Abstract
Algal biomass synthesised nanocomposites have a higher surface area and reusability advantages. This study aimed to synthesise and characterise ZnMgO and silica-supported graphene with ZnMgO (G-ZnMgO) nanocomposites from Kappaphycusalvarezii and evaluate their potential in the application of photocatalysis to remove Rhodamine-B (RhB) and methylene blue (MB) dyes from their aqueous medium by maximising the percentage removal using response surface methodology (RSM) modelling. Nanocomposites were synthesised and characterised by biogenic and instrumental (Powder X-ray diffraction (P-XRD), electron microscopic analysis (SEM and TEM), Fourier transform infrared spectroscopy (FTIR), Energy dispersive analysis of X-rays (EDAX). and UV-visible diffuse reflectance spectroscopy (UV-DRS)) methods, respectively; modelling predicted the optimal conditions to be photocatalyst dosage and contact time of 1 g/L and 90 min, respectively, to obtain maximum MB dye removal of 80% using G-ZnMgO. The results showed the best fit between experimental and RSM predicted values. Thus, the obtained results conclude that the algal biomass synthesised nanocomposites were found to be one of the potential photocatalysts for the removal of RhB and MB dyes from their aqueous solution.
1. Introduction
Unrefined dyes are predominant pollutants in wastewater treatment because they are frequently utilised in numerous industrialised applications and released into the ecosystem. The majority of chemicals are refractory, strong, coloring agents, or even poisonous and cancerous. Researchers have developed simple and efficient dye sewage treatment technologies as a consequence of the negative effects of dyes on public health and environments [1].
Even though widely used methods are available for wastewater treatment, photocatalysis has been studied for decades as a possible solution. It is also used for the synthesis of chemical compounds and energy-related advancement techniques. It allows the use of sunlight as a renewable and sustainable energy source. This method relies on the existence of microelectronics that could be activated by illumination of higher potency than its bandgap energy, producing pairs of electrons with a high level of energy that can be employed in electrochemical reactions to degrade dyes. Photocatalysis is often used for the activation of a unit process by a solid (photocatalyst) irradiated by a light (UV/visible) source. Due to their great photosensitivity and stability, semiconductor metal oxide nanocomposites have been used as photocatalysts [2]. Titanium dioxide (TiO2) is the most extensively utilised photocatalyst for the breakdown of organic contaminants among the many semiconductor materials. However, TiO2 requires solar light for efficient photocatalysis [3].
In the visible-light photocatalytic area, ZnO and MgO are potential low-cost and narrow bandgap materials. Zn-doped MgO (ZnMgO) can absorb the most visible light. The formation of nano-sized ZnMgO particles can be accomplished in a number of approaches, including the sol-gel technique, chemically gaseous phase deposition, sensor evaporation, hydrothermal method, and combustible aerosols production, in accordance with the research. Green synthesis of ZnMgO using seaweed has been reported [4], but it has not been widely exploited yet.
Response surface methodology develops a quadratic model with minimum trials and errors. Additionally, it is used to study the interaction between independent factors [5]. It has two designs: Box–Wilson and Box–Behnken design. According to their principle, the number of trials required to conduct for the Box–Wilson design is 2f + 2*f + m and 2*f*(f−1) + m for the Box–Behnken design, where f is the quantity of individual factors and m is the quantity of midpoint [6]. Normally, the Box–Wilson design requires more experiments and produces more accurate results than the Box–Behnken design. Box–Wilson design starts with analysis for a minimum of two independent factors, whereas the Box–Behnken design starts with a minimum of three factors.
In this work, Zn50Mg50O and G-Zn50Mg50O (silica-supported graphene with Zn50Mg50O) nanocomposites were synthesised using red algae Kappaphycus alvarezii as a stabilising agent by co-precipitation method to improve the ultraviolet receptive capability of metallic photo-catalysts (doping with other elements) by altering the physical and chemical properties of the metal oxides used in this study. The properties of nanocomposite were characterised by powder X-ray diffraction (P-XRD), UV-visible diffuse reflectance spectroscopy (UV-DRS), scanning electron microscopy (SEM) along with energy dispersive analysis of X-rays (EDAX), Transmission electron microscope (TEM), and Fourier transform infrared spectroscopy (FTIR) for their crystallinity, surface morphology, elemental composition, optical direct band gap energy, and functional groups respectively. The photocatalytic activities of nanocomposites (Zn50Mg50O and G-Zn50Mg50O) were studied under visible light for the decomposition of RhB and MB dye molecules.
