Control of the Drying Patterns for Complex Colloidal Solutions and Their Applications
Abstract
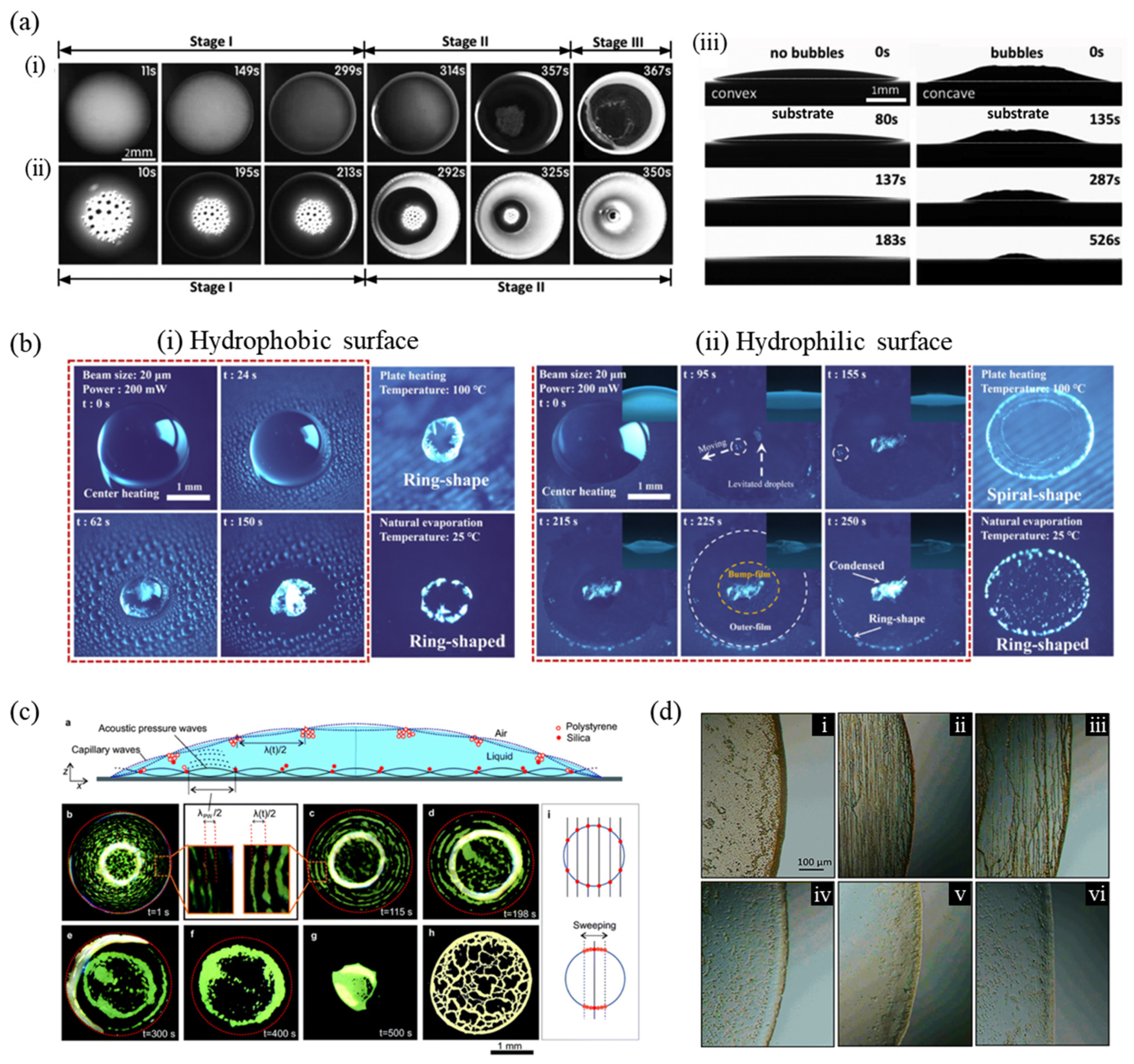
1. Introduction
2. Evaporation of a Sessile Droplet
2.1. Evaporation Mechanism of a Sessile Droplet Pinned on a Substrate
2.2. Marangoni Flow
2.3. Effect of Surface Wetting Characteristics on the Evaporation Process
3. Chemical Additives
3.1. Effect of Polymer Additives
3.2. Effect of Surfactants
3.3. Effect of Salt Addition
3.4. Effect of Variation in pH
4. External Factors without Chemical Additives
4.1. External Vapor Source
4.2. Relative Humidity
4.3. Temperature
4.4. Other Factors
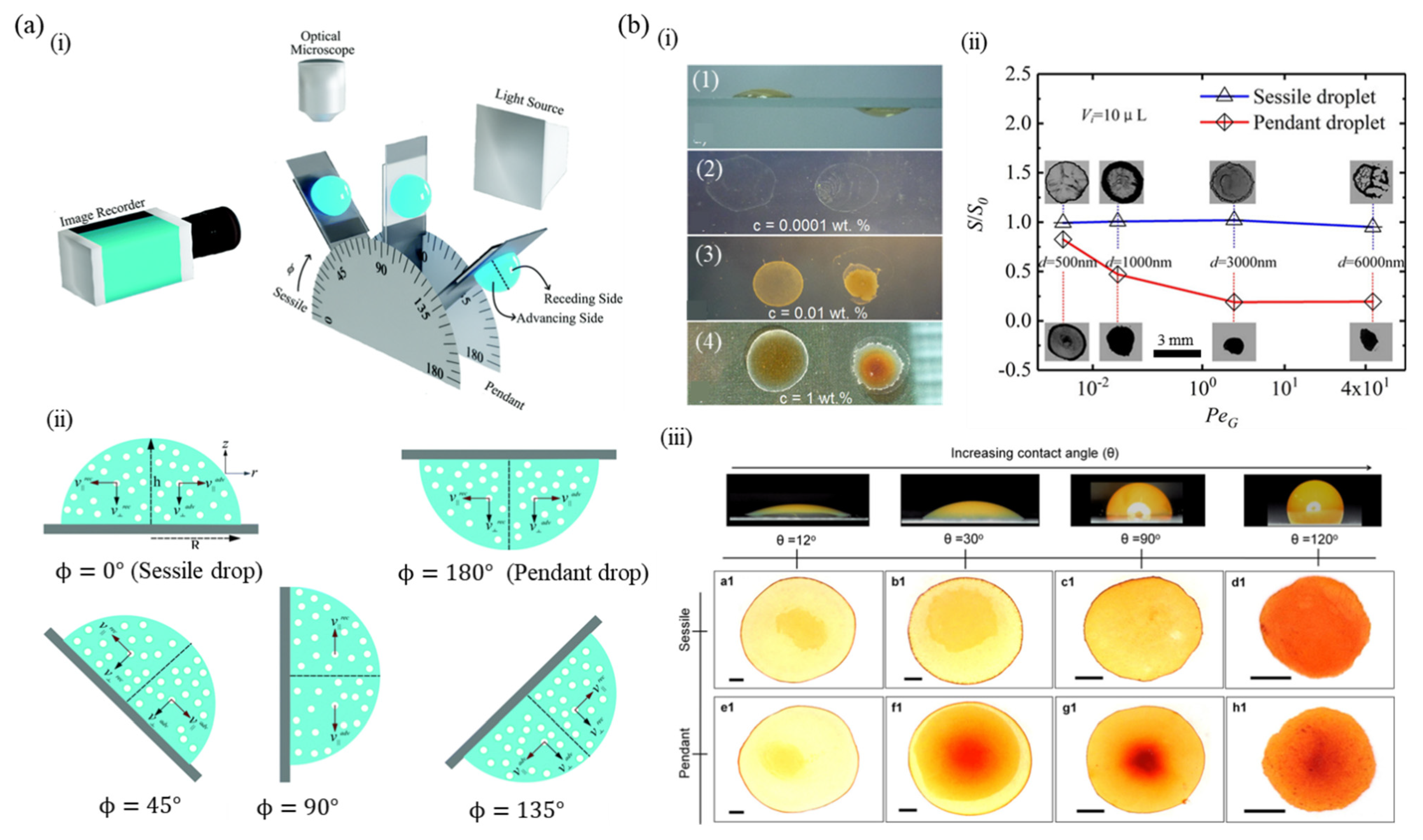
5. Effect of Geometry
5.1. Inclined Substrate
5.2. Pendant Drop
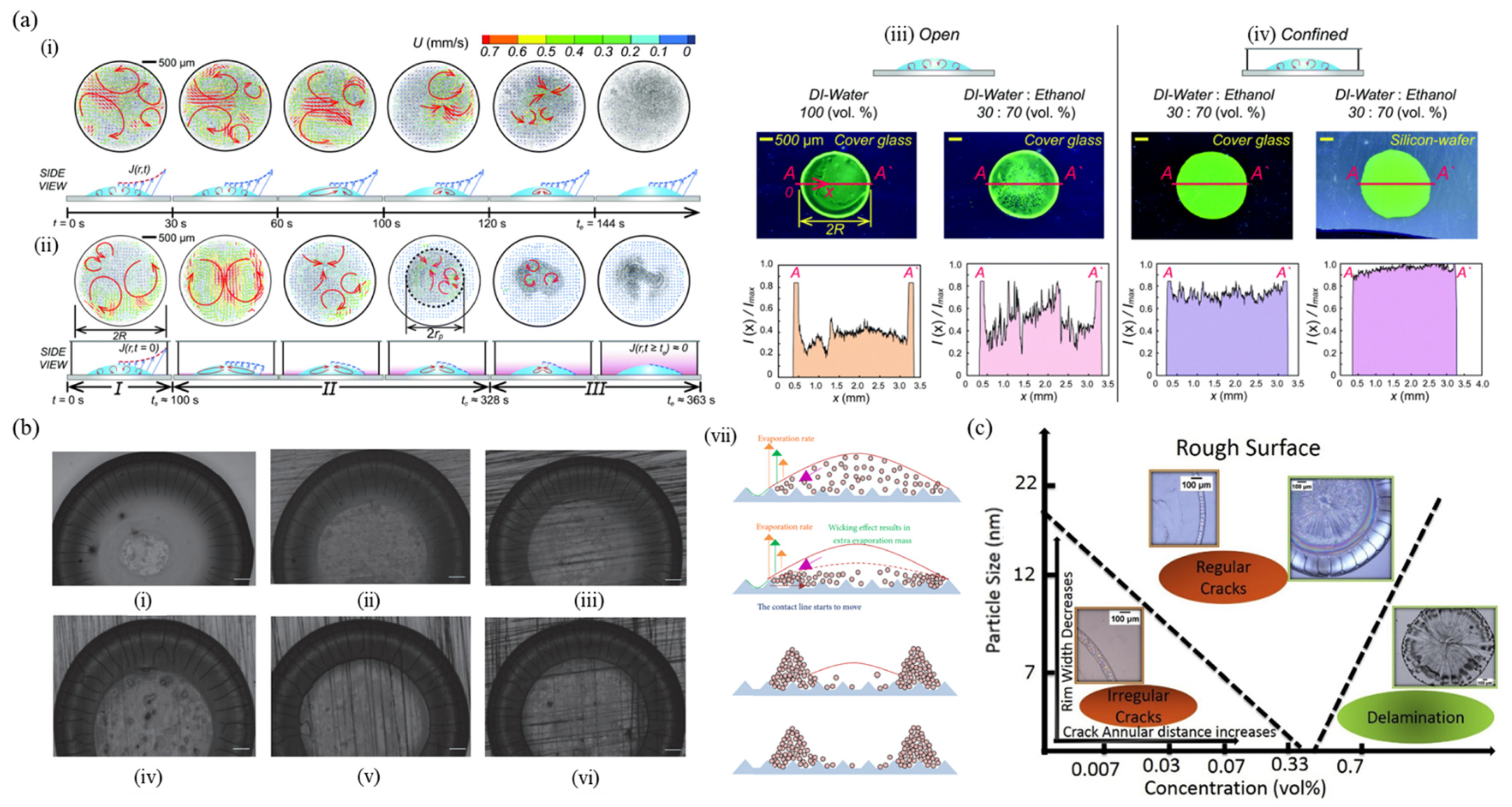
5.3. Confined Geometry
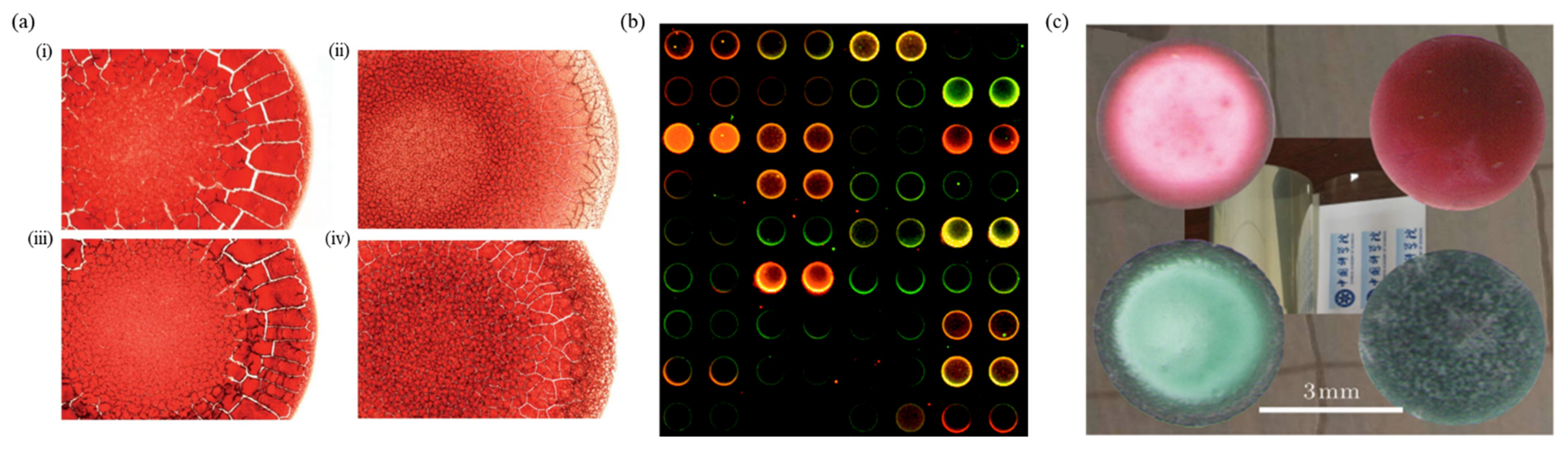
5.4. Substrate Roughness
6. Summary
7. Applications
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Liu, S.; Zhang, X.; Yin, M.; Feng, H.; Zhang, J.; Zhang, L.; Xie, W. Coffee-ring-free ultrasonic spray coating single-emission layers for white organic light-emitting devices and their energy-transfer mechanism. ACS Appl. Energy Mater. 2017, 1, 103–112. [Google Scholar] [CrossRef]
- Deng, Y.; Wang, Q.; Yuan, Y.; Huang, J. Vividly colorful hybrid perovskite solar cells by doctor-blade coating with perovskite photonic nanostructures. Mater. Horiz. 2015, 2, 578–583. [Google Scholar] [CrossRef]
- Jiang, C.; Ng, S.M.; Leung, C.W.; Pong, P.W. Magnetically assembled iron oxide nanoparticle coatings and their integration with pseudo-spin-valve thin films. J. Mater. Chem. C 2017, 5, 252–263. [Google Scholar] [CrossRef]
- Tiara, A.; Moon, H.; Cho, G.; Lee, J. Fully roll-to-roll gravure printed electronics: Challenges and the way to integrating logic gates. Jpn. J. Appl. Phys. 2022, 61, SE0802. [Google Scholar] [CrossRef]
- He, P.; Derby, B. Controlling coffee ring formation during drying of inkjet printed 2D inks. Adv. Mater. Interfaces 2017, 4, 1700944. [Google Scholar] [CrossRef]
- Jiang, C.; Zhong, Z.; Liu, B.; He, Z.; Zou, J.; Wang, L.; Wang, J.; Peng, J.; Cao, Y. Coffee-ring-free quantum dot thin film using inkjet printing from a mixed-solvent system on modified ZnO transport layer for light-emitting devices. ACS Appl. Mater. Interfaces 2016, 8, 26162–26168. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Yu, H.; Zou, W.; Wang, Z.; Liu, L. High-resolution and controllable nanodeposition pattern of Ag nanoparticles by electrohydrodynamic jet printing combined with coffee ring effect. Adv. Mater. Interfaces 2019, 6, 1900912. [Google Scholar] [CrossRef]
- Kuang, M.; Wang, L.; Song, Y. Controllable printing droplets for high-resolution patterns. Adv. Mater. 2014, 26, 6950–6958. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Duan, Y.; Shao, Z.; Zhang, G.; Li, H.; Huang, Y.; Yin, Z. High-Resolution Pixelated Light Emitting Diodes Based on Electrohydrodynamic Printing and Coffee-Ring-Free Quantum Dot Film. Adv. Mater. Technol. 2020, 5, 2000401. [Google Scholar] [CrossRef]
- Zhang, G.; Zhang, H.; Yu, R.; Duan, Y.; Huang, Y.; Yin, Z. Critical Size/Viscosity for Coffee-Ring-Free Printing of Perovskite Micro/Nanopatterns. ACS Appl. Mater. Interfaces 2022, 14, 14712–14720. [Google Scholar] [CrossRef] [PubMed]
- Gao, A.; Yan, J.; Wang, Z.; Liu, P.; Wu, D.; Tang, X.; Fang, F.; Ding, S.; Li, X.; Sun, J. Printable CsPbBr3 perovskite quantum dot ink for coffee ring-free fluorescent microarrays using inkjet printing. Nanoscale 2020, 12, 2569–2577. [Google Scholar] [CrossRef]
- Friederich, A.; Binder, J.R.; Bauer, W. Rheological control of the coffee stain effect for inkjet printing of ceramics. J. Am. Ceram. Soc. 2013, 96, 2093–2099. [Google Scholar] [CrossRef]
- Park, J.; Moon, J. Control of colloidal particle deposit patterns within picoliter droplets ejected by ink-jet printing. Langmuir 2006, 22, 3506–3513. [Google Scholar] [CrossRef]
- Sun, J.; Bao, B.; He, M.; Zhou, H.; Song, Y. Recent advances in controlling the depositing morphologies of inkjet droplets. ACS Appl. Mater. Interfaces 2015, 7, 28086–28099. [Google Scholar] [CrossRef]
- Al-Milaji, K.N.; Secondo, R.R.; Ng, T.N.; Kinsey, N.; Zhao, H. Interfacial Self-Assembly of Colloidal Nanoparticles in Dual-Droplet Inkjet Printing. Adv. Mater. Interfaces 2018, 5, 1701561. [Google Scholar] [CrossRef]
