Size-Dependent Structural, Magnetic and Magnetothermal Properties of Y3Fe5O12 Fine Particles Obtained by SCS
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Methods
3. Results
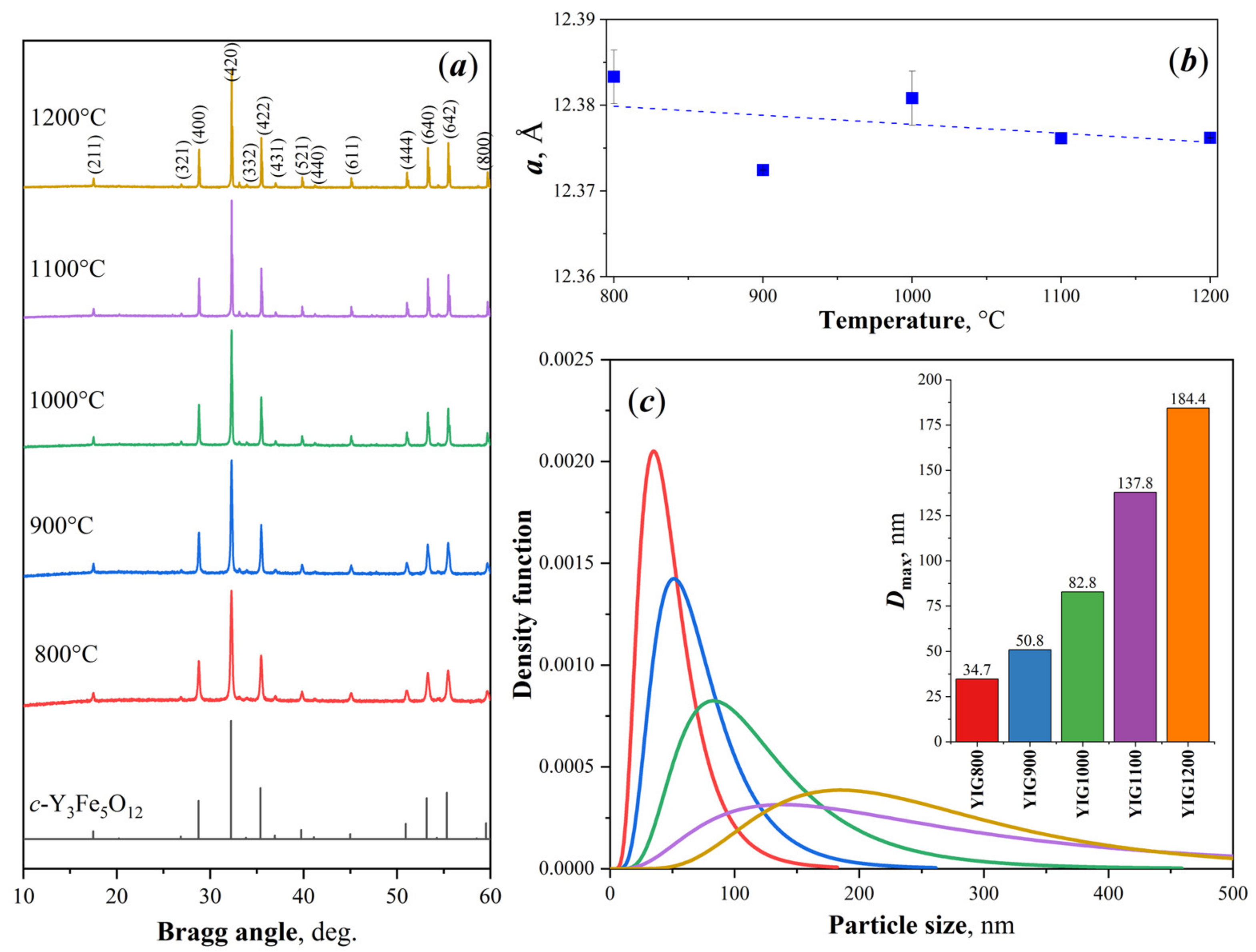
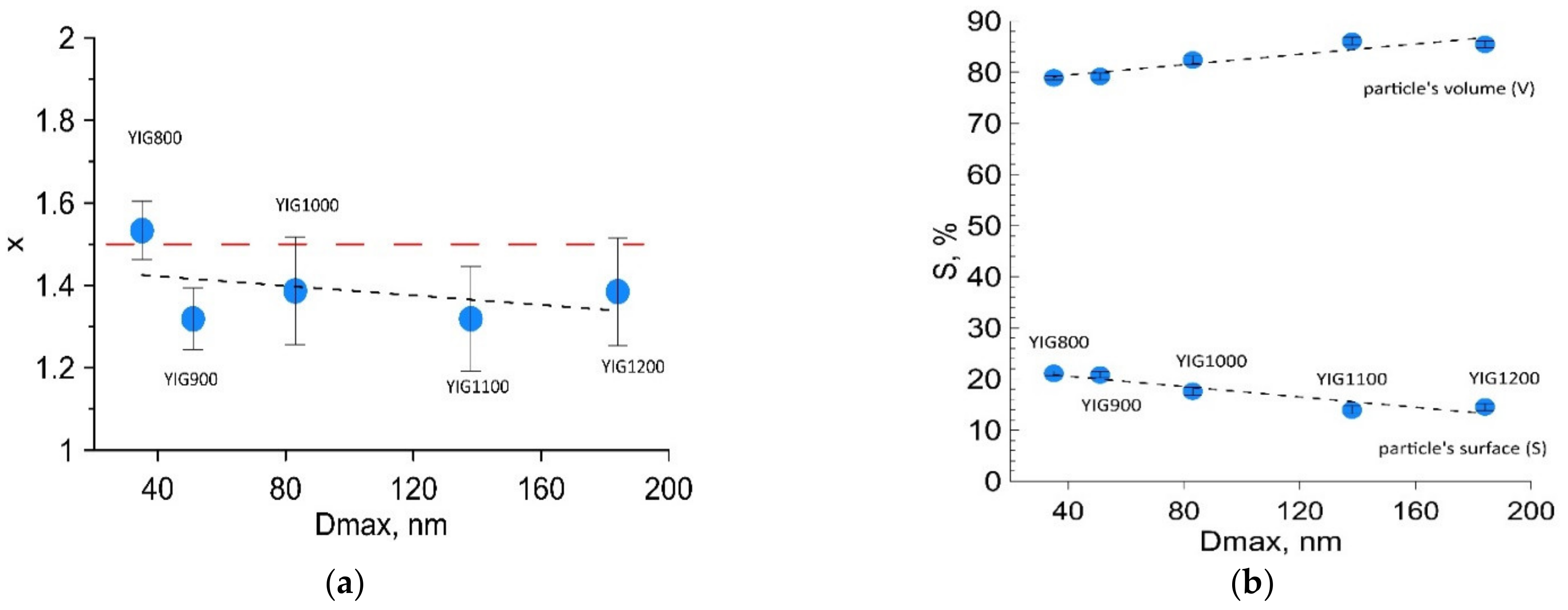
3.1. Scanning Electron Microscopy
3.2. X-ray Diffraction
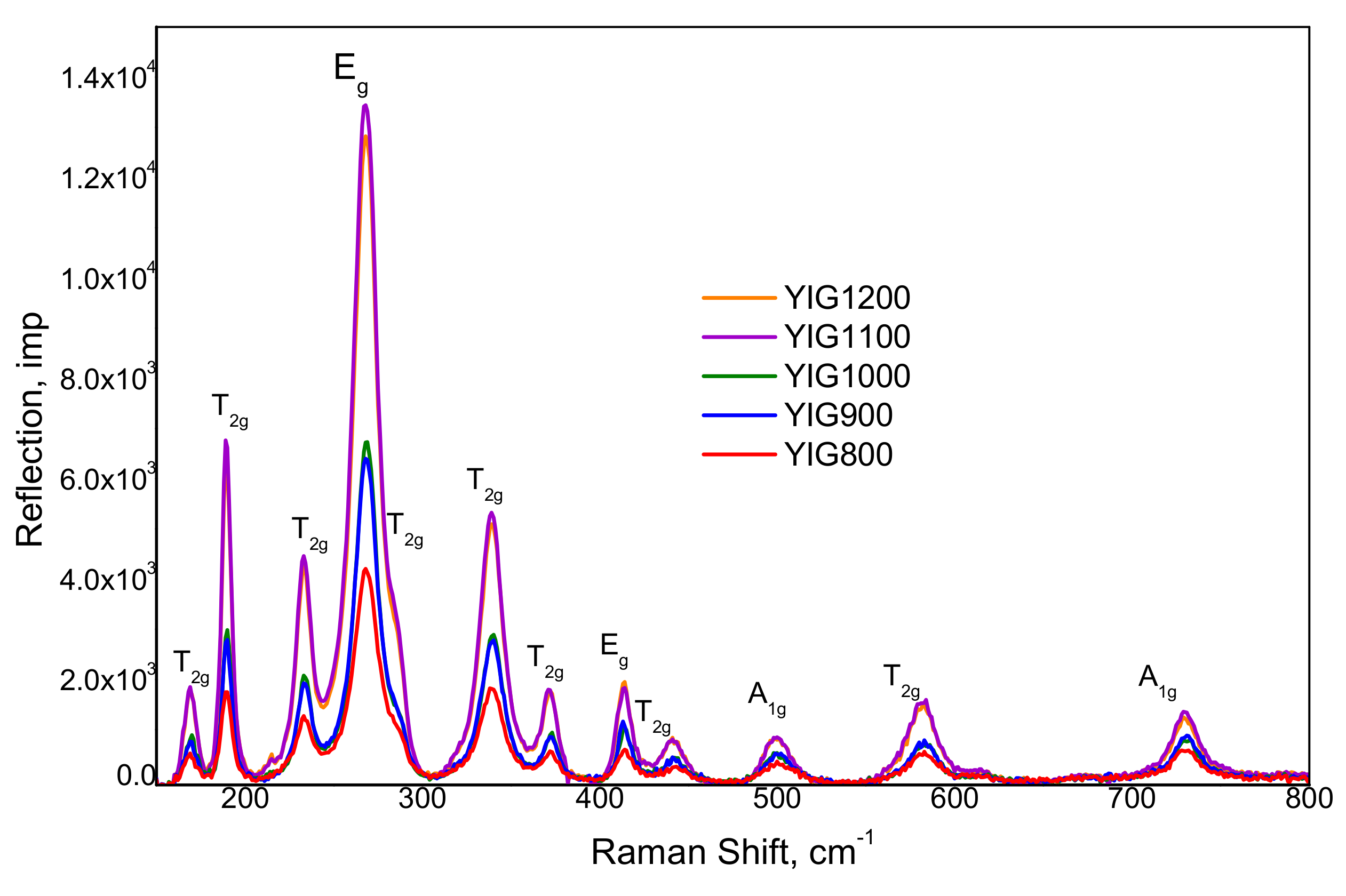
3.3. Raman Spectroscopy
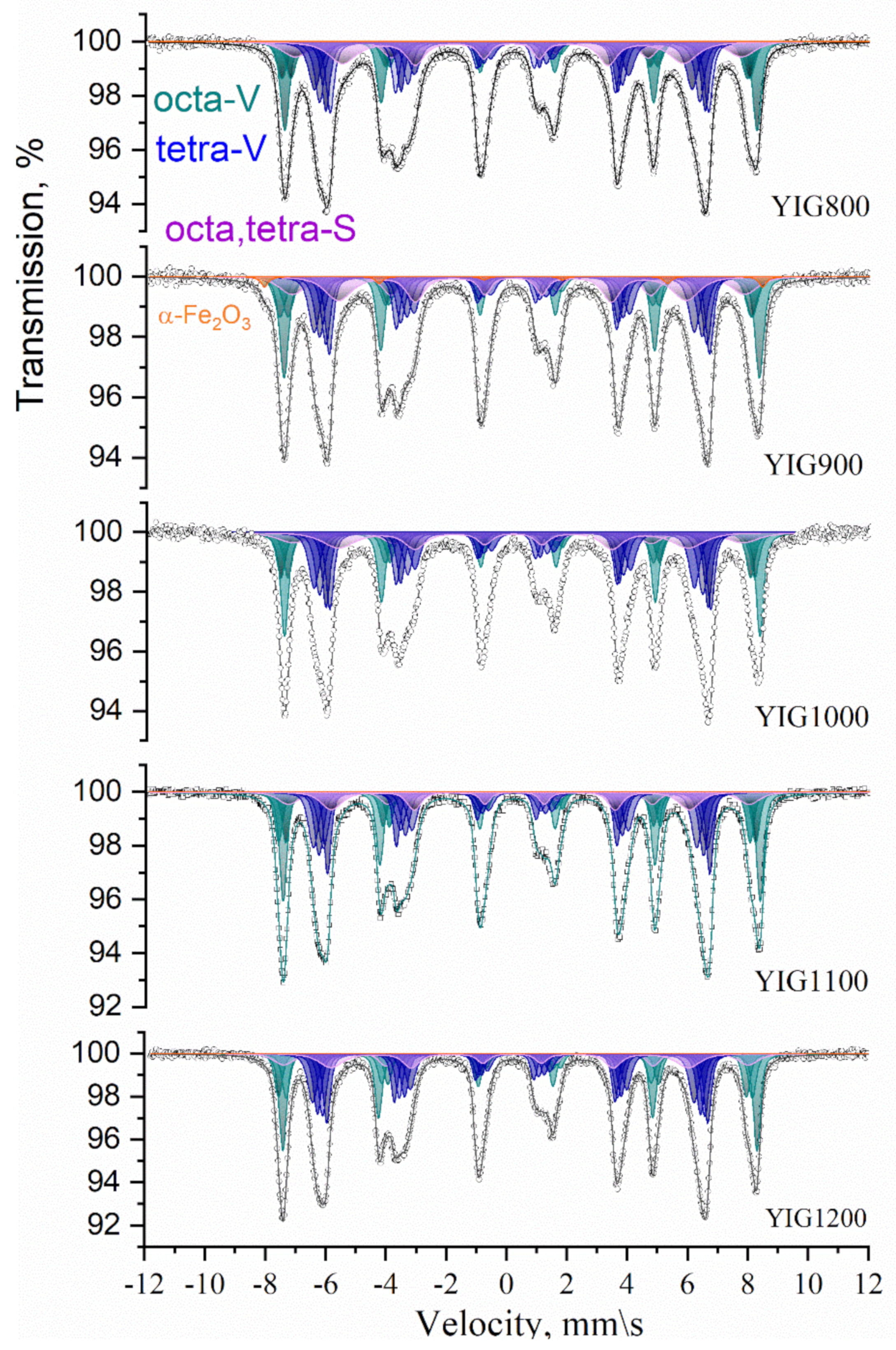
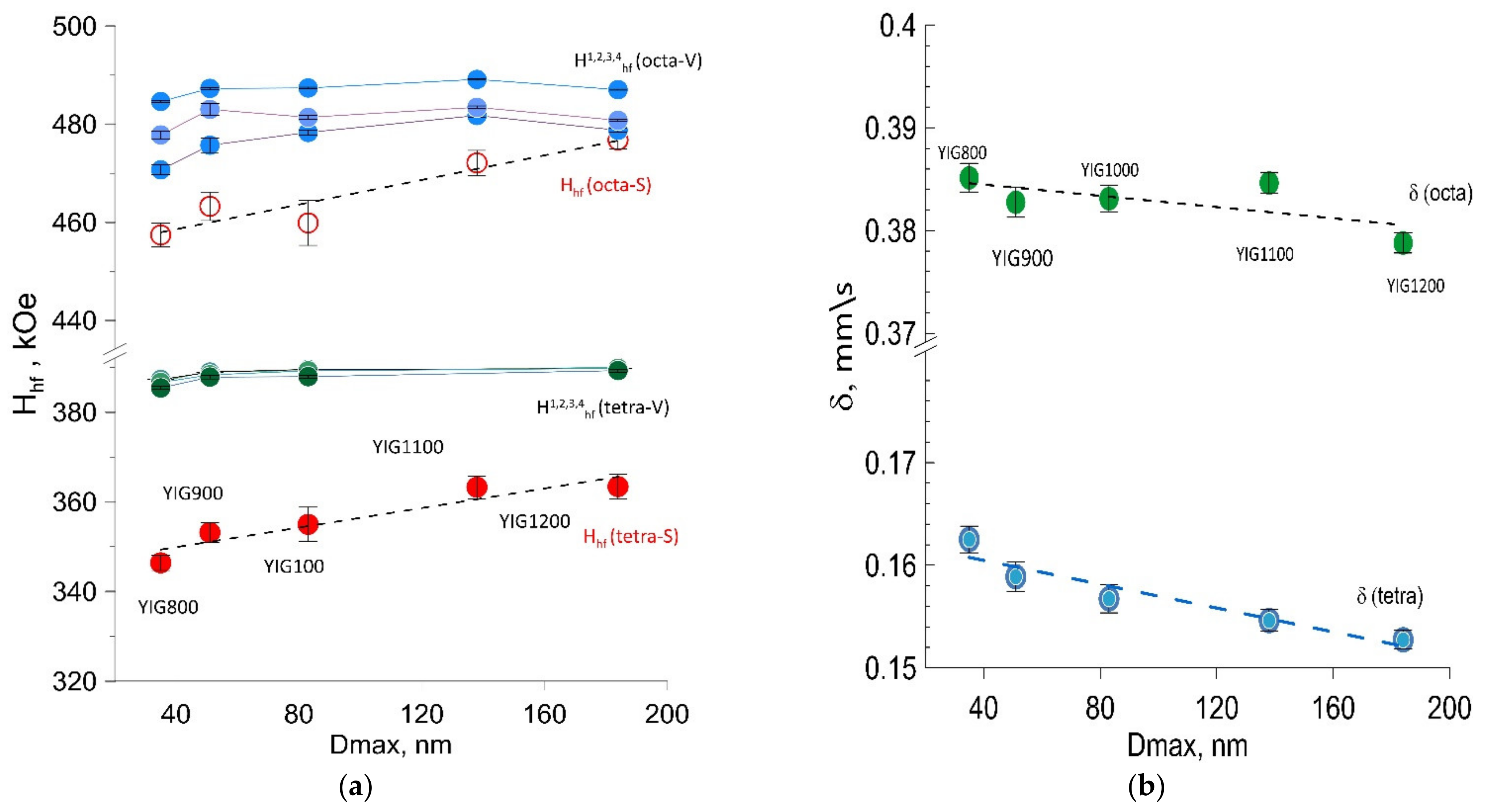
3.4. Mossbauer Spectroscopy
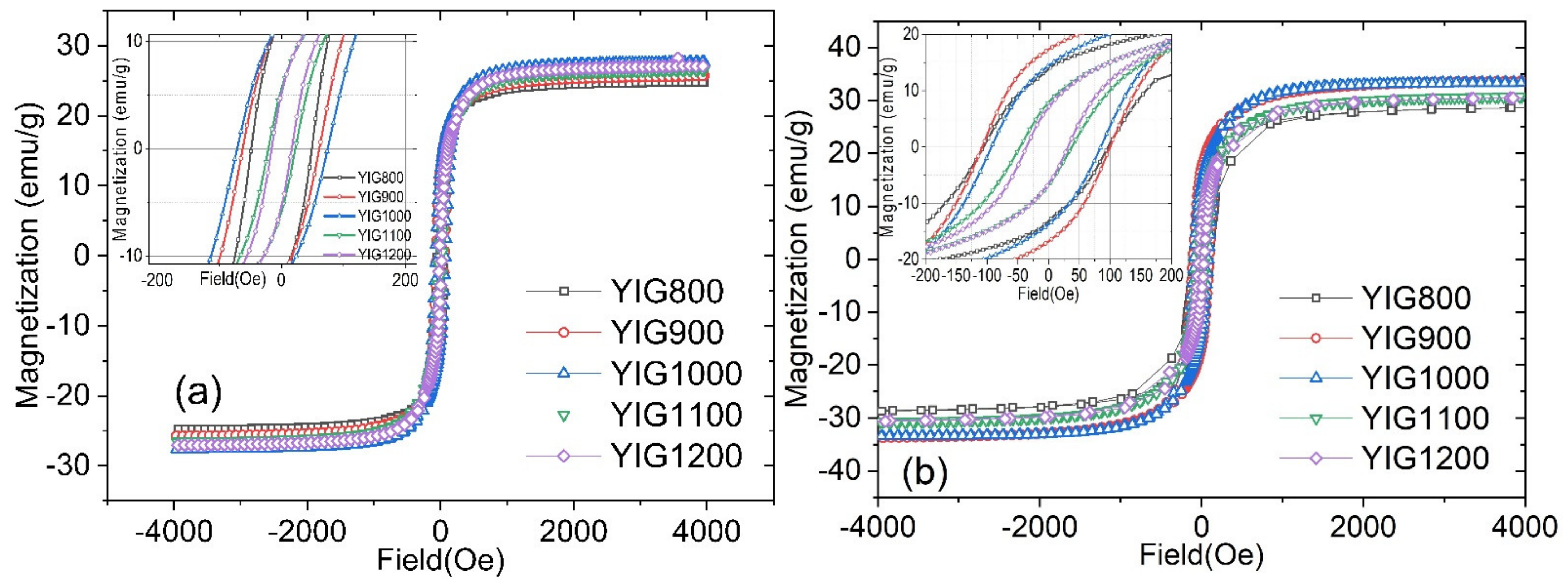
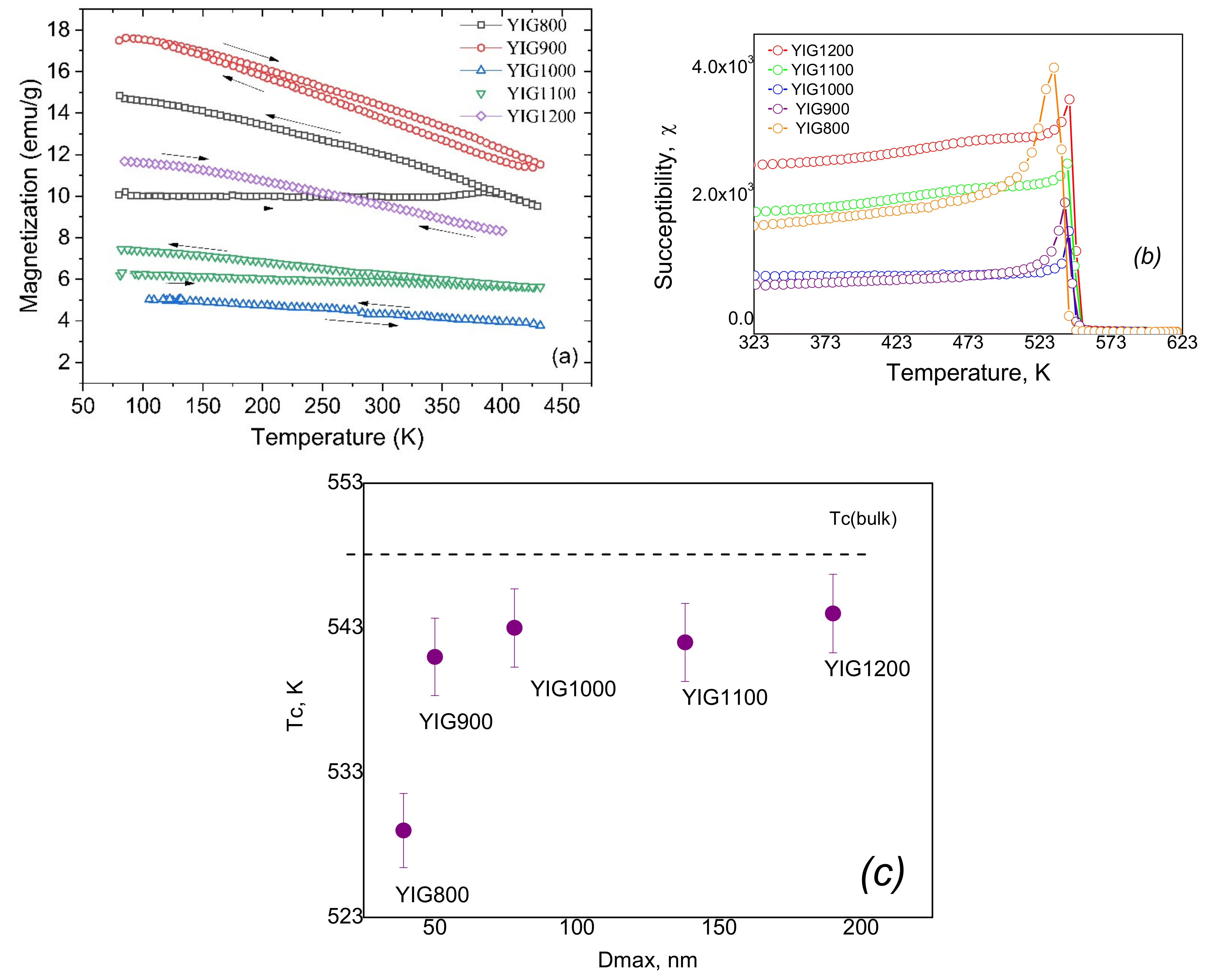
3.5. Magnetic Measurements
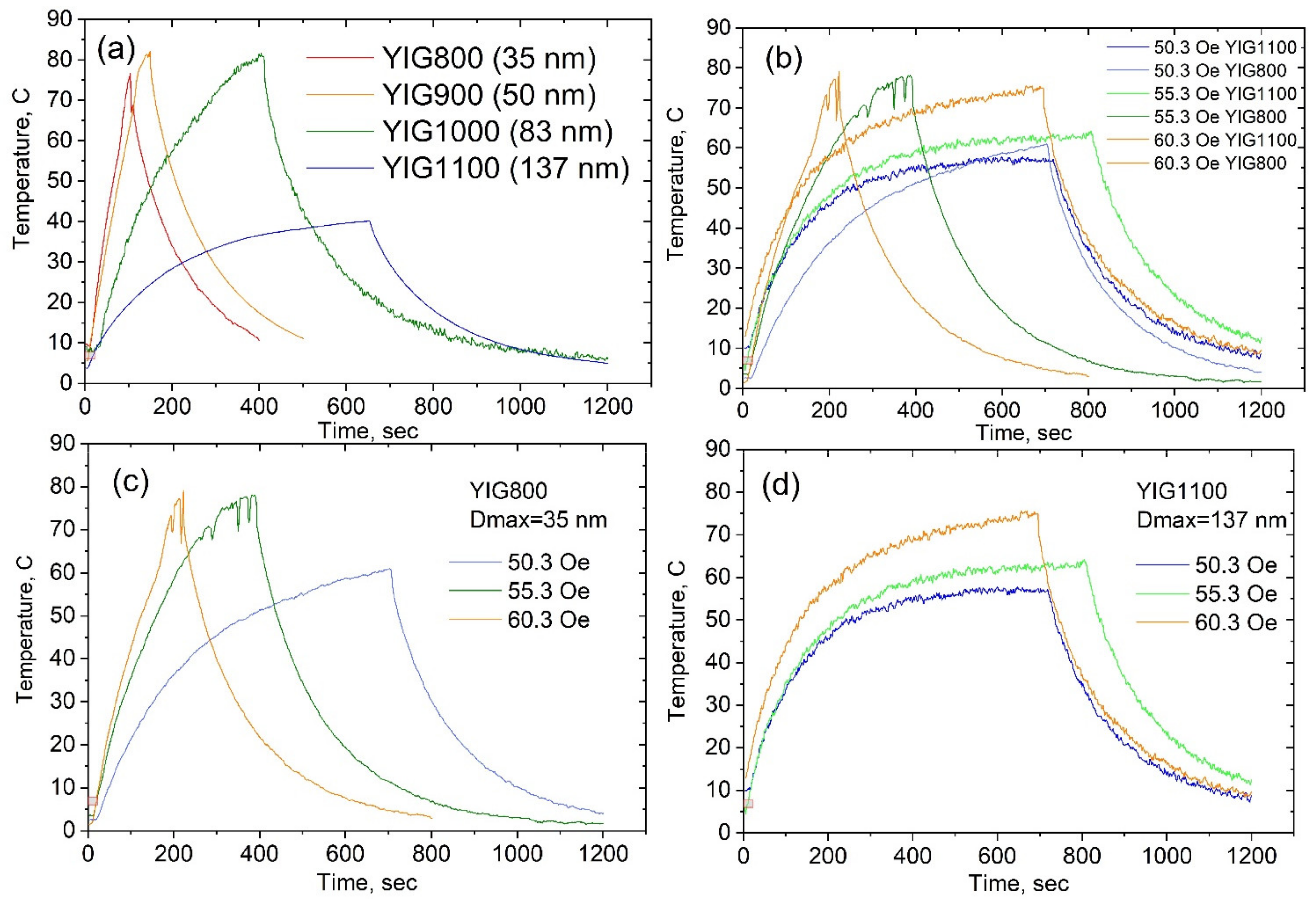
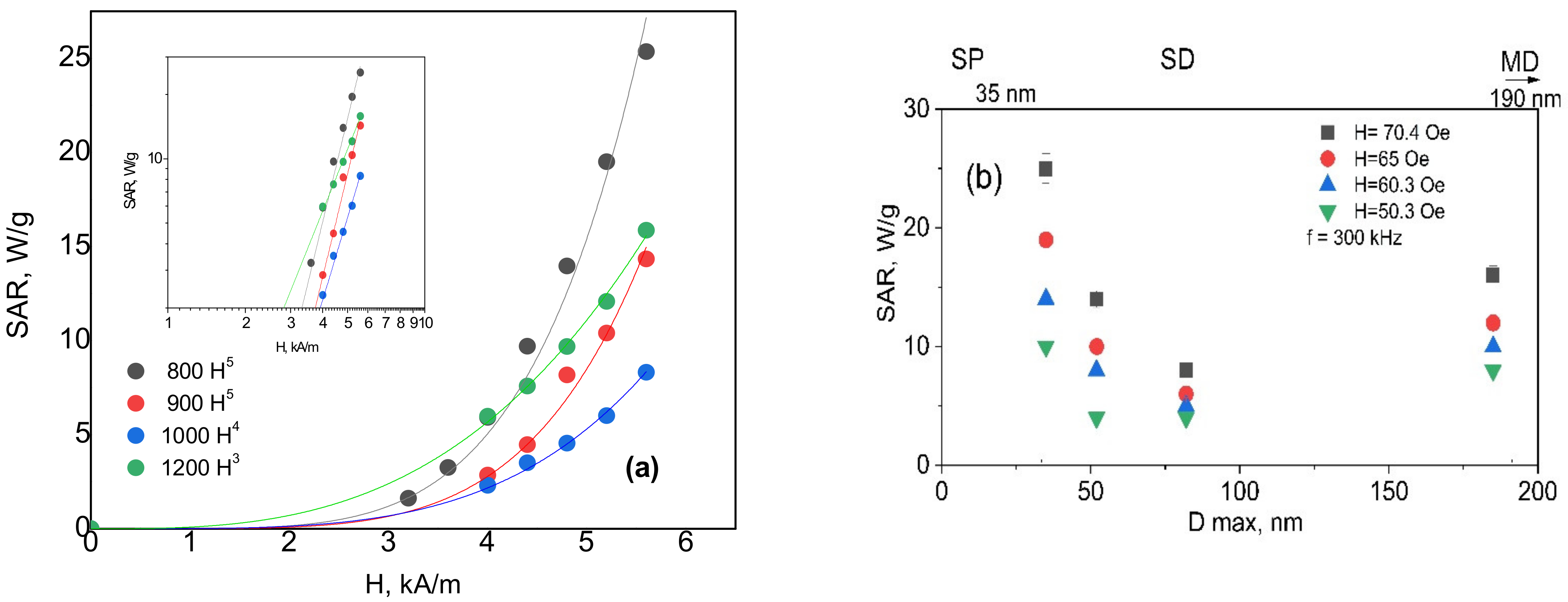
3.6. Heat Generation in AC Magnetic Field
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cherepanov, V.; Kolokolov, I.; L’vov, V. The Saga of YIG: Spectra, Thermodynamics, Interaction and Relaxation of Magnons in a Complex Magnet. Phys. Rep. 1993, 229, 81–144. [Google Scholar] [CrossRef]
- Dionne, G.F. Magnetic Oxides; Springer: New York, NY, USA; Dordrecht, The Nertherlands; Heidelberg, Germany; London, UK, 2009; 474p, ISBN 978-1-4419-0053-1. [Google Scholar]
- Mallmann, E.J.J.; Sombra, A.S.B.; Goes, J.C.; Fechine, P.B.A. Yttrium Iron Garnet: Properties and Applications Review. Solid State Phenom. 2013, 202, 65–96. [Google Scholar] [CrossRef]
- Kiseleva, T.; Grigoreva, T.; Tyapkin, P.; Ivanenko, I.; Devyatkina, E.; Markov, G.; Deleg, S.; Ilyushin, A.; Lyakhov, N. Mossbauer and Raman Spectroscopy Study of Y-Garnet Particles’ Magnetic Properties Tune-up through Mechanochemically Synthesized Precursors. Hyperfine Interact. 2021, 242, 57. [Google Scholar]
