Graphene-Delivered Insecticides against Cotton Bollworm
Abstract
:1. Introduction
2. Results and Discussion
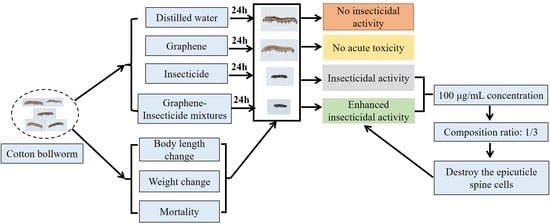
2.1. Workflow of the Synergistic Mechanism of Graphene-Delivered Insecticide
2.2. Characterizations of Graphene, Insecticides, and Graphene–Insecticide Mixtures
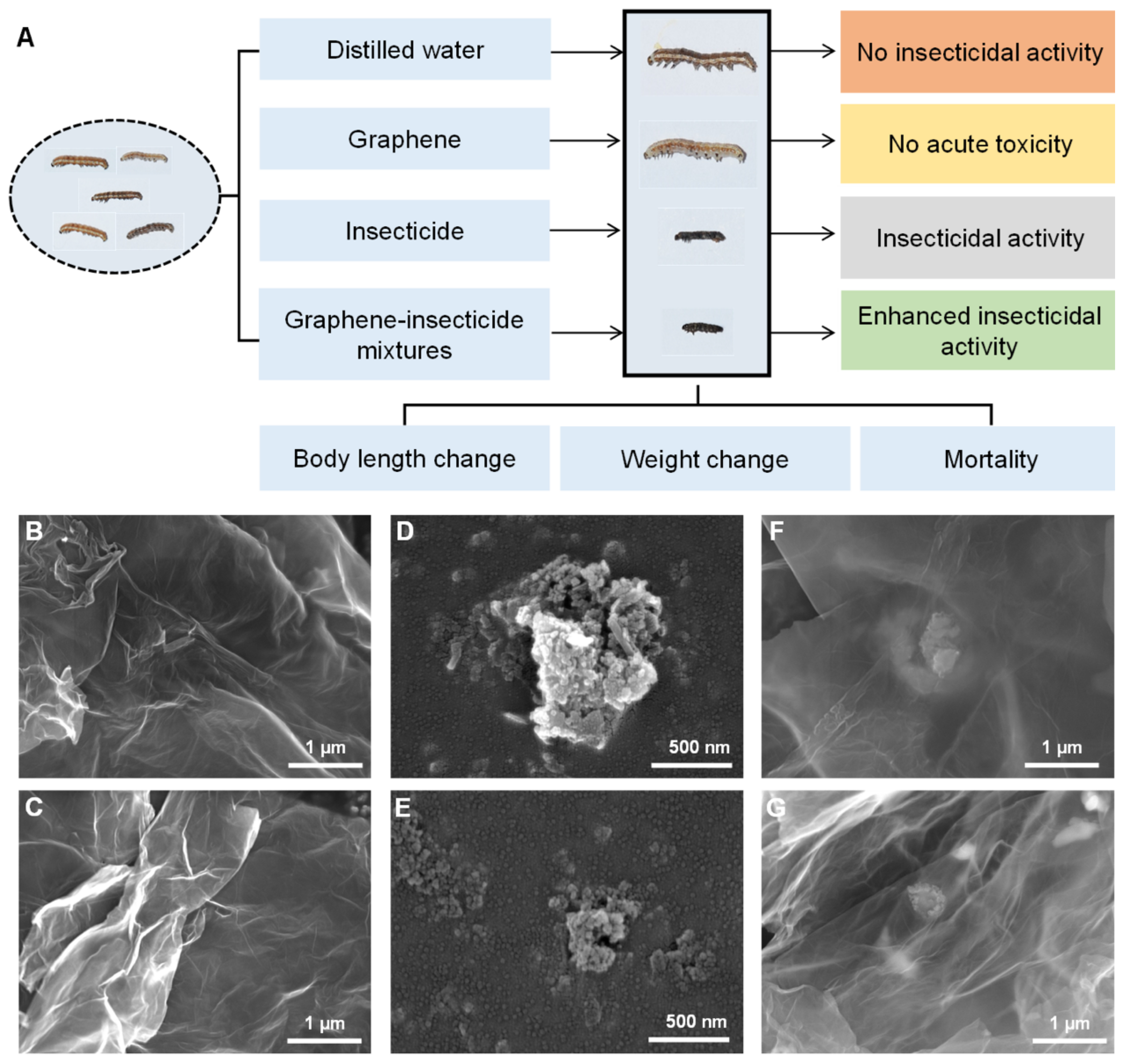
2.3. Characterization of Surface Morphology of Graphene
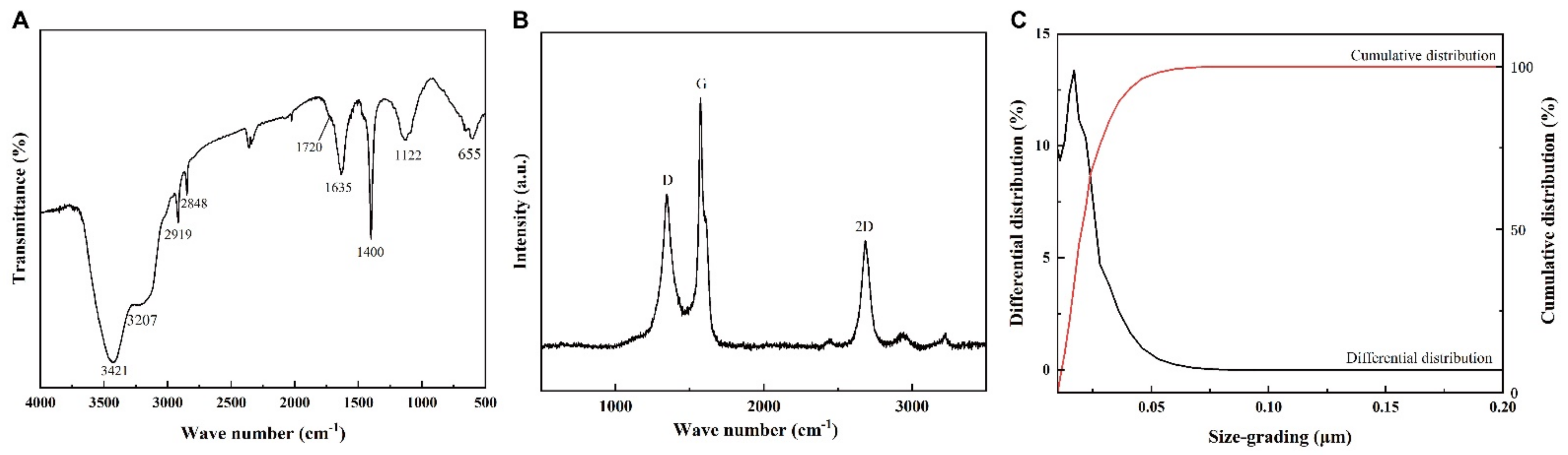
2.4. Composition and Structure Analyses of Graphene
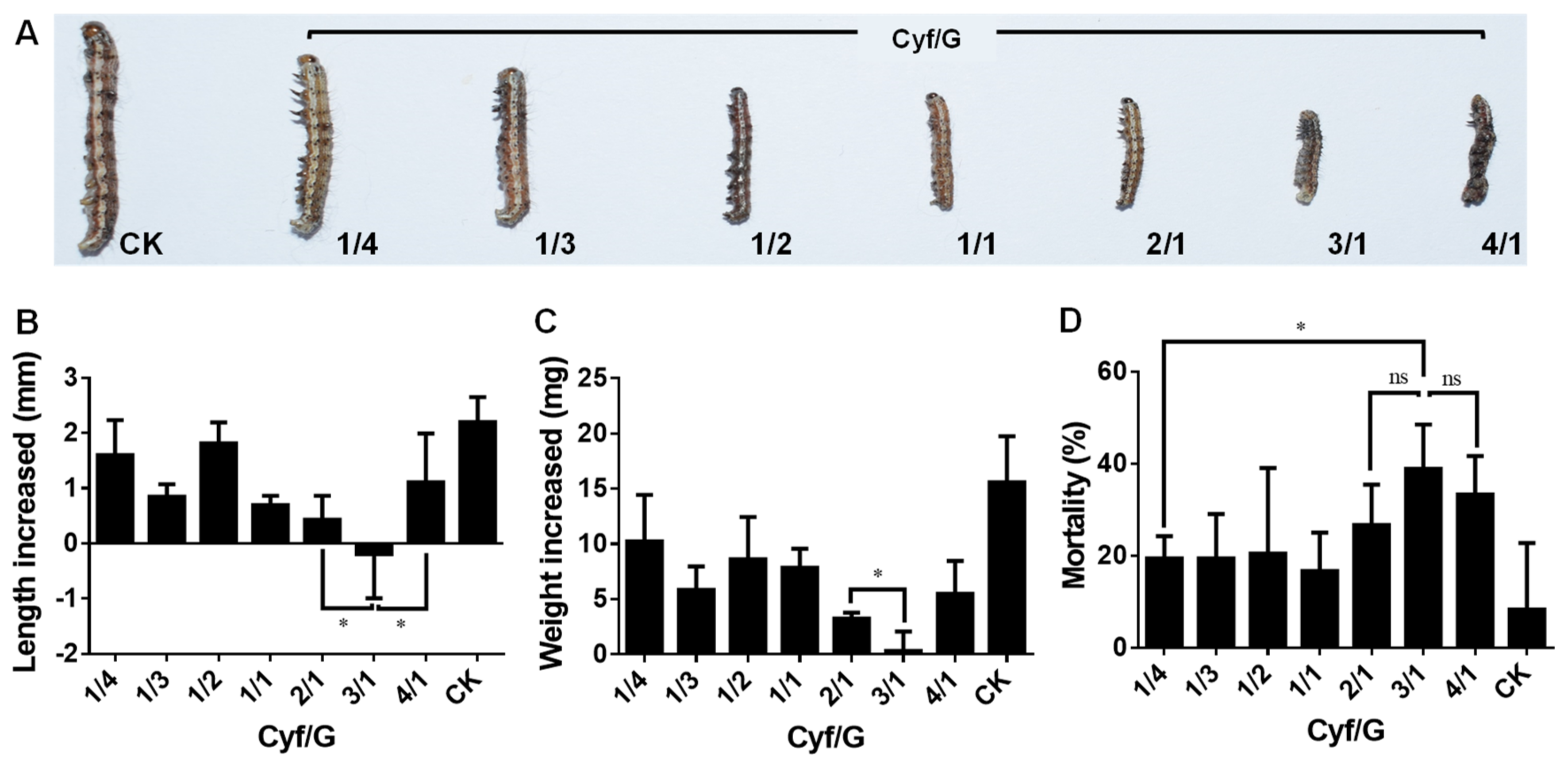
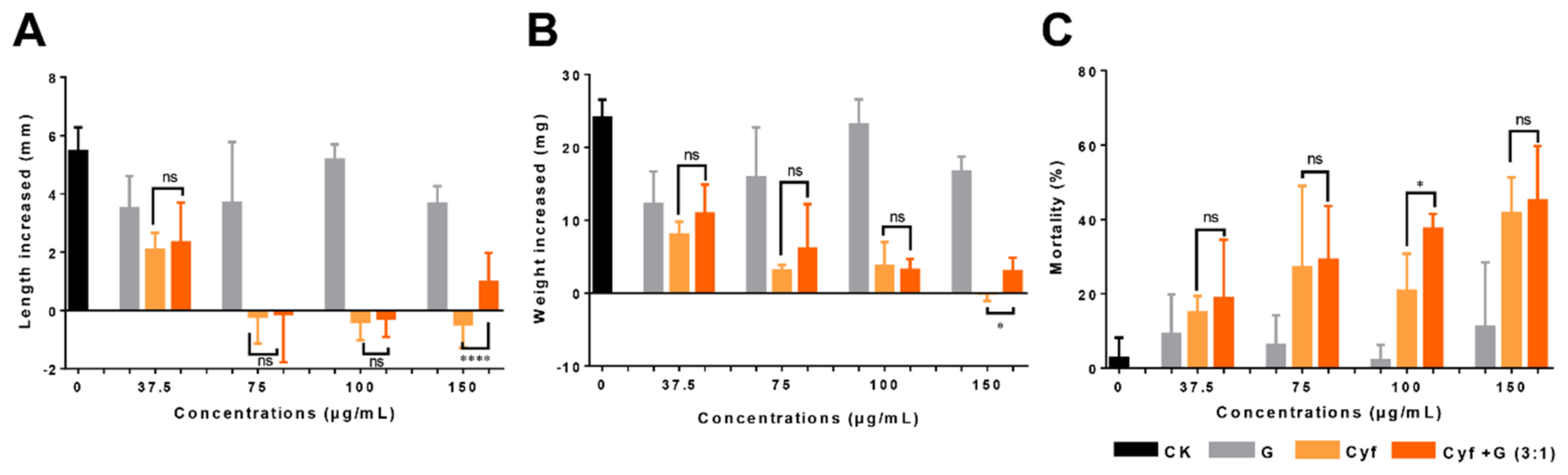
2.5. Insecticidal Activity of Graphene–Insecticide Mixtures against Cotton Bollworm
2.6. Insecticidal Activity of Graphene, Insecticides, and Graphene–Insecticide Mixtures
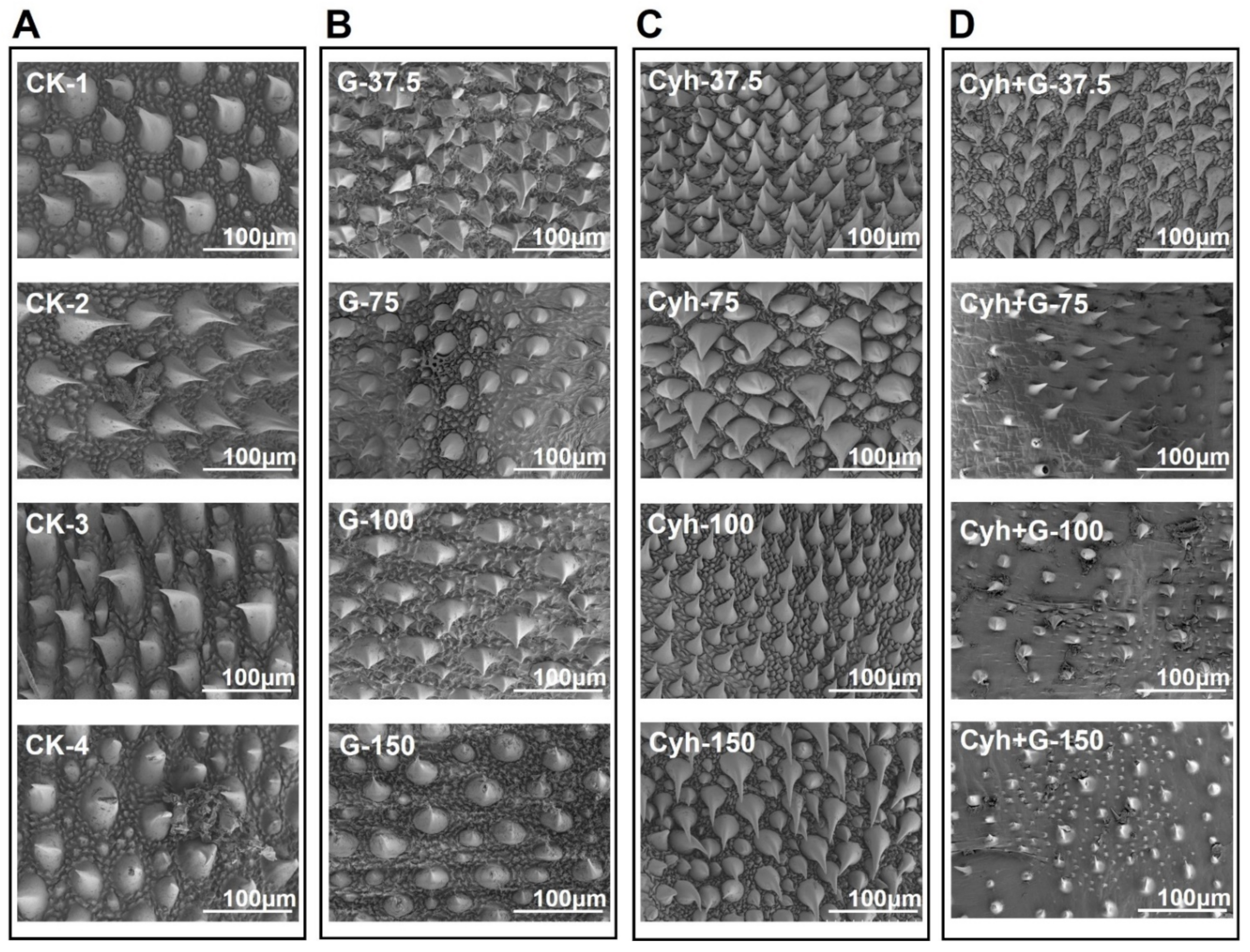
2.7. Graphene–Cyh Mixture Effectively Destroys the Epicuticular Spines of Cotton Bollworm
3. Potential Application and Prospects
4. Materials and Methods
4.1. Preparation and Characterization of Graphene and Graphene–Insecticide Mixtures
4.2. Acquisition of Insecticides and Cotton Bollworm
4.3. Cotton Bollworm Culture and Insecticide Spraying
4.4. Identification of Morphological Changes in Cotton Bollworm Epicuticle Cells
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Willett, W.; Rockstrom, J.; Loken, B.; Springmann, M.; Lang, T.; Vermeulen, S.; Garnett, T.; Tilman, D.; DeClerck, F.; Wood, M.; et al. Food in the Anthropocene: The Eat-Lancet Commission on Healthy Diets from Sustainable Food Systems. Lancet 2019, 393, 447–492. [Google Scholar] [CrossRef]