2. Materials and Methods
2.1. Chemicals
All the chemicals utilised in this investigation were of the highest purity and analytical grade. Sugarcane juice was bought at a nearby market. Silica gel, Rhodamine (RhB), Methylene Blue (MB), Zinc acetate (Zn(CH₃CO2)2), magnesium nitrate (Mg(NO3)2), Sodium hydroxide (NaOH), Sodium sulfate, and Triton X-100 were purchased from Sigma Aldrich (St. Louis, MO, USA). The overall study was performed with Milli-Q water. An electronic balance was used to weigh each chemical (Shimadzu, Kyoto, Japan).
2.2. Collection and Preparation of Algae Extract
Kappaphycus alvarezii, marine red macroalgae, was collected in Mandapam, Rameswaram, Tamil Nadu, India. The algal product was often rinsed, dehydrated, and pulverised before being used. A total of 5 g of sample was mixed with 500 mL of Milli-Q water and soaked overnight. The aqueous filtrate was collected. Furthermore, the filtrate was utilised to produce nanocomposite, Zn50Mg50O.
2.3. Synthesis of Zn50Mg50O and Silica-Supported Graphene with Zn50Mg50O Nanocomposites
The ZnMgO nanocomposite (Zn50Mg50O) was prepared by using 0.1 M of molar ratio of substrates (Zn(CH₃CO2)2: Mg(NO3)2, 1:4), the solution with the addition of Kappaphycus alvarezii extract and sodium hydroxide (NaOH-0.2 M) acting as the reducing agent. It is designated as Zn50Mg50O. Concentrated sugarcane juice (100 mL) was mixed with 200 g of silica gel and added with 300 g of prepared Zn50Mg50O nanocomposite, and the mixture was dried at 100 °C for 1 h. After drying the composite, calcination was continued for 3 h at 350 °C in N2 atmosphere in a furnace. It is designated as G-Zn50Mg50O.
2.4. Characterisation of Nanocomposites
The characteristics of nanoparticles and nanocomposites were characterised by P-XRD, UV-visible diffuse reflectance spectroscopy (UV-DRS), electron microscopic analysis (SEM), EDAX, and Fourier transform infrared spectroscopy (FTIR) for their crystallinity, surface morphology, elemental composition, optical direct band gap energy, and functional groups, respectively.
2.5. Batch Photocatalytic Studies
Nanocomposites (Zn50Mg50O and G-Zn50Mg50O) were utilised for the degradation of RhB and MB dyes using UV illumination (100 W Tungsten halogen lamp). A measured dosage of catalytic material was applied to 75 mL of dye (RhB or MB) solution in a standard experimental setup. The suspension was extensively stirred in the dark before light irradiation to create an adsorption–desorption equilibrium of dye onto the catalyst. Three mL of aliquot was obtained at regular intervals during the procedure and filtered to eliminate the catalyst particles. A UV spectrophotometer was used to calculate the absorption of RhB and MB at regular intervals at their distinctive absorption wavelengths of 554 and 664 nm, respectively. The illumination was from a 100 W halogen lamp, and the electrolyte solution was an aqueous 0.1 M Na2SO4 solution. The working electrode prepared using 10 mg of the prepared nanomaterials were combined with Triton X-100 (20 µL) and purified water (40 µL) to create a slurry, which was then coated on an FTO glass substrate using the doctor blade technique. The electrode’s active surface area was 0.5 × 0.5 cm2, and the sticky tape served as a separator for a triple-layered homogeneous coating. It was then dried for 3 h at 120 °C, following the method of Theerthagiri et al. [7].
2.6. Response Surface Methodology
The surface response modelling and analysis were carried out using Stat-Ease, Inc.’s original Design-Expert 13 programme. In this study, the Box–Wilson technique was chosen to optimise the amount of dye removal at the appropriate photocatalyst dosage and contact time. All investigations were carried out in accordance with the Box–Wilson parametric study, and the significance of the experimental data’s fit to linear, 2FI (two-factor interaction), quadratic, and cubic models was investigated. The variables were again matched to the important quadratic formula to analyse the impact of independent factors on the response, which is depicted in Formula (1), and model coefficients were assessed using Formula (2). The coefficients were then evaluated to examine the difference among experimental and simulation results in Equation (1). Analysis of variance (ANOVA) was used to determine the model’s significance using the highest F-value, lowest p-value, and a 95% confidence level. The goodness-of-fit between predicted and experimental values was also evaluated using the regression coefficient R2, the difference between modified and projected R2, and predicted residual error sum of squares (PRESS) values.
where: β0 is an endpoint, βi, βii, βij are quadratic, square, and interactions factors, Y is the response, respectively, Xi and Xj are significant indicators, and ε is a random variable.
