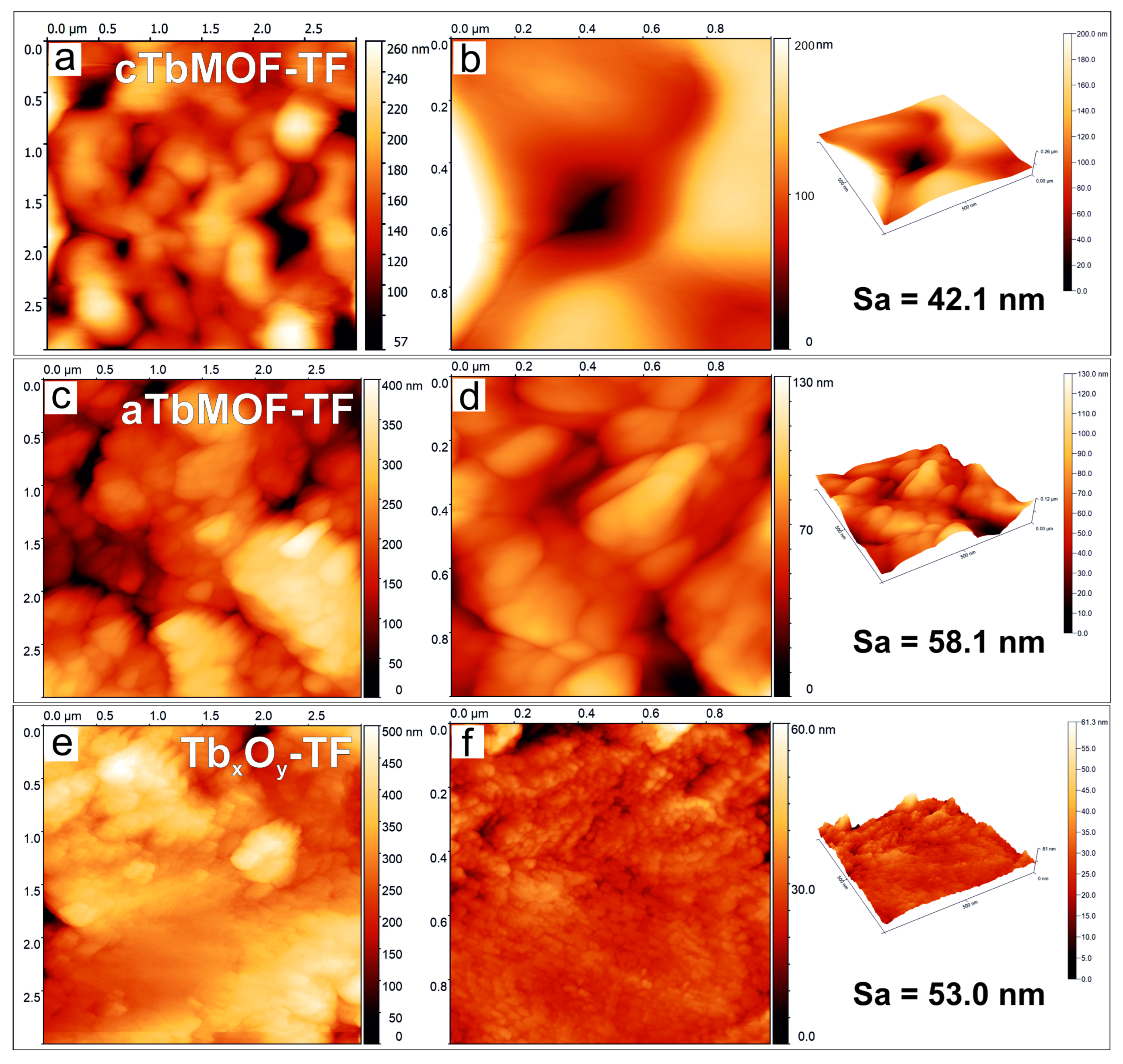
3.5. Topography Characterization of Films
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Nasruddin, N.; Zulys, A.; Fayza, Y.; Buhori, A.; Naufal, M.; Ghiyats, M.; Saha, B.B. Synthesis and characterization of a novel microporous lanthanide based metal-organic framework (MOF) using napthalenedicarboxylate acid. J. Mater. Res. Technol. 2020, 9, 7409–7417. [Google Scholar] [CrossRef]
- Zhong, M.; Kong, L.; Zhao, K.; Zhang, Y.H.; Li, N.; Bu, X.H. Recent Progress of Nanoscale Metal-Organic Frameworks in Synthesis and Battery Applications. Adv. Sci. 2021, 8, 2001980. [Google Scholar] [CrossRef] [PubMed]
- Wei, K.; Wang, X.; Jiao, X.; Li, C.; Chen, D. Self-supported three-dimensional macroporous amorphous NiFe bimetallic-organic frameworks for enhanced water oxidation. Appl. Surf. Sci. 2021, 550, 149323. [Google Scholar] [CrossRef]
- Fonseca, J.; Gong, T.; Jiao, L.; Jiang, H.L. Metal-organic frameworks (MOFs) beyond crystallinity: Amorphous MOFs, MOF liquids and MOF glasses. J. Mater. Chem. A 2021, 9, 10562. [Google Scholar] [CrossRef]
- Bennett, T.D.; Cheetham, A.K. Amorphous Metal-Organic Frameworks. Acc. Chem. Res. 2014, 47, 1555–1562. [Google Scholar] [CrossRef]
- Liu, C.; Wang, J.; Wan, J.; Cheng, Y.; Huang, R.; Zhang, C.; Hu, W.; Wei, G.; Yu, C. Amorphous Metal-Organic Framework-Dominated Nanocomposites with Both Compositional and Structural Heterogeneity for Oxygen Evolution. Angew. Chem. Int. Ed. 2019, 132, 14587. [Google Scholar] [CrossRef]
- Yang, F.; Li, W.; Tang, B. Facile Synthesis of Amorphous UiO-66 (Zr-MOF) for Supercapacitor Application. J. Alloys Compd. 2018, 733, 8–14. [Google Scholar] [CrossRef]
- Sapnik, A.F.; Bechis, I.; Collins, S.M.; Johnstone, D.N.; Divitini, G.; Smith, A.J.; Chater, P.A.; Addicoat, M.A.; Johnson, T.; Keen, D.A.; et al. Mixed hierarchical local structure in a disordered metal–organic framework. Nat. Commun. 2021, 12, 2062. [Google Scholar] [CrossRef]
- Colodrero, R.M.P.; Papathanasiou, K.E.; Stavgianoudaki, N.; Olivera-Pastor, P.; Losilla, E.R.; Aranda, M.A.G.; León-Reina, L.; Sanz, J.; Sobrados, I.; Choquesillo-Lazarte, D.; et al. Multifunctional Luminescent and Proton-Conducting Lanthanide Carboxyphosphonate Open-Framework Hybrids Exhibiting Crystalline-to-Amorphous-to-Crystalline Transformations. Chem. Mater. 2012, 24, 3780–3792. [Google Scholar] [CrossRef]
- Cai, D.; Guo, H.; Wen, L.; Liu, C. Fabrication of hierarchical architectures of Tb-MOF by a ‘‘green coordination modulation method’’ for the sensing of heavy metal ions. Cryst. Eng. Comm. 2013, 15, 6702–6708. [Google Scholar] [CrossRef]