- Lowry, G.V.; Avellan, A.; Gilbertson, L.M. Opportunities and challenges for nanotechnology in the agri-tech revolution. Nat. Nanotechnol. 2019, 14, 517–522. [Google Scholar] [CrossRef] [PubMed]
- Khan, H.A.A.; Akram, W.; Shad, S.A. Genetics, cross-resistance and mechanism of resistance to spinosad in a field strain of Musca domestica L. (Diptera: Muscidae). Acta Trop. 2014, 130, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Kristensen, M.; Huang, J.; Qiao, C.L.; Jespersen, J.B. Variation of Musca domestica L. acetylcholinesterase in Danish housefly populations. Pest Manag. Sci. 2006, 62, 738–745. [Google Scholar] [CrossRef] [PubMed]
- Chang, K.S.; Kim, H.C.; Klein, T.A.; Ju, Y.R. Insecticide resistance and cytochrome-P450 activation in unfed and blood-fed laboratory and field populations of Culex pipiens pallens. J. Pest. Sci. 2017, 90, 759–771. [Google Scholar] [CrossRef] [Green Version]
- Klarich, K.L.; Pflug, N.C.; Dewald, E.M.; Hladik, M.L.; Kolpin, D.W.; Cwiertny, D.M.; Lefevre, G.H. Occurrence of neonicotinoid insecticides in finished drinking water and fate during drinking water treatment. Environ. Sci. Technol. Lett. 2017, 4, 168–173. [Google Scholar] [CrossRef]
