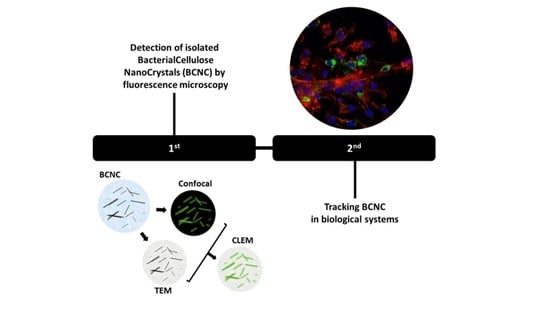
Tracking Bacterial Nanocellulose in Animal Tissues by Fluorescence Microscopy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Preparation of Bacterial Nanocellulose
2.2.2. Production of Bacterial Cellulose Nanocrystals
2.3. Characterization of the BCNC
2.3.1. Zeta Potential
2.3.2. Fourier-Transform Infrared Spectroscopy (FTIR)
2.3.3. Transmission Electron Microscopy (TEM)
2.4. GFP-CBM3A Adsorption onto BNC and BCNC
2.5. Correlative Light Electron Microscopy (CLEM)
2.6. In Vitro Assays
2.6.1. Cell Line and Cell Culture
2.6.2. Bone-Marrow-Derived Macrophage (BMMΦ) Differentiation
2.6.3. Cytotoxicity Testing
2.6.4. Uptake of BCNC by BMMΦ Primary Cells
2.7. Cellulose Tracking in Animal Tissues
2.7.1. Animals, Housing and Feeding Conditions
2.7.2. Test Substance and Dosing Concentration
2.7.3. In Vivo Study
2.7.4. Staining and Microscopic Observations
2.8. Statistical Analysis
3. Results and Discussion
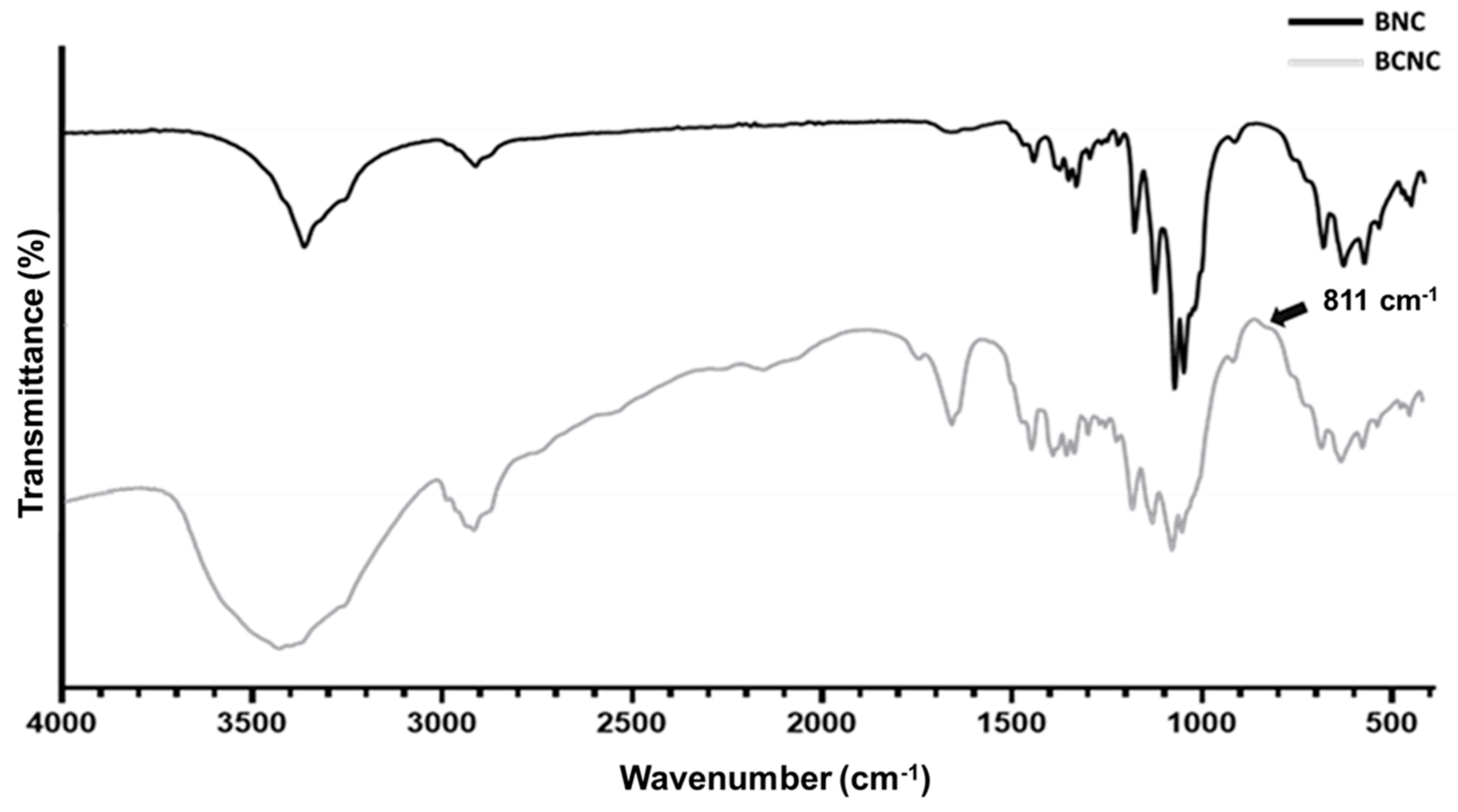
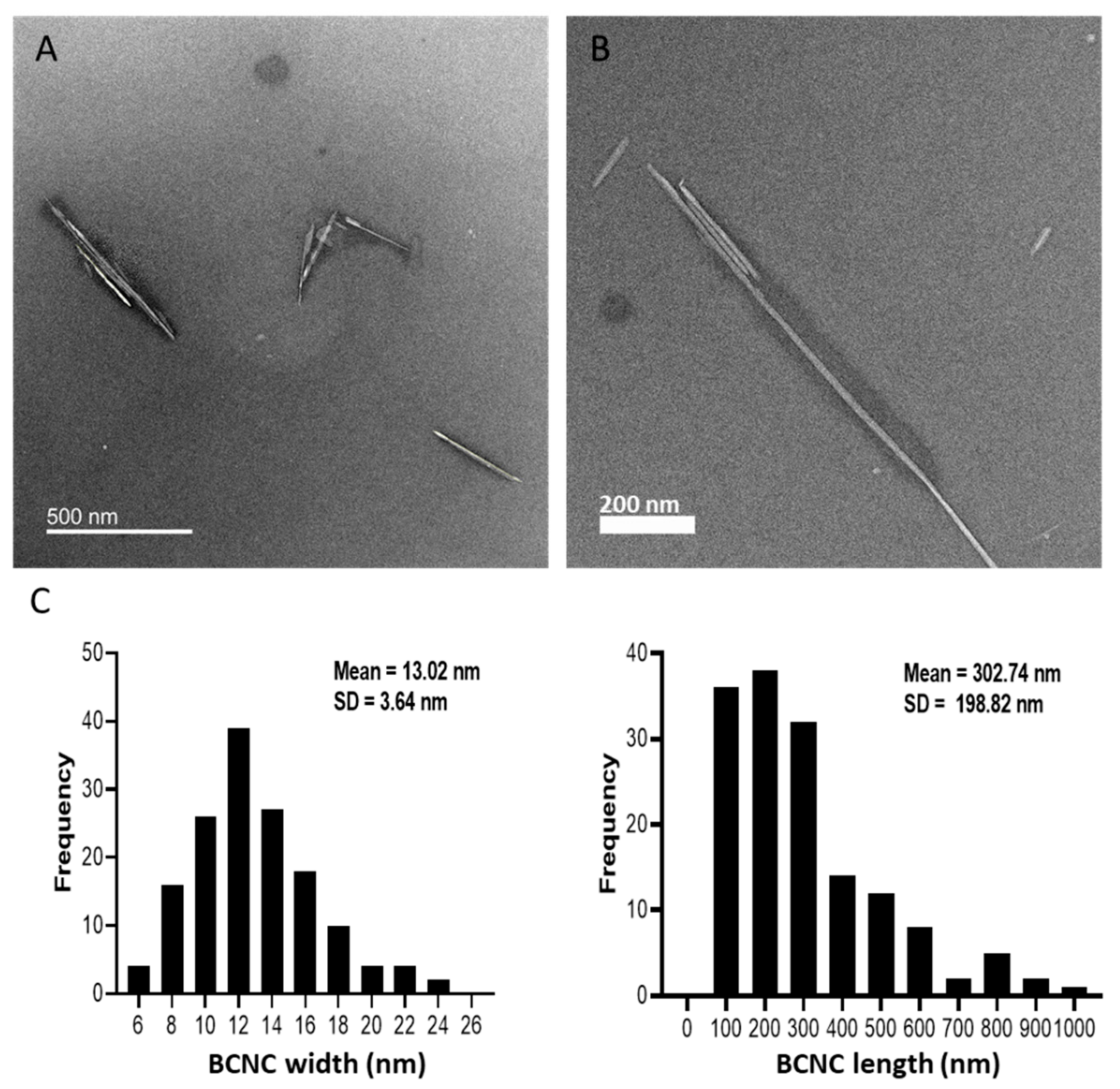
3.1. BCNC Production and Characterization
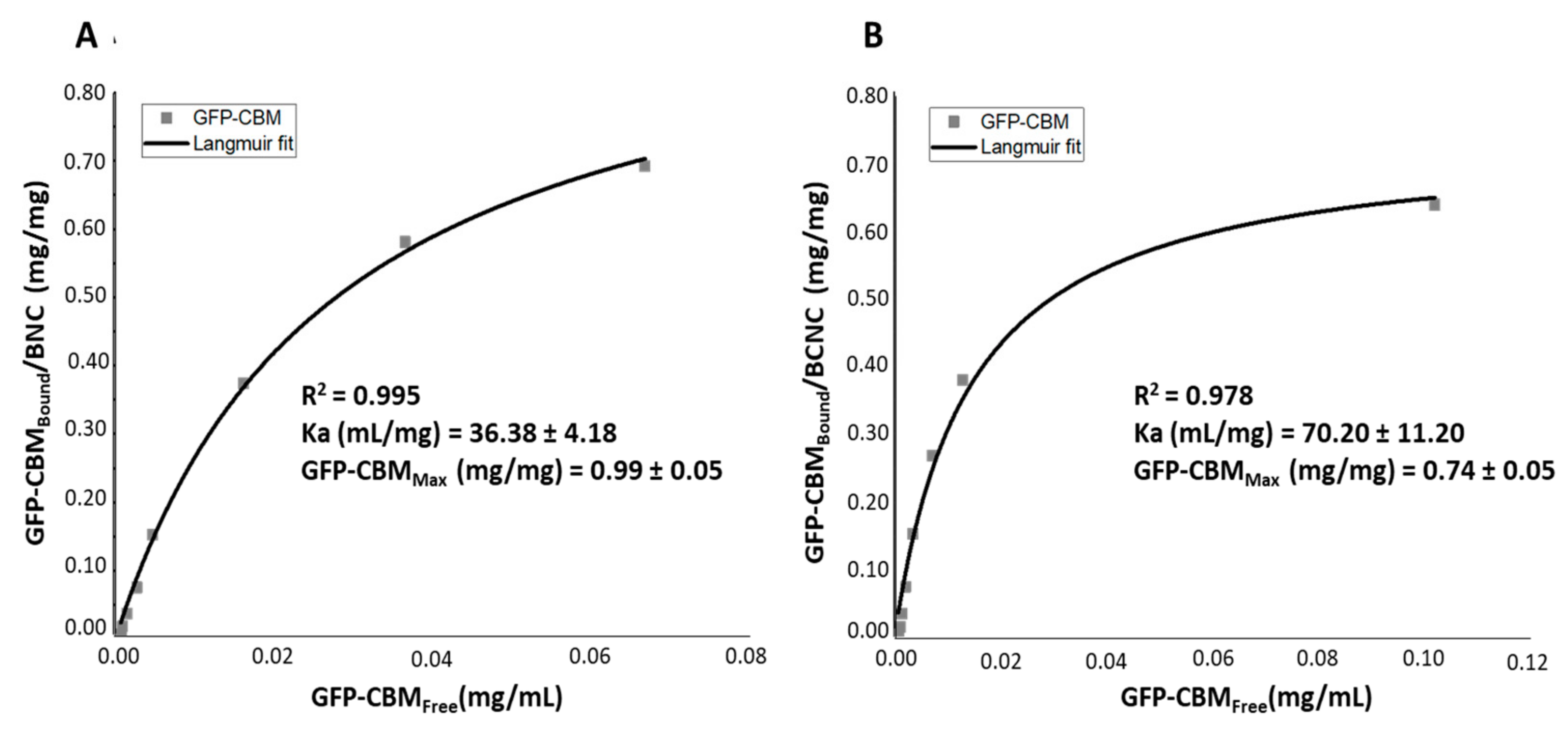
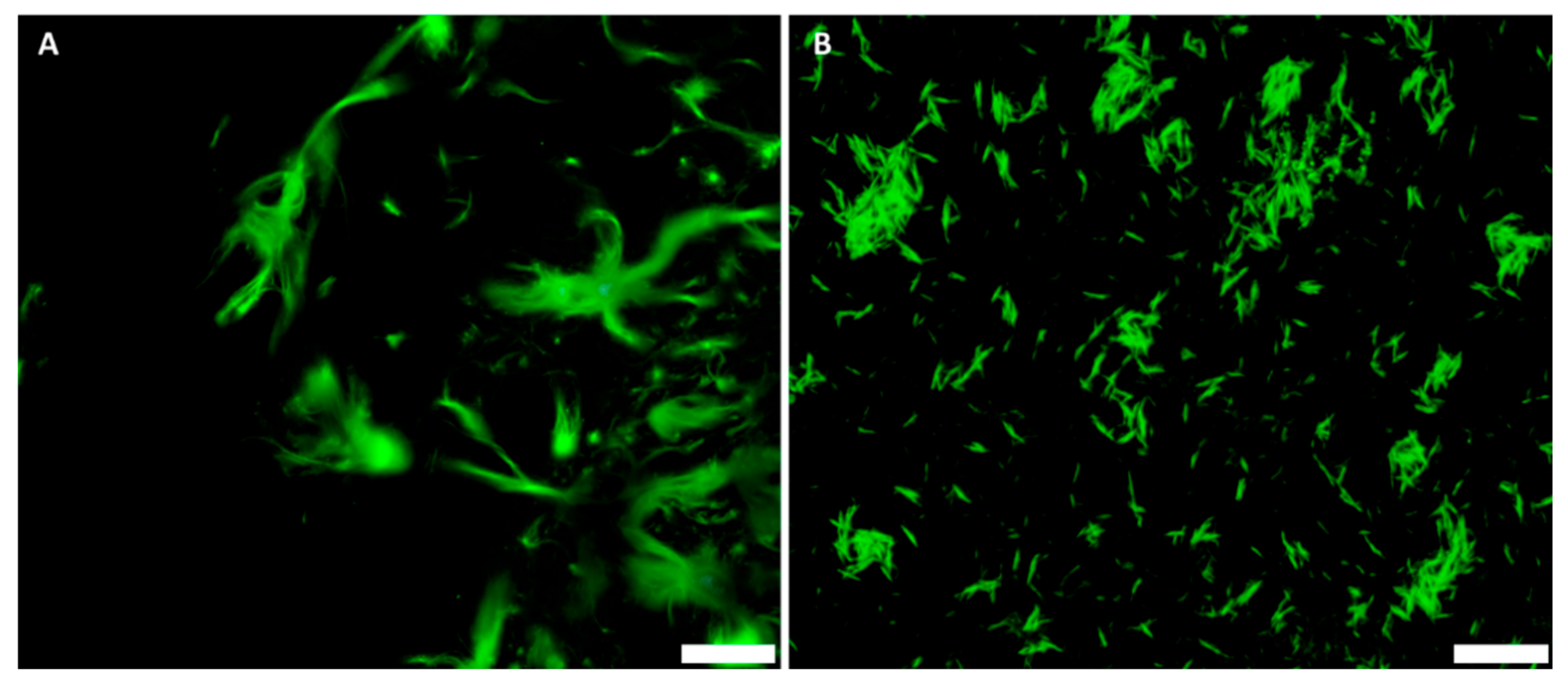
3.2. GFP–CBM Adsorption onto BNC and BCNC
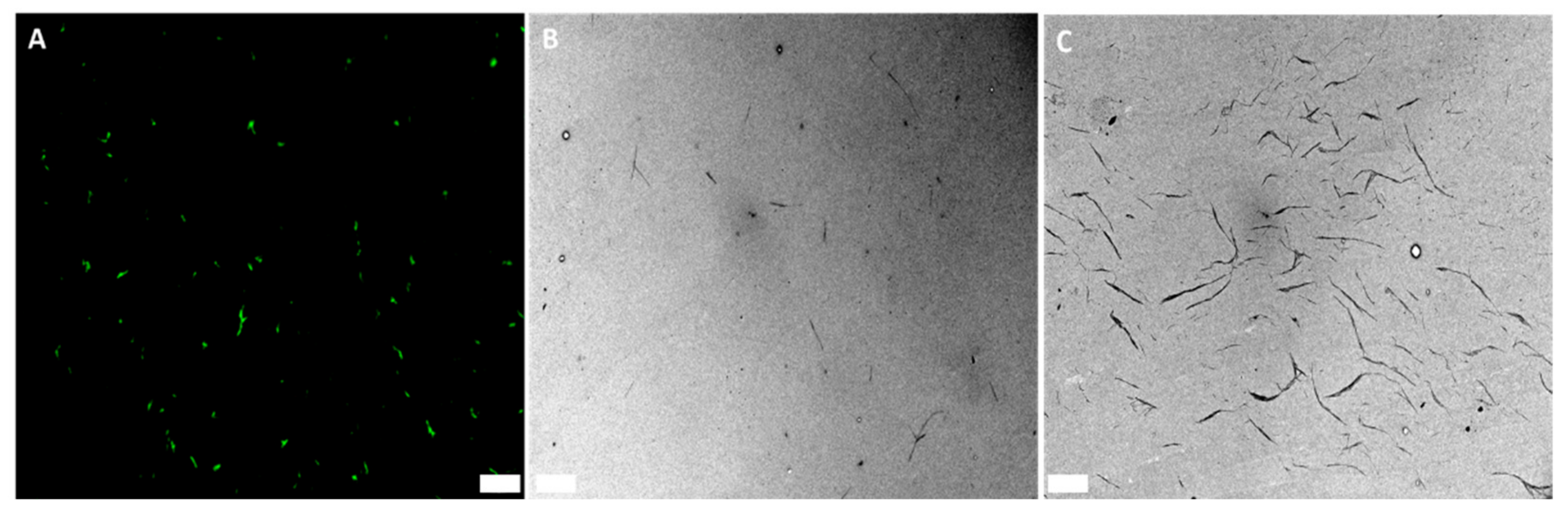
3.3. Considerations for Correlative Light Electron Micoscopy
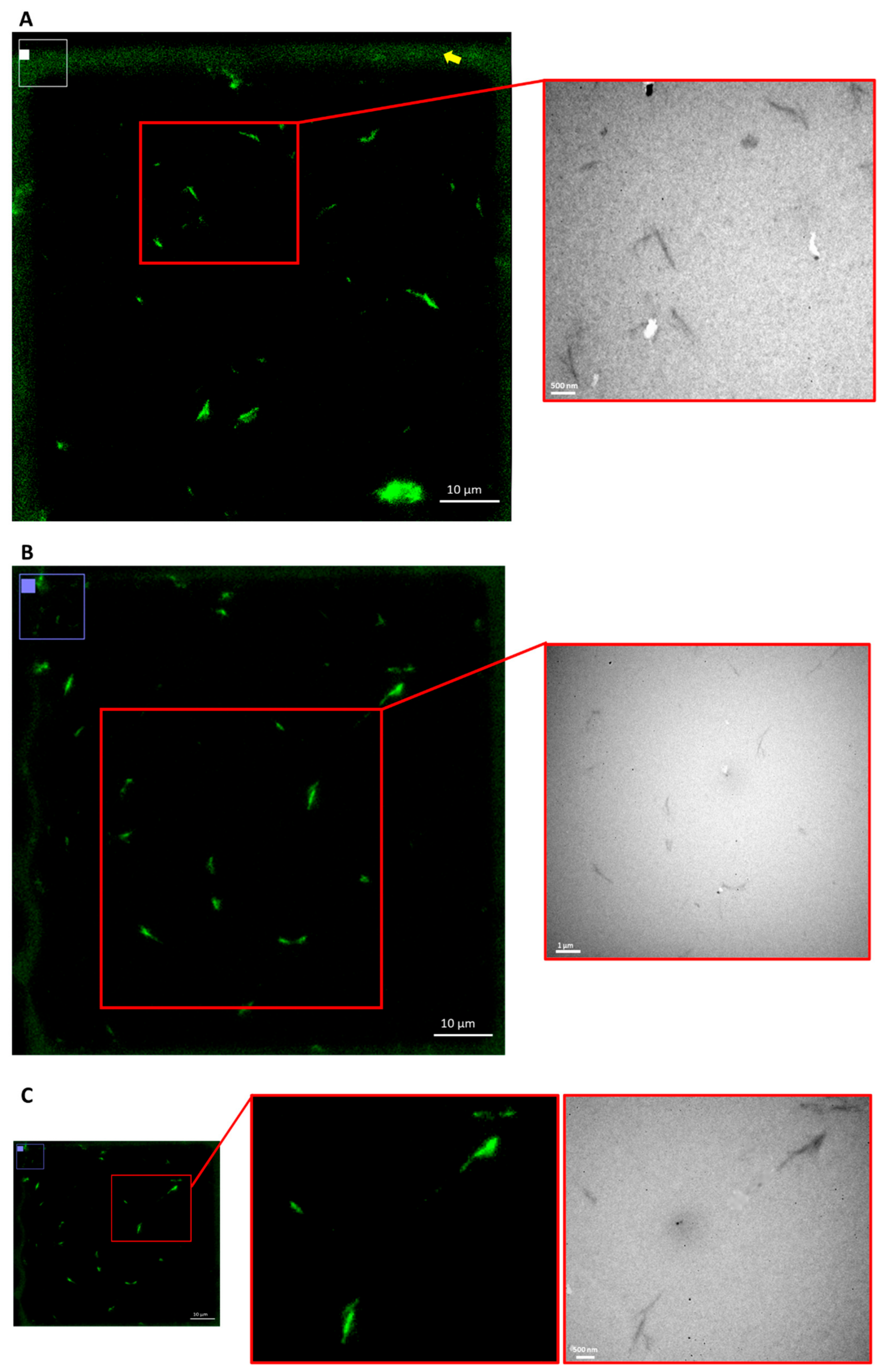
3.4. Correlative Light Electron Microscopy
3.5. In Vitro Assays
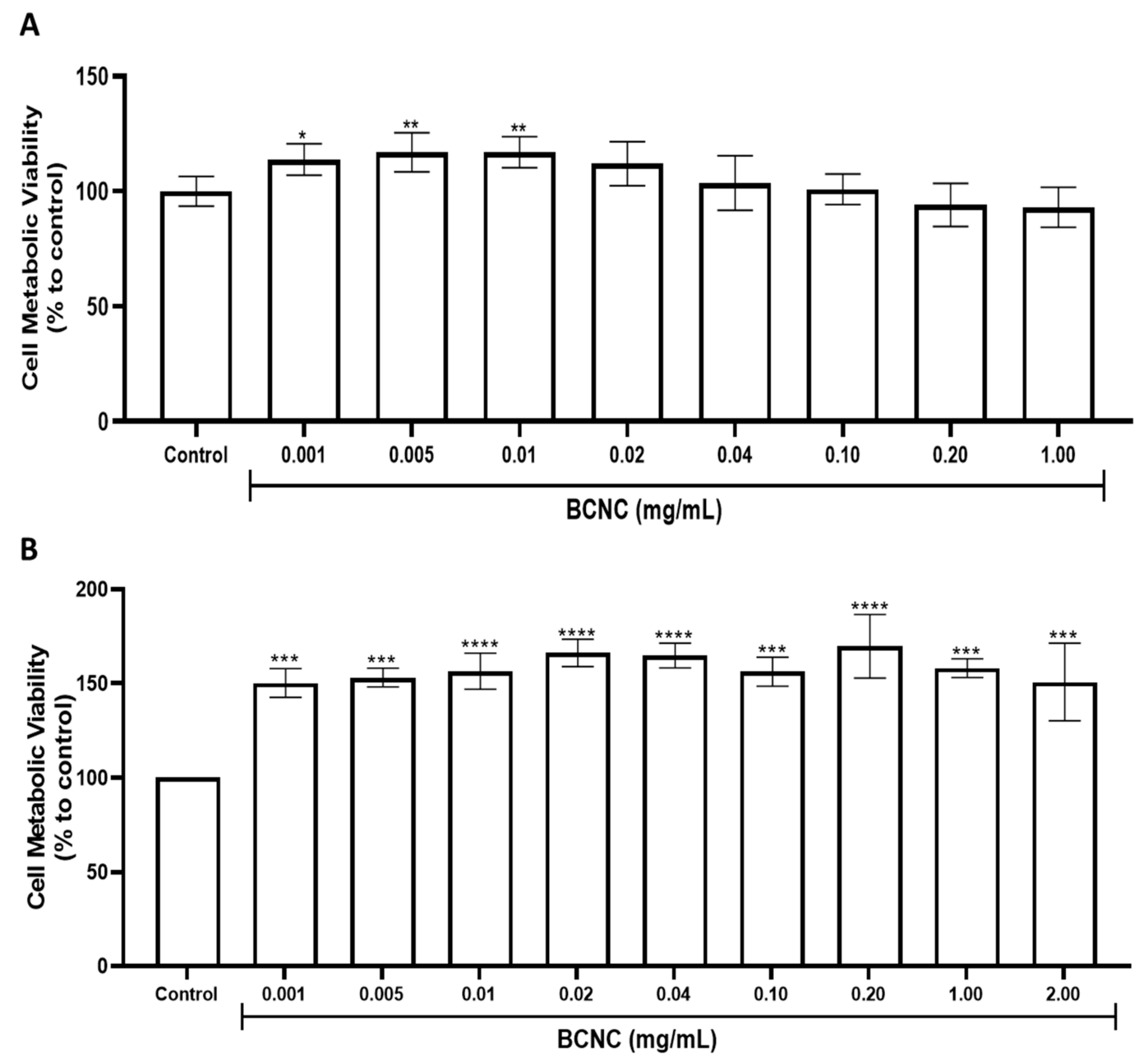
3.5.1. Cytotoxicity Evaluation
3.5.2. Uptake of BCNC by BMMΦ Primary Cells
3.6. Nanocellulose Tracking in Animal Tissues
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ma, T.; Hu, X.; Lu, S.; Liao, X.; Song, Y.; Hu, X. Nanocellulose: A promising green treasure from food wastes to available food materials. Crit. Rev. Food Sci. Nutr. 2022, 62, 989–1002. [Google Scholar] [CrossRef]