- Zhang, Y.; Chang, C.H. Metal-Organic Framework Thin Films: Fabrication, Modification, and Patterning. Processes 2020, 8, 377. [Google Scholar] [CrossRef]
- Hu, M.; Shu, Y.; Kirillov, A.; Liu, W.; Yang, L.; Dou, W. Epoxy Functional Composites Based on Lanthanide Metal−Organic Frameworks for Luminescent Polymer Materials. ACS Appl. Mater. Interfaces 2021, 13, 7625–7634. [Google Scholar] [CrossRef]
- Tsuruoka, T.; Furukawa, S.; Takashima, Y.; Yoshida, K.; Isoda, S.; Kitagawa, S. Nanoporous nanorods fabricated by coordination modulation and oriented attachment growth. Angew. Chem. Int. Ed. 2009, 48, 4739–4743. [Google Scholar] [CrossRef] [PubMed]
- Horcajada, P.; Serre, C.; Grosso, D.; Boissière, C.; Perruchas, S.; Sanchez, C.; Férey, G. Colloidal Route for Preparing Optical Thin Films of Nanoporous Metal–Organic Frameworks. Adv. Mater. 2009, 21, 1931–1935. [Google Scholar] [CrossRef]
- Li, X.; Lu, S.; Tu, D.; Zheng, W.; Chen, X. Luminescent lanthanide metal-organic framework nanoprobes: From fundamentals to bioapplications. Nanoscale 2020, 12, 15021–15035. [Google Scholar] [CrossRef] [PubMed]
- Shan, B.; James, J.B.; Armstrong, M.R.; Close, E.C.; Letham, P.A.; Nikkhah, K.; Lin, Y.S.; Mu, B. Influences of Deprotonation and Modulation on Nucleation and Growth of UiO-66: Intergrowth and Orientation. J. Phys. Chem. C 2018, 122, 2200–2206. [Google Scholar] [CrossRef]
- Fursikov, P.V.; Abdusalyamova, M.N.; Makhmudov, F.A.; Shairmardanov, E.N.; Kovalev, I.D.; Kovalev, D.Y.; Morgunov, R.B.; Koplak, O.V.; Volodin, A.A.; Khodos, I.I.; et al. Structural features and magnetic behavior of nanocrystalline powders of terbium oxide prepared by the thermal decomposition of terbium acetate in air. J. Alloys Compd. 2016, 657, 163–173. [Google Scholar] [CrossRef]
- Balabanov, S.S.; Permin, D.A.; Rostokina, E.Y.; Egorov, S.V.; Sorokinb, A.A.; Kuznetsov, D.D. Synthesis and structural characterization of ultrafine terbium oxide powders. Ceram. Int. 2017, 43, 16569–16574. [Google Scholar] [CrossRef]
- Zhao, L.; Sun, K.; Youliwasi, N.; Guo, H.; Yang, G.; Jiao, F.; Dong, B.; Chai, Y.; Mintova, S.; Liu, C. Highly Sensitive H2O2 Sensor Based on Porous Bimetallic Oxide Ce1−xTbxOy Derived from Homeotypic Ln-MOFs. Appl. Surf. Sci. 2019, 470, 91–98. [Google Scholar] [CrossRef]
- Veber, P.; Velázquez, M.; Gadret, G.; Rytz, D.; Peltz, M.; Decourt, R. Flux growth at 1230 °C of cubic Tb2O3 single crystals and characterization of their optical and magnetic properties. Cryst. Eng. Comm. 2015, 17, 492–497. [Google Scholar] [CrossRef]
- Abu-Zied, B.M.; Mohamed, A.R.N.; Asiri, A.M. Effect of Thermal Treatment on the Formation, Textural and Electrical Conductivity Properties of Nanocrystalline Tb4O7. J. Nanosci. Nanotechnol. 2015, 15, 4487–4492. [Google Scholar] [CrossRef] [PubMed]