- Beketov, M.A.; Kefford, B.J.; Schafer, R.B.; Liess, M. Pesticides Reduce Regional Biodiversity of Stream Invertebrates. Proc. Natl. Acad. Sci. USA 2013, 110, 11039–11043. [Google Scholar] [CrossRef] [Green Version]
- Pecenka, J.R.; Ingwell, L.L.; Foster, R.E.; Krupke, C.H.; Kaplan, I. Ipm Reduces Insecticide Applications by 95% While Maintaining or Enhancing Crop Yields through Wild Pollinator Conservation. Proc. Natl. Acad. Sci. USA 2021, 118, e2108429118. [Google Scholar] [CrossRef]
- Long, S.; Ting, D.; Hu, C.; Chen, J.; Lu, J.; Lu, Z.; Han, H. Antibacterial Activity of Graphene Oxide/G-C3n4 Composite through Photocatalytic Disinfection under Visible Light. ACS Sustain. Chem. Eng. 2017, 5, 8693–8701. [Google Scholar]
- Duhan, J.S.; Kumar, R.; Kumar, N.; Kaur, P.; Nehra, K.; Duhan, S. Nanotechnology: The new perspective in precision agriculture. Biotechnol. Rep. 2017, 15, 11–23. [Google Scholar] [CrossRef]
- Kah, M.; Beulke, S.; Tiede, K.; Hofmann, T. Nanopesticides: State of knowledge, environmental fate, and exposure modeling. Crit. Rev. Environ. Sci. Technol. 2013, 43, 1823–1867. [Google Scholar] [CrossRef]
- Gogos, A.; Knauer, K.; Bucheli, T.D. Nanomaterials in plant protection and fertilization: Current state, foreseen applications, and research priorities. J. Agric. Food Chem. 2012, 60, 9781–9792. [Google Scholar] [CrossRef]
- Choudhury, S.R.; Nair, K.K.; Kumar, R.; Gogoi, R.; Srivastava, C.; Gopal, M.; Subhramanyam, B.S.; Devakumar, C.; Goswami, A. Nanosulfur: A potent fungicide against food pathogen, Aspergillus niger. AIP Conf. Proc. 2010, 1276, 154–157. [Google Scholar]