- Nakashima, H.; Pradipto, A.-M.; Akiyama, T.; Ito, T.; Nakamura, K. Electron Correlation Effects and Magneto-Optical Properties of Yttrium Iron Garnet. AIP Adv. 2020, 10, 045029. [Google Scholar] [CrossRef]
- McCloy, J.S.; Walsh, B. Sublattice Magnetic Relaxation in Rare Earth Iron Garnets. IEEE Trans. Magn. 2013, 49, 4253–4256. [Google Scholar] [CrossRef]
- Kim, T.-Y.; Yamazaki, Y.; Hong, Y.-D.; Hirano, T. Magneto-Optical Properties of Bi-YIG Nanoparticle with Polymethacrylate Matrix Materials. In Proceedings of the 2003 IEEE International Magnetics Conference (INTERMAG), Boston, MA, USA, 30 March–3 April 2003; p. EQ-04. [Google Scholar] [CrossRef]
- Jeon, Y.H.; Lee, J.W.; Oh, J.H.; Lee, J.C.; Choi, S.C. Magneto-Optical Properties of Bi-YIG Nanoparticles/Epoxy Hybrid Materials. Phys. Status Solidi (a) 2004, 201, 1893–1896. [Google Scholar] [CrossRef]
- Hirazawa, H.; Matsumoto, R.; Sakamoto, M.; Enkhnaran, U.; Sangaa, D.; Kiseleva, T.Y.; Yano, J.; Fukuoka, H.; Aono, H. Heat Generation in the AC Magnetic Field of Fine Y3Fe5O12 Powder Materials Prepared by Modifying Co-Precipitation Synthesis. J. Ceram. Soc. Jpn. 2021, 129, 579–583. [Google Scholar] [CrossRef]
- Aono, H.; Ebara, H.; Senba, R.; Naohara, T.; Maehara, T.; Hirazawa, H.; Watanabe, Y. High Heat Generation Ability in AC Magnetic Field of Y3Fe5O12 Powder Prepared Using Bead Milling. J. Am. Ceram. Soc. 2011, 94, 4116–4119. [Google Scholar] [CrossRef]
- Liang, Y.-J.; Xie, J.; Yu, J.; Zheng, Z.; Liu, F.; Yang, A. Recent Advances of High Performance Magnetic Iron Oxide Nanoparticles: Controlled Synthesis, Properties Tuning and Cancer Theranostics. Nano Sel. 2021, 2, 216–250. [Google Scholar] [CrossRef]