- Serpa, A.; Velásquez-Cock, J.; Gañán, P.; Castro, C.; Vélez, L.; Zuluaga, R. Vegetable nanocellulose in food science: A review. Food Hydrocoll. 2016, 57, 178–186. [Google Scholar] [CrossRef]
- Endes, C.; Camarero-Espinosa, S.; Mueller, S.; Foster, E.J.; Petri-Fink, A.; Rothen-Rutishauser, B.; Weder, C.; Clift, M.J.D. A critical review of the current knowledge regarding the biological impact of nanocellulose. J. Nanobiotechnol. 2016, 14, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Da Gama, F.M.P.; Dourado, F. Bacterial NanoCellulose: What future? Bioimpacts 2018, 8, 1. [Google Scholar] [CrossRef] [PubMed]
- Thomas, P.; Duolikun, T.; Rumjit, N.P.; Moosavi, S.; Lai, C.W.; Johan, M.R.B.; Fen, L.B. Comprehensive review on nanocellulose: Recent developments, challenges and future prospects. J. Mech. Behav. Biomed. Mater. 2020, 110, 103884. [Google Scholar] [CrossRef] [PubMed]
- Dufresne, A. Nanocellulose processing properties and potential applications. Curr. For. Rep. 2019, 5, 76–89. [Google Scholar] [CrossRef]
- Statista. Available online: https://www.statista.com/statistics/1192542/global-nanocellulose-market-size/ (accessed on 12 April 2022).
- EFSA Panel on Food Additives and Nutrient Sources Added to Food (ANS); Younes, M.; Aggett, P.; Aguilar, F.; Crebelli, R.; Di Domenico, A.; Dusemund, B.; Filipič, M.; Frutos, M.J.; Galtier, P.; et al. Re-evaluation of celluloses E 460 (i), E 460 (ii), E 461, E 462, E 463, E 464, E 465, E 466, E 468 and E 469 as food additives. EFSA J. 2018, 16, e05047. [Google Scholar] [CrossRef] [Green Version]
- Dourado, F.; Leal, M.; Martins, D.; Fontão, A.; Rodrigues, A.C.; Gama, M. Celluloses as food ingredients/additives: Is there a room for BNC? In Bacterial Nanocellulose; Gama, M., Dourado, F., Bielecki, S., Eds.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 123–133. [Google Scholar] [CrossRef]
- Turbak, A.F.; Snyder, F.W.; Sandberg, K.R. Microfibrillated cellulose, a new cellulose product: Properties, uses, and commercial potential. J. Appl. Polym. Sci. Appl. Polym. Symp. 1983, 37, 815–827. [Google Scholar]