- Kumar, S.; Nehra, M.; Dilbaghi, N.; Marrazza, G.; Hassan, A.A.; Kim, K.H. Nano-based smart pesticide formulations: Emerging opportunities for agriculture. J. Control Release 2019, 294, 131–153. [Google Scholar] [CrossRef]
- Palomino-Asencio, L.; Ramirez-Torres, A.; Avelar, J.; Garza, J.; Garcia-Hernandez, E. Functionalized Graphene Pieces to Trap the Insecticide Imidacloprid: A Theoretical Analysis. J. Mol. Model. 2019, 25, 117. [Google Scholar] [CrossRef]
- Sharma, S.; Singh, S.; Ashok, K.G.; Shanmugam, V. Anti-Drift Nano-Stickers Made of Graphene Oxide for Targeted Pesticide Delivery and Crop Pest Control. Carbon 2017, 115, 781–790. [Google Scholar] [CrossRef]
- Yujia, T.; Shao, L.; Li, X.; Lu, J.; Sun, H.; Xiang, S.; Zhang, Z.; Wu, Y.; Wu, X. Adhesive and Stimulus-Responsive Polydopamine-Coated Graphene Oxide System for Pesticide-Loss Control. J. Agric. Food Chem. 2018, 66, 2616–2622. [Google Scholar]
- Wang, X.; Xie, H.; Wang, Z.; He, K. Graphene Oxide as a Pesticide Delivery Vector for Enhancing Acaricidal Activity against Spider Mites. Colloids Surf. B Biointerfaces 2019, 173, 632–638. [Google Scholar] [CrossRef]
- Xiuping, W.; Xie, H.; Wang, Z.; He, K.; Jing, D. Graphene Oxide as a Multifunctional Synergist of Insecticides against Lepidopteran Insect. Environ. Sci. Nano 2019, 6, 75–84. [Google Scholar]