- Wang, S.-W.; Lin, H.-Y.; Lin, C.-C.; Kao, T.S.; Chen, K.-J.; Han, H.-V.; Li, J.-R.; Lee, P.-T.; Chen, H.-M.; Hong, M.-H. Pulsed-laser micropatterned quantum-dot array for white light source. Sci. Rep. 2016, 6, 23563. [Google Scholar] [CrossRef] [PubMed]
- Ammosova, L.; Jiang, Y.; Suvanto, M.; Pakkanen, T.A. Precise micropatterning of silver nanoparticles on plastic substrates. Appl. Surf. Sci. 2017, 401, 353–361. [Google Scholar] [CrossRef]
- Xu, T.; Xu, L.-P.; Zhang, X.; Wang, S. Bioinspired superwettable micropatterns for biosensing. Chem. Soc. Rev. 2019, 48, 3153–3165. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.; Chen, Z.; Dong, Z.; Fan, Y.; Chen, Y.; Liu, B.; Yu, C.; Li, C.; Dai, H.; Li, H. Uniform Spread of High-Speed Drops on Superhydrophobic Surface by Live-Oligomeric Surfactant Jamming. Adv. Mater. 2019, 31, 1904475. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Gong, C.; Akakuru, O.U.; Su, Z.; Wu, A.; Wei, G. The design and biomedical applications of self-assembled two-dimensional organic biomaterials. Chem. Soc. Rev. 2019, 48, 5564–5595. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, V.; Zito, G.; Arrabito, G.; Cataldo, S.; Scopelliti, M.; Giordano, C.; Vetri, V.; Pignataro, B. Aqueous processed biopolymer interfaces for single-cell microarrays. ACS Biomater. Sci. Eng. 2020, 6, 3174–3186. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Yin, Y.; Ali, M.U.; Peng, W.; Zhang, S.; Li, D.; Zou, T.; Li, Y.; Jiao, S.; Chen, S.-j. Inkjet printed uniform quantum dots as color conversion layers for full-color OLED displays. Nanoscale 2020, 12, 2103–2110. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.; Woo, J.Y.; Lee, D.C.; Lee, J.; Jeong, S.; Kim, D. Continuous purification of colloidal quantum dots in large-scale using porous electrodes in flow channel. Sci. Rep. 2017, 7, 43581. [Google Scholar] [CrossRef] [PubMed]
- Shimoni, A.; Azoubel, S.; Magdassi, S. Inkjet printing of flexible high-performance carbon nanotube transparent conductive films by “coffee ring effect”. Nanoscale 2014, 6, 11084–11089. [Google Scholar] [CrossRef]
- He, P.; Cao, J.; Ding, H.; Liu, C.; Neilson, J.; Li, Z.; Kinloch, I.A.; Derby, B. Screen-printing of a highly conductive graphene ink for flexible printed electronics. ACS Appl. Mater. Interfaces 2019, 11, 32225–32234. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, X.; Xin, Z.; Deng, M.; Wen, Y.; Song, Y. Controlled inkjetting of a conductive pattern of silver nanoparticles based on the coffee-ring effect. Adv. Mat. 2013, 25, 6714–6718. [Google Scholar] [CrossRef]
- Lee, M.; Parajuli, S.; Moon, H.; Song, R.; Lee, S.; Shrestha, S.; Park, J.; Yang, H.; Jung, Y.; Cho, G.; et al. Characterization of silver nanoparticle inks toward stable roll-to-roll gravure printing. Flex. Print. Electron. 2022, 7, 014003. [Google Scholar] [CrossRef]
- Jafry, A.T.; Lee, H.; Tenggara, A.P.; Lim, H.; Moon, Y.; Kim, S.-H.; Lee, Y.; Kim, S.-M.; Park, S.; Byun, D.; et al. Double-sided electrohydrodynamic jet printing of two-dimensional electrode array in paper-based digital microfluidics. Sens. Actuators B Chem. 2019, 282, 831–837. [Google Scholar] [CrossRef]
- Deegan, R.D. Pattern formation in drying drops. Phys. Rev. E 2000, 61, 475. [Google Scholar] [CrossRef]
- Deegan, R.D.; Bakajin, O.; Dupont, T.F.; Huber, G.; Nagel, S.R.; Witten, T.A. Contact line deposits in an evaporating drop. Phys. Rev. E 2000, 62, 756. [Google Scholar] [CrossRef]
- Hu, H.; Larson, R.G. Evaporation of a sessile droplet on a substrate. J. Phys. Chem. B 2002, 106, 1334–1344. [Google Scholar] [CrossRef]
- Nguyen, T.A.; Hampton, M.A.; Nguyen, A.V. Evaporation of nanoparticle droplets on smooth hydrophobic surfaces: The inner coffee ring deposits. J. Phys. Chem. C 2013, 117, 4707–4716. [Google Scholar] [CrossRef]
- Li, Y.-F.; Sheng, Y.-J.; Tsao, H.-K. Evaporation stains: Suppressing the coffee-ring effect by contact angle hysteresis. Langmuir 2013, 29, 7802–7811. [Google Scholar] [CrossRef]
- Patil, N.D.; Bange, P.G.; Bhardwaj, R.; Sharma, A. Effects of substrate heating and wettability on evaporation dynamics and deposition patterns for a sessile water droplet containing colloidal particles. Langmuir 2016, 32, 11958–11972. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y.; Liu, Y. On-demand evaporation mode of sessile droplet by designing substrate surface wettability patterns. Phys. Fluids 2021, 33, 122015. [Google Scholar] [CrossRef]