- DeLoid, G.M.; Sohal, I.S.; Lorente, L.R.; Molina, R.M.; Pyrgiotakis, G.; Stevanovic, A.; Zhang, R.; McClements, D.J.; Geitner, N.K.; Bousfield, D.W.; et al. Reducing intestinal digestion and absorption of fat using a nature-derived biopolymer: Interference of triglyceride hydrolysis by nanocellulose. ACS Nano 2018, 12, 6469–6479. [Google Scholar] [CrossRef]
- Tarhan, Ö. Safety and regulatory issues of nanomaterials in foods. In Handbook of Food Nanotechnology; Jafari, S.M., Ed.; Academic Press: Cambrige, MA, USA, 2020; pp. 655–703. [Google Scholar] [CrossRef]
- EFSA Scientific Committee; Hardy, A.; Benford, D.; Halldorsson, T.; Jeger, M.J.; Knutsen, H.K.; More, S.; Naegeli, H.; Noteborn, H.; Ockleford, C.; et al. Guidance on risk assessment of the application of nanoscience and nanotechnologies in the food and feed chain: Part 1, human and animal health. EFSA J. 2018, 16, e05327. [Google Scholar] [CrossRef] [Green Version]
- Stoudmann, N.; Schmutz, M.; Hirsch, C.; Nowack, B.; Som, C. Human hazard potential of nanocellulose: Quantitative insights from the literature. Nanotoxicology 2020, 14, 1241–1257. [Google Scholar] [CrossRef]
- Lu, Q.; Yu, X.; Yagoub, A.E.A.; Wahia, H.; Zhou, C. Application and challenge of nanocellulose in the food industry. Food Biosci. 2021, 43, 101285. [Google Scholar] [CrossRef]
- Singh, G.; Saquib, S.; Gupta, A. Environmental, legal, health, and safety issue of nanocellulose. In Nanocellulose Materials; Oraon, R., Raetani, D., Singh, P., Hussain, C.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 265–288. [Google Scholar]
- Hillyer, J.F.; Albrecht, R.M. Gastrointestinal persorption and tissue distribution of differently sized colloidal gold nanoparticles. J. Pharm. Sci. 2021, 90, 1927–1936. [Google Scholar] [CrossRef] [PubMed]