- Fopase, R.; Saxena, V.; Seal, P.; Borah, J.P.; Pandey, L.M. Yttrium Iron Garnet for Hyperthermia Applications: Synthesis, Characterization and in-Vitro Analysis. Mater. Sci. Eng. C 2020, 116, 111163. [Google Scholar] [CrossRef]
- Komlev, A.S.; Zverev, V.I. Chapter 14-Magnetocaloric Effect for Medical Applications. In Magnetic Materials and Technologies for Medical Applications; Tishin, A.M., Ed.; Woodhead Publishing Series in Electronic and Optical Materials; Woodhead Publishing: Kidlington, UK; Elsevier: Sawston, UK, 2022; pp. 437–467. ISBN 978-0-12-822532-5. [Google Scholar]
- Davydov, A.S.; Belousov, A.V.; Krusanov, G.A.; Kolyvanova, M.A.; Kovalev, B.B.; Komlev, A.S.; Krivoshapkin, P.V.; Morozov, V.N.; Zverev, V.I. Promising Magnetic Nanoradiosensitizers for Combination of Tumor Hyperthermia and X-ray Therapy: Theoretical Calculation. J. Appl. Phys. 2021, 129, 033902. [Google Scholar] [CrossRef]
- Vavaev, E.S.; Novoselova, M.; Shchelkunov, N.M.; German, S.; Komlev, A.S.; Mokrousov, M.D.; Zelepukin, I.V.; Burov, A.M.; Khlebtsov, B.N.; Lyubin, E.V.; et al. CaCO3 Nanoparticles Coated with Alternating Layers of Poly-L-Arginine Hydrochloride and Fe3O4 Nanoparticles as Navigable Drug Carriers and Hyperthermia Agents. ACS Appl. Nano Mater. 2022, 5, 2994–3006. [Google Scholar] [CrossRef]
- Griaznova, O.Y.; Belyaev, I.B.; Sogomonyan, A.S.; Zelepukin, I.V.; Tikhonowski, G.V.; Popov, A.A.; Komlev, A.S.; Nikitin, P.I.; Gorin, D.A.; Kabashin, A.V.; et al. Laser Synthesized Core-Satellite Fe-Au Nanoparticles for Multimodal In Vivo Imaging and In Vitro Photothermal Therapy. Pharmaceutics 2022, 14, 994. [Google Scholar] [CrossRef] [PubMed]