- Höcker, J.; Cartas, W.; Schaefer, A.; Bäumer, M.; Weaver, J.F.; Falta, J.; Flege, J.I. Growth, Structure, and Stability of the High-index TbOx(112) Surface on Cu(111). J. Phys. Chem. C 2015, 119, 14175–14184. [Google Scholar] [CrossRef]
- Belaya, S.V.; Bakovets, V.V.; Boronin, A.I.; Koshcheev, S.V.; Lobzareva, M.N.; Korolkov, I.V.; Stabnikov, P.A. Terbium oxide films grown by chemical vapor deposition from terbium(III) dipivaloylmethanate. Inorg. Mater. 2014, 50, 379–386. [Google Scholar] [CrossRef]
- Belaya, S.V.; Bakovets, V.V.; Asanov, I.P.; Korolkov, I.V.; Sulyaeva, V.S. MOCVD Synthesis of Terbium Oxide Films and their Optical Properties. Chem. Vap. Depos. 2015, 21, 150–155. [Google Scholar] [CrossRef]
- Lee, C.J.; Mehar, V.; Weaver, J.F. Growth and Structure of Tb2O3(111) Films on Pt(111). J. Phys. Chem. C 2018, 122, 9997–10005. [Google Scholar] [CrossRef]
- Lee, C.J.; Sayal, A.; Vashishtha, S.; Weaver, J.F. Redox-mediated transformation of a Tb2O3(111) thin film from the cubic fluorite to bixbyite structure. Phys. Chem. Chem. Phys. 2020, 22, 379–390. [Google Scholar] [CrossRef]
- Brunckova, H.; Mudra, E.; Rocha, L.; Nassar, E.; Nascimento, W.; Kolev, H.; Kovalcikova, A.; Molcanova, Z.; Podobova, M.; Medvecky, L. Preparation, and characterization of isostructural lanthanide Eu/Gd/Tb metal-organic framework thin film for luminescent applications. Appl. Surf. Sci. 2021, 542, 148731. [Google Scholar] [CrossRef]
- Brunckova, H.; Mudra, E.; Rocha, L.; Nassar, E.; Nascimento, W.; Kolev, H.; Lisnichuk, M.; Kovalcikova, A.; Molcanova, Z.; Streckova, M.; et al. Nanostructure and Luminescent Properties of Bimetallic Lanthanide Eu/Gd, Tb/Gd and Eu/Tb Coordination Polymers. Inorganics 2021, 9, 77. [Google Scholar] [CrossRef]
- Guo, H.; Zhu, Y.; Wang, S.; Su, S.; Zhou, L.; Zhang, H. Combining Coordination Modulation with Acid-Base Adjustment for the Control over Size of Metal-Organic Frameworks. Chem. Mater. 2012, 24, 444–450. [Google Scholar] [CrossRef]
- Utochnikova, V.V.; Pietraszkiewicz, O.; Kozbiał, M.; Gierycz, P.; Pietraszkiewicz, M.; Kuzmina, N.P. Mixed-ligand terbium terephthalates: Synthesis, photophysical and thermal properties and use for luminescent terbium terephthalate thin film deposition. J. Photochem. Photobiol. A Chem. 2013, 253, 72–80. [Google Scholar] [CrossRef]
- Siqueira, A.B.; Carvalho, C.T.; Ionashiro, E.Y.; Bannach, G.; Rodrigues, E.C.; Ionashiro, M. Synthesis, characterization and thermal behavior of solid 2-methoxybenzoates of trivalent metals. J. Therm. Anal. Calorim. 2008, 92, 945–951. [Google Scholar] [CrossRef]