- Song, R.; Lee, M.; Moon, H.; Lee, S.; Shin, S.; Kim, D.; Kim, Y.; Oh, B.; Lee, J. Particle dynamics in drying colloidal solution using discrete particle method. Flex. Print. Electron. 2021, 6, 044007. [Google Scholar] [CrossRef]
- Deegan, R.D.; Bakajin, O.; Dupont, T.F.; Huber, G.; Nagel, S.R.; Witten, T.A. Capillary flow as the cause of ring stains from dried liquid drops. Nature 1997, 389, 827–829. [Google Scholar] [CrossRef]
- Hu, H.; Larson, R.G. Analysis of the microfluid flow in an evaporating sessile droplet. Langmuir 2005, 21, 3963–3971. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Larson, R.G. Marangoni effect reverses coffee-ring depositions. J. Phys. Chem. B 2006, 110, 7090–7094. [Google Scholar] [CrossRef] [PubMed]
- Larson, R.G. Transport and deposition patterns in drying sessile droplets. AIChE J. 2014, 60, 1538–1571. [Google Scholar] [CrossRef]
- Al-Milaji, K.N.; Zhao, H. New Perspective of mitigating the coffee-ring effect: Interfacial assembly. J. Phys. Chem. C 2019, 123, 12029–12041. [Google Scholar] [CrossRef]
- Anyfantakis, M.; Baigl, D. Manipulating the Coffee-Ring Effect: Interactions at Work. Chemphyschem 2015, 16, 2726–2734. [Google Scholar] [CrossRef] [PubMed]
- Anyfantakis, M.; Geng, Z.; Morel, M.; Rudiuk, S.; Baigl, D. Modulation of the coffee-ring effect in particle/surfactant mixtures: The importance of particle–interface interactions. Langmuir 2015, 31, 4113–4120. [Google Scholar] [CrossRef] [PubMed]
- Yunker, P.J.; Still, T.; Lohr, M.A.; Yodh, A. Suppression of the coffee-ring effect by shape-dependent capillary interactions. Nature 2011, 476, 308–311. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yang, Q.; Li, M.; Song, Y. Rate-dependent interface capture beyond the coffee-ring effect. Sci. Rep. 2016, 6, 24628. [Google Scholar] [CrossRef] [PubMed]
- Anyfantakis, M.; Baigl, D.; Binks, B.P. Evaporation of drops containing silica nanoparticles of varying hydrophobicities: Exploiting particle–particle interactions for additive-free tunable deposit morphology. Langmuir 2017, 33, 5025–5036. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Li, C.Y.; Sun, Y. From multi-ring to spider web and radial spoke: Competition between the receding contact line and particle deposition in a drying colloidal drop. Soft Matter 2014, 10, 4458–4463. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.-H.; Shen, T.-Z.; Song, J.-K. Water front recession and the formation of various types of wrinkles in dried graphene oxide droplets. Carbon 2016, 105, 297–304. [Google Scholar] [CrossRef]
- Shmuylovich, L.; Shen, A.Q.; Stone, H.A. Surface morphology of drying latex films: Multiple ring formation. Langmuir 2002, 18, 3441–3445. [Google Scholar] [CrossRef]
- Wu, M.; Man, X.; Doi, M. Multi-ring deposition pattern of drying droplets. Langmuir 2018, 34, 9572–9578. [Google Scholar] [CrossRef]
- Weon, B.M.; Je, J.H. Capillary force repels coffee-ring effect. Phys. Rev. E 2010, 82, 015305. [Google Scholar] [CrossRef] [PubMed]
- Mampallil, D.; Reboud, J.; Wilson, R.; Wylie, D.; Klug, D.R.; Cooper, J.M. Acoustic suppression of the coffee-ring effect. Soft Matter 2015, 11, 7207–7213. [Google Scholar] [CrossRef] [PubMed]
- Mampallil, D.; Eral, H.B. A review on suppression and utilization of the coffee-ring effect. Adv. Colloid. Interf. Sci. 2018, 252, 38–54. [Google Scholar] [CrossRef] [PubMed]
- Marin, A.G.; Gelderblom, H.; Lohse, D.; Snoeijer, J.H. Order-to-disorder transition in ring-shaped colloidal stains. Phys. Rev. Lett. 2011, 107, 085502. [Google Scholar] [CrossRef]
- Cui, L.; Zhang, J.; Zhang, X.; Huang, L.; Wang, Z.; Li, Y.; Gao, H.; Zhu, S.; Wang, T.; Yang, B. Suppression of the coffee ring effect by hydrosoluble polymer additives. ACS Appl. Mater. Interfaces 2012, 4, 2775–2780. [Google Scholar] [CrossRef] [PubMed]
- Eales, A.D.; Routh, A.F.; Dartnell, N.; Simon, G. Evaporation of pinned droplets containing polymer–an examination of the important groups controlling final shape. AIChE J. 2015, 61, 1759–1767. [Google Scholar] [CrossRef]