3. Results and Discussion
3.1. Powder XRD Analysis
Zn50Mg50O and G-Zn50Mg50O nanocomposites were analyzed for their crystallinity using powder X-ray diffractometry (P-XRD), as shown in Figure 1. Nanocomposites’ diffraction patterns were evaluated according to the simultaneous analyses shown in JCPDS Nos. 01-079-0207 and.00-004-0821, respectively, and are closely aligned with the reported 2θ values. CuKα1 radiation (λ = 1.5406 Å), 40 kV–40 mA, 2θ scanning technique was used to analyze the X-ray diffraction peaks of prepared nanocomposites samples. The data were collected between 20 and 80 degrees. The diffraction patterns were detected using the miller indexes (h,k,l), and the resulting P-XRD spectra revealed that the prepared nanocomposites were all nanocrystals in nature. The sharp peaks of P-XRD demonstrate the reactive component of MgO and ZnO. The actual percentage proportion of the nanomaterials corresponded with data peaks by P-XRD when there were less impurities. The nanocomposites’ purity and crystalline size were determined to be excellent [8,9].
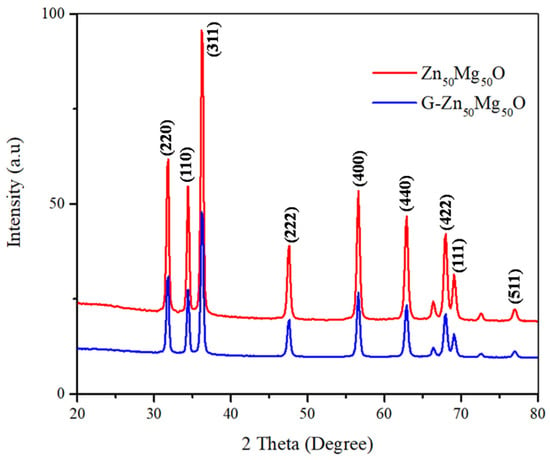
Figure 1.
XRD spectra of Zn50Mg50O and G-Zn50Mg50O.
3.2. UV-DRS
Applying the Tauc relationship [10] given in Equation (3), the optic band gap energy (Eg) of the biogenic produced nanocomposites was determined.
where hν—the photon energy, α—the absorption co-efficient, A—constant and n = ½ for direct transition and n = 2 for indirect transition [11]. If the velocity of the conduction band’s electrons and electron holes are equal, an electron will emit a photon in the straight transfer. The direct band difference energy is equivalent to the photon’s energy [12,13]. A plot between (αhυ)2 and hυ is drawn in order to derive the photonic direct band gap energies from the UV-DRS absorption spectra. Figure 2′s straight-line extrapolation to the (αhν)2 = 0 axis yields the optical direct band gap energy value. The predicted optical direct band gap energies of green synthesised Zn50Mg50O and G-Zn50Mg50O were 3.08 and 4.71 eV, respectively. The energy gap for semiconductors lies between conductors and insulators. The visible light absorption capacity decreased as the graphene addition increased.
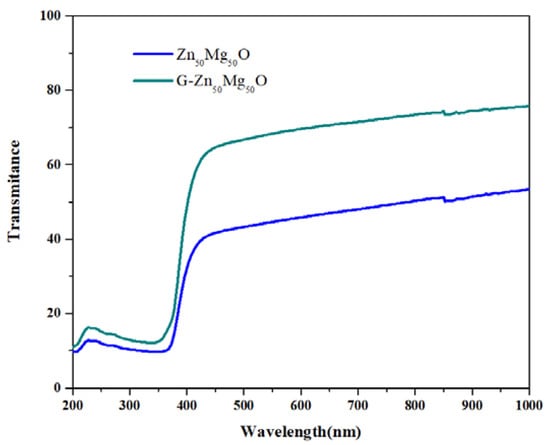
Figure 2.
UV-DR spectra of Zn50Mg50O and G-Zn50Mg50O.