- Rahimi-Nasrabadi, M.; Pourmortazavi, S.M.; Ganjali, M.R.; Norouzi, P. Nanosized terbium carbonate, and oxide particles: Optimized synthesis, and application as photodegradation catalyst. J. Mater. Sci. Mater Electron 2018, 29, 2988–2998. [Google Scholar] [CrossRef]
- Malasi, A.; Taz, H.; Farah, A.; Patel, M.; Lawrie, B.; Pooser, R.; Baddorf, A.; Duscher, G.; Kalyanaraman, R. Novel Iron-based ternary amorphous oxide semiconductor with very high transparency, electronic conductivity, and mobility. Sci. Rep. 2015, 5, 18157. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Hope, G.A. Raman and Fluorescence Spectroscopy of CeO2, Er2O3, Nd2O3, Tm2O3, Yb2O3, La2O3, and Tb4O7. J. Spectrosc. 2015, 2015, 940172. [Google Scholar] [CrossRef]
- Gadipelli, S.; Guo, Z. Postsynthesis Annealing of MOF-5 Remarkably Enhances the Framework Structural Stability and CO2 Uptake. Chem. Mater. 2014, 26, 6333–6338. [Google Scholar] [CrossRef]
- Li, Z.; Zhu, G.; Guo, X.; Zhao, X.; Jin, Z.; Qiu, S. Synthesis, Structure, and Luminescent and Magnetic Properties of Novel Lanthanide Metal-Organic Frameworks with Zeolite-like Topology. Inorg. Chem. 2007, 46, 5174–5178. [Google Scholar] [CrossRef]
- Laurikenas, A.; Beganskiene, A.; Kareiva, A. On the Synthesis and Characterization of Lanthanide Metal-Organic Frameworks. Ceramics 2018, 1, 6. [Google Scholar] [CrossRef]
- Garduńo-Wilches, I.A.; Alarcón-Flores, G.; Carro-Gastélum, A.; Carmona-Téllez, S.; Aguilar-Frutis, M.A.; Loera-Serna, S. Enhanced photoluminescence quantum yield of terbium nano-MOFs synthesized by microwave assisted solvothermal method. Nano-Struct. Nano-Objects 2021, 26, 100736. [Google Scholar] [CrossRef]
- Zhang, Y.; Deng, J.; Zhang, H.; Liu, Y.; Dai, H. Three-dimensionally ordered macroporous Pr6O11 and Tb4O7 with mesoporous walls: Preparation, characterization, and catalytic activity for CO oxidation. Catal. Today 2015, 245, 28–36. [Google Scholar] [CrossRef]
- Tuenge, R.T.; Eyring, L.J. On the structures of the intermediate phases in the terbium oxide system. Solid State Chem. 1982, 41, 75–89. [Google Scholar] [CrossRef]
- Jeong, S.Y.; Moon, Y.K.; Kim, J.K.; Park, S.W.; Jo, Y.K.; Kang, Y.C.; Lee, J.H. A General Solution to Mitigate Water Poisoning of Oxide Chemiresistors: Bilayer Sensors with Tb4O7 Overlayer. Adv. Funct. Mater. 2021, 31, 2007895. [Google Scholar] [CrossRef]
- Zhu, C.; Lv, C.; Jiang, M.; Zhou, J.; Li, D.; Ma, X.; Yang, D. Green electroluminescence from Tb4O7 films on silicon: Impact excitation of Tb3+ ions by hot carriers. Appl. Phys. Lett. 2016, 108, 051113. [Google Scholar] [CrossRef]
- Lonappan, D.; Shekar, N.C.; Sahu, P.; Kumar, J.; Paul, R.; Paul, P. Unusually large structural stability of terbium oxide phase under high pressure. J. Alloys Compd. 2010, 490, 47–49. [Google Scholar] [CrossRef]
