Effects of PEG Chain Length on Relaxometric Properties of Iron Oxide Nanoparticles-Based MRI Contrast Agent
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Hydrophobic IONPs
2.3. Ligand Exchange
2.4. Characterizations
2.5. Relaxivity Measurements
3. Results and Discussion
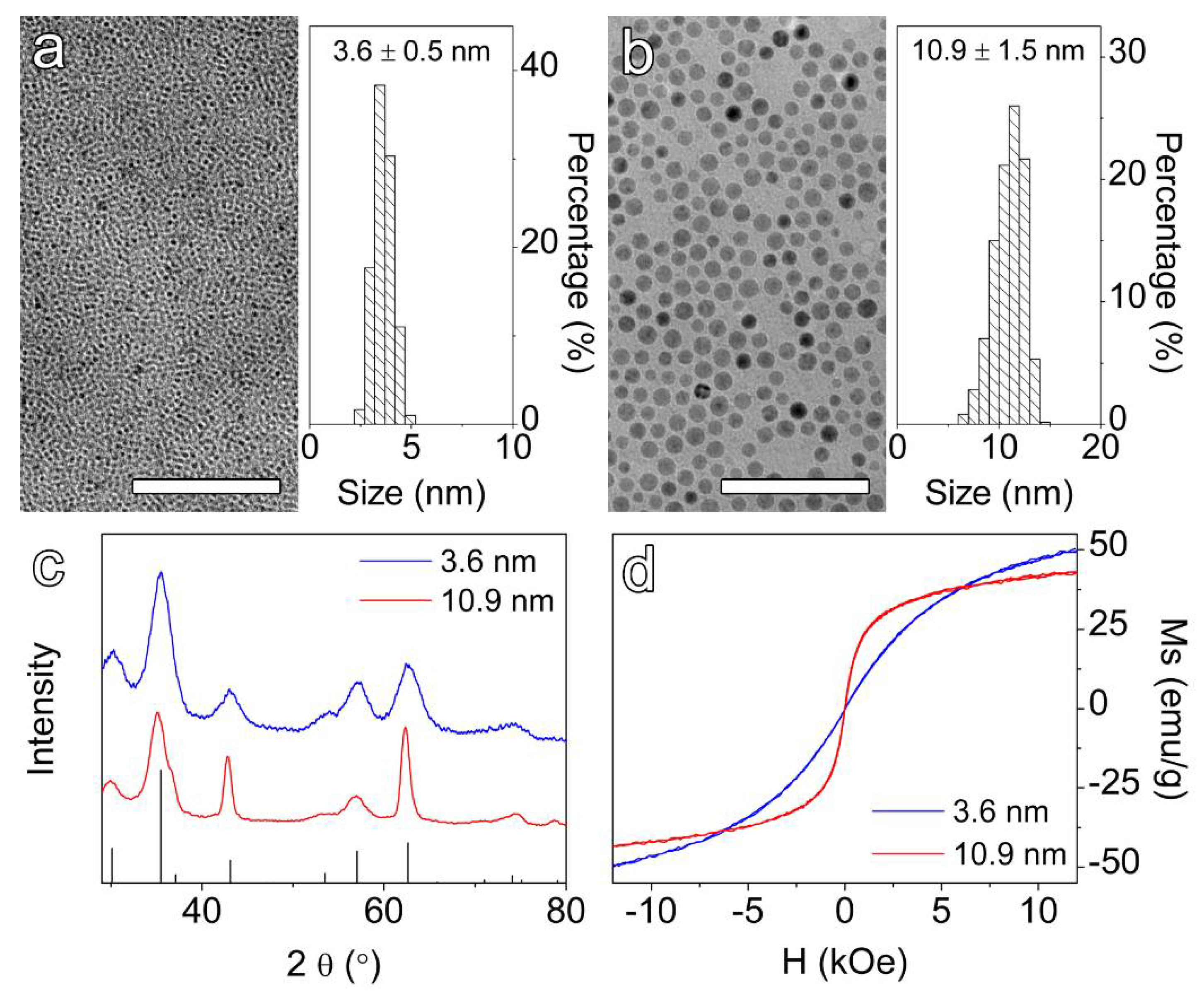
3.1. Synthesis of Hydrophobic IONPs
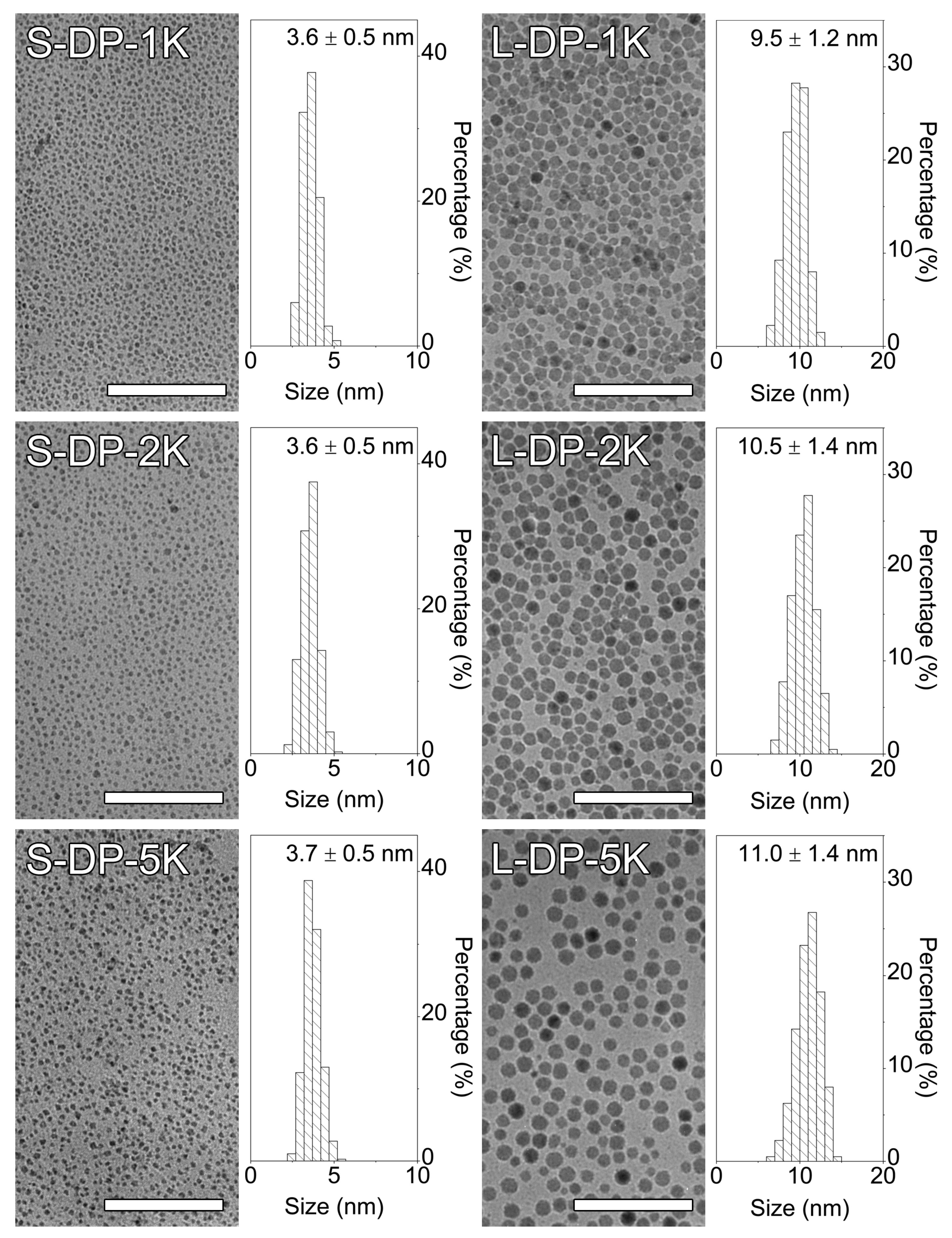
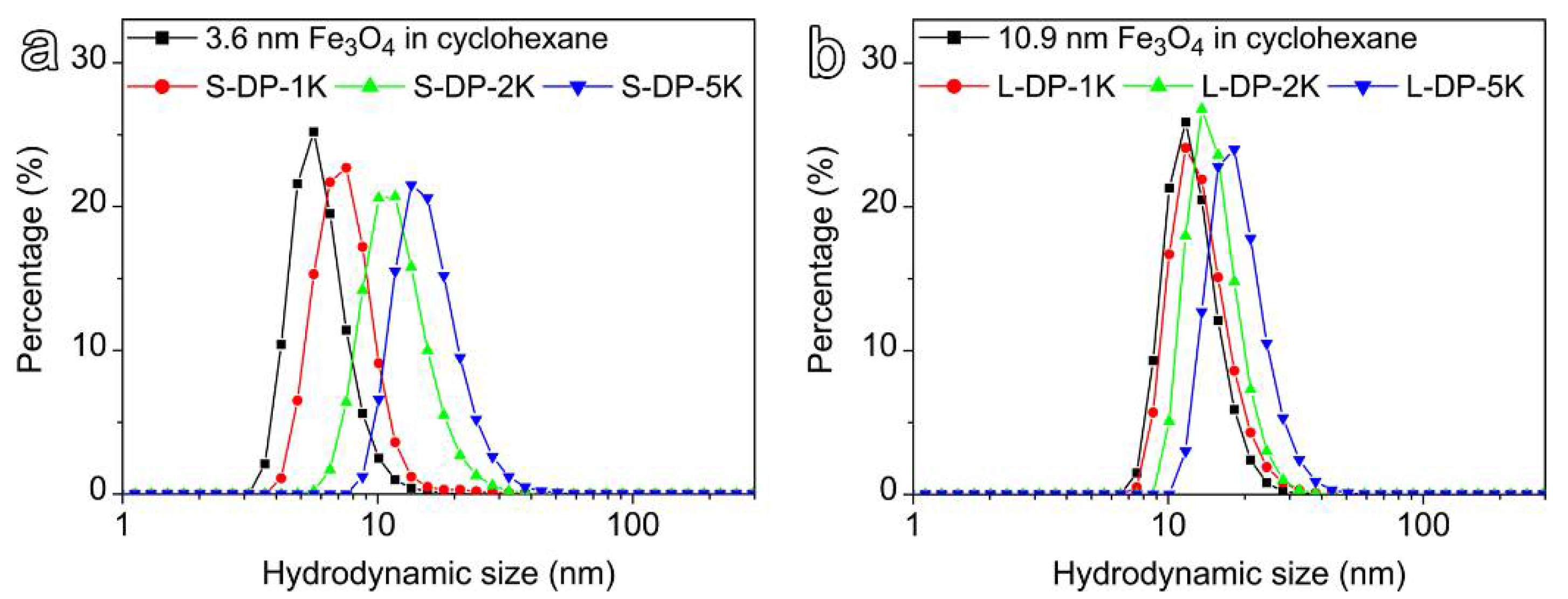
3.2. Ligand Exchange of Hydrophobic IONPs
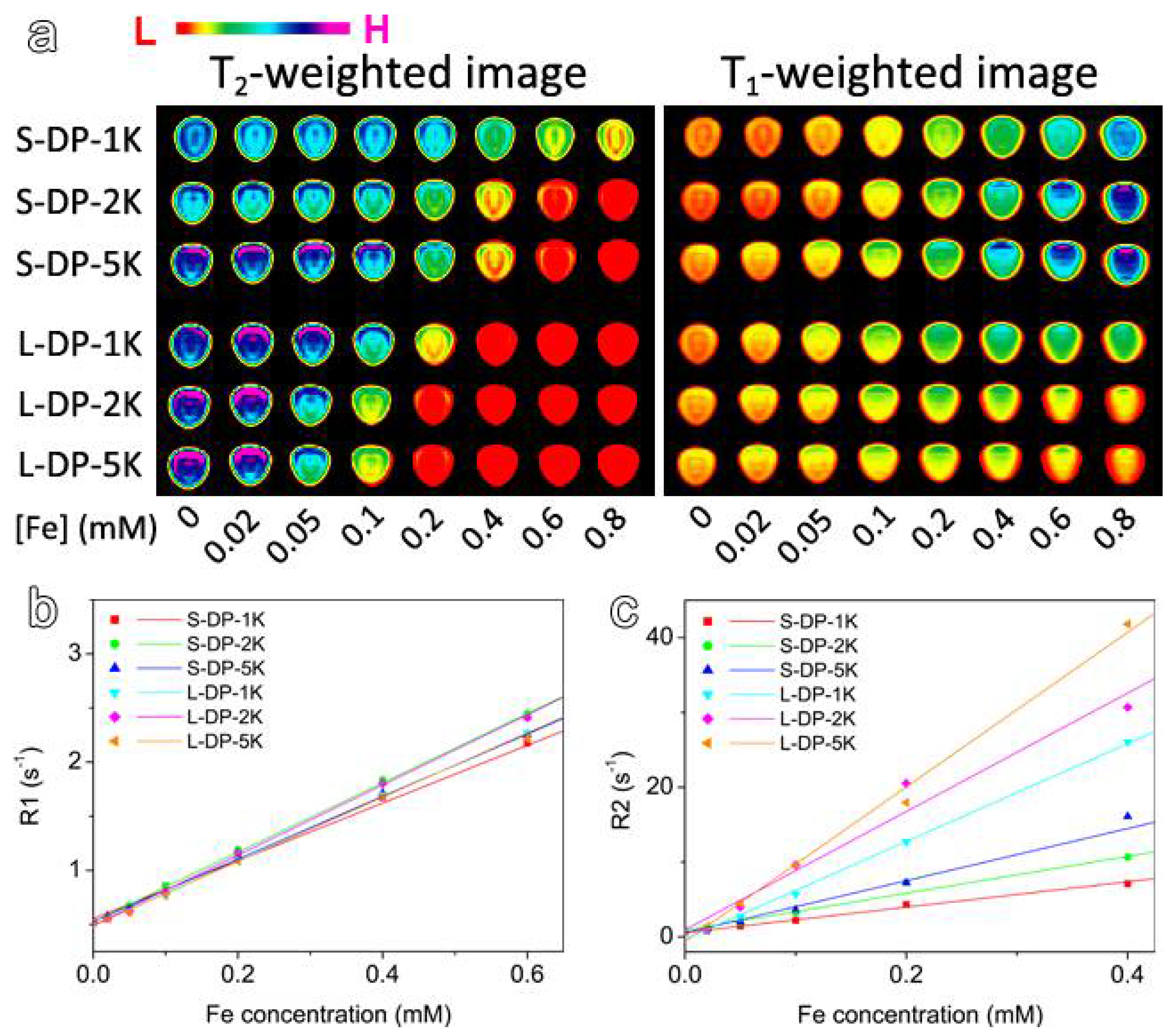
3.3. MRI Enhancement Effects of the PEGylated IONPs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Shin, T.H.; Kim, P.K.; Kang, S.; Cheong, J.; Kim, S.; Lim, Y.; Shin, W.; Jung, J.Y.; Lah, J.D.; Choi, B.W.; et al. High-resolution T-1 MRI via renally clearable dextran nanoparticles with an iron oxide shell. Nat. Biomed. Eng. 2021, 5, 252–263. [Google Scholar] [CrossRef]
- Kim, B.H.; Lee, N.; Kim, H.; An, K.; Park, Y.I.; Choi, Y.; Shin, K.; Lee, Y.; Kwon, S.G.; Na, H.B.; et al. Large-Scale Synthesis of Uniform and Extremely Small-Sized Iron Oxide Nanoparticles for High-Resolution T1 Magnetic Resonance Imaging Contrast Agents. J. Am. Chem. Soc. 2011, 133, 12624–12631. [Google Scholar] [CrossRef]
- Zhou, H.G.; Guo, M.Y.; Li, J.Y.; Qin, F.L.; Wang, Y.Q.; Liu, T.; Liu, J.; Sabet, Z.F.; Wang, Y.L.; Liu, Y.; et al. Hypoxia-Triggered Self-Assembly of Ultrasmall Iron Oxide Nanoparticles to Amplify the Imaging Signal of a Tumor. J. Am. Chem. Soc. 2021, 143, 1846–1853. [Google Scholar] [CrossRef] [PubMed]
- Weissleder, R.; Nahrendorf, M.; Pittet, M.J. Imaging macrophages with nanoparticles. Nat. Mater. 2014, 13, 125–138. [Google Scholar] [CrossRef]
- Yan, S.; Hu, K.; Zhang, M.; Sheng, J.; Xu, X.; Tang, S.; Li, Y.; Yang, S.; Si, G.; Mao, Y.; et al. Extracellular magnetic labeling of biomimetic hydrogel-induced human mesenchymal stem cell spheroids with ferumoxytol for MRI tracking. Bioact. Mater. 2022, 19, 418–428. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.Z.; Li, B.; Qiao, Y.S. Fe3O4 Nanoparticles in Targeted Drug/Gene Delivery Systems. Materials 2018, 11, 324. [Google Scholar] [CrossRef] [Green Version]
- Luther, D.C.; Huang, R.; Jeon, T.; Zhang, X.Z.; Lee, Y.W.; Nagaraj, H.; Rotello, V.M. Delivery of drugs, proteins, and nucleic acids using inorganic nanoparticles. Adv. Drug Deliv. Rev. 2020, 156, 188–213. [Google Scholar] [CrossRef]
- Hu, F.Q.; Wei, L.; Zhou, Z.; Ran, Y.L.; Li, Z.; Gao, M.Y. Preparation of biocompatible magnetite nanocrystals for in vivo magnetic resonance detection of cancer. Adv. Mater. 2006, 18, 2553–2556. [Google Scholar] [CrossRef]
- Li, Z.; Yi, P.W.; Sun, Q.; Lei, H.; Zhao, H.L.; Zhu, Z.H.; Smith, S.C.; Lan, M.B.; Lu, G.Q. Ultrasmall Water-Soluble and Biocompatible Magnetic Iron Oxide Nanoparticles as Positive and Negative Dual Contrast Agents. Adv. Funct. Mater. 2012, 22, 2387–2393. [Google Scholar] [CrossRef]
- Lu, J.X.; Sun, J.H.; Li, F.Y.; Wang, J.; Liu, J.N.; Kim, D.; Fan, C.H.; Hyeon, T.; Ling, D.S. Highly Sensitive Diagnosis of Small Hepatocellular Carcinoma Using pH-Responsive Iron Oxide Nanocluster Assemblies. J. Am. Chem. Soc. 2018, 140, 10071–10074. [Google Scholar] [CrossRef]
- Sandiford, L.; Phinikaridou, A.; Protti, A.; Meszaros, L.K.; Cui, X.; Yan, Y.; Frodsham, G.; Williamson, P.A.; Gaddum, N.; Botnar, R.M.; et al. Bisphosphonate-Anchored PEGylation and Radiolabeling of Superparamagnetic Iron Oxide: Long-Circulating Nanoparticles for in Vivo Multimodal (T1 MRI-SPECT) Imaging. ACS Nano 2013, 7, 500–512. [Google Scholar] [CrossRef] [PubMed]
- Ge, J.X.; Chen, L.; Huang, B.X.; Gao, Y.; Zhou, D.D.; Zhou, Y.; Chen, C.; Wen, L.; Li, Q.; Zeng, J.F.; et al. Anchoring Group-Mediated Radiolabeling of Inorganic Nanoparticles-A Universal Method for Constructing Nuclear Medicine Imaging Nanoprobes. ACS Appl. Mater. Interfaces 2022, 14, 8838–8846. [Google Scholar] [CrossRef] [PubMed]
- Lee, N.; Cho, H.R.; Oh, M.H.; Lee, S.H.; Kim, K.; Kim, B.H.; Shin, K.; Ahn, T.Y.; Choi, J.W.; Kim, Y.W.; et al. Multifunctional Fe3O4/TaOx Core/Shell Nanoparticles for Simultaneous Magnetic Resonance Imaging and X-ray Computed Tomography. J. Am. Chem. Soc. 2012, 134, 10309–10312. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.L.; Jiang, H.; Ye, J.; Zhao, C.Q.; Gao, S.P.; Wu, C.Y.; Li, C.H.; Li, J.C.; Wang, X.M. Nitrogen-Doped Carbon Quantum Dot Stabilized Magnetic Iron Oxide Nanoprobe for Fluorescence, Magnetic Resonance, and Computed Tomography Triple-Modal In Vivo Bioimaging. Adv. Funct. Mater. 2016, 26, 8694–8706. [Google Scholar] [CrossRef]
- Lee, J.E.; Lee, N.; Kim, H.; Kim, J.; Choi, S.H.; Kim, J.H.; Kim, T.; Song, I.C.; Park, S.P.; Moon, W.K.; et al. Uniform Mesoporous Dye-Doped Silica Nanoparticles Decorated with Multiple Magnetite Nanocrystals for Simultaneous Enhanced Magnetic Resonance Imaging, Fluorescence Imaging, and Drug Delivery. J. Am. Chem. Soc. 2010, 132, 552–557. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.; Hou, Y.; Zeng, J.; Liu, C.; Zhang, P.; Jing, L.; Shangguan, D.; Gao, M. Dual-Ratiometric Target-Triggered Fluorescent Probe for Simultaneous Quantitative Visualization of Tumor Microenvironment Protease Activity and pH & ITin Vivo & IT. J. Am. Chem. Soc. 2018, 140, 211–218. [Google Scholar]
- Lin, L.S.; Cong, Z.X.; Cao, J.B.; Ke, K.M.; Peng, Q.L.; Gao, J.H.; Yang, H.H.; Liu, G.; Chen, X.Y. Multifunctional Fe3O4@Polydopamine Core-Shell Nanocomposites for Intracellular mRNA Detection and Imaging-Guided Photothermal Therapy. ACS Nano 2014, 8, 3876–3883. [Google Scholar] [CrossRef] [Green Version]
- Liu, N.; Wu, L.; Zuo, W.; Lin, Q.; Liu, J.; Jin, Q.; Xiao, Z.; Chen, L.; Zhao, Y.; Zhou, J.; et al. pH/Thermal-Sensitive Nanoplatform Capable of On-Demand Specific Release to Potentiate Drug Delivery and Combinational Hyperthermia/Chemo/Chemodynamic Therapy. ACS Appl. Mater. Interfaces 2022, 14, 29668–29678. [Google Scholar] [CrossRef]
- Cheng, G.H.; Zong, W.; Guo, H.Z.; Li, F.Y.; Zhang, X.; Yu, P.; Ren, F.X.; Zhang, X.L.; Shi, X.E.; Gao, F.; et al. Programmed Size-Changeable Nanotheranostic Agents for Enhanced Imaging-Guided Chemo/Photodynamic Combination Therapy and Fast Elimination. Adv. Mater. 2021, 33, 2100398. [Google Scholar] [CrossRef]
- Vuong, Q.L.; Berret, J.-F.; Fresnais, J.; Gossuin, Y.; Sandre, O. A Universal Scaling Law to Predict the Efficiency of Magnetic Nanoparticles as MRI T2-Contrast Agents. Adv. Healthc. Mater. 2012, 1, 502–512. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Z.J.; Yang, L.J.; Gao, J.H.; Chen, X.Y. Structure-Relaxivity Relationships of Magnetic Nanoparticles for Magnetic Resonance Imaging. Adv. Mater. 2019, 31, 180456. [Google Scholar] [CrossRef] [PubMed]
- Jeon, M.; Halbert, M.V.; Stephen, Z.R.; Zhang, M.Q. Iron Oxide Nanoparticles as T-1 Contrast Agents for Magnetic Resonance Imaging: Fundamentals, Challenges, Applications, and Prospectives. Adv. Mater. 2021, 33, 1906539. [Google Scholar] [CrossRef] [PubMed]
- Smolensky, E.D.; Park, H.Y.E.; Berquo, T.S.; Pierre, V.C. Surface functionalization of magnetic iron oxide nanoparticles for MRI applications—Effect of anchoring group and ligand exchange protocol. Contrast Media Mol. Imaging 2011, 6, 189–199. [Google Scholar] [CrossRef] [Green Version]
- Tong, S.; Hou, S.; Zheng, Z.; Zhou, J.; Bao, G. Coating Optimization of Superparamagnetic Iron Oxide Nanoparticles for High T2 Relaxivity. Nano Lett. 2010, 10, 4607–4613. [Google Scholar] [CrossRef] [Green Version]
- Zeng, J.F.; Jing, L.H.; Hou, Y.; Jiao, M.X.; Qiao, R.R.; Jia, Q.J.; Liu, C.Y.; Fang, F.; Lei, H.; Gao, M.Y. Anchoring Group Effects of Surface Ligands on Magnetic Properties of Fe304 Nanoparticles: Towards High Performance MRI Contrast Agents. Adv. Mater. 2014, 26, 2694–2698. [Google Scholar] [CrossRef]
- Stepanov, A.; Burilov, V.; Pinus, M.; Mustafina, A.; Rümmeli, M.H.; Mendez, R.G.; Amirov, R.; Lukashenko, S.; Zvereva, E.; Katsuba, S.; et al. Water transverse relaxation rates in aqueous dispersions of superparamagnetic iron oxide nanoclusters with diverse hydrophilic coating. Coll. Surf. A Physicochem. Eng. Aspects 2014, 443, 450–458. [Google Scholar] [CrossRef]
- Pöselt, E.; Kloust, H.; Tromsdorf, U.; Janschel, M.; Hahn, C.; Maßlo, C.; Weller, H. Relaxivity Optimization of a PEGylated Iron-Oxide-Based Negative Magnetic Resonance Contrast Agent for T2-Weighted Spin–Echo Imaging. ACS Nano 2012, 6, 1619–1624. [Google Scholar] [CrossRef]
- Gao, Z.; Hou, Y.; Zeng, J.; Chen, L.; Liu, C.; Yang, W.; Gao, M. Tumor Microenvironment-Triggered Aggregation of Antiphagocytosis 99mTc-Labeled Fe3O4 Nanoprobes for Enhanced Tumor Imaging In Vivo. Adv. Mater. 2017, 29, 1701095. [Google Scholar] [CrossRef]
- Zhang, P.S.; Zeng, J.F.; Li, Y.Y.; Yang, C.; Meng, J.L.; Hou, Y.; Gao, M.Y. Quantitative Mapping of Glutathione within Intracranial Tumors through Interlocked MRI Signals of a Responsive Nanoprobe. Angew. Chem.-Int. Ed. 2021, 60, 8130–8138. [Google Scholar] [CrossRef]
- Fedorenko, S.; Stepanov, A.; Zairov, R.; Kaman, O.; Amirov, R.; Nizameev, I.; Kholin, K.; Ismaev, I.; Voloshina, A.; Sapunova, A.; et al. One-pot embedding of iron oxides and Gd(III) complexes into silica nanoparticles—Morphology and aggregation effects on MRI dual contrasting ability. Coll. Surf. A Physicochem. Eng. Aspects 2018, 559, 60–67. [Google Scholar] [CrossRef]
- Joshi, H.M.; Lin, Y.P.; Aslam, M.; Prasad, P.V.; Schultz-Sikma, E.A.; Edelman, R.; Meade, T.; Dravid, V.P. Effects of Shape and Size of Cobalt Ferrite Nanostructures on Their MRI Contrast and Thermal Activation. J. Phys. Chem. C 2009, 113, 17761–17767. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cotin, G.; Blanco-Andujar, C.; Perton, F.; Asin, L.; de la Fuente, J.M.; Reichardt, W.; Schaffner, D.; Ngyen, D.V.; Mertz, D.; Kiefer, C.; et al. Unveiling the role of surface, size, shape and defects of iron oxide nanoparticles for theranostic applications. Nanoscale 2021, 13, 14552–14571. [Google Scholar] [CrossRef]
- Xie, Y.X.; Jiang, J.N.; Tang, Q.Y.; Zou, H.B.; Zhao, X.; Liu, H.M.; Ma, D.; Cai, C.L.; Zhou, Y.; Chen, X.J.; et al. Iron Oxide Nanoparticles as Autophagy Intervention Agents Suppress Hepatoma Growth by Enhancing Tumoricidal Autophagy. Adv. Sci. 2020, 7, 1903323. [Google Scholar] [CrossRef] [PubMed]
- Jia, Q.J.; Zeng, J.F.; Qjao, R.R.; Jing, L.H.; Peng, L.; Gu, F.L.; Gao, M.Y. Gelification: An Effective Measure for Achieving Differently Sized Biocompatible Fe3O4 Nanocrystals through a Single Preparation Recipe. J. Am. Chem. Soc. 2011, 133, 19512–19523. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Ge, J.; Huang, B.; Zhou, D.; Huang, G.; Zeng, J.; Gao, M. Anchoring Group Mediated Radiolabeling for Achieving Robust Nanoimaging Probes. Small 2021, 17, 2104977. [Google Scholar] [CrossRef]
- Wang, X.; Chen, L.; Ge, J.; Afshari, M.J.; Yang, L.; Miao, Q.; Duan, R.; Cui, J.; Liu, C.; Zeng, J.; et al. Rational Constructed Ultra-Small Iron Oxide Nanoprobes Manifesting High Performance for T1-Weighted Magnetic Resonance Imaging of Glioblastoma. Nanomaterials 2021, 11, 2601. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.Y.; Niu, M.; Qiao, R.R.; Gao, M.Y. Superdispersible PVP-Coated Fe3O4 Nanocrystals Prepared by a “One-Pot” Reaction. J. Phys. Chem. B 2008, 112, 14390–14394. [Google Scholar] [CrossRef] [PubMed]
- Santra, S.; Tapec, R.; Theodoropoulou, N.; Dobson, J.; Hebard, A.; Tan, W.H. Synthesis and characterization of silica-coated iron oxide nanoparticles in microemulsion: The effect of nonionic surfactants. Langmuir 2001, 17, 2900–2906. [Google Scholar] [CrossRef]
- Lu, C.W.; Hung, Y.; Hsiao, J.K.; Yao, M.; Chung, T.H.; Lin, Y.S.; Wu, S.H.; Hsu, S.C.; Liu, H.M.; Mou, C.Y.; et al. Bifunctional magnetic silica nanoparticles for highly efficient human stem cell labeling. Nano Lett. 2007, 7, 149–154. [Google Scholar] [CrossRef]
- Tromsdorf, U.I.; Bigall, N.C.; Kaul, M.G.; Bruns, O.T.; Nikolic, M.S.; Mollwitz, B.; Sperling, R.A.; Reimer, R.; Hohenberg, H.; Parak, W.J.; et al. Size and Surface Effects on the MRI Relaxivity of Manganese Ferrite Nanoparticle Contrast Agents. Nano Lett. 2007, 7, 2422–2427. [Google Scholar] [CrossRef]
- Brero, F.; Basini, M.; Avolio, M.; Orsini, F.; Arosio, P.; Sangregorio, C.; Innocenti, C.; Guerrini, A.; Boucard, J.; Ishow, E.; et al. Coating Effect on the H-1-NMR Relaxation Properties of Iron Oxide Magnetic Nanoparticles. Nanomaterials 2020, 10, 1660. [Google Scholar] [CrossRef]
- Patsula, V.; Horak, D.; Kucka, J.; Mackova, H.; Lobaz, V.; Francova, P.; Herynek, V.; Heizer, T.; Paral, P.; Sefc, L. Synthesis and modification of uniform PEG-neridronate-modified magnetic nanoparticles determines prolonged blood circulation and biodistribution in a mouse preclinical model. Sci. Rep. 2019, 9, 10765. [Google Scholar] [CrossRef] [PubMed]
- Stanicki, D.; Larbanoix, L.; Boutry, S.; Vangijzegem, T.; Ternad, I.; Garifo, S.; Muller, R.N.; Laurent, S. Impact of the chain length on the biodistribution profiles of PEGylated iron oxide nanoparticles: A multimodal imaging study. J. Mater. Chem. B 2021, 9, 5055–5068. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; An, K.J.; Hwang, Y.S.; Park, J.G.; Noh, H.J.; Kim, J.Y.; Park, J.H.; Hwang, N.M.; Hyeon, T. Ultra-large-scale syntheses of monodisperse nanocrystals. Nat. Mater. 2004, 3, 891–895. [Google Scholar] [CrossRef]
- Sun, S.H.; Zeng, H.; Robinson, D.B.; Raoux, S.; Rice, P.M.; Wang, S.X.; Li, G.X. Monodisperse MFe2O4 (M = Fe, Co, Mn) nanoparticles. J. Am. Chem. Soc. 2004, 126, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Sperling, R.A.; Liedl, T.; Duhr, S.; Kudera, S.; Zanella, M.; Lin, C.A.J.; Chang, W.H.; Braun, D.; Parak, W.J. Size Determination of (Bio)conjugated Water-Soluble Colloidal Nanoparticles: A Comparison of Different Techniques. J. Phys. Chem. C 2007, 111, 11552–11559. [Google Scholar] [CrossRef]
- Panich, A.M.; Salti, M.; Goren, S.D.; Yudina, E.B.; Aleksenskii, A.E.; Vul’, A.Y.; Shames, A.I. Gd(III)-Grafted Detonation Nanodiamonds for MRI Contrast Enhancement. J. Phys. Chem. C 2019, 123, 2627–2631. [Google Scholar] [CrossRef]
- Panich, A.M.; Salti, M.; Prager, O.; Swissa, E.; Kulvelis, Y.V.; Yudina, E.B.; Aleksenskii, A.E.; Goren, S.D.; Vul', A.Y.; Shames, A.I. PVP-coated Gd-grafted nanodiamonds as a novel and potentially safer contrast agent for in vivo MRI. Magn. Reason. Med. 2021, 86, 935–942. [Google Scholar] [CrossRef] [PubMed]
- Koenig, S.H.; Kellar, K.E. Theory of 1/T1 and 1/T2 NMRD profiles of solutions of magnetic nanoparticles. Magn. Reason. Med. 1995, 34, 227–233. [Google Scholar] [CrossRef]
- Brooks, R.A.; Moiny, F.; Gillis, P. On T-2-shortening by weakly magnetized particles: The chemical exchange model. Magn. Reason. Med. 2001, 45, 1014–1020. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muller, R.N.; Vander Elst, L.; Roch, A.; Peters, J.A.; Csajbok, E.; Gillis, P.; Gossuin, Y. Relaxation by Metal-Containing Nanosystems. In Advances in Inorganic Chemistry; Academic Press: Cambridge, MA, USA, 2005; Volume 57, pp. 239–292. [Google Scholar]
- Laurent, S.; Forge, D.; Port, M.; Roch, A.; Robic, C.; Elst, L.V.; Muller, R.N. Magnetic iron oxide nanoparticles: Synthesis, stabilization, vectorization, physicochemical characterizations, and biological applications. Chem. Rev. 2008, 108, 2064–2110. [Google Scholar] [CrossRef] [PubMed]
- Hanot, C.C.; Choi, Y.S.; Anani, T.B.; Soundarrajan, D.; David, A.E. Effects of Iron-Oxide Nanoparticle Surface Chemistry on Uptake Kinetics and Cytotoxicity in CHO-K1 Cells. Int. J. Mol. Sci. 2016, 17, 54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| 3.6 nm IONPs | 10.9 nm IONPs | ||||
|---|---|---|---|---|---|
| Sample | r2 (mM−1 s −1) | r1 (mM−1 s −1) | Sample | r2 (mM −1 s −1) | r1 (mM−1 s −1) |
| S-DP-1K | 16.97 | 2.67 | L-DP-1K | 65.37 | 2.96 |
| S-DP-2K | 24.64 | 3.21 | L-DP-2K | 79.07 | 3.24 |
| S-DP-5K | 34.82 | 2.89 | L-DP-5K | 103.28 | 2.97 |
| 3.6 nm IONPs | 10.9 nm IONPs | ||
|---|---|---|---|
| Sample | MS (emu/g) | Sample | MS (emu/g) |
| S-DP-1K | 7.69 | L-DP-1K | 11.69 |
| S-DP-2K | 12.97 | L-DP-2K | 19.79 |
| S-DP-5K | 17.99 | L-DP-5K | 28.13 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ge, J.; Li, C.; Wang, N.; Zhang, R.; Afshari, M.J.; Chen, C.; Kou, D.; Zhou, D.; Wen, L.; Zeng, J.; et al. Effects of PEG Chain Length on Relaxometric Properties of Iron Oxide Nanoparticles-Based MRI Contrast Agent. Nanomaterials 2022, 12, 2673. https://doi.org/10.3390/nano12152673
Ge J, Li C, Wang N, Zhang R, Afshari MJ, Chen C, Kou D, Zhou D, Wen L, Zeng J, et al. Effects of PEG Chain Length on Relaxometric Properties of Iron Oxide Nanoparticles-Based MRI Contrast Agent. Nanomaterials. 2022; 12(15):2673. https://doi.org/10.3390/nano12152673
Chicago/Turabian StyleGe, Jianxian, Cang Li, Ning Wang, Ruru Zhang, Mohammad Javad Afshari, Can Chen, Dandan Kou, Dandan Zhou, Ling Wen, Jianfeng Zeng, and et al. 2022. "Effects of PEG Chain Length on Relaxometric Properties of Iron Oxide Nanoparticles-Based MRI Contrast Agent" Nanomaterials 12, no. 15: 2673. https://doi.org/10.3390/nano12152673
APA StyleGe, J., Li, C., Wang, N., Zhang, R., Afshari, M. J., Chen, C., Kou, D., Zhou, D., Wen, L., Zeng, J., & Gao, M. (2022). Effects of PEG Chain Length on Relaxometric Properties of Iron Oxide Nanoparticles-Based MRI Contrast Agent. Nanomaterials, 12(15), 2673. https://doi.org/10.3390/nano12152673