3.3. SEM-EDAX
HR-SEM images of Zn50Mg50O and G-Zn50Mg50O nanocomposite materials are shown in Figure 3a–d, which clearly illustrate the surface characteristics of nanomaterials synthesised by the biogenic co-precipitation technique. ZnO NPs were rod-shaped, with diameters ranging from 57 to 68 nm, as seen in Figure 3a. EDAX findings revealed the existence of major elements: Zn, Mg, and O (Figure 3b) [14,15]. Figure 3c shows the surface morphology of G-Zn50Mg50O nanocomposite.
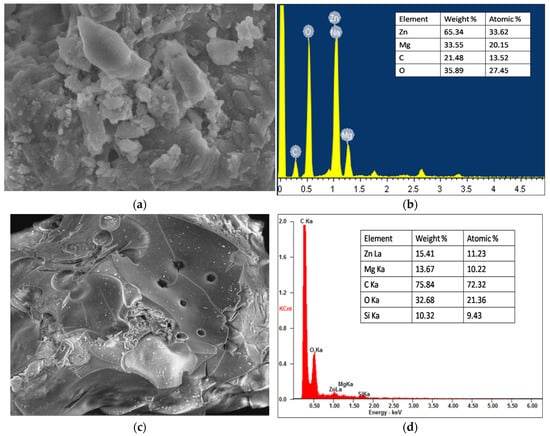
Figure 3.
SEM images of (a) Zn50Mg50O and (c) G-Zn50Mg50O and EDAX spectra of (b) Zn50Mg50O and (d) G-Zn50Mg50O.
In Figure 3c, MgO and ZnO particles were randomly coated on the surface of graphene, and micrographs revealed a trace content of ZnO nanorods and particles with hexagonal MgO nanopellets. The large accumulation of particles on the surface of graphene is due to the possibility of carbon-based bonds forming during the synthesis. Notably, the addition of ZnO and graphene, as well as thermal processes during nanocomposites synthesis, had no impact on the morphological properties of MgO. The EDAX spectra analysis was used to evaluate the degree of distribution of various elements on the nanocomposite surface (Figure 3d), which revealed a standardised distribution of major elements (Mg, O, Zn, Si, and C) on the catalyst surface. This discovery revealed that the synthesis process was completed successfully, with no ashes or impurities present in the samples.
3.4. TEM
Transmission Electron Microscope (TEM) analysis is a simple as well as an effective way to examine the morphology of nanoparticles and nanocomposites. Figure 4a,b shows the structural and morphological characteristics of Zn50Mg50O and G-Zn50Mg50O nanocomposites, which display hexagonal, rod, and irregular morphology with sizes of 55–70 and 60–70 nm, respectively [16,17].
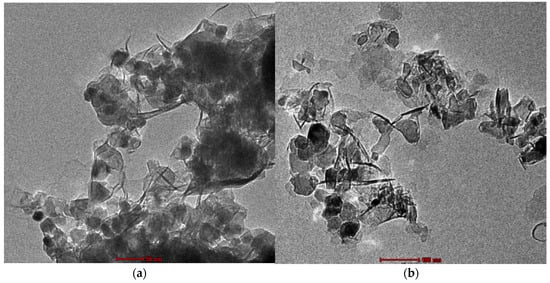
Figure 4.
TEM images of (a) Zn50Mg50O and (b) G-Zn50Mg50O.
3.5. FTIR
FTIR analysis showed a broad peak at 3452 cm−1 and 2960 cm−1 due to the major alcoholic O-H functional group, which is the important shifted metal ion absorption point [13,18,19,20,21]. Peaks were produced by the excitations of -CN and CH3 at 1752 cm−1 and 1457 cm−1, respectively (Figure 5). The peak at 1187 cm−1 could be shifted by the OH bond’s bending vibration, which is associated with the water surface that was absorbed by the synthetic ZnO/MgO nanocomposites. The Mg–O and Zn–O bindings that serve to shape pure and composite types of synthetic metal oxides may be responsible for the peaks at 813 cm−1 and 620 cm−1.