- Tseng, K.P.; Yang, Q.; McCormack, S.J.; Kriven, W.M. High-entropy, phase-constrained, lanthanide sesquioxide. J. Am. Ceram. Soc. 2020, 103, 569–576. [Google Scholar] [CrossRef]
- Smirnov, M.Y.; Vovk, E.I.; Kalinkin, A.V.; Pashis, A.V.; Bukhtiyarov, V.I. An XPS Study of the Oxidation of Noble Metal Particles Evaporated onto the Surface of an Oxide Support in Their Reaction with NOx. Kinet. Catal. 2012, 53, 117–124. [Google Scholar] [CrossRef]
- Kaur, H.; Shrivastav, V.; Kumar, M.; Sharma, A.L.; Deep, A. Investigations on optoelectronic properties of metal (Terbium)-organic framework / tris(8-hydroxyquinolinato) aluminium composite for potential device applications. Mater. Chem. Phys. 2020, 255, 123569. [Google Scholar] [CrossRef]
- Jhang, J.H.; Schaefer, A.; Cartas, W.; Epuri, S.; Baumer, M.; Weaver, J.F. Growth and Partial Reduction of Sm2O3(111) Thin Films on Pt(111): Evidence for the Formation of SmO(100). J. Phys. Chem. C 2013, 117, 21396–21406. [Google Scholar] [CrossRef]
- Lu, X.H.; Li, G.R.; Yu, X.L.; Tong, Y.X. Electrochemical Synthesis and Characterization of TbO2−x Flowerlike Nanostructures. Electrochem. Solid-State Lett. 2008, 11, K85–K88. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brunckova, H.; Mudra, E.; Streckova, M.; Medvecky, L.; Sopcak, T.; Shepa, I.; Kovalcikova, A.; Lisnichuk, M.; Kolev, H. Transformation of Amorphous Terbium Metal–Organic Framework on Terbium Oxide TbOx(111) Thin Film on Pt(111) Substrate: Structure of TbxOy Film. Nanomaterials 2022, 12, 2817. https://doi.org/10.3390/nano12162817
Brunckova H, Mudra E, Streckova M, Medvecky L, Sopcak T, Shepa I, Kovalcikova A, Lisnichuk M, Kolev H. Transformation of Amorphous Terbium Metal–Organic Framework on Terbium Oxide TbOx(111) Thin Film on Pt(111) Substrate: Structure of TbxOy Film. Nanomaterials. 2022; 12(16):2817. https://doi.org/10.3390/nano12162817
Chicago/Turabian StyleBrunckova, Helena, Erika Mudra, Magdalena Streckova, Lubomir Medvecky, Tibor Sopcak, Ivan Shepa, Alexandra Kovalcikova, Maksym Lisnichuk, and Hristo Kolev. 2022. "Transformation of Amorphous Terbium Metal–Organic Framework on Terbium Oxide TbOx(111) Thin Film on Pt(111) Substrate: Structure of TbxOy Film" Nanomaterials 12, no. 16: 2817. https://doi.org/10.3390/nano12162817
APA StyleBrunckova, H., Mudra, E., Streckova, M., Medvecky, L., Sopcak, T., Shepa, I., Kovalcikova, A., Lisnichuk, M., & Kolev, H. (2022). Transformation of Amorphous Terbium Metal–Organic Framework on Terbium Oxide TbOx(111) Thin Film on Pt(111) Substrate: Structure of TbxOy Film. Nanomaterials, 12(16), 2817. https://doi.org/10.3390/nano12162817