- De Gans, B.-J.; Schubert, U.S. Inkjet printing of well-defined polymer dots and arrays. Langmuir 2004, 20, 7789–7793. [Google Scholar] [CrossRef] [PubMed]
- Seo, C.; Jang, D.; Chae, J.; Shin, S. Altering the coffee-ring effect by adding a surfactant-like viscous polymer solution. Sci. Rep. 2017, 7, 500. [Google Scholar] [CrossRef] [PubMed]
- Poulard, C.; Damman, P. Control of spreading and drying of a polymer solution from Marangoni flows. EPL (Europhys. Lett.) 2007, 80, 64001. [Google Scholar] [CrossRef]
- Nguyen, V.X.; Stebe, K.J. Patterning of small particles by a surfactant-enhanced Marangoni-Bénard instability. Phys. Rev. Lett. 2002, 88, 164501. [Google Scholar] [CrossRef]
- Marin, A.; Liepelt, R.; Rossi, M.; Kähler, C.J. Surfactant-driven flow transitions in evaporating droplets. Soft Matter 2016, 12, 1593–1600. [Google Scholar] [CrossRef] [PubMed]
- Kajiya, T.; Kobayashi, W.; Okuzono, T.; Doi, M. Controlling the drying and film formation processes of polymer solution droplets with addition of small amount of surfactants. J. Phys. Chem. B 2009, 113, 15460–15466. [Google Scholar] [CrossRef] [PubMed]
- Still, T.; Yunker, P.J.; Yodh, A.G. Surfactant-induced Marangoni eddies alter the coffee-rings of evaporating colloidal drops. Langmuir 2012, 28, 4984–4988. [Google Scholar] [CrossRef] [PubMed]
- Jung, N.; Seo, H.W.; Leo, P.H.; Kim, J.; Kim, P.; Yoo, C.S. Surfactant effects on droplet dynamics and deposition patterns: A lattice gas model. Soft Matter 2017, 13, 6529–6541. [Google Scholar] [CrossRef] [PubMed]
- Kaya, D.; Belyi, V.; Muthukumar, M. Pattern formation in drying droplets of polyelectrolyte and salt. J. Chem. Phys. 2010, 133, 114905. [Google Scholar] [CrossRef]
- Pathak, B.; Christy, J.; Sefiane, K.; Gozuacik, D. Complex pattern formation in solutions of protein and mixed salts using dehydrating sessile droplets. Langmuir 2020, 36, 9728–9737. [Google Scholar] [CrossRef]
- Yang, H.; Yang, Y.; Sheng, S.; Wen, B.; Sheng, N.; Liu, X.; Wan, R.; Yan, L.; Hou, Z.; Lei, X. Controlling the coffee ring effect on graphene and polymer by cations. Chin. Phys. Lett. 2020, 37, 028103. [Google Scholar] [CrossRef]
- Ma, J.C.; Dougherty, D.A. The cation–π interaction. Chem. Rev. 1997, 97, 1303–1324. [Google Scholar] [CrossRef]
- Shi, G.; Liu, J.; Wang, C.; Song, B.; Tu, Y.; Hu, J.; Fang, H. Ion enrichment on the hydrophobic carbon-based surface in aqueous salt solutions due to cation-π interactions. Sci. Rep. 2013, 3, 3436. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Shi, G.; Shen, J.; Peng, B.; Zhang, B.; Wang, Y.; Bian, F.; Wang, J.; Li, D.; Qian, Z. Ion sieving in graphene oxide membranes via cationic control of interlayer spacing. Nature 2017, 550, 380–383. [Google Scholar] [CrossRef]
- Liu, X.; Sheng, S.; Yang, H.; He, Z.; Yang, Y.; Sheng, N.; Fang, H.; Shi, G. Uniform, Anticorrosive, and Antiabrasive Coatings on Metallic Surfaces for Cation–Metal and Cation–π Interactions. ACS Appl. Mater. Interfaces 2020, 12, 38638–38646. [Google Scholar] [CrossRef] [PubMed]
- Paria, S.; Chaudhuri, R.G.; Jason, N.N. Self-assembly of colloidal sulfur particles on a glass surface from evaporating sessile drops: Influence of different salts. New J. Chem. 2014, 38, 5943–5951. [Google Scholar] [CrossRef]
- Du, F.; Zhang, L.; Shen, W. Controllable dried patterns of colloidal drops. J. Colloid Interface Sci. 2022, 606, 758–767. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Wang, W.; Vikesland, P.J. Implications of the coffee-ring effect on virus infectivity. Langmuir 2021, 37, 11260–11268. [Google Scholar] [CrossRef] [PubMed]
- Zeid, W.B.; Brutin, D. Influence of relative humidity on spreading, pattern formation and adhesion of a drying drop of whole blood. Colloid Surf. A-Physicochem. Eng. Asp. 2013, 430, 1–7. [Google Scholar] [CrossRef]
- Girard, F.; Antoni, M.; Faure, S.; Steinchen, A. Influence of heating temperature and relative humidity in the evaporation of pinned droplets. Colloid Surf. A-Physicochem. Eng. Asp. 2008, 323, 36–49. [Google Scholar] [CrossRef]
- Ristenpart, W.; Kim, P.; Domingues, C.; Wan, J.; Stone, H.A. Influence of substrate conductivity on circulation reversal in evaporating drops. Phys. Rev. Lett. 2007, 99, 234502. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Lv, C.; Li, Z.; Quéré, D.; Zheng, Q. From coffee rings to coffee eyes. Soft Matter 2015, 11, 4669–4673. [Google Scholar] [CrossRef]