- Schleh, C.; Semmler-Behnke, M.; Lipka, J.; Wenk, A.; Hirn, S.; Schäffler, M.; Schmid, G.; Simon, U.; Kreyling, W.G. Size and surface charge of gold nanoparticles determine absorption across intestinal barriers and accumulation in secondary target organs after oral administration. Nanotoxicology 2012, 6, 36–46. [Google Scholar] [CrossRef] [Green Version]
- Kim, K.S.; Suzuki, K.; Cho, H.; Youn, Y.S.; Bae, Y.H. Oral nanoparticles exhibit specific high-efficiency intestinal uptake and lymphatic transport. ACS Nano 2018, 12, 8893–8900. [Google Scholar] [CrossRef] [PubMed]
- Tortella, G.R.; Rubilar, O.; Durán, N.; Diez, M.C.; Martínez, M.; Parada, J.; Seabra, A.B. Silver nanoparticles: Toxicity in model organisms as an overview of its hazard for human health and the environment. J. Hazard. Mater. 2020, 390, 121974. [Google Scholar] [CrossRef]
- Chong, C.L.; Fang, C.M.; Pung, S.Y.; Ong, C.E.; Pung, Y.F.; Kong, C.; Pan, Y. Current updates on the in vivo assessment of zinc oxide nanoparticles toxicity using animal models. Bionanoscience 2021, 11, 590–620. [Google Scholar] [CrossRef]
- Brohi, R.D.; Wang, L.; Talpur, H.S.; Wu, D.; Khan, F.A.; Bhattarai, D.; Rehman, Z.-U.; FarmanUllah, F.; Huo, L.-J. Toxicity of nanoparticles on the reproductive system in animal models: A review. Front. Pharmacol. 2017, 8, 606. [Google Scholar] [CrossRef]
- Juhr, N.C.; Franke, J. A method for estimating the available energy of incompletely digested carbohydrates in rats. J. Nutr. 1992, 122, 1425–1433. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Zhu, C.; Yang, J.; Nie, Y.; Chen, C.; Sun, D. Recent advances in bacterial cellulose. Cellulose 2014, 21, 1–30. [Google Scholar] [CrossRef]
- Joint FAO/WHO Expert Committee on Food Additives; WHO Expert Committee on Food Additives. Safety Evaluation of Certain Food Additives and Contaminants; WHO Press: Geneva, Switzerland, 2002. [Google Scholar]
- Kotkoskie, L.A.; Butt, M.T.; Selinger, E.; Freeman, C.; Weiner, M.L. Qualitative investigation of uptake of fine particle size microcrystalline cellulose following oral administration in rats. J. Anat. 1996, 189, 531. [Google Scholar] [PubMed]
- Nagele, W.; Müller, N.; Brugger-Pichler, E.; Nagele, J. Persorption Of Plant Microparticles After Oral Plant Food Intake. Int. J. Herb. Med. 2013, 2, 1–8. [Google Scholar]
- Kelleher, J.; Walters, M.P.; Srinivasan, T.R.; Hart, G.; Findlay, J.M.; Losowsky, M.S. Degradation of cellulose within the gastrointestinal tract in man. Gut 1984, 25, 811–815. [Google Scholar] [CrossRef] [Green Version]
- Winkler, H.C.; Suter, M.; Naegeli, H. Critical review of the safety assessment of nano-structured silica additives in food. J. Nanobiotechnol. 2016, 14, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jafarizadeh-Malmiri, H.; Sayyar, Z.; Anarjan, N.; Berenjian, A. Nano-additives for food industries. In Nanobiotechnology in Food: Concepts, Applications and Perspectives; Springer: Cham, Switzerland, 2019; pp. 41–68. [Google Scholar] [CrossRef]
- Blasco, C.; Pico, Y. Determining nanomaterials in food. TrAC Trends Anal. Chem. 2011, 30, 84–99. [Google Scholar] [CrossRef]
- Dasgupta, N.; Ranjan, S. Nano-Food Toxicity and Regulations. In An Introduction to Food Grade Nanoemulsions; Environmental Chemistry for a Sustainable World; Springer: Singapore, 2018; pp. 151–179. [Google Scholar] [CrossRef]
- Dourado, F.; Gama, M.; Rodrigues, A.C. A review on the toxicology and dietetic role of bacterial cellulose. Toxicol. Rep. 2017, 4, 543–553. [Google Scholar] [CrossRef] [Green Version]
- Cortese, K.; Diaspro, A.; Tacchetti, C. Advanced correlative light/electron microscopy: Current methods and new developments using Tokuyasu cryosections. J. Histochem. Cytochem. 2009, 57, 1103–1112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Timmermans, F.J.; Otto, C. Contributed review: Review of integrated correlative light and electron microscopy. Rev. Sci. Instrum. 2015, 86, 011501. [Google Scholar] [CrossRef] [PubMed]
- Sjollema, K.A.; Schnell, U.; Kuipers, J.; Kalicharan, R.; Giepmans, B.N. Correlated light microscopy and electron microscopy. Methods Cell Biol. 2012, 111, 157–173. [Google Scholar] [CrossRef] [PubMed]
- De Boer, P.; Hoogenboom, J.P.; Giepmans, B.N. Correlated light and electron microscopy: Ultrastructure lights up! Nat. Methods 2015, 12, 503–513. [Google Scholar] [CrossRef] [PubMed]
- Ventura, C.; Pinto, F.; Lourenço, A.F.; Ferreira, P.J.; Louro, H.; Silva, M.J. On the toxicity of cellulose nanocrystals and nanofibrils in animal and cellular models. Cellulose 2020, 27, 5509–5544. [Google Scholar] [CrossRef]
- Vasconcelos, N.F.; Feitosa, J.P.A.; da Gama, F.M.P.; Morais, J.P.S.; Andrade, F.K.; de Souza, M.D.S.M.; de Freitas Rosa, M. Bacterial cellulose nanocrystals produced under different hydrolysis conditions: Properties and morphological features. Carbohydr. Polym. 2017, 155, 425–431. [Google Scholar] [CrossRef] [PubMed]
- Blanco, A.; Monte, M.C.; Campano, C.; Balea, A.; Merayo, N.; Negro, C. Nanocellulose for industrial use: Cellulose nanofibers (CNF), cellulose nanocrystals (CNC), and bacterial cellulose (BC). In Handbook of Nanomaterials for Industrial Applications; Hussain, C.M., Ed.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 74–126. [Google Scholar] [CrossRef]
- Knudsen, K.B.; Kofoed, C.; Espersen, R.; Højgaard, C.; Winther, J.R.; Willemoës, M.; Wedin, I.; Nuopponen, M.; Vilske, S.; Aimonen, K.; et al. Visualization of nanofibrillar cellulose in biological tissues using a biotinylated carbohydrate binding module of β-1, 4-Glycanase. Chem. Res. Toxicol. 2015, 28, 1627–1635. [Google Scholar] [CrossRef] [Green Version]
- Rasband, W.S.; ImageJ, U.S. National Institutes of Health, Bethesda, MD, USA. 1997–2022. Available online: https://imagej.nih.gov/ij/ (accessed on 25 July 2022).
- Kim, D.W.; Jang, Y.H.; Kim, C.S.; Lee, N.S. Effect of metal ions on the degradation and adsorption of two cellobiohydrolases on microcrystalline cellulose. Bull. Korean Chem. Soc. 2001, 22, 716–720. [Google Scholar] [CrossRef]
- Liu, C.H.; Tsao, M.H.; Sahoo, S.L.; Wu, W.C. Magnetic nanoparticles with fluorescence and affinity for DNA sensing and nucleus staining. RSC Adv. 2017, 7, 5937–5947. [Google Scholar] [CrossRef] [Green Version]
- Origin(Pro), Version 9.5.1.195. OriginLab Corporation: Northampton, MA, USA. Available online: https://www.originlab.com/ (accessed on 25 July 2022).
- Silva-Carvalho, R.; Fidalgo, J.; Melo, K.R.; Queiroz, M.F.; Leal, S.; Rocha, H.A.; Cruz, T.; Parpot, P.; Tomás, A.; Gama, M. Development of dextrin-amphotericin B formulations for the treatment of Leishmaniasis. Int. J. Biol. Macromol. 2020, 153, 276–288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’brien, J.; Wilson, I.; Orton, T.; Pognan, F. Investigation of the Alamar Blue (resazurin) fluorescent dye for the assessment of mammalian cell cytotoxicity. Eur. J. Biochem. 2000, 267, 5421–5426. [Google Scholar] [CrossRef]
- Viegas, M.S.; Martins, T.C.; Seco, F.; Do Carmo, A. An improved and cost-effective methodology for the reduction of autofluorescence in direct immunofluorescence studies on formalin-fixed paraffin-embedded tissues. Eur. J. Histochem. 2007, 51, 59–66. [Google Scholar]
- Mirhosseini, H.; Tan, C.P.; Hamid, N.S.; Yusof, S. Effect of Arabic gum, xanthan gum and orange oil contents on ζ-potential, conductivity, stability, size index and pH of orange beverage emulsion. Colloids Surf. A Physicochem. Eng. Asp. 2008, 315, 47–56. [Google Scholar] [CrossRef]
- Campano, C.; Balea, A.; Blanco, Á.; Negro, C. A reproducible method to characterize the bulk morphology of cellulose nanocrystals and nanofibers by transmission electron microscopy. Cellulose 2020, 27, 4871–4887. [Google Scholar] [CrossRef]
- Roman, M.; Winter, W.T. Effect of sulfate groups from sulfuric acid hydrolysis on the thermal degradation behavior of bacterial cellulose. Biomacromolecules 2004, 5, 1671–1677. [Google Scholar] [CrossRef]
- Listyanda, R.F.; Wildan, M.W.; Ilman, M.N. Preparation and characterization of cellulose nanocrystal extracted from ramie fibers by sulfuric acid hydrolysis. Heliyon 2020, 6, e05486. [Google Scholar] [CrossRef]
- Shang, Z.; An, X.; Seta, F.T.; Ma, M.; Shen, M.; Dai, L.; Liu, H.; Ni, Y. Improving dispersion stability of hydrochloric acid hydrolyzed cellulose nano-crystals. Carbohydr. Polym. 2019, 222, 115037. [Google Scholar] [CrossRef] [PubMed]
- Vanderfleet, O.M.; Cranston, E.D. Production routes to tailor the performance of cellulose nanocrystals. Nat. Rev. Mater. 2021, 6, 124–144. [Google Scholar] [CrossRef]
- Liu, L.; Kong, F. The behavior of nanocellulose in gastrointestinal tract and its influence on food digestion. J. Food Eng. 2021, 292, 110346. [Google Scholar] [CrossRef]
- Ong, E.; Gilkes, N.R.; Miller, R.C., Jr.; Warren, R.A.J.; Kilburn, D.G. The cellulose-binding domain (CBDCex) of an exoglucanase from Cellulomonas fimi: Production in Escherichia coli and characterization of the polypeptide. Biotechnol. Bioeng. 1993, 42, 401–409. [Google Scholar] [CrossRef]
- Boraston, A.B.; Bolam, D.N.; Gilbert, H.J.; Davies, G.J. Carbohydrate-binding modules: Fine-tuning polysaccharide recognition. Biochem. J. 2004, 382, 769–781. [Google Scholar] [CrossRef] [PubMed]
- Lehtiö, J.; Sugiyama, J.; Gustavsson, M.; Fransson, L.; Linder, M.; Teeri, T.T. The binding specificity and affinity determinants of family 1 and family 3 cellulose binding modules. Proc. Natl. Acad. Sci. USA 2003, 100, 484–489. [Google Scholar] [CrossRef] [Green Version]
- Creagh, A.L.; Ong, E.; Jervis, E.; Kilburn, D.G.; Haynes, C.A. Binding of the cellulose-binding domain of exoglucanase Cex from Cellulomonas fimi to insoluble microcrystalline cellulose is entropically driven. Proc. Natl. Acad. Sci. USA 1996, 93, 12229–12234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakagawa, K.; Surassmo, S.; Min, S.G.; Choi, M.J. Dispersibility of freeze-dried poly (epsilon-caprolactone) nanocapsules stabilized by gelatin and the effect of freezing. J. Food Eng. 2011, 102, 177–188. [Google Scholar] [CrossRef]
- Anarjan, N.; Nehdi, I.A.; Sbihi, H.M.; Al-Resayes, S.I.; Malmiri, H.J.; Tan, C.P. Preparation of astaxanthin nanodispersions using gelatin-based stabilizer systems. Molecules 2014, 19, 14257–14265. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Likos, C.N.; Vaynberg, K.A.; Löwen, H.; Wagner, N.J. Colloidal stabilization by adsorbed gelatin. Langmuir 2000, 16, 4100–4108. [Google Scholar] [CrossRef]
- Bin Ahmad, M.; Lim, J.J.; Shameli, K.; Ibrahim, N.A.; Tay, M.Y. Synthesis of silver nanoparticles in chitosan, gelatin and chitosan/gelatin bionanocomposites by a chemical reducing agent and their characterization. Molecules 2011, 16, 7237–7248. [Google Scholar] [CrossRef] [Green Version]
- Cox, G.; Sheppard, C.J. Practical limits of resolution in confocal and non-linear microscopy. Microsc. Res. Tech. 2004, 63, 18–22. [Google Scholar] [CrossRef]
- Kim, G.D.; Yang, H.; Park, H.R.; Park, C.S.; Park, Y.S.; Lee, S.E. Evaluation of immunoreactivity of in vitro and in vivo models against bacterial synthesized cellulose to be used as a prosthetic biomaterial. Biochip J. 2013, 7, 201–209. [Google Scholar] [CrossRef]
- Lopes, V.R.; Sanchez-Martinez, C.; Strømme, M.; Ferraz, N. In vitro biological responses to nanofibrillated cellulose by human dermal, lung and immune cells: Surface chemistry aspect. Part. Fibre Toxicol. 2017, 14, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Vartiainen, J.; Pöhler, T.; Sirola, K.; Pylkkänen, L.; Alenius, H.; Hokkinen, J.; Tapper, U.; Lahtinen, P.; Kapanen, A.; Putkisto, K.; et al. Health and environmental safety aspects of friction grinding and spray drying of microfibrillated cellulose. Cellulose 2011, 18, 775–786. [Google Scholar] [CrossRef]
- Grainger, J.R.; Konkel, J.E.; Zangerle-Murray, T.; Shaw, T.N. Macrophages in gastrointestinal homeostasis and inflammation. Pflug. Arch. Eur. J. Physiol. 2017, 469, 527–539. [Google Scholar] [CrossRef] [PubMed]
- Mendes, R.G.; Mandarino, A.; Koch, B.; Meyer, A.K.; Bachmatiuk, A.; Hirsch, C.; Gemming, T.; Schmidt, O.G.; Liu, Z.; Rümmeli, M.H. Size and time dependent internalization of label-free nano-graphene oxide in human macrophages. Nano Res. 2017, 10, 1980–1995. [Google Scholar] [CrossRef]
- Erdem, J.S.; Alswady-Hoff, M.; Ervik, T.K.; Skare, Ø.; Ellingsen, D.G.; Zienolddiny, S. Cellulose nanocrystals modulate alveolar macrophage phenotype and phagocytic function. Biomaterials 2019, 203, 31–42. [Google Scholar] [CrossRef]
- Petithory, T.; Pieuchot, L.; Josien, L.; Ponche, A.; Anselme, K.; Vonna, L. Size-Dependent Internalization Efficiency of Macrophages from Adsorbed Nanoparticle-Based Monolayers. Nanomaterials 2021, 11, 1963. [Google Scholar] [CrossRef] [PubMed]
- Gustafson, H.H.; Holt-Casper, D.; Grainger, D.W.; Ghandehari, H. Nanoparticle uptake: The phagocyte problem. Nano Today 2015, 10, 487–510. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wizenty, J.; Ashraf, M.I.; Rohwer, N.; Stockmann, M.; Weiss, S.; Biebl, M.; Pratschke, J.; Aigner, F.; Wuensch, T. Autofluorescence: A potential pitfall in immunofluorescence-based inflammation grading. J. Immunol. Methods 2018, 456, 28–37. [Google Scholar] [CrossRef] [PubMed]
- Mackie, A.; Gourcy, S.; Rigby, N.; Moffat, J.; Capron, I.; Bajka, B. The fate of cellulose nanocrystal stabilised emulsions after simulated gastrointestinal digestion and exposure to intestinal mucosa. Nanoscale 2019, 11, 2991–2998. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Torcello-Gómez, A.; Foster, T.J. Interactions between cellulose ethers and a bile salt in the control of lipid digestion of lipid-based systems. Carbohydr. Polym. 2014, 113, 53–61. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mota, R.; Rodrigues, A.C.; Silva-Carvalho, R.; Costa, L.; Martins, D.; Sampaio, P.; Dourado, F.; Gama, M. Tracking Bacterial Nanocellulose in Animal Tissues by Fluorescence Microscopy. Nanomaterials 2022, 12, 2605. https://doi.org/10.3390/nano12152605
Mota R, Rodrigues AC, Silva-Carvalho R, Costa L, Martins D, Sampaio P, Dourado F, Gama M. Tracking Bacterial Nanocellulose in Animal Tissues by Fluorescence Microscopy. Nanomaterials. 2022; 12(15):2605. https://doi.org/10.3390/nano12152605
Chicago/Turabian StyleMota, Renato, Ana Cristina Rodrigues, Ricardo Silva-Carvalho, Lígia Costa, Daniela Martins, Paula Sampaio, Fernando Dourado, and Miguel Gama. 2022. "Tracking Bacterial Nanocellulose in Animal Tissues by Fluorescence Microscopy" Nanomaterials 12, no. 15: 2605. https://doi.org/10.3390/nano12152605
APA StyleMota, R., Rodrigues, A. C., Silva-Carvalho, R., Costa, L., Martins, D., Sampaio, P., Dourado, F., & Gama, M. (2022). Tracking Bacterial Nanocellulose in Animal Tissues by Fluorescence Microscopy. Nanomaterials, 12(15), 2605. https://doi.org/10.3390/nano12152605