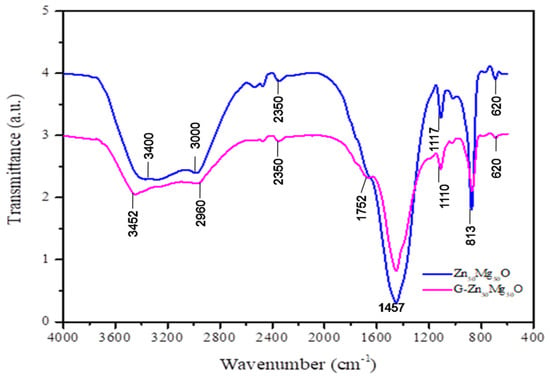
Figure 5.
FTIR spectra of Zn50Mg50O and G-Zn50Mg50O.
3.6. RSM Analysis for Photocatalytic Studies
The independent variables and values used for the photocatalytic removal of dyes using nanocomposites are shown in Table 1. Independent variables and quantities were chosen from early experimental investigation and a review of the literature. In the Box–Wilson design, there are (2f + 2*f + m) experiments, where f is the number of independent numeric factors and m is the number of mid-point. Hence, the number of experiments carried out was (22 + 2*2 + 3) = 11. The Box–Wilson matrix of 11 trials for photocatalytic dye removal using nanocomposites is shown in Table 2.

Table 1.
Independent variables and quantities utilised for photocatalytic removal of dyes using nanocomposites.

Table 2.
Box–Wilson design matrix for photocatalytic removal of dyes using nanocomposites.
The numerous models, linear, 2FI, quadratic, and cubic models, were analyzed for photocatalytic removal of dyes using nanocomposites. The values of F, p, R2, and the difference between modified and expected R2 and PRESS were utilised to verify the significance of the model. A high F-value of 56.75, a low p-value of <0.05, R2 > 0.8, the difference between modified and expected R2 < 0.2, and the least value of PRESS implied the important model and suitable for photocatalytic removal. The model fitting technique revealed that the quadratic equation fitted well with the investigational data of photocatalytic degradation of dyes using nanocomposites.
Equations (4)–(7) represent the results of fitting the experimental data to the quadratic model.
The quadratic models describing the photocatalytic degradation of RhB and MB dyes using Zn50Mg50O are represented by Equations (4) and (5), respectively. The quadratic models describing the photocatalytic degradation of RhB and MB dyes using G-Zn50Mg50O nanocomposite are represented by Equations (6) and (7), respectively. The reaction generally decreased when negative coefficient terms increased and vice versa. In a similar manner, a decrease in positive coefficient terms tended to reduce the dependent factor and vice versa [22]. According to the maxima and minima principle, positive coefficient values in linear terms and negative coefficient values in quadratic terms tend to maximise the response. Equations (3)–(6) thus showed that the linear and quadratic terms’ positive and negative coefficients tended to optimise dye removal.
Table 3 show the analysis of variance (ANOVA) for the quadratic model developed for photocatalytic removal of dyes using nanocomposites. From the ANOVA table, it is evident that the model was significant, with F-values ranging between 80.19 and 188.68 and a low p-value of <0.05. For photocatalytic degradation of RhB and MB dyes using Zn50Mg50O, the interaction between photocatalyst dosage and time was insignificant, with a p-value > 0.05. Linear terms for photocatalytic removal were significant with a p-value < 0.05. This means that the linear terms have more effect on dye removal. For photocatalytic degradation of RhB and MB dyes using Zn50Mg50O, quadratic terms were significant with a p-value > 0.05. For photocatalytic degradation of RhB and MB dyes using G-Zn50Mg50O, the quadratic term on time was significant with a p-value > 0.05.

Table 3.
Analysis of variance (ANOVA) for the quadratic model developed for photocatalytic dye removal.
Figure 6 shows the interactive effect of photocatalyst dosage and contact time on photocatalytic removal of (a) RhB and (b) MB dyes using Zn50Mg50O, and (c) RhB and (d) MB dyes using G-Zn50Mg50O. From Figure 6a, the photocatalytic removal of 17.7% was obtained for RhB dye using Zn50Mg50O at the lowest photocatalyst dosage and contact time of 0.2 g/L and 15 min, respectively. At this point, when the photocatalyst dosage increased to 0.6 g/L, the dye removal enhanced to 26.2%. Further increment of photocatalyst dosage to 1 g/L led to an increase in dye removal to 34.2%. Similarly, the dye removal increased to 51% at the photocatalyst dosage and contact time of 0.2 g/L and 90 min, respectively. The dye removal increased to 78.5% when the contact time increased to 165 min at the same photocatalyst dosage of 0.2 g/L. Additionally, the dye removals of 72% and 100% were obtained at a contact time of 90 and 165 min at the photocatalyst dosage of 0.6 g/L. Finally, the dye removals of 74.8% and 100% were observed at contact time values of 90 and 165 min at the photocatalyst dosage of 1 g/L. From the interactive effect between photocatalyst dosage and contact time, the optimal conditions were found to be photocatalyst dosage and contact time of 0.6 g/L and 165 min, respectively, to obtain dye removal of 100%.