- Malla, L.K.; Bhardwaj, R.; Neild, A. Colloidal deposit of an evaporating sessile droplet on a non-uniformly heated substrate. Colloid Surf. A-Physicochem. Eng. Asp. 2020, 584, 124009. [Google Scholar] [CrossRef]
- Parsa, M.; Harmand, S.; Sefiane, K.; Bigerelle, M.; Deltombe, R. Effect of substrate temperature on pattern formation of nanoparticles from volatile drops. Langmuir 2015, 31, 3354–3367. [Google Scholar] [CrossRef]
- Lama, H.; Basavaraj, M.G.; Satapathy, D.K. Tailoring crack morphology in coffee-ring deposits via substrate heating. Soft Matter 2017, 13, 5445–5452. [Google Scholar] [CrossRef]
- Carreón, Y.J.; Ríos-Ramírez, M.; Vázquez-Vergara, P.; Salinas-Almaguer, S.; Cipriano-Urbano, I.; Briones-Aranda, A.; Díaz-Hernández, O.; Santos, G.J.E.; González-Gutiérrez, J. Effects of substrate temperature on patterns produced by dried droplets of proteins. Colloids Surf. B Biointerfaces 2021, 203, 111763. [Google Scholar] [CrossRef]
- Qi, W.; Li, J.; Weisensee, P.B. Evaporation of sessile water droplets on horizontal and vertical biphobic patterned surfaces. Langmuir 2019, 35, 17185–17192. [Google Scholar] [CrossRef] [PubMed]
- Devlin, N.R.; Loehr, K.; Harris, M.T. The importance of gravity in droplet evaporation: A comparison of pendant and sessile drop evaporation with particles. AIChE J. 2016, 62, 947–955. [Google Scholar] [CrossRef]
- Espín, L.; Kumar, S. Sagging of evaporating droplets of colloidal suspensions on inclined substrates. Langmuir 2014, 30, 11966–11974. [Google Scholar] [CrossRef] [PubMed]
- Xue, C.; Lott, J.T.; Kolachalama, V.B. Estimation of size and contact angle of evaporating sessile liquid drops using texture analysis. Langmuir 2019, 35, 3672–3679. [Google Scholar] [CrossRef]
- Thampi, S.P.; Basavaraj, M.G. Beyond coffee rings: Drying drops of colloidal dispersions on inclined substrates. ACS Omega 2020, 5, 11262–11270. [Google Scholar] [CrossRef] [PubMed]
- Bigioni, T.P.; Lin, X.-M.; Nguyen, T.T.; Corwin, E.I.; Witten, T.A.; Jaeger, H.M. Kinetically driven self assembly of highly ordered nanoparticle monolayers. Nat. Mater. 2006, 5, 265–270. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Ingrosso, C.; Fakhfouri, V.; Striccoli, M.; Agostiano, A.; Curri, M.L.; Brugger, J. Inkjet-printed multicolor arrays of highly luminescent nanocrystal-based nanocomposites. Small 2009, 5, 1051–1057. [Google Scholar] [CrossRef] [PubMed]
- Joshi, A.S.; Sun, Y. Wetting dynamics and particle deposition for an evaporating colloidal drop: A lattice Boltzmann study. Phys. Rev. E 2010, 82, 041401. [Google Scholar] [CrossRef] [PubMed]
- Bansal, L.; Hatte, S.; Basu, S.; Chakraborty, S. Universal evaporation dynamics of a confined sessile droplet. Appl. Phys. Lett. 2017, 111, 101601. [Google Scholar] [CrossRef]
- Bansal, L.; Chakraborty, S.; Basu, S. Confinement-induced alterations in the evaporation dynamics of sessile droplets. Soft Matter 2017, 13, 969–977. [Google Scholar] [CrossRef] [PubMed]
- Basu, S.; Rao, D.C.K.; Chattopadhyay, A.; Chakraborty, J. Dissolution dynamics of a vertically confined sessile droplet. Phys. Rev. E 2021, 103, 013101. [Google Scholar] [CrossRef]
- Pyeon, J.; Kim, H. Controlling uniform patterns by evaporation of multi-component liquid droplets in a confined geometry. Soft Matter 2021, 17, 3578–3585. [Google Scholar] [CrossRef]
- Li, J.; Shan, L.; Ma, B.; Jiang, X.; Solomon, A.; Iyengar, M.; Padilla, J.; Agonafer, D. Investigation of the confinement effect on the evaporation behavior of a droplet pinned on a micropillar structure. J. Colloid Interface Sci. 2019, 555, 583–594. [Google Scholar] [CrossRef] [PubMed]
- Mondal, R.; Basavaraj, M.G. Patterning of colloids into spirals via confined drying. Soft Matter 2020, 16, 3753–3761. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.; Zhang, J.; Zhang, X.; Li, Y.; Wang, Z.; Gao, H.; Wang, T.; Zhu, S.; Yu, H.; Yang, B. Avoiding coffee ring structure based on hydrophobic silicon pillar arrays during single-drop evaporation. Soft Matter 2012, 8, 10448–10456. [Google Scholar] [CrossRef]
- Dou, R.; Derby, B. Formation of coffee stains on porous surfaces. Langmuir 2012, 28, 5331–5338. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, X.; Liu, F.; Li, L.; Dai, J.; Liu, T. Enhanced coffee-ring effect via substrate roughness in evaporation of colloidal droplets. Adv. Condens. Matter Phys. 2018, 2018, 9795654. [Google Scholar] [CrossRef]
- Lohani, D.; Basavaraj, M.G.; Satapathy, D.K.; Sarkar, S. Coupled effect of concentration, particle size and substrate morphology on the formation of coffee rings. Colloid Surf. A-Physicochem. Eng. Asp. 2020, 589, 124387. [Google Scholar] [CrossRef]
- Shaikeea, A.; Basu, S.; Hatte, S.; Bansal, L. Insights into vapor-mediated interactions in a nanocolloidal droplet system: Evaporation dynamics and affects on self-assembly topologies on macro-to microscales. Langmuir 2016, 32, 10334–10343. [Google Scholar] [CrossRef] [PubMed]
- Malinowski, R.; Volpe, G.; Parkin, I.P.; Volpe, G. Dynamic control of particle deposition in evaporating droplets by an external point source of vapor. J. Phys. Chem. Lett. 2018, 9, 659–664. [Google Scholar] [CrossRef] [PubMed]
- Kabi, P.; Pal, R.; Basu, S. Moses effect: Splitting a sessile droplet using a vapor-mediated marangoni effect leading to designer surface patterns. Langmuir 2020, 36, 1279–1287. [Google Scholar] [CrossRef]
- Hegde, O.; Basu, S. Spatio-temporal modulation of self-assembled central aggregates of buoyant colloids in sessile droplets using vapor mediated interactions. J. Colloid Interface Sci. 2021, 598, 136–146. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Ryu, J.; Sung, H.J.; Kim, H. Control of solutal Marangoni-driven vortical flows and enhancement of mixing efficiency. J. Colloid Interface Sci. 2020, 561, 408–415. [Google Scholar] [CrossRef] [PubMed]
- Ryu, J.; Kim, J.; Park, J.; Kim, H. Analysis of vapor-driven solutal Marangoni flows inside a sessile droplet. Int. J. Heat Mass Transf. 2021, 164, 120499. [Google Scholar] [CrossRef]
- Jadav, M.; Patel, R.; Mehta, R. Influence of magnetic field on evaporation of a ferrofluid droplet. J. Appl. Phys. 2017, 122, 145302. [Google Scholar] [CrossRef]
- Saroj, S.K.; Panigrahi, P.K. Magnetic suppression of the coffee ring effect. J. Magn. Magn. Mater. 2020, 513, 167199. [Google Scholar] [CrossRef]
- Sanyal, A.; Basu, S.; Chaudhuri, S. Controlling particle deposit morphologies in drying nano-particle laden sessile droplets using substrate oscillations. Phys. Chem. Chem. Phys. 2016, 18, 14549–14560. [Google Scholar] [CrossRef]
- Kim, S.J.; Kang, K.H.; Lee, J.-G.; Kang, I.S.; Yoon, B.J. Control of particle-deposition pattern in a sessile droplet by using radial electroosmotic flow. Anal. Chem. 2006, 78, 5192–5197. [Google Scholar] [CrossRef] [PubMed]
- Scriven, L.; Sternling, C. The marangoni effects. Nature 1960, 187, 186–188. [Google Scholar] [CrossRef]
- Sempels, W.; De Dier, R.; Mizuno, H.; Hofkens, J.; Vermant, J. Auto-production of biosurfactants reverses the coffee ring effect in a bacterial system. Nat. Commun. 2013, 4, 1757. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Larson, R.G. Analysis of the effects of Marangoni stresses on the microflow in an evaporating sessile droplet. Langmuir 2005, 21, 3972–3980. [Google Scholar] [CrossRef]
- Karpitschka, S.; Liebig, F.; Riegler, H. Marangoni contraction of evaporating sessile droplets of binary mixtures. Langmuir 2017, 33, 4682–4687. [Google Scholar] [CrossRef] [PubMed]
- Hasan, M.S.; Sobolev, K.; Nosonovsky, M. Evaporation of droplets capable of bearing viruses airborne and on hydrophobic surfaces. J. Appl.Phys. 2021, 129, 024703. [Google Scholar] [CrossRef]
- Picknett, R.; Bexon, R. The evaporation of sessile or pendant drops in still air. J. Colloid Interface Sci. 1977, 61, 336–350. [Google Scholar] [CrossRef]
- Bhardwaj, R.; Fang, X.; Somasundaran, P.; Attinger, D. Self-assembly of colloidal particles from evaporating droplets: Role of DLVO interactions and proposition of a phase diagram. Langmuir 2010, 26, 7833–7842. [Google Scholar] [CrossRef] [PubMed]
- Al-Milaji, K.N.; Radhakrishnan, V.; Kamerkar, P.; Zhao, H. pH-modulated self-assembly of colloidal nanoparticles in a dual-droplet inkjet printing process. J. Colloid Interface Sci. 2018, 529, 234–242. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Boulogne, F.; Um, E.; Jacobi, I.; Button, E.; Stone, H.A. Controlled uniform coating from the interplay of Marangoni flows and surface-adsorbed macromolecules. Phys. Rev. Lett. 2016, 116, 124501. [Google Scholar] [CrossRef] [PubMed]