- Gu, B.G.; Wang, H.M.; Chen, W.L.; Cai, D.J.; Shan, Z.J. Risk Assessment of Lambda-Cyhalothrin on Aquatic Organisms in Paddy Field in China. Regul. Toxicol. Pharmacol. 2007, 48, 69–74. [Google Scholar] [CrossRef]
- Abbas, N.; Shad, S.A. Assessment of Resistance Risk to Lambda-Cyhalothrin and Cross-Resistance to Four Other Insecticides in the House Fly, Musca domestica L. (Diptera: Muscidae). Parasitol. Res. 2015, 114, 2629–2637. [Google Scholar] [CrossRef] [PubMed]
- Piechowicz, B.J.; Sienko, M.J.; Grodzicki, P.; Podbielska, M.; Szpyrka, E.; Zareba, L.; Piechowicz, I.; Sadlo, S. Assessment of Risk to Honey Bees and Honey Consumers Resulting from the Insect Exposure to Captan, Thiacloprid, Penthiopyrad, and Lambda-Cyhalothrin Used in a Commercial Apple Orchard. Environ. Monit. Assess. 2021, 193, 129. [Google Scholar] [CrossRef] [PubMed]
- Kalaiselvi, R.A. Regupathy, and Sendha Krishnamoorthy. Bioefficacy of Lambda-Cyhalothrin 5 Cs against Bollworm Complex in Cotton. Pestology 2006, 30, 28–30. [Google Scholar]
- Kah, M.; Tufenkji, N.; White, J.C. Nano-Enabled Strategies to Enhance Crop Nutrition and Protection. Nat. Nanotechnol. 2019, 14, 532–540. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Jianguo, Z.; Cao, J.; Zhao, Y.; Huang, J.; Zheng, Z.; Li, W.; Jiang, S.; Qiao, J.; Xing, B.; et al. Opportunities for Graphene, Single-Walled and Multi-Walled Carbon Nanotube Applications in Agriculture: A Review. Crop. Design. 2022, 1, 100006. [Google Scholar] [CrossRef]