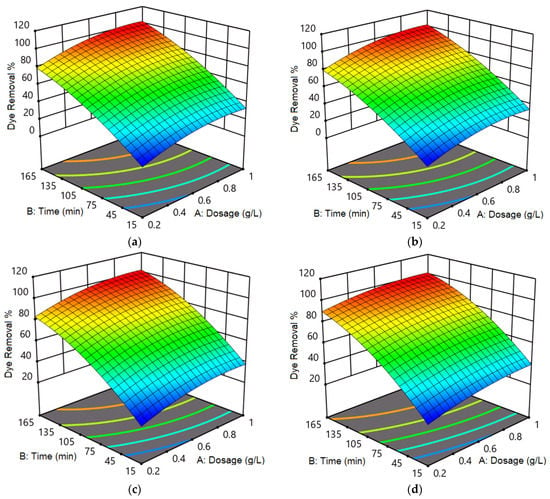
Figure 6.
Interactive effect of photocatalyst dosage and contact time on photocatalytic removal of (a) RhB and (b) MB dyes using Zn50Mg50O, and (c) RhB and (d) MB dyes using G-Zn50Mg50O.
From Figure 6b, the photocatalytic removal of 24.7% was obtained for MB dye using Zn50Mg50O at the lowest photocatalyst dosage and contact time of 0.2 g/L and 15 min, respectively. At this point, when the photocatalyst dosage increased to 0.6 g/L, the dye removal enhanced to 29.2%. Further increment of photocatalyst dosage to 1 g/L led to an increase in dye removal to 39.2%. Similarly, the dye removal increased to 56.1% at the photocatalyst dosage and contact time of 0.2 g/L and 90 min, respectively. The dye removal increased to 83.6% when the contact time increased to 165 min at the same photocatalyst dosage of 0.2 g/L. Additionally, the dye removals of 76.7% and 100% were obtained at a contact time of 90 and 165 min at the photocatalyst dosage of 0.6 g/L. Finally, the dye removals of 79.8% and 100% were observed at contact time values of 90 and 165 min at the photocatalyst dosage of 1 g/L. From the interactive effect between photocatalyst dosage and contact time, the optimal conditions were found to be photocatalyst dosage and contact time of 0.6 g/L and 165 min, respectively, to obtain dye removal of 100%.
From Figure 6c, the photocatalytic removal of 19.7% was obtained for RhB dye using G-Zn50Mg50O at the lowest photocatalyst dosage and contact time of 0.2 g/L and 15 min, respectively. At this point, when the photocatalyst dosage increased to 0.6 g/L, the dye removal enhanced to 28.2%. Further increment of photocatalyst dosage to 1 g/L led to an increase in dye removal to 35.2%. Similarly, the dye removal increased to 52% at the photocatalyst dosage and contact time of 0.2 g/L and 90 min, respectively. The dye removal increased to 79.5% when the contact time increased to 165 min at the same photocatalyst dosage of 0.2 g/L. Additionally, the dye removals of 72.9% and 100% were obtained at a contact time of 90 and 165 min at the photocatalyst dosage of 0.6 g/L. Finally, the dye removals of 75.8% and 100% were observed at contact time values of 90 and 165 min at the photocatalyst dosage of 1 g/L. From the interactive effect between photocatalyst dosage and contact time, the optimal conditions were found to be photocatalyst dosage and contact time of 0.6 g/L and 165 min, respectively, to obtain dye removal of 100%.
From Figure 6d, the photocatalytic removal of 26.7% was obtained for MB dye using G-Zn50Mg50O at the lowest photocatalyst dosage and contact time of 0.2 g/L and 15 min, respectively. At this point, when the photocatalyst dosage increased to 0.6 g/L, the dye removal enhanced to 32.2%. Further increment of photocatalyst dosage to 1 g/L led to an increase in dye removal to 40.2%. Similarly, the dye removal increased to 63% at the photocatalyst dosage and contact time of 0.2 g/L and 90 min, respectively. The dye removal increased to 90.5% when the contact time increased to 165 min at the same photocatalyst dosage of 0.2 g/L. Additionally, the dye removals of 77.8% and 100% were obtained at a contact time of 90 and 165 min at the photocatalyst dosage of 0.6 g/L. Finally, the dye removals of 80.8% and 100% were observed at contact time values of 90 and 165 min at the photocatalyst dosage of 1 g/L. From the interactive effect between photocatalyst dosage and contact time, the optimal conditions were found to be photocatalyst dosage and contact time of 0.6 g/L and 165 min, respectively, to obtain dye removal of 100%.
Experiments were conducted under different production settings given in Table 2 to evaluate the validity of the proposed model and the resulting predicted results. The deviation between the investigational and expected values was 3.43, which is lesser than ±5%. The values obtained were not significantly different from the expected values, according to the coefficient of variation (CV), which was 6.56%. Additionally, it was demonstrated that the difference between observed and expected values is small. The analysis of the data was supported by the fact that all of the data points were within 10% of the expected and experimental values.
4. Conclusions
The current investigation aimed to prepare and investigate ZnMgO and silica-supported graphene with ZnMgO (G-ZnMgO) nanocomposites and evaluate their potential in the application of photocatalysis to remove RhB and MB dyes from their Kappaphycus alvarezii aqueous extract by maximising the percentage removal using response surface methodology (RSM) modelling. RSM modelling predicted the optimal conditions were found to be photocatalyst dosage and contact time of 0.56 g/L and 165 min, respectively, to obtain maximum MB dye removal of 10% using G-Zn50Mg50O. For RhB using Zn50Mg50O and G-Zn50Mg50Oand MB using Zn50Mg50O, 100% removal was achieved at photocatalyst dosage between 0.58 and 0.62 g/L and 165 min. The results showed the best fit between experimental and RSM predicted values. Thus, the obtained results conclude that the algal biomass synthesised nanocomposites were found to be one of the potential photocatalysts for the degradation of RhB and MB dyes from Kappaphycus alvarezii aqueous solution.
Author Contributions
Conceptualisation and investigation, G.N.; methodology and data analysis J.P. and S.S.; writing original and revising draft G.N., R.G., M.T., M.D., P.B., A.P. and V.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Carmen, Z.; Daniela, S. Textile Organic Dyes-Characteristics, Polluting Effects and Separation/Elimination Procedures from Industrial Effluents-A Critical Overview; IntechOpen: London, UK, 2012; pp. 55–86. [Google Scholar]
- Belver, C.; Bedia, J.; Gómez-Avilés, A.; Peñas-Garzón, M.; Rodriguez, J.J. Semiconductor photocatalysis for water purification. In Nanoscale Materials in Water Purification; Elsevier: Amsterdam, The Netherlands, 2019; pp. 581–651. [Google Scholar]
- Asahi, R.; Morikawa, T.; Irie, H.; Ohwaki, T. Nitrogen-doped titanium dioxide as visible-light-sensitive photocatalyst: Designs, developments, and prospects. Chem. Rev. 2014, 114, 9824–9852. [Google Scholar] [CrossRef] [PubMed]
- Nethavhanani, T. Synthesis of zinc oxide nanoparticles by a green process and the investigation of their physical properties. AIP Conf. Proc. 2018, 1962, 040007. [Google Scholar] [CrossRef]
- Ferreira, S.C.; Bruns, R.E.; Ferreira, H.S.; Matos, G.D.; David, J.M.; Brandão, G.C.; Dos Santos, W.N.L. Box-Behnken design: An alternative for the optimization of analytical methods. Anal. Chim. Acta 2007, 597, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Montgomery, D.C. Design and Analysis of Experiments, 8th ed.; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2013. [Google Scholar]
- Theerthagiri, J.; Senthil, R.A.; Malathi, A.; Selvi, A.; Madhavan, J.; Ashokkumar, M. Synthesis and characterization of a CuS–WO3 composite photocatalyst for enhanced visible light photocatalytic activity. RSC Adv. 2015, 5, 52718–52725. [Google Scholar] [CrossRef]
- Chawla, S.; Jayanthi, K.; Chander, H.; Haranath, D.; Halder, S.K.; Kar, M. Synthesis and optical properties of ZnO/MgO nanocomposite. J. Alloys Compd. 2008, 459, 457–460. [Google Scholar] [CrossRef]
- Zhang, Y.; Qi, H.; Zhang, L.; Wang, Y.; Zhong, L.; Zheng, Y.; Xue, J. A RGO aerogel/TiO2/MoS2 composite photocatalyst for the removal of organic dyes by the cooperative action of adsorption and photocatalysis. Environ. Sci. Pollut. Res. 2022, 29, 8980–8995. [Google Scholar] [CrossRef] [PubMed]
- Anand, K.V.; Chinnu, M.K.; Kumar, R.M.; Mohan, R.; Jayavel, R. Formation of zinc sulfide nanoparticles in HMTA matrix. Appl. Surf. Sci. 2009, 255, 8879–8882. [Google Scholar] [CrossRef]
- Rufus, A.; Sreeju, N.; Philip, D. Synthesis of biogenic hematite (α-Fe2O3) nanoparticles for antibacterial and nanofluid applications. RSC Adv. 2016, 6, 94206–94217. [Google Scholar] [CrossRef]
- Gajendiran, J.; Rajendran, V. Synthesis and characterization of coupled semiconductor metal oxide (ZnO/CuO) nanocomposite. Mater. Lett. 2014, 116, 311–313. [Google Scholar] [CrossRef]
- Jangid, N.K.; Jadoun, S.; Yadav, A.; Srivastava, M.; Kaur, N. Polyaniline-TiO2-based photocatalysts for dyes degradation. Polym. Bull. 2021, 78, 4743–4777. [Google Scholar] [CrossRef]
- Panchal, P.; Paul, D.R.; Sharma, A.; Hooda, D.; Yadav, R.; Meena, P.; Nehra, S.P. Phytoextract mediated ZnO/MgO nanocomposites for photocatalytic and antibacterial activities. J. Photochem. Photobiol. A Chem. 2019, 385, 112049. [Google Scholar] [CrossRef]
- Fagier, M.A. Plant-mediated biosynthesis and photocatalysis activities of zinc oxide nanoparticles: A prospect towards dyes mineralization. J. Nanotechnol. 2021, 2021, 6629180. [Google Scholar] [CrossRef]
- Govindaraju, K.; Anand, K.V.; Anbarasu, S.; Theerthagiri, J.; Revathy, S.; Krupakar, P.; Subramanian, K.S. Seaweed (Turbinariaornata)-assisted green synthesis of magnesium hydroxide [Mg(OH)2] nanomaterials and their anti-mycobacterial activity. Mater. Chem. Phys. 2020, 239, 122007. [Google Scholar] [CrossRef]
- Tahir, M.B.; Tufail, S.; Ahmad, A.; Rafique, M.; Iqbal, T.; Abrar, M.; Ijaz, M. Semiconductor nanomaterials for the detoxification of dyes in real wastewater under visible-light photocatalysis. Int. J. Environ. Anal. Chem. 2021, 101, 1735–1749. [Google Scholar] [CrossRef]
- Thakkar, K.N.; Mhatre, S.S.; Parikh, R.Y. Biological synthesis of metallic nanoparticles. Nanomed. Nanotechnol. Biol. Med. 2010, 6, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Malik, R.; Tomer, V.K.; Chaudhary, V.; Dahiya, M.S.; Rana, P.S.; Nehra, S.P.; Duhan, S. Facile synthesis of hybridized mesoporousAu@TiO2/SnO2 as efficient photocatalyst and selective VOC sensor. ChemistrySelect 2016, 1, 3247–3258. [Google Scholar] [CrossRef]
- Ahmaruzzaman, M.; Gupta, V.K. Rice husk and its ash as low-cost adsorbents in water and wastewater treatment. Ind. Eng. Chem. Res. 2011, 50, 13589–13613. [Google Scholar] [CrossRef]
- La, D.D.; Tran, C.V.; Hoang, N.T.; Ngoc, M.D.D.; Nguyen, T.P.; Vo, H.T.; Nguyen, D.D. Efficient photocatalysis of organic dyes under simulated sunlight irradiation by a novel magnetic CuFe2O4@ porphyrin nanofiber hybrid material fabricated via self-assembly. Fuel 2020, 281, 118655. [Google Scholar] [CrossRef]
- Tripathi, P.; Srivastava, V.C.; Kumar, A. Optimization of an azo dye batch adsorption parameters using Box–Behnken design. Desalination 2009, 249, 1273–1279. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).