- Sefiane, K. Patterns from drying drops. Adv. Colloid Interface Sci. 2014, 206, 372–381. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, J.; Tam, C.; Askounis, A.; Qi, S. Suppression of the coffee-ring effect by tailoring the viscosity of pharmaceutical sessile drops. Colloid Surf. A-Physicochem. Eng. Asp. 2021, 614, 126144. [Google Scholar] [CrossRef]
- Krieger, I.M.; Dougherty, T.J. A mechanism for non-Newtonian flow in suspensions of rigid spheres. Trans. Soc. Rheol. 1959, 3, 137–152. [Google Scholar] [CrossRef]
- Eales, A.D.; Dartnell, N.; Goddard, S.; Routh, A.F. The impact of trough geometry on film shape. A theoretical study of droplets containing polymer, for P-OLED display applications. J. Colloid Interface Sci. 2015, 458, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Berry, G.C.; Fox, T.G. The viscosity of polymers and their concentrated solutions. In Fortschritte der Hochpolymeren-Forschung; Springer: Berlin/Heidelberg, Germany, 1968; pp. 261–357. [Google Scholar] [CrossRef]
- Vega, J.F.; Otegui, J.; Ramos, J.; Martínez-Salazar, J. Effect of molecular weight distribution on Newtonian viscosity of linear polyethylene. Rheol. Acta 2012, 51, 81–87. [Google Scholar] [CrossRef]
- Xavier, J.; Sharma, S.; Seo, Y.; Isseroff, R.; Koga, T.; White, H.; Ulman, A.; Shin, K.; Satija, S.; Sokolov, J. Effect of nanoscopic fillers on dewetting dynamics. Macromolecules 2006, 39, 2972–2980. [Google Scholar] [CrossRef]
- Bahadur, J.; Sen, D.; Mazumder, S.; Paul, B.; Bhatt, H.; Singh, S. Control of buckling in colloidal droplets during evaporation-induced assembly of nanoparticles. Langmuir 2012, 28, 1914–1923. [Google Scholar] [CrossRef] [PubMed]
- Whitby, C.P.; Hermant, A. Concentration of deposit patterns by nanoparticles modified with short amphiphiles. Colloid Surf. A-Physicochem. Eng. Asp. 2020, 594, 124648. [Google Scholar] [CrossRef]
- Yan, Q.; Gao, L.; Sharma, V.; Chiang, Y.-M.; Wong, C. Particle and substrate charge effects on colloidal self-assembly in a sessile drop. Langmuir 2008, 24, 11518–11522. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.M.; Jiang, X.; Kimerling, L.C.; Hammond, P.T. Selective self-organization of colloids on patterned polyelectrolyte templates. Langmuir 2000, 16, 7825–7834. [Google Scholar] [CrossRef]
- Deleurence, R.; Parneix, C.; Monteux, C. Mixtures of latex particles and the surfactant of opposite charge used as interface stabilizers–influence of particle contact angle, zeta potential, flocculation and shear energy. Soft Matter 2014, 10, 7088–7095. [Google Scholar] [CrossRef]
- Aizenberg, J.; Braun, P.V.; Wiltzius, P. Patterned colloidal deposition controlled by electrostatic and capillary forces. Phys. Rev. Lett. 2000, 84, 2997. [Google Scholar] [CrossRef] [PubMed]
- Guha, R.; Mohajerani, F.; Mukhopadhyay, A.; Collins, M.D.; Sen, A.; Velegol, D. Modulation of spatiotemporal particle patterning in evaporating droplets: Applications to diagnostics and materials science. ACS Appl. Mater. Interfaces 2017, 9, 43352–43362. [Google Scholar] [CrossRef] [PubMed]
- Moraila-Martínez, C.L.; Cabrerizo-Vílchez, M.A.; Rodríguez-Valverde, M.A. The role of the electrostatic double layer interactions in the formation of nanoparticle ring-like deposits at driven receding contact lines. Soft Matter 2013, 9, 1664–1673. [Google Scholar] [CrossRef]
- Yang, Q.; Lv, C.; Hao, P.; He, F.; Ouyang, Y.; Niu, F. Air bubble-triggered suppression of the coffee-ring effect. Colloid Interface Sci. Commun. 2020, 37, 100284. [Google Scholar] [CrossRef]
- Li, D.; Chen, R.; Zhu, X.; Liao, Q.; Ye, D.; Yang, Y.; Li, W.; Li, H.; Yang, Y. Light-Fueled Beating Coffee-Ring Deposition for Droplet Evaporative Crystallization. Anal. Chem. 2021, 93, 8817–8825. [Google Scholar] [CrossRef] [PubMed]
- Prevo, B.G.; Velev, O.D. Controlled, rapid deposition of structured coatings from micro-and nanoparticle suspensions. Langmuir 2004, 20, 2099–2107. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Xia, J.; Lin, Z. Evaporation-induced self-assembly of nanoparticles from a sphere-on-flat geometry. Angew. Chem. Int. Ed. 2007, 46, 1860–1863. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Liao, W.-S.; Chen, X.; Yang, T.; Wark, S.E.; Son, D.H.; Batteas, J.D.; Cremer, P.S. Evaporation-induced assembly of quantum dots into nanorings. ACS Nano 2009, 3, 173–180. [Google Scholar] [CrossRef]