- Giraldo, J.P.; Wu, H.G.; Newkirk, M.; Kruss, S. Nanobiotechnology Approaches for Engineering Smart Plant Sensors. Nat. Nanotechnol. 2019, 14, 541–553. [Google Scholar] [CrossRef]
- Chen, Z.; Zhao, J.; Qiao, J.; Li, W.; Guan, Z.; Liu, Z.; Bai, X.; Xing, B.; Zhang, J.; Li, J.; et al. Graphene-Mediated Antioxidant Enzyme Activity and Respiration in Plant Roots. ACS Agricult. Sci. Technol. 2022, 2, 646–660. [Google Scholar] [CrossRef]
- Zhang, X.; Cao, H.; Zhao, J.; Wang, H.; Xing, B.; Chen, Z.; Li, X.; Zhang, J. Graphene Oxide Exhibited Positive Effects on the Growth of Aloe Vera L. Physiol. Mol. Biol. Plants 2021, 27, 815–824. [Google Scholar] [CrossRef]
- Chen, Z.; Zhao, J.; Song, J.; Han, S.; Du, Y.; Qiao, Y.; Liu, Z.; Qiao, J.; Li, W.; Li, J.; et al. Influence of Graphene on the Multiple Metabolic Pathways of Zea Mays Roots Based on Transcriptome Analysis. PLoS ONE 2021, 16, e0244856. [Google Scholar] [CrossRef]
- Zhang, X.; Cao, H.; Wang, H.; Zhao, J.; Gao, K.; Qiao, J.; Li, J.; Ge, S. The Effects of Graphene-Family Nanomaterials on Plant Growth: A Review. Nanomaterials 2022, 12, 936. [Google Scholar] [CrossRef]
- Cătălin Balaure, P.; Gudovan, D.; Gudovan, I. 4-Nanopesticides: A new paradigm in crop protection. In New Pesticides and Soil Sensors; Grumezescu, A.M., Ed.; Academic Press: Cambridge, MA, USA, 2017; pp. 129–192. [Google Scholar]
- Kah, M.; Hofmann, T. Nanopesticide Research: Current Trends and Future Priorities. Environ. Int. 2014, 63, 224–235. [Google Scholar] [CrossRef]
- Loha, K.M.; Shakil, N.A.; Kumar, J.; Singh, M.K.; Adak, T.; Jain, S. Release Kinetics of Beta-Cyfluthrin from Its Encapsulated Formulations in Water. J. Environ. Sci. Health B 2011, 46, 201–206. [Google Scholar] [CrossRef]
- Pradhan, S.; Roy, I.; Lodh, G.; Patra, P.; Choudhury, S.R.; Samanta, A.; Goswami, A. Entomotoxicity and Biosafety Assessment of Pegylated Acephate Nanoparticles: A Biologically Safe Alternative to Neurotoxic Pesticides. J. Environ. Sci. Health B 2013, 48, 559–569. [Google Scholar] [CrossRef]
- Kumari, M.; Tomar, S.; Singh, D.P.; Solankey, S.S. Nanopesticides: A New Paradigm in Crop Protection. In New Pesticides and Soil Sensors; Academic Press: Cambridge, MA, USA, 2020; Volume 20, pp. 104–109. [Google Scholar]
- Salles De Carvalho, S.; Vendramim, J.D.; de Sá, I.C.G.; Ribeiro, L.D.P.; Forim, M.R.; Forim, M.R. Systemic Insecticidal Effect of Neem-Based Nanoformulations against Bemisia tabaci (Hemiptera: Aleyrodidae) Biotype B in Tomato. Bragantia 2015, 74, 298–306. [Google Scholar] [CrossRef] [Green Version]
- Xiuping, W.; Zhang, T.; Xie, H.; Wang, Z.; Jing, D.; He, K.; Gao, X. Phenotypic Responses and Potential Genetic Mechanism of Lepidopteran Insects under Exposure to Graphene Oxide. Ecotoxicol. Environ. Saf. 2021, 228, 113008. [Google Scholar]
- Guo, X.; Zhao, J.; Wang, R.; Zhang, H.; Xing, B.; Naeem, M.; Yao, T.; Li, R.; Xu, R.; Zhang, Z.; et al. Effects of Graphene Oxide on Tomato Growth in Different Stages. Plant Physiol. Biochem. 2021, 162, 447–455. [Google Scholar] [CrossRef]
- Zhang, X.; Cao, H.; Wang, H.; Zhang, R.; Jia, H.; Huang, J.; Zhao, J.; Yao, J. Effects of Graphene on Morphology, Microstructure and Transcriptomic Profiling of Pinus Tabuliformis Carr. Roots. PLoS ONE 2021, 16, e0253812. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Z.; Zhao, J.; Liu, Z.; Bai, X.; Li, W.; Guan, Z.; Zhou, M.; Zhu, H. Graphene-Delivered Insecticides against Cotton Bollworm. Nanomaterials 2022, 12, 2731. https://doi.org/10.3390/nano12162731
Chen Z, Zhao J, Liu Z, Bai X, Li W, Guan Z, Zhou M, Zhu H. Graphene-Delivered Insecticides against Cotton Bollworm. Nanomaterials. 2022; 12(16):2731. https://doi.org/10.3390/nano12162731
Chicago/Turabian StyleChen, Zhiwen, Jianguo Zhao, Zehui Liu, Xiuli Bai, Weijia Li, Zhifang Guan, Ming Zhou, and Hongwei Zhu. 2022. "Graphene-Delivered Insecticides against Cotton Bollworm" Nanomaterials 12, no. 16: 2731. https://doi.org/10.3390/nano12162731
APA StyleChen, Z., Zhao, J., Liu, Z., Bai, X., Li, W., Guan, Z., Zhou, M., & Zhu, H. (2022). Graphene-Delivered Insecticides against Cotton Bollworm. Nanomaterials, 12(16), 2731. https://doi.org/10.3390/nano12162731