- Soleimani, H.; Abbas, Z.; Yahya, N.; Shameli, K.; Soleimani, H.; Shabanzadeh, P. Reflection and Transmission Coefficient of Yttrium Iron Garnet Filled Polyvinylidene Fluoride Composite Using Rectangular Waveguide at Microwave Frequencies. Int. J. Mol. Sci. 2012, 13, 8540–8548. [Google Scholar] [CrossRef]
- Komlev, A.S.; Gimaev, R.R.; Zverev, V.I. Smart Magnetocaloric Coatings for Implants: Controlled Drug Release for Targeted Delivery. Phys. Open 2021, 7, 100063. [Google Scholar] [CrossRef]
- Winkler, H.; Eisberg, R.; Alp, E.; Rüffer, R.; Gerdau, E.; Lauer, S.; Trautwein, A.X.; Grodzicki, M.; Vera, A. Pure Nuclear Reflexes and Combined Hyperfine Interactions in YIG. Z. Phys. B Condens. Matter 1983, 49, 331–341. [Google Scholar] [CrossRef]
- Gilleo, M.A.; Geller, S. Magnetic and crystallographic properties of substituted Yttrium-iron garnet. Phys. Rev. 1958, 110, 73–78. [Google Scholar] [CrossRef]
- Vandormael, D.; Grandjean, F.; Hautot, D.; Long, G.J. Mössbauer Spectral Evidence for Rhombohedral Symmetry in R3Fe5O12 Garnets with R = Y, Eu and Dy. J. Phys. Condens. Matter 2001, 13, 1759. [Google Scholar] [CrossRef]
- Sawatzky, G.A.; Van Der Woude, F.; Morrish, A.H. Recoilless-Fraction Ratios for Octahedral and Tetrahedral Sites of a Spinel and a Garnet. Phys. Rev. 1969, 183, 383–386. [Google Scholar] [CrossRef]
- Haneda, K.; Morrish, A. Mössbauer Study of Magnetism in YIG Small Particles. J. Magn. Soc. Jpn. 1998, 22, 255–257. [Google Scholar] [CrossRef] [Green Version]
- Niyaifar, M.; Mohammadpour, H.; Dorafshani, M.; Hasanpour, A. Size Dependence of Non-Magnetic Thickness in YIG Nanoparticles. J. Magn. Magn. Mater. 2016, 409, 104–110. [Google Scholar] [CrossRef]
- Nguyet, D.T.T.; Duong, N.P.; Satoh, T.; Anh, L.N.; Hien, T.D. Temperature-Dependent Magnetic Properties of Yttrium Iron Garnet Nanoparticles Prepared by Citrate Sol–Gel. J. Alloys Compd. 2012, 541, 18–22. [Google Scholar] [CrossRef]
- Kitayama, K.; Sakaguchi, M.; Takahara, Y.; Endo, H.; Ueki, H. Phase Equilibrium in the System Y–Fe–O at 1100 C. J. Solid State Chem. 2004, 177, 1933–1938. [Google Scholar] [CrossRef]
- Popkov, V.I.; Almjasheva, O.V.; Panchuk, V.V.; Semenov, V.G.; Gusarov, V.V. The Role of Pre-Nucleus States in Formation of Nanocrystalline Yttrium Orthoferrite. Dokl. Chem. 2016, 473, 356–359. [Google Scholar] [CrossRef]
- Noun, W.; Popova, E.; Bardelli, F.; Dumont, Y.; Bertacco, R.; Tagliaferri, A.; Tessier, M.; Guyot, M.; Berini, B.; Keller, N. Determination of Yttrium Iron Garnet Superexchange Parameters as a Function of Oxygen and Cation Stoichiometry. Phys. Rev. B 2010, 81, 054411. [Google Scholar] [CrossRef]
- Jacob, K.T.; Rajitha, G. Nonstoichiometry, Defects and Thermodynamic Properties of YFeO3, YFe2O4 and Y3Fe5O12. Solid State Ion. 2012, 224, 32–40. [Google Scholar] [CrossRef]
- Sadhana, K.; Murthy, S.R.; Praveena, K. Structural and Magnetic Properties of Dy3+ Doped Y3Fe5O12 for Microwave Devices. Mater. Sci. Semicond. Process. 2015, 34, 305–311. [Google Scholar] [CrossRef]
- Kum, J.S.; Kim, S.J.; Shim, I.B.; Kim, C.S. Magnetic Properties and Mössbauer Studies of Y3−x Cex Fe5O12 Fabricated using a sol-gel method. In Proceedings of the Program of the 2003 IEEE International Magnetics Conference (Intermag Conference), Boston, MA, USA, 30 March–3 April 2003; p. EU-09. [Google Scholar]
- Niaz Akhtar, M.; Azhar Khan, M.; Ahmad, M.; Murtaza, G.; Raza, R.; Shaukat, S.F.; Asif, M.H.; Nasir, N.; Abbas, G.; Nazir, M.S.; et al. Y3Fe5O12 Nanoparticulate Garnet Ferrites: Comprehensive Study on the Synthesis and Characterization Fabricated by Various Routes. J. Magn. Magn. Mater. 2014, 368, 393–400. [Google Scholar] [CrossRef]
- Abbas, R.; Martinson, K.D.; Kiseleva, T.Y.; Markov, G.P.; Tyapkin, P.Y.; Popkov, V.I. Effect of Fuel Type on the Solution Combustion Synthesis, Structure, and Magnetic Properties of YIG Nanocrystals. Mater. Today Commun. 2022, 32, 103866. [Google Scholar] [CrossRef]
- Apostolov, A.T.; Apostolova, I.N.; Wesselinowa, J.M. Application of Ion-Doped Y3Fe5O12 Nanoparticles for Self-Controlled Magnetic Hyperthermia. Phys. Status Solidi (B) 2022, 259, 2100545. [Google Scholar] [CrossRef]
- Chistyakova, N.; Antonova, A.; Elizarov, I.; Fabritchnyi, P.; Afanasov, M.; Korolenko, M.; Gracheva, M.; Pchelina, D.; Sergueev, I.; Leupold, O.; et al. Mössbauer, Nuclear Forward Scattering, and Raman Spectroscopic Approaches in the Investigation of Bioinduced Transformations of Mixed-Valence Antimony Oxide. J. Phys. Chem. A 2021, 125, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Matsnev, M.E.; Rusakov, V.S. SpectrRelax: An Application for Mössbauer Spectra Modeling and Fitting. AIP Conf. Proc. 2012, 1489, 178–185. [Google Scholar] [CrossRef]
- Voyer, C.; Ryan, D. A Complete Solution to the Mössbauer Problem, All in One Place. Hyperfine Interact. 2006, 170, 91–104. [Google Scholar] [CrossRef]
- Liu, N.N.; Pyatakov, A.P.; Zharkov, M.N.; Pyataev, N.A.; Sukhorukov, G.B.; Alekhina, Y.A.; Perov, N.S.; Gun’ko, Y.K.; Tishin, A.M. Optimization of Zn–Mn Ferrite Nanoparticles for Low Frequency Hyperthermia: Exploiting the Potential of Superquadratic Field Dependence of Magnetothermal Response. Appl. Phys. Lett. 2022, 120, 102403. [Google Scholar] [CrossRef]
- Ferreira, M.C.; Pimentel, B.; Andrade, V.; Zverev, V.; Gimaev, R.R.; Pomorov, A.S.; Pyatakov, A.; Alekhina, Y.; Komlev, A.; Makarova, L.; et al. Understanding the Dependence of Nanoparticles Magnetothermal Properties on Their Size for Hyperthermia Applications: A Case Study for La-Sr Manganites. Nanomaterials 2021, 11, 1826. [Google Scholar] [CrossRef]
- Munoz-Menendez, C.; Conde-Leboran, I.; Serantes, D.; Chantrell, R.; Chubykalo-Fesenko, O.; Baldomir, D. Distinguishing between Heating Power and Hyperthermic Cell-Treatment Efficacy in Magnetic Fluid Hyperthermia. Soft Matter 2016, 12, 8815–8818. [Google Scholar] [CrossRef] [Green Version]
- Natividad, E.; Castro, M.; Goglio, G.; Andreu, I.; Epherre, R.; Duguet, É.; Mediano, A. New Insights into the Heating Mechanisms and Self-Regulating Abilities of Manganite Perovskite Nanoparticles Suitable for Magnetic Fluid Hyperthermia. Nanoscale 2012, 4, 3954–3962. [Google Scholar] [CrossRef]
- Sánchez, R.D.; Rivas, J.; Vaqueiro, P.; López-Quintela, M.A.; Caeiro, D. Particle Size Effects on Magnetic Properties of Yttrium Iron Garnets Prepared by a Sol–Gel Method. J. Magn. Magn. Mater. 2002, 247, 92–98. [Google Scholar] [CrossRef]


| Samples | Synthesis Temperature, T, °C | Particles Size, Dmax, nm | Saturation Magnetization, Is, emu/g | Curie Temperature, Tc, °C | SAR, W/g (H = 70 Oe f = 300 kHz) |
|---|---|---|---|---|---|
| YIG800 | 800 | 35 | 24.2 | 256 | 25 |
| YIG900 | 900 | 51 | 25.0 | 267 | 14 |
| YIG1000 | 1000 | 83 | 27.0 | 270 | 8 |
| YIG1100 | 1100 | 137 | 25.7 | 269 | 16 |
| YIG1200 | 1200 | 184 | 26.3 | 272 | 25 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kiseleva, T.; Abbas, R.; Martinson, K.; Komlev, A.; Lazareva, E.; Tyapkin, P.; Solodov, E.; Rusakov, V.; Pyatakov, A.; Tishin, A.; et al. Size-Dependent Structural, Magnetic and Magnetothermal Properties of Y3Fe5O12 Fine Particles Obtained by SCS. Nanomaterials 2022, 12, 2733. https://doi.org/10.3390/nano12162733
Kiseleva T, Abbas R, Martinson K, Komlev A, Lazareva E, Tyapkin P, Solodov E, Rusakov V, Pyatakov A, Tishin A, et al. Size-Dependent Structural, Magnetic and Magnetothermal Properties of Y3Fe5O12 Fine Particles Obtained by SCS. Nanomaterials. 2022; 12(16):2733. https://doi.org/10.3390/nano12162733
Chicago/Turabian StyleKiseleva, Tatiana, Rashad Abbas, Kirill Martinson, Aleksei Komlev, Evgenia Lazareva, Pavel Tyapkin, Evgeniy Solodov, Vyacheslav Rusakov, Alexander Pyatakov, Alexander Tishin, and et al. 2022. "Size-Dependent Structural, Magnetic and Magnetothermal Properties of Y3Fe5O12 Fine Particles Obtained by SCS" Nanomaterials 12, no. 16: 2733. https://doi.org/10.3390/nano12162733
APA StyleKiseleva, T., Abbas, R., Martinson, K., Komlev, A., Lazareva, E., Tyapkin, P., Solodov, E., Rusakov, V., Pyatakov, A., Tishin, A., Perov, N., Uyanga, E., Sangaa, D., & Popkov, V. (2022). Size-Dependent Structural, Magnetic and Magnetothermal Properties of Y3Fe5O12 Fine Particles Obtained by SCS. Nanomaterials, 12(16), 2733. https://doi.org/10.3390/nano12162733