- Hampton, M.A.; Nguyen, T.A.; Nguyen, A.V.; Xu, Z.P.; Huang, L.; Rudolph, V. Influence of surface orientation on the organization of nanoparticles in drying nanofluid droplets. J. Colloid Interface Sci. 2012, 377, 456–462. [Google Scholar] [CrossRef]
- Sandu, I.; Fleaca, C.T. The influence of gravity on the distribution of the deposit formed onto a substrate by sessile, hanging, and sandwiched hanging drop evaporation. J. Colloid Interface Sci. 2011, 358, 621–625. [Google Scholar] [CrossRef] [PubMed]
- Leng, J. Drying of a colloidal suspension in confined geometry. Phys. Rev. E 2010, 82, 021405. [Google Scholar] [CrossRef]
- Mondal, R.; Semwal, S.; Kumar, P.L.; Thampi, S.P.; Basavaraj, M.G. Patterns in drying drops dictated by curvature-driven particle transport. Langmuir 2018, 34, 11473–11483. [Google Scholar] [CrossRef]
- Semenov, S.; Starov, V.; Rubio, R.; Agogo, H.; Velarde, M. Evaporation of sessile water droplets: Universal behaviour in presence of contact angle hysteresis. Colloid Surf. A-Physicochem. Eng. Asp. 2011, 391, 135–144. [Google Scholar] [CrossRef]
- Kim, J.Y.; Hwang, I.G.; Weon, B.M. Evaporation of inclined water droplets. Sci. Rep. 2017, 7, 42848. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Deegan, R. Ring formation on an inclined surface. J. Fluid Mech. 2015, 775, R3. [Google Scholar] [CrossRef]
- Kumar, P.L.; Thampi, S.P.; Basavaraj, M.G. Patterns from drops drying on inclined substrates. Soft Matter 2021, 17, 7670. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.-Q.; Brutin, D.; Liu, Q.-S.; Wang, Y.; Mourembles, A.; Xie, J.-C.; Tadrist, L. Experimental investigation of pendant and sessile drops in microgravity. Microgravity Sci. Technol. 2010, 22, 339–345. [Google Scholar] [CrossRef][Green Version]
- Li, W.; Ji, W.; Sun, H.; Lan, D.; Wang, Y. Pattern formation in drying sessile and pendant droplet: Interactions of gravity settling, interface shrinkage, and capillary flow. Langmuir 2018, 35, 113–119. [Google Scholar] [CrossRef]
- Kumar, P.L.; Thampi, S.P.; Basavaraj, M.G. Particle size and substrate wettability dependent patterns in dried pendant drops. J. Phys. Condens. Matter 2020, 33, 024003. [Google Scholar] [CrossRef] [PubMed]
- Kuncicky, D.M.; Bose, K.; Costa, K.D.; Velev, O.D. Sessile droplet templating of miniature porous hemispheres from colloid crystals. Chem. Mater. 2007, 19, 141–143. [Google Scholar] [CrossRef]
- Sharifi-Mood, N.; Liu, I.; Stebe, K. Capillary interactions on fluid interfaces: Opportunities for directed assembly. Soft Matter Self-Assembly 2016, 193, 165. [Google Scholar]
- Song, R.; Eo, S.; Lee, M.; Lee, J. Physics-informed machine learning for optimizing the coating conditions of blade coating. Phys. Fluids 2022, in press. [CrossRef]
- Masoud, H.; Howell, P.D.; Stone, H.A. Evaporation of multiple droplets. J. Fluid Mech. 2021, 927, R4. [Google Scholar] [CrossRef]
- Brutin, D.; Sobac, B.; Loquet, B.; Sampol, J. Pattern formation in drying drops of blood. J. Fluid Mech. 2011, 667, 85–95. [Google Scholar] [CrossRef]
- Kimovich, M.A.; Zimin, Y.; Bochkareva, A. Morphology of dried blood serum specimens of viral hepatitis. Hepat. Mon. 2007, 7, 207–210. [Google Scholar]
- Zhang, M.; Meng, L.; Deng, H.; Liu, H. Controllable patterning of nanoparticles via solution transfer processes. Mater. Chem. Front. 2021, 5, 5247–5256. [Google Scholar] [CrossRef]
- Shao, J.J.; Lv, W.; Yang, Q.H. Self-assembly of graphene oxide at interfaces. Adv. Mater. 2014, 26, 5586–5612. [Google Scholar] [CrossRef]
- Zou, J.; Kim, F. Diffusion driven layer-by-layer assembly of graphene oxide nanosheets into porous three-dimensional macrostructures. Nat. Commun. 2014, 5, 5254. [Google Scholar] [CrossRef]
- Park, J.; Moon, J.; Shin, H.; Wang, D.; Park, M. Direct-write fabrication of colloidal photonic crystal microarrays by ink-jet printing. J. Colloid Interface Sci. 2006, 298, 713–719. [Google Scholar] [CrossRef] [PubMed]
- Han, W.; Lin, Z. Learning from “Coffee Rings”: Ordered Structures Enabled by Controlled Evaporative Self-Assembly. Angew. Chem. Int. Ed. 2012, 51, 1534–1546. [Google Scholar] [CrossRef] [PubMed]
- Dugas, V.; Broutin, J.; Souteyