Ameliorative Effects by Hexagonal Boron Nitride Nanoparticles against Beta Amyloid Induced Neurotoxicity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of hBN-NPs
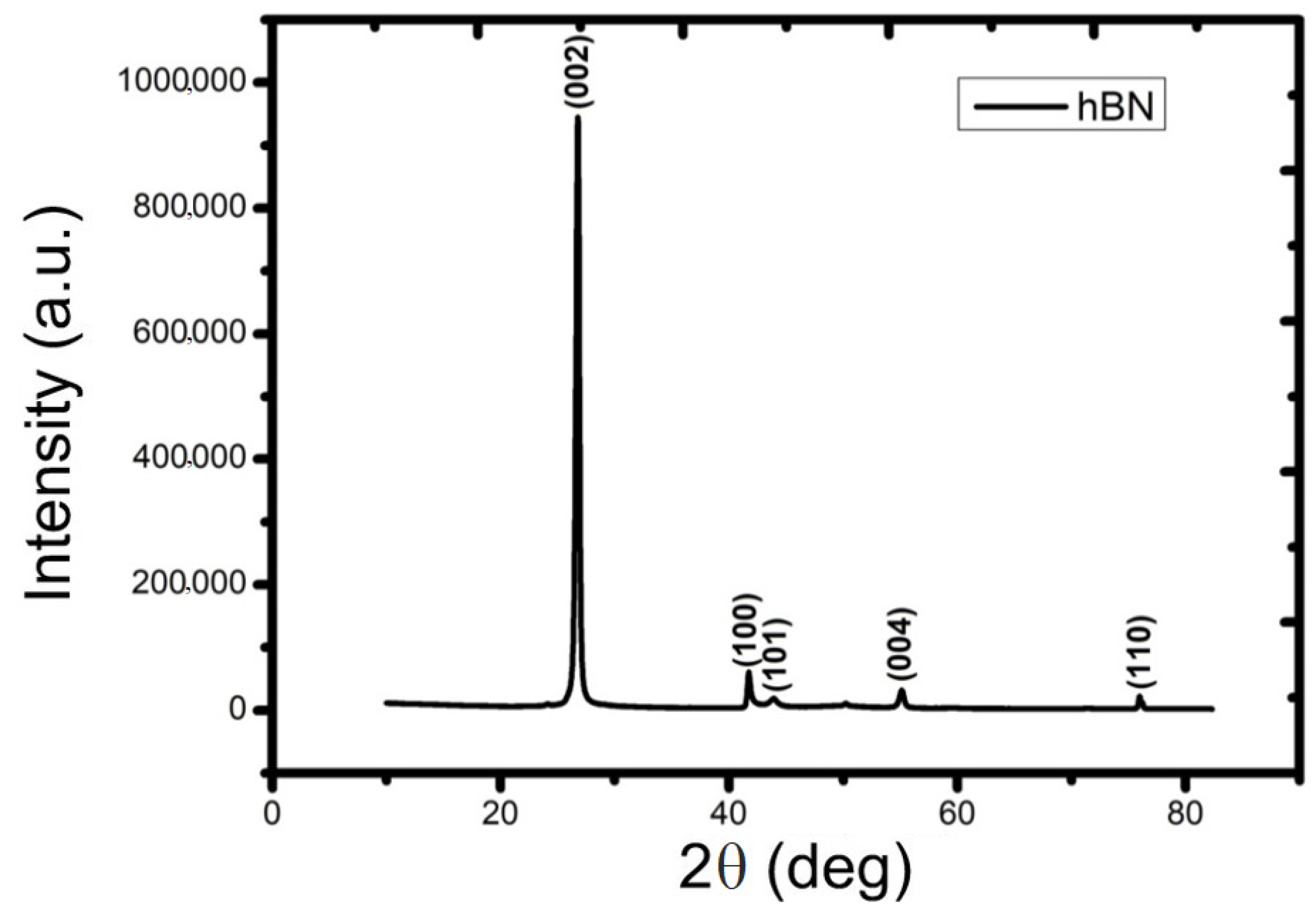
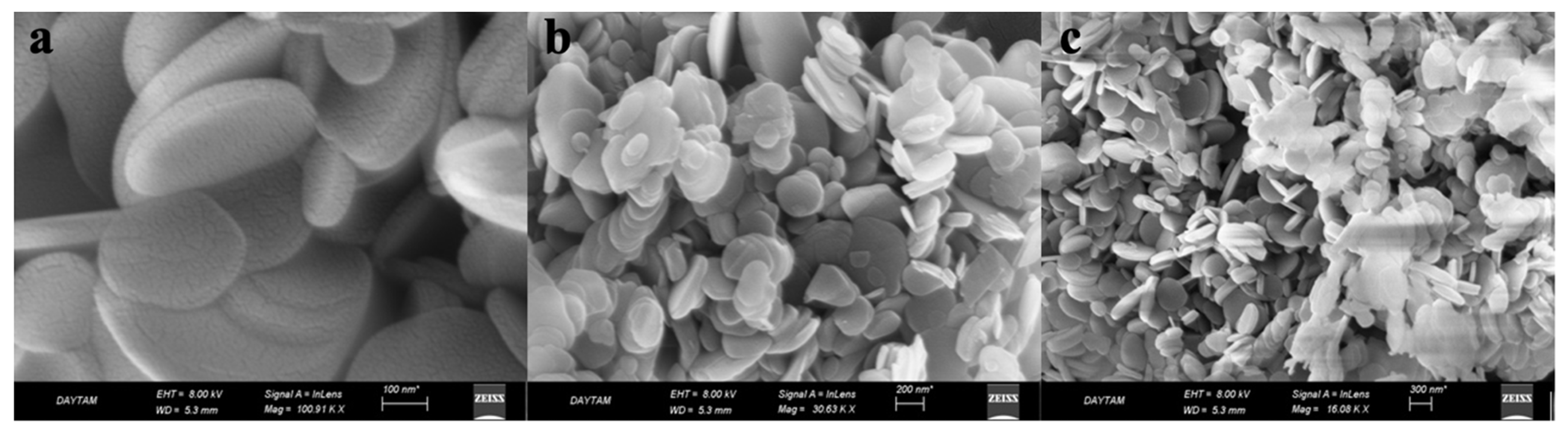
2.2. Characterization of hBN-NPs
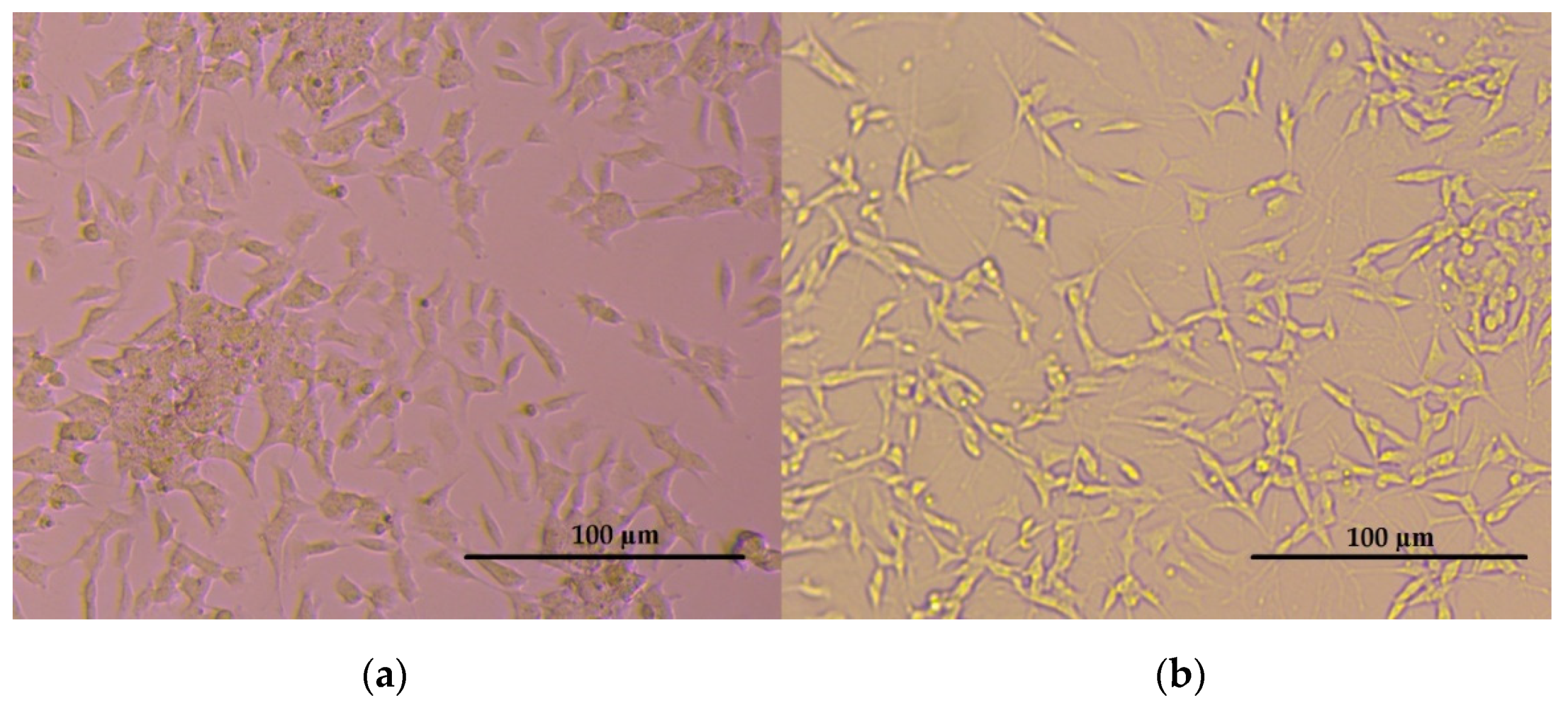
2.3. Cell Cultures
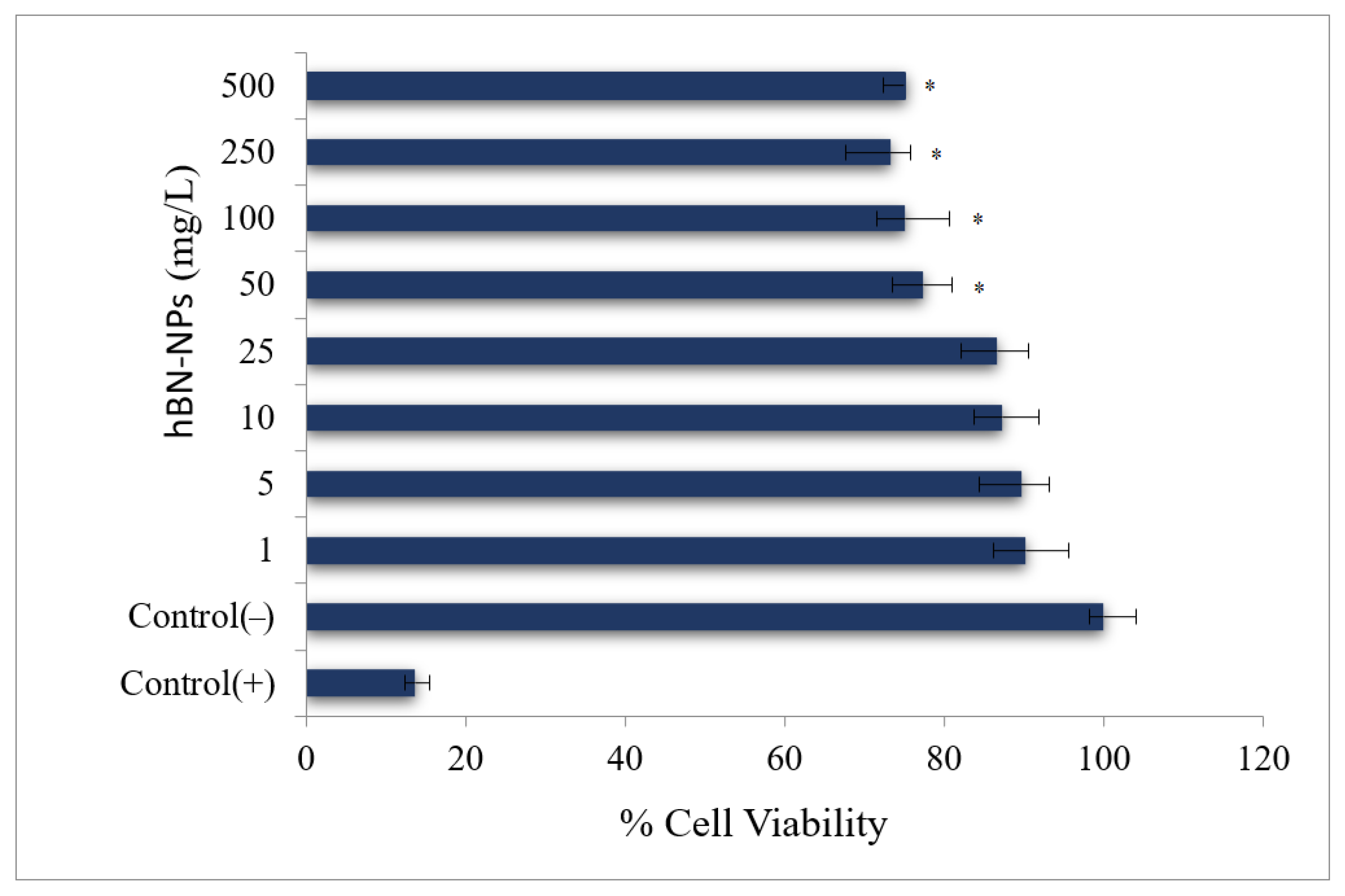
2.4. Cell Viability Testing
2.4.1. MTT Assay
2.4.2. LDH Assay
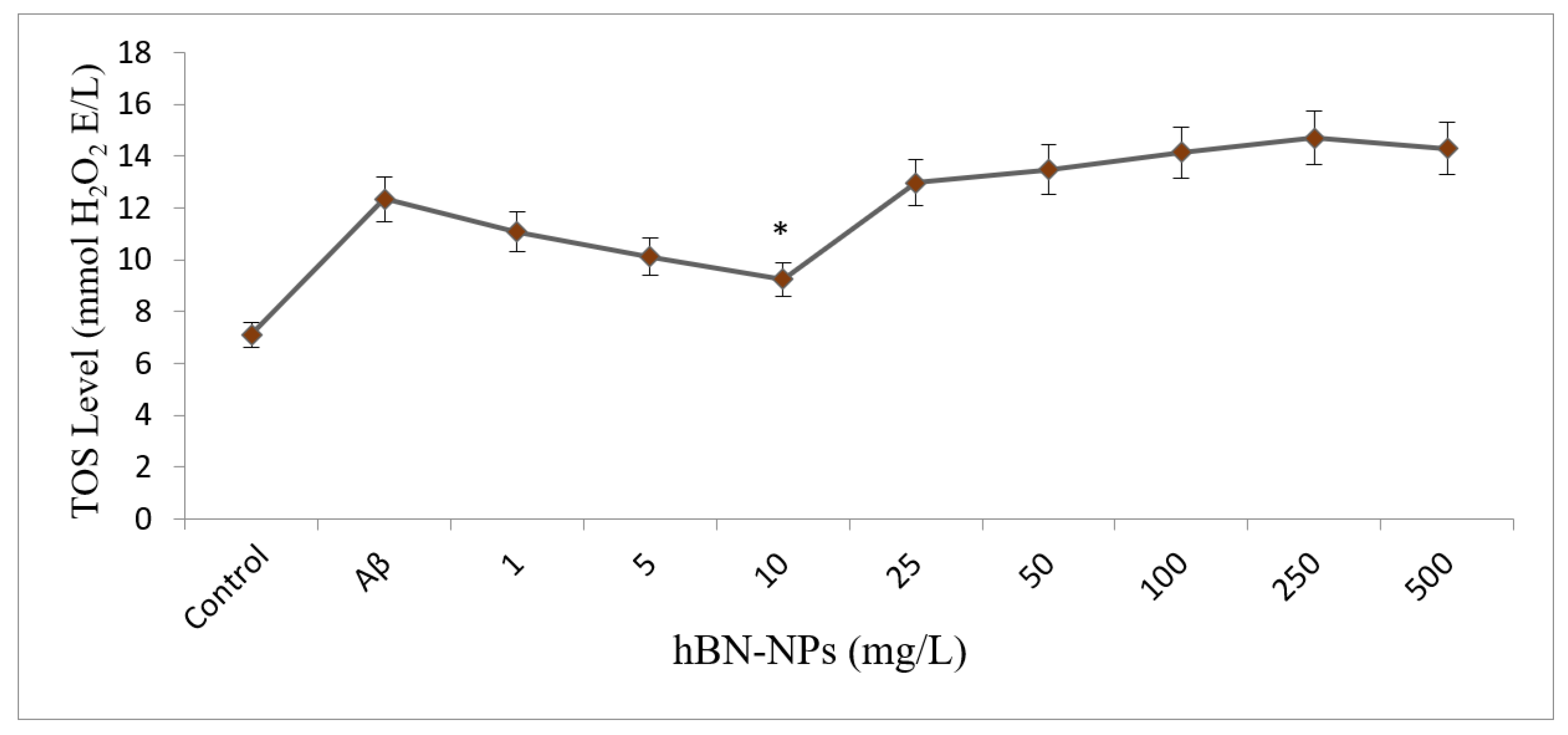
2.4.3. Assays for Oxidative Stress and Antioxidant Status
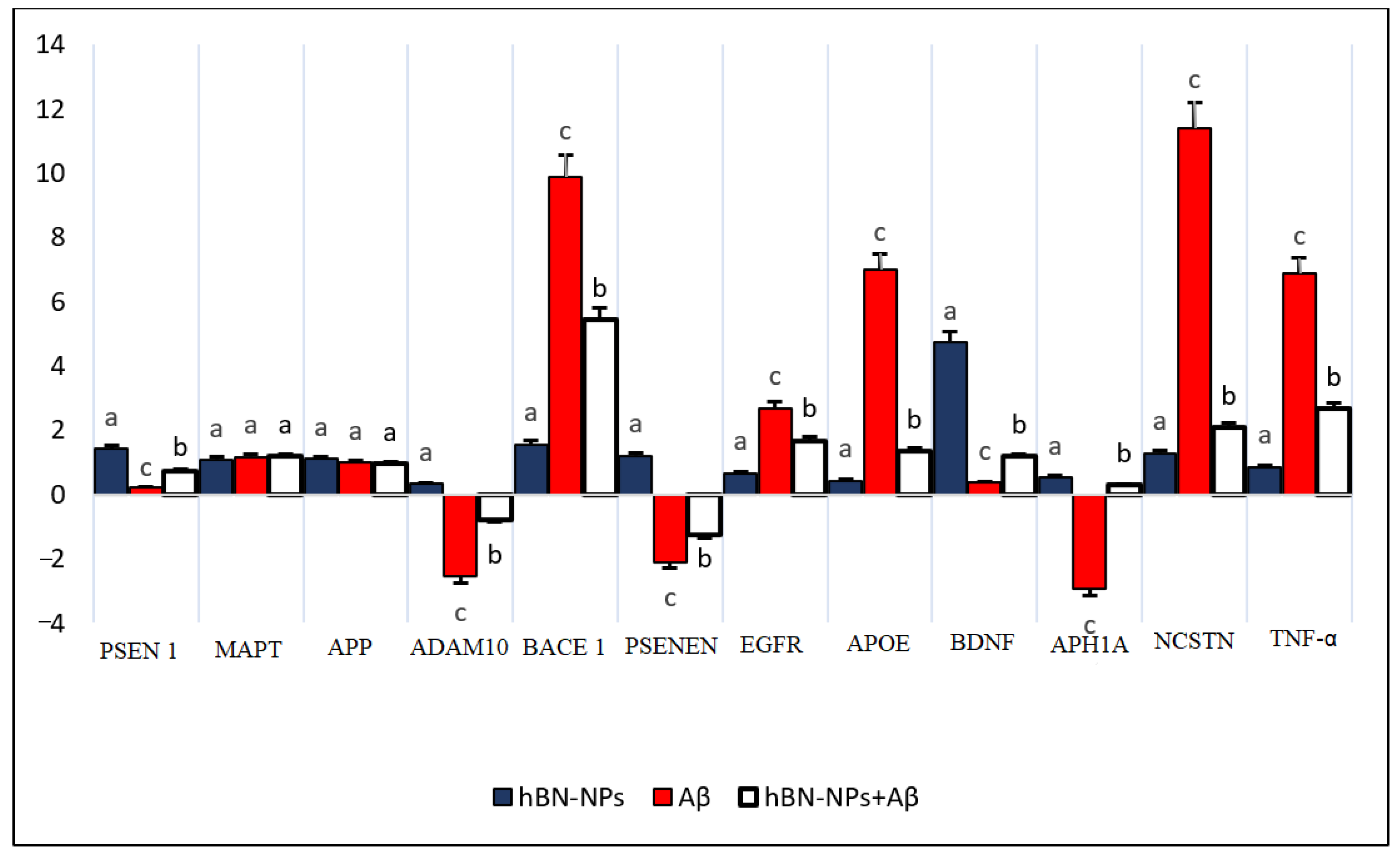
2.4.4. RNA Isolation
2.4.5. Hoechst 33258 Fluorescent Staining
3. Statistical Analysis
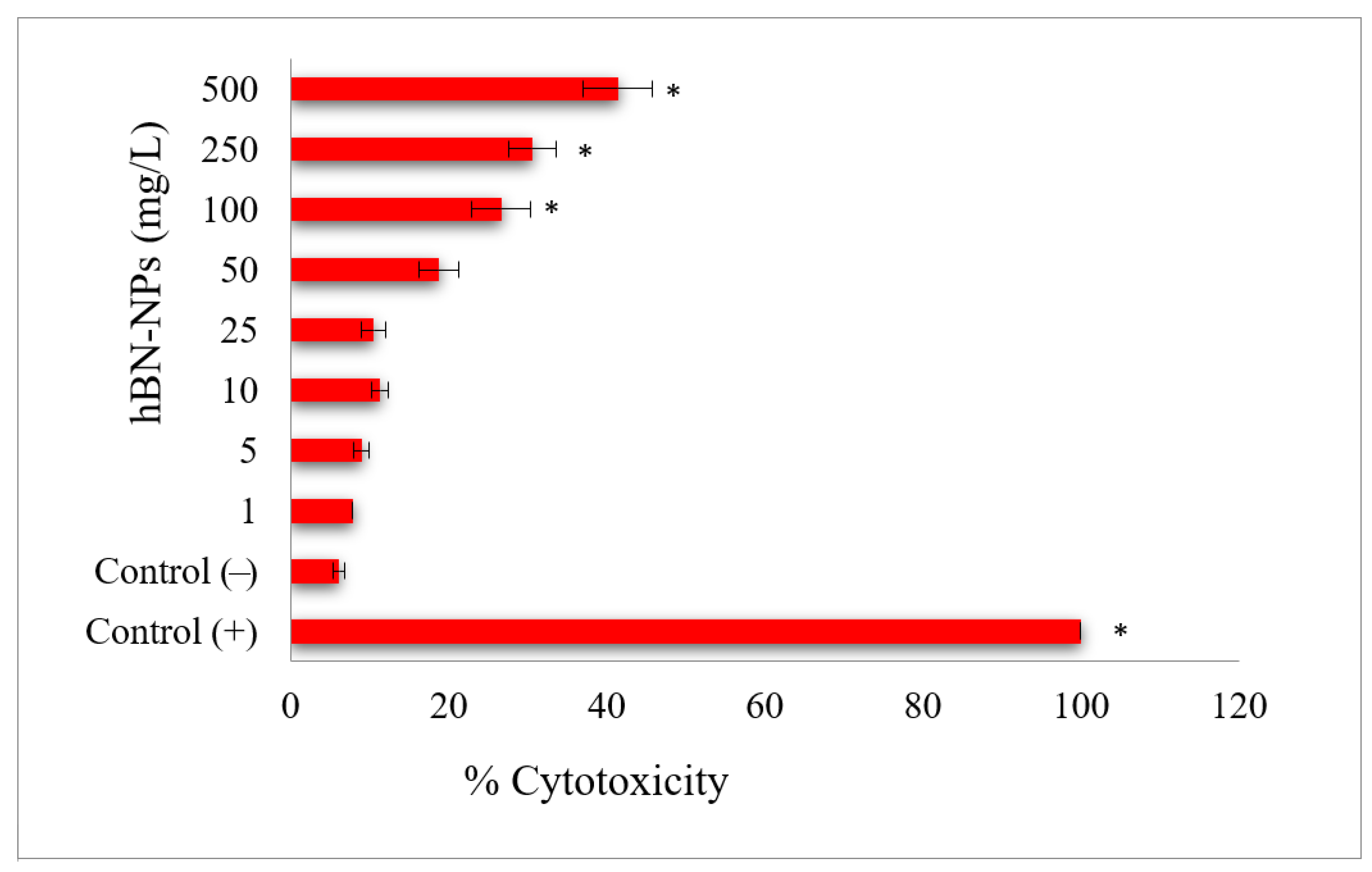
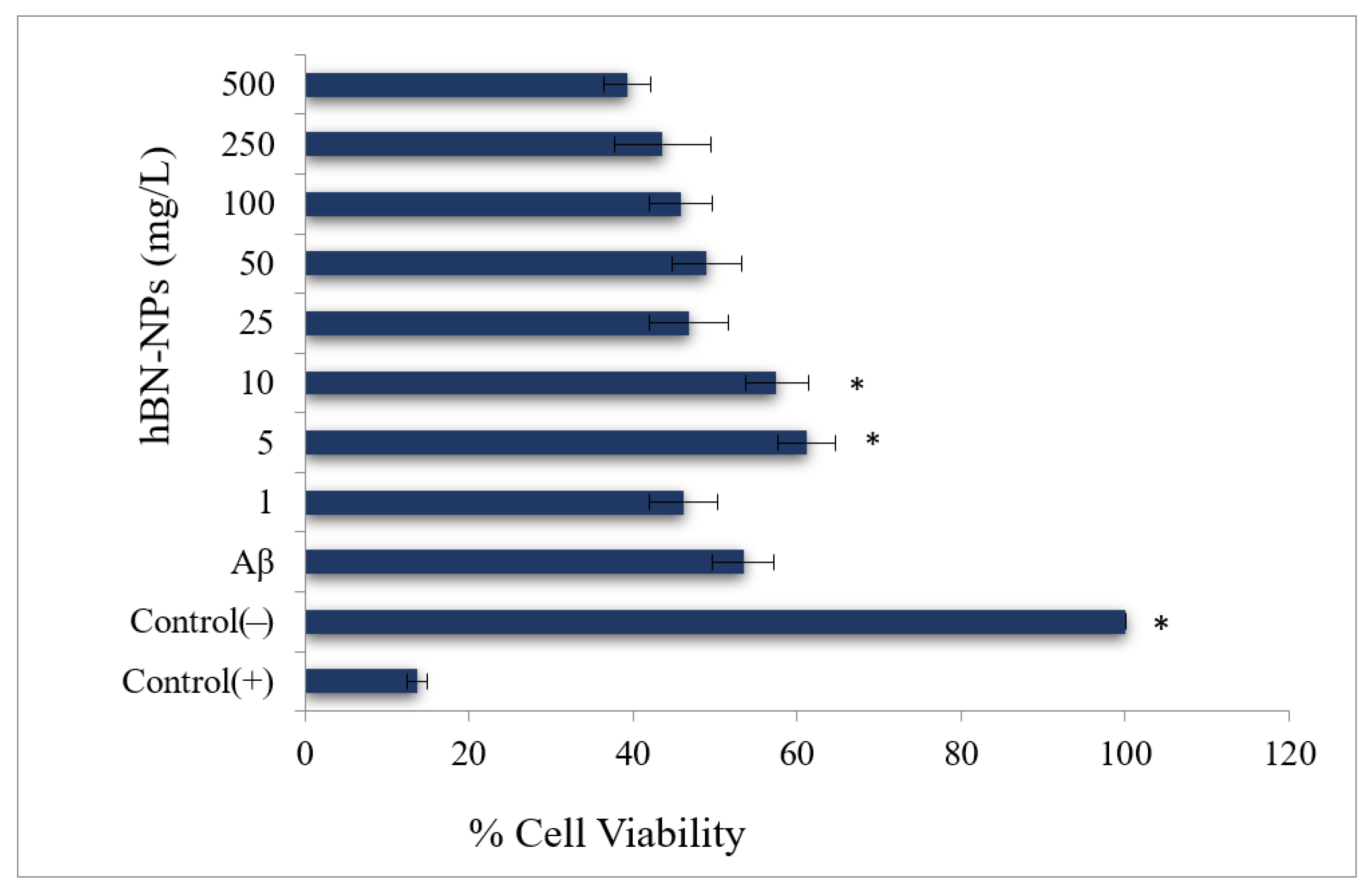
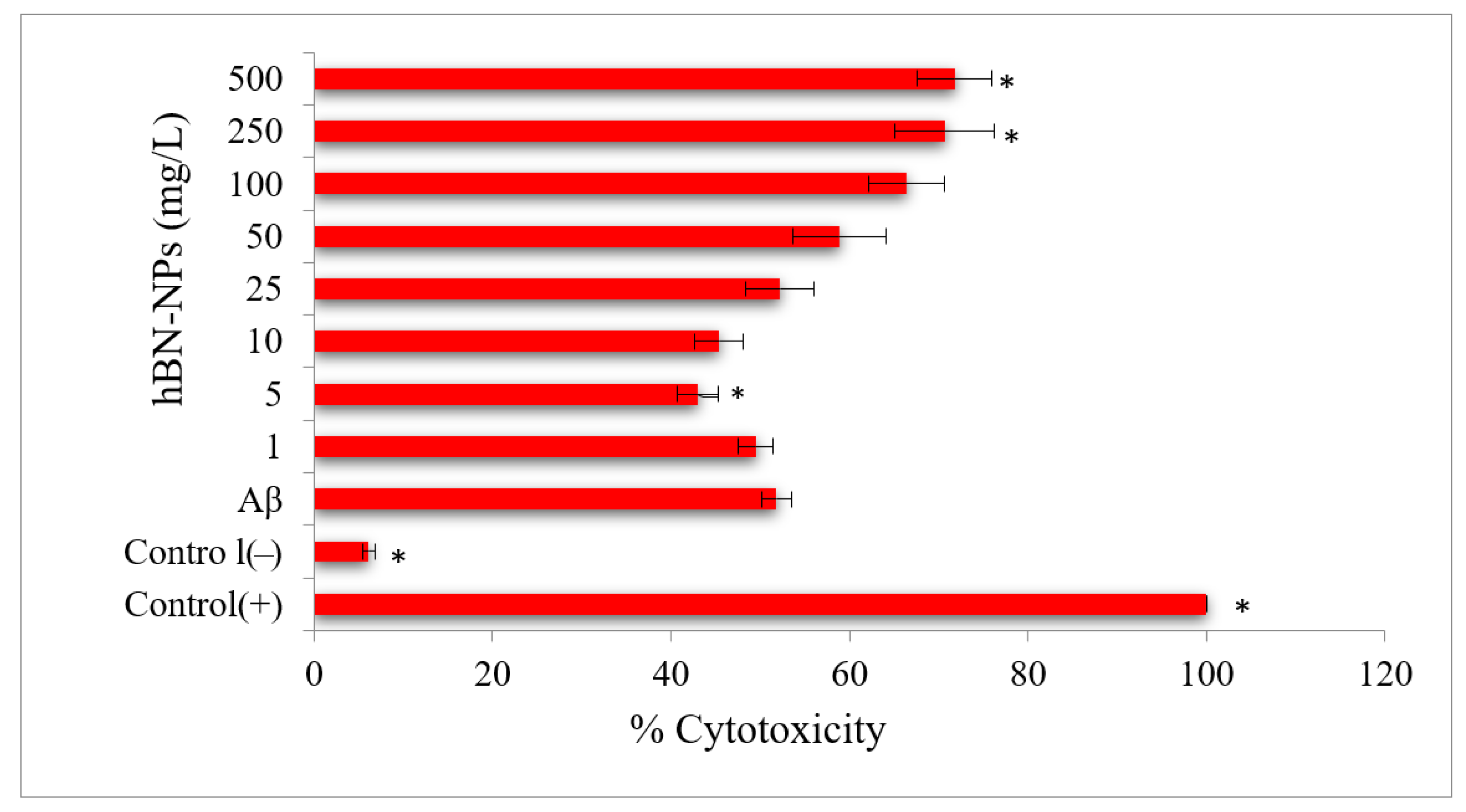
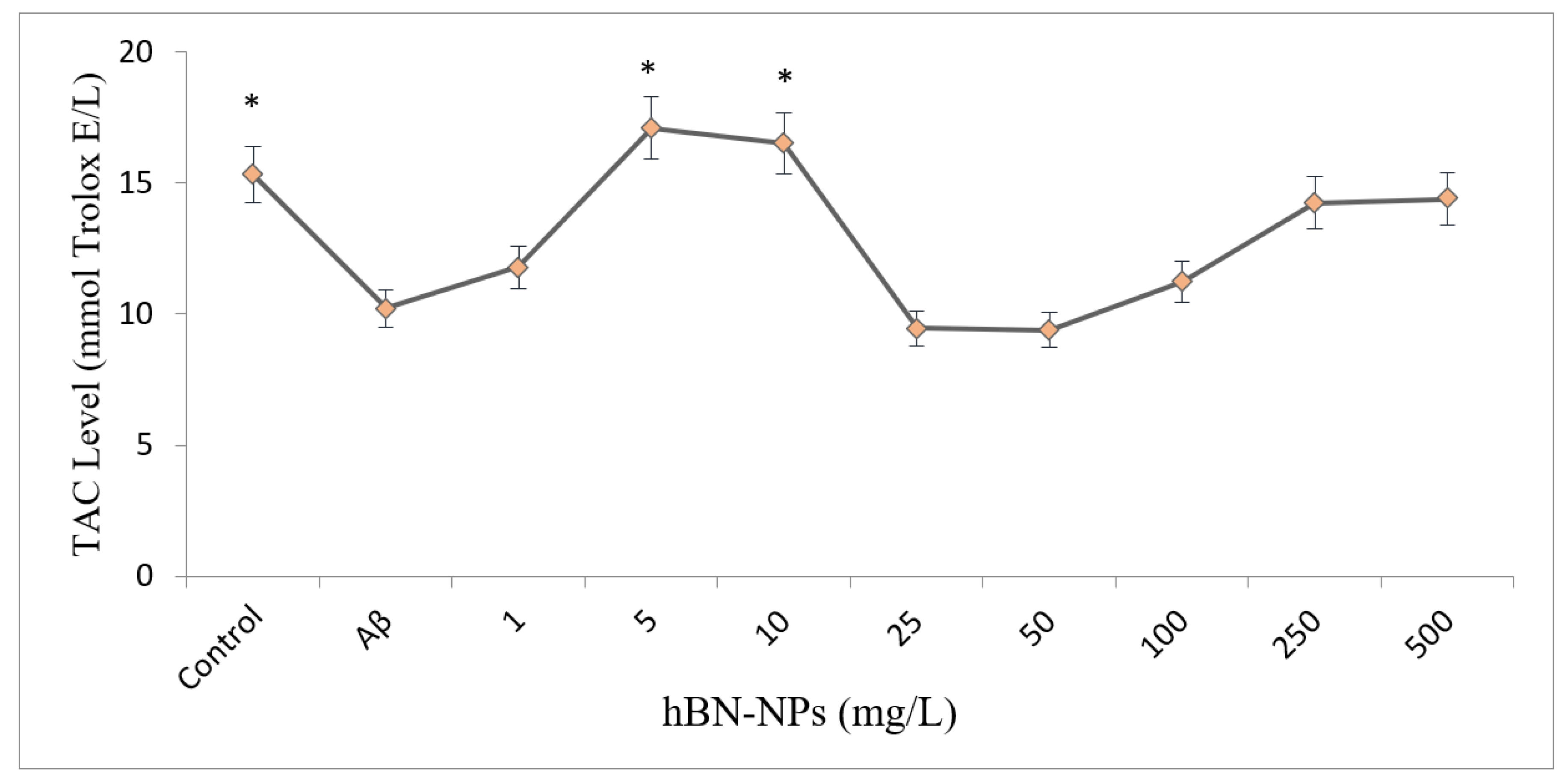
4. Results
5. Discussion
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Geng, J.; Qu, K.; Ren, J.; Qu, X. Rapid and efficient screening of Alzheimer’s disease β-amyloid inhibitors using label-free gold nanoparticles. Mol. Biosyst. 2010, 6, 2389–2391. [Google Scholar] [CrossRef] [PubMed]
- Moore, K.A.; Pate, K.M.; Soto-Ortega, D.D.; Lohse, S.; van der Munnik, N.; Lim, M.; Jackson, K.S.; Lyles, V.D.; Jones, L.; Glassgow, N.; et al. Influence of gold nanoparticle surface chemistry and diameter upon Alzheimer’s disease amyloid-β protein aggregation. J. Biol. Eng. 2017, 11, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moreira, P.; Honda, K.; Liu, Q.; Aliev, G.; Oliveira, C.; Santos, M.; Zhu, X.; Smith, M.; Perry, G. Alzheimers Disease and Oxidative Stress: The Old Problem Remains Unsolved. Curr. Med. Chem. Nerv. Syst. Agents 2005, 5, 51–62. [Google Scholar] [CrossRef]
- Brambilla, D.; Le Droumaguet, B.; Nicolas, J.; Hashemi, S.H.; Wu, L.-P.; Moghimi, S.M.; Couvreur, P.; Andrieux, K. Nanotechnologies for Alzheimer’s disease: Diagnosis, therapy, and safety issues. Nanomed. Nanotechnol. Biol. Med. 2011, 7, 521–540. [Google Scholar] [CrossRef] [PubMed]
- Turkez, H.; Cacciatore, I.; Arslan, M.E.; Fornasari, E.; Marinelli, L.; Di Stefano, A.; Mardinoglu, A. Histidyl-Proline Diketopiperazine Isomers as Multipotent Anti-Alzheimer Drug Candidates. Biomolecules 2020, 10, 737. [Google Scholar] [CrossRef] [PubMed]
- Mathew, A.; Fukuda, T.; Nagaoka, Y.; Hasumura, T.; Morimoto, H.; Yoshida, Y.; Maekawa, T.; Venugopal, K.; Kumar, D.S. Curcumin loaded-PLGA nanoparticles conjugated with Tet-1 peptide for potential use in Alzheimer’s disease. PLoS ONE 2012, 7, e32616. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.-J.; Wang, P.-W.; Yang, I.-H.; Wu, C.-L.; Chuang, J.-H. Amyloid-beta mediates the receptor of advanced glycation end product-induced pro-inflammatory response via toll-like receptor 4 signaling pathway in retinal ganglion cell line RGC-5. Int. J. Biochem. Cell Biol. 2015, 64, 1–10. [Google Scholar] [CrossRef]
- Yu, X.L.; Li, Y.N.; Zhang, H.; Su, Y.J.; Zhou, W.W.; Zhang, Z.P.; Wang, S.W.; Xu, P.X.; Wang, Y.J.; Liu, R.T. Rutin inhibits amylin-induced neurocytotoxicity and oxidative stress. Food Funct. 2015, 6, 3296–3306. [Google Scholar] [CrossRef]
- Lleó, A.; Greenberg, S.M.; Growdon, J.H. Current pharmacotherapy for Alzheimer’s disease. Annu. Rev. Med. 2006, 57, 513–533. [Google Scholar] [CrossRef]
- Kim, Y.; Park, J.-H.; Lee, H.; Nam, J.-M. How do the size, charge and shape of nanoparticles affect amyloid β aggregation on brain lipid bilayer? Sci. Rep. 2016, 6, 19548. [Google Scholar] [CrossRef]
- Yu, L.; Wang, S.; Chen, X.; Yang, H.; Li, X.; Xu, Y.; Zhu, X. Orientin alleviates cognitive deficits and oxidative stress in Aβ1–42-induced mouse model of Alzheimer’s disease. Life Sci. 2015, 121, 104–109. [Google Scholar] [CrossRef] [PubMed]
- Han, S.-H.; Chang, Y.J.; Jung, E.S.; Kim, J.-W.; Na, D.L.; Jung, I.M. Effective screen for amyloid β aggregation inhibitor using amyloid β-conjugated gold nanoparticles. Int. J. Nanomed. 2010, 6, 1–12. [Google Scholar]
- Sozio, P.; Marinelli, L.; Cacciatore, I.; Fontana, A.; Türkez, H.; Giorgioni, G.; Ambrosini, D.; Barbato, F.; Grumetto, L.; Pacella, S.; et al. New Flurbiprofen Derivatives: Synthesis, Membrane Affinity and Evaluation of in Vitro Effect on β-Amyloid Levels. Molecules 2013, 18, 10747–10767. [Google Scholar] [CrossRef]
- Azam, F.; Alabdullah, N.H.; Ehmedat, H.M.; Abulifa, A.R.; Taban, I.; Upadhyayula, S. NSAIDs as potential treatment option for preventing amyloid β toxicity in Alzheimer’s disease: An investigation by docking, molecular dynamics, and DFT studies. J. Biomol. Struct. Dyn. 2018, 36, 2099–2117. [Google Scholar] [CrossRef] [PubMed]
- Lam, S.; Bayraktar, A.; Zhang, C.; Turkez, H.; Nielsen, J.; Boren, J.; Shoaie, S.; Uhlen, M.; Mardinoglu, A. A systems biology approach for studying neurodegenerative diseases. Drug Discov. Today 2020, 25, 1146–1159. [Google Scholar] [CrossRef] [PubMed]
- Turkez, H.; Arslan, M.E.; Barboza, J.N.; Kahraman, C.Y.; de Sousa, D.P.; Mardinoğlu, A. Therapeutic Potential of Ferulic Acid in Alzheimer’s Disease. Curr. Drug Deliv. 2021, 19, 860–873. [Google Scholar] [CrossRef]
- Rondanelli, M.; Faliva, M.A.; Peroni, G.; Infantino, V.; Gasparri, C.; Iannello, G.; Perna, S.; Riva, A.; Petrangolini, G.; Tartara, A. Pivotal role of boron supplementation on bone health: A narrative review. J. Trace Elem. Med. Biol. 2020, 62, 126577. [Google Scholar] [CrossRef]
- Marinelli, L.; Fornasari, E.; Di Stefano, A.; Turkez, H.; Arslan, M.E.; Eusepi, P.; Ciulla, M.; Cacciatore, I. (R)-α-Lipoyl-Gly-l-Pro-l-Glu dimethyl ester as dual acting agent for the treatment of Alzheimer’s disease. Neuropeptides 2017, 66, 52–58. [Google Scholar] [CrossRef]
- Turkez, H. Effects of boric acid and borax on titanium dioxide genotoxicity. J. Appl. Toxicol. 2008, 28, 658–664. [Google Scholar] [CrossRef]
- Das, B.C.; Nandwana, N.K.; Das, S.; Nandwana, V.; Shareef, M.A.; Das, Y.; Saito, M.; Weiss, L.M.; Almaguel, F.; Hosmane, N.S.; et al. Boron Chemicals in Drug Discovery and Development: Synthesis and Medicinal Perspective. Molecules 2022, 27, 2615. [Google Scholar] [CrossRef]
- Ucar, A.; Parlak, V.; Ozgeris, F.B.; Yeltekin, A.C.; Arslan, M.E.; Alak, G.; Turkez, H.; Kocaman, E.M.; Atamanalp, M. Magnetic nanoparticles-induced neurotoxicity and oxidative stress in brain of rainbow trout: Mitigation by ulexite through modulation of antioxidant, anti-inflammatory, and antiapoptotic activities. Sci. Total Environ. 2022, 838, 155718. [Google Scholar] [CrossRef] [PubMed]
- Turkez, H.; Arslan, M.E.; Tatar, A.; Mardinoglu, A. Promising potential of boron compounds against Glioblastoma: In Vitro antioxidant, anti-inflammatory and anticancer studies. Neurochem. Int. 2021, 149, 105137. [Google Scholar] [CrossRef] [PubMed]
- Küçükdoğru, R.; Türkez, H.; Arslan, M.E.; Tozlu, Ö.Ö.; Sönmez, E.; Mardinoğlu, A.; Cacciatore, I.; Di Stefano, A. Neuroprotective effects of boron nitride nanoparticles in the experimental Parkinson’s disease model against MPP+ induced apoptosis. Metab. Brain Dis. 2020, 35, 947–957. [Google Scholar] [CrossRef] [PubMed]
- Turkez, H.; Geyikoglu, F.; Tatar, A.; Keles, M.S.; Kaplan, I. The effects of some boron compounds against heavy metal toxicity in human blood. Exp. Toxicol. Pathol. 2012, 64, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Bernard, S.; Miele, P. Nanostructured and architectured boron nitride from boron, nitrogen and hydrogen-containing molecular and polymeric precursors. Mater. Today 2014, 17, 443–450. [Google Scholar] [CrossRef]
- Kim, J.H.; Pham, T.V.; Hwang, J.H.; Kim, C.S.; Kim, M.J. Boron nitride nanotubes: Synthesis and applications. Nano Converg. 2018, 5, 17. [Google Scholar] [CrossRef] [Green Version]
- Sharker, S.M.; Alam, M.A.; Shill, M.C.; Rahman, G.M.S.; Reza, H.M. Functionalized hBN as targeted photothermal chemotherapy for complete eradication of cancer cells. Int. J. Pharm. 2017, 534, 206–212. [Google Scholar] [CrossRef]
- Chen, X.; Wu, P.; Rousseas, M.; Okawa, D.; Gartner, Z.; Zettl, A.; Bertozzi, C.R. Boron nitride nanotubes are noncytotoxic and can be functionalized for interaction with proteins and cells. J. Am. Chem. Soc. 2009, 131, 890–891. [Google Scholar] [CrossRef] [Green Version]
- Izyumskaya, N.; Demchenko, D.O.; Das, S.; Özgür, Ü.; Avrutin, V.; Morkoç, H. Recent development of boron nitride towards electronic applications. Adv. Electron. Mater. 2017, 3, 1600485. [Google Scholar] [CrossRef]
- Merlo, A.; Mokkapati, V.R.S.S.; Pandit, S.; Mijakovic, I. Boron nitride nanomaterials: Biocompatibility and bio-applications. Biomater. Sci. 2018, 6, 2298–2311. [Google Scholar] [CrossRef] [Green Version]
- Xiong, C.; Tu, W. Synthesis of water-dispersible boron nitride nanoparticles. Eur. J. Inorg. Chem. 2014, 2014, 3010–3015. [Google Scholar] [CrossRef]
- Weng, Q.; Wang, X.; Wang, X.; Bando, Y.; Golberg, D. Functionalized hexagonal boron nitride nanomaterials: Emerging properties and applications. Chem. Soc. Rev. 2016, 45, 3989–4012. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rohani, P.; Kim, S.; Swihart, M.T. Boron Nanoparticles for Room-Temperature Hydrogen Generation from Water. Adv. Energy Mater. 2016, 6, 1502550. [Google Scholar] [CrossRef]
- Türkez, H.; Arslan, M.E.; Tatar, A.; Özdemir, Ö.; Sönmez, E.; Çadirci, K.; Hacimüftüoğlu, A.; Ceylan, B.; Açikyildiz, M.; Kahraman, C.Y.; et al. Molecular Genetics and Cytotoxic Responses to Titanium Diboride and Zinc Borate Nanoparticles on Cultured Human Primary Alveolar Epithelial Cells. Materials 2022, 15, 2359. [Google Scholar] [CrossRef] [PubMed]
- Türkez, H.; Arslan, M.E.; Sönmez, E.; Tatar, A.; Açikyildiz, M.; Geyikoğlu, F. Toxicogenomic responses of human alveolar epithelial cells to tungsten boride nanoparticles. Chem. Biol. Interact. 2017, 273, 257–265. [Google Scholar] [CrossRef]
- Türkez, H.; Arslan, M.E.; Sönmez, E.; Tatar, A.; Geyikoğlu, F.; Açikyildiz, M.; Mardinoğlu, A. Safety Assessments of Nickel Boride Nanoparticles on the Human Pulmonary Alveolar Cells by Using Cell Viability and Gene Expression Analyses. Biol. Trace Elem. Res. 2021, 199, 2602–2611. [Google Scholar] [CrossRef]
- Türkez, H.; Arslan, M.E.; Sönmez, E.; Geyikoğlu, F.; Açıkyıldız, M.; Tatar, A. Microarray assisted toxicological investigations of boron carbide nanoparticles on human primary alveolar epithelial cells. Chem. Biol. Interact. 2019, 300, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Mondal, S.; Banthia, A.K. Low-temperature synthetic route for boron carbide. J. Eur. Ceram. Soc. 2005, 25, 287–291. [Google Scholar] [CrossRef]
- Türkez, H.; Arslan, M.E.; Sönmez, E.; Açikyildiz, M.; Tatar, A.; Geyikoğlu, F. Synthesis, characterization and cytotoxicity of boron nitride nanoparticles: Emphasis on toxicogenomics. Cytotechnology 2019, 71, 351–361. [Google Scholar] [CrossRef]
- Sukhorukova, I.V.; Zhitnyak, I.Y.; Kovalskii, A.M.; Matveev, A.T.; Lebedev, O.I.; Li, X.; Gloushankova, N.A.; Golberg, D.; Shtansky, D.V. Boron Nitride Nanoparticles with a Petal-Like Surface as Anticancer Drug-Delivery Systems. ACS Appl. Mater. Interfaces 2015, 7, 17217–17225. [Google Scholar] [CrossRef]
- Dibandjo, P.; Bois, L.; Chassagneux, F.; Cornu, D.; Letoffe, J.-M.; Toury, B.; Babonneau, F.; Miele, P. Synthesis of Boron Nitride with Ordered Mesostructure. Adv. Mater. 2005, 17, 571–574. [Google Scholar] [CrossRef]
- Şen, Ö.; Emanet, M.; Çulha, M. One-Step Synthesis of Hexagonal Boron Nitrides, Their Crystallinity and Biodegradation. Front. Bioeng. Biotechnol. 2018, 6, 83. [Google Scholar] [CrossRef] [PubMed]
- Cheung, Y.T.; Lau, W.K.W.; Yu, M.S.; Lai, C.S.W.; Yeung, S.C.; So, K.F.; Chang, R.C.C. Effects of all-trans-retinoic acid on human SH-SY5Y neuroblastoma as in vitro model in neurotoxicity research. Neurotoxicology 2009, 30, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Pagoni, A.; Marinelli, L.; Di Stefano, A.; Ciulla, M.; Turkez, H.; Mardinoglu, A.; Vassiliou, S.; Cacciatore, I. Novel anti-Alzheimer phenol-lipoyl hybrids: Synthesis, physico-chemical characterization, and biological evaluation. Eur. J. Med. Chem. 2020, 186, 111880. [Google Scholar] [CrossRef]
- Arslan, M.E.; Türkez, H.; Mardinoğlu, A. In vitro neuroprotective effects of farnesene sesquiterpene on alzheimer’s disease model of differentiated neuroblastoma cell line. Int. J. Neurosci. 2020, 131, 745–754. [Google Scholar] [CrossRef]
- Parra, M.; Jung, J.; Boone, T.D.; Tran, L.; Blaber, E.A.; Brown, M.; Chin, M.; Chinn, T.; Cohen, J.; Doebler, R.; et al. Microgravity validation of a novel system for RNA isolation and multiplex quantitative real time PCR analysis of gene expression on the International Space Station. PLoS ONE 2017, 12, e0183480. [Google Scholar] [CrossRef] [Green Version]
- ARSLAN, M.E. Anticarcinogenic properties of malic acid on glioblastoma cell line through necrotic cell death mechanism. MANAS J. Eng. 2021, 9, 22–29. [Google Scholar] [CrossRef]
- Zhu, L.; Lian, G.; Tan, M.; Wang, Q.; Zhao, X.; Cui, D.; Tao, X. Reaction of Hexagonal Boron Nitride Nano-crystals under Mild Hydrothermal Conditions. Z. Nat. B 2008, 63, 742–746. [Google Scholar] [CrossRef]
- Moghimi, S.M.; Hunter, A.C.; Murray, J.C. Nanomedicine: Current status and future prospects. FASEB J. 2005, 19, 311–330. [Google Scholar] [CrossRef] [Green Version]
- Albanese, A.; Tang, P.S.; Chan, W.C.W. The effect of nanoparticle size, shape, and surface chemistry on biological systems. Annu. Rev. Biomed. Eng. 2012, 14, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Khawaja, A.M. The legacy of nanotechnology: Revolution and prospects in neurosurgery. Int. J. Surg. 2011, 9, 608–614. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferreira, T.H.; Silva, P.R.O.; Santos, R.G.; Sousa, E.M.B. A novel synthesis route to produce boron nitride nanotubes for bioapplications. J. Biomater. Nanobiotechnol. 2011, 2, 426–434. [Google Scholar] [CrossRef] [Green Version]
- Thareja, S.; Zhu, M.; Ji, X.; Wang, B. Boron-based small molecules in disease detection and treatment (2013–2016). Heterocycl. Commun. 2017, 23, 137–153. [Google Scholar] [CrossRef]
- Rasel, M.A.I. Experimental Exploration of Boron Nitride Nanoparticle Interaction with Living Cells. Ph.D. Thesis, Queensland University of Technology, Brisbane, Australia, 2017. [Google Scholar]
- Singh, B.; Kaur, G.; Singh, P.; Singh, K.; Kumar, B.; Vij, A.; Kumar, M.; Bala, R.; Meena, R.; Singh, A.; et al. Nanostructured Boron Nitride with High Water Dispersibility for Boron Neutron Capture Therapy. Sci. Rep. 2016, 6, 35535. [Google Scholar] [CrossRef] [Green Version]
- Ciofani, G.; Raffa, V.; Menciassi, A.; Dario, P. Preparation of Boron Nitride Nanotubes Aqueous Dispersions for Biological Applications. J. Nanosci. Nanotechnol. 2008, 8, 6223–6231. [Google Scholar] [CrossRef]
- Ciofani, G.; Danti, S.; D’Alessandro, D.; Moscato, S.; Menciassi, A. Assessing cytotoxicity of boron nitride nanotubes: Interference with the MTT assay. Biochem. Biophys. Res. Commun. 2010, 394, 405–411. [Google Scholar] [CrossRef]
- Varadarajan, S.; Yatin, S.; Aksenova, M.; Butterfield, D.A. Review: Alzheimer’s amyloid β-peptide-associated free radical oxidative stress and neurotoxicity. J. Struct. Biol. 2000, 130, 184–208. [Google Scholar] [CrossRef] [PubMed]
- Butterfield, D.A.; Reed, T.; Newman, S.F.; Sultana, R. Roles of amyloid β-peptide-associated oxidative stress and brain protein modifications in the pathogenesis of Alzheimer’s disease and mild cognitive impairment. Free Radic. Biol. Med. 2007, 43, 658–677. [Google Scholar] [CrossRef] [Green Version]
- Markesbery, W.R. Oxidative stress hypothesis in Alzheimer’s disease. Free Radic. Biol. Med. 1997, 23, 134–147. [Google Scholar] [CrossRef]
- Zhao, L.N.; Long, H.W.; Mu, Y.; Chew, L.Y. The toxicity of amyloid β oligomers. Int. J. Mol. Sci. 2012, 13, 7303–7327. [Google Scholar] [CrossRef] [Green Version]
- Su, B.; Wang, X.; Nunomura, A.; Moreira, P.; Lee, H.-G.; Perry, G.; Smith, M.; Zhu, X. Oxidative stress signaling in Alzheimer’s disease. Curr. Alzheimer Res. 2008, 5, 525–532. [Google Scholar] [CrossRef]
- Jo, D.-G.; Arumugam, T.V.; Woo, H.-N.; Park, J.-S.; Tang, S.-C.; Mughal, M.; Hyun, D.-H.; Park, J.-H.; Choi, Y.-H.; Gwon, A.-R.; et al. Evidence that γ-secretase mediates oxidative stress-induced β-secretase expression in Alzheimer’s disease. Neurobiol. Aging 2010, 31, 917–925. [Google Scholar] [CrossRef] [Green Version]
- Kurz, A.; Perneczky, R. Novel insights for the treatment of Alzheimer’s disease. Prog. Neuropsychopharmacol. Biol. Psychiatry 2011, 35, 373–379. [Google Scholar] [CrossRef] [PubMed]
- Muthaiyah, B.; Essa, M.M.; Chauhan, V.; Chauhan, A. Protective effects of walnut extract against amyloid beta peptide-induced cell death and oxidative stress in PC12 cells. Neurochem. Res. 2011, 36, 2096–2103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yıldırım, Ö.Ç.; Arslan, M.E.; Öner, S.; Cacciatore, I.; Di Stefano, A.; Mardinoglu, A.; Turkez, H. Boron Nitride Nanoparticles Loaded with a Boron-Based Hybrid as a Promising Drug Carrier System for Alzheimer’s Disease Treatment. Int. J. Mol. Sci. 2022, 23, 8249. [Google Scholar] [CrossRef]
- Kar, F.; Hacıoğlu, C.; Göncü, Y.; Söğüt, İ.; Şenturk, H.; Burukoğlu Dönmez, D.; Kanbak, G.; Ay, N. In Vivo Assessment of the Effect of Hexagonal Boron Nitride Nanoparticles on Biochemical, Histopathological, Oxidant and Antioxidant Status. J. Clust. Sci. 2021, 32, 517–529. [Google Scholar] [CrossRef]
- Behl, C.; Davis, J.B.; Klier, F.G.; Schubert, D. Amyloid beta peptide induces necrosis rather than apoptosis. Brain Res. 1994, 645, 253–264. [Google Scholar] [CrossRef]
- Akama, K.T.; Van Eldik, L.J. β-amyloid stimulation of inducible nitric-oxide synthase in astrocytes is interleukin-1β- and tumor necrosis factor-α (TNFα)-dependent, and involves a TNFα receptor-associated factor- and NFκB-inducing kinase- dependent signaling mechanism. J. Biol. Chem. 2000, 275, 7918–7924. [Google Scholar] [CrossRef] [Green Version]
- Ethell, D.W.; Buhler, L.A. Fas ligand-mediated apoptosis in degenerative disorders of the brain. J. Clin. Immunol. 2003, 23, 439–446. [Google Scholar] [CrossRef]
- Castorina, A.; Tiralongo, A.; Giunta, S.; Carnazza, M.L.; Scapagnini, G.; D’Agata, V. Early effects of aluminum chloride on beta-secretase mRNA expression in a neuronal model of beta-amyloid toxicity. Cell Biol. Toxicol. 2010, 26, 367–377. [Google Scholar] [CrossRef]
- Marcello, E.; Borroni, B.; Pelucchi, S.; Gardoni, F.; Di Luca, M. ADAM10 as a therapeutic target for brain diseases: From developmental disorders to Alzheimer’s disease. Expert Opin. Ther. Targets 2017, 21, 1017–1026. [Google Scholar] [CrossRef] [PubMed]
- Peron, R.; Vatanabe, I.; Manzine, P.; Camins, A.; Cominetti, M. Alpha-secretase ADAM10 regulation: Insights into Alzheimer’s disease treatment. Pharmaceuticals 2018, 11, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sathya, M.; Premkumar, P.; Karthick, C.; Moorthi, P.; Jayachandran, K.S.; Anusuyadevi, M. BACE1 in Alzheimer’s disease. Clin. Chim. Acta 2012, 414, 171–178. [Google Scholar] [CrossRef]
- De Strooper, B.; Beullens, M.; Contreras, B.; Levesque, L.; Craessaerts, K.; Cordell, B.; Moechars, D.; Bollen, M.; Fraser, P.; St. George-Hyslop, P.; et al. Phosphorylation, subcellular localization, and membrane orientation of the Alzheimer’s disease-associated presenilins. J. Biol. Chem. 1997, 272, 3590–3598. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cai, Y.; An, S.S.A.; Kim, S. Mutations in presenilin 2 and its implications in Alzheimer’s disease and other dementia-associated disorders. Clin. Interv. Aging 2015, 10, 1163–1172. [Google Scholar]
- Çinleti, B.; Yardimci, N.; Aytürk, Z.; Ilhan, A.; Kaya, G.; Acar, M.; Koç, E.R.; Gündüz, E.; Gündüz, M. The effects and interactions of APOE and APH-1A polymorphisms in Alzheimer disease. Turk. J. Med. Sci. 2015, 45, 1098–1105. [Google Scholar] [CrossRef] [PubMed]
- Kelleher, R.J.; Shen, J. Presenilin-1 mutations and Alzheimer’s disease. Proc. Natl. Acad. Sci. USA 2017, 114, 629–631. [Google Scholar] [CrossRef] [Green Version]
- Phillips, M.C. Apolipoprotein E isoforms and lipoprotein metabolism. IUBMB Life 2014, 66, 616–623. [Google Scholar] [CrossRef]
- Huang, Y.; Liu, X.Q.; Wyss-Coray, T.; Brecht, W.J.; Sanan, D.A.; Mahley, R.W. Apolipoprotein E fragments present in Alzheimer’s disease brains induce neurofibrillary tangle-like intracellular inclusions in neurons. Proc. Natl. Acad. Sci. USA 2001, 98, 8838–8843. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.-C.; Kanekiyo, T.; Xu, H.; Bu, G. Apolipoprotein E and Alzheimer disease: Risk, mechanisms and therapy. Nat. Rev. Neurol. 2013, 9, 106–118. [Google Scholar] [CrossRef] [Green Version]
- Deane, R.; Sagare, A.; Hamm, K.; Parisi, M.; Lane, S.; Finn, M.B.; Holtzman, D.M.; Zlokovic, B.V. apoE isoform–specific disruption of amyloid β peptide clearance from mouse brain. J. Clin. Investig. 2008, 118, 4002–4013. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, G.-D.; Bi, R.; Zhang, D.-F.; Xu, M.; Luo, R.; Wang, D.; Fang, Y.; Li, T.; Zhang, C.; Yao, Y.-G. Female-specific effect of the BDNF gene on Alzheimer’s disease. Neurobiol. Aging 2017, 53, 192.e11–192.e19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garzon, D.J.; Fahnestock, M. Oligomeric Amyloid Decreases Basal Levels of Brain-Derived Neurotrophic factor (BDNF) mRNA via Specific Downregulation of BDNF Transcripts IV and V in Differentiated Human Neuroblastoma Cells. J. Neurosci. 2007, 27, 2628–2635. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wee, P.; Wang, Z. Epidermal Growth Factor Receptor Cell Proliferation Signaling Pathways. Cancers 2017, 9, 52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Janelsins, M.C.; Mastrangelo, M.A.; Park, K.M.; Sudol, K.L.; Narrow, W.C.; Oddo, S.; LaFerla, F.M.; Callahan, L.M.; Federoff, H.J.; Bowers, W.J. Chronic neuron-specific tumor necrosis factor-alpha expression enhances the local inflammatory environment ultimately leading to neuronal death in 3xTg-AD Mice. Am. J. Pathol. 2008, 173, 1768–1782. [Google Scholar] [CrossRef] [Green Version]
- Liu, Q.; Chen, Y.; Shen, C.; Xiao, Y.; Wang, Y.; Liu, Z.; Liu, X. Chicoric acid supplementation prevents systemic inflammation-induced memory impairment and amyloidogenesis via inhibition of NF-κB. FASEB J. 2017, 31, 1494–1507. [Google Scholar] [CrossRef] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aydin, N.; Turkez, H.; Tozlu, O.O.; Arslan, M.E.; Yavuz, M.; Sonmez, E.; Ozpolat, O.F.; Cacciatore, I.; Di Stefano, A.; Mardinoglu, A. Ameliorative Effects by Hexagonal Boron Nitride Nanoparticles against Beta Amyloid Induced Neurotoxicity. Nanomaterials 2022, 12, 2690. https://doi.org/10.3390/nano12152690
Aydin N, Turkez H, Tozlu OO, Arslan ME, Yavuz M, Sonmez E, Ozpolat OF, Cacciatore I, Di Stefano A, Mardinoglu A. Ameliorative Effects by Hexagonal Boron Nitride Nanoparticles against Beta Amyloid Induced Neurotoxicity. Nanomaterials. 2022; 12(15):2690. https://doi.org/10.3390/nano12152690
Chicago/Turabian StyleAydin, Nursah, Hasan Turkez, Ozlem Ozdemir Tozlu, Mehmet Enes Arslan, Mehmet Yavuz, Erdal Sonmez, Ozgur Fırat Ozpolat, Ivana Cacciatore, Antonio Di Stefano, and Adil Mardinoglu. 2022. "Ameliorative Effects by Hexagonal Boron Nitride Nanoparticles against Beta Amyloid Induced Neurotoxicity" Nanomaterials 12, no. 15: 2690. https://doi.org/10.3390/nano12152690
APA StyleAydin, N., Turkez, H., Tozlu, O. O., Arslan, M. E., Yavuz, M., Sonmez, E., Ozpolat, O. F., Cacciatore, I., Di Stefano, A., & Mardinoglu, A. (2022). Ameliorative Effects by Hexagonal Boron Nitride Nanoparticles against Beta Amyloid Induced Neurotoxicity. Nanomaterials, 12(15), 2690. https://doi.org/10.3390/nano12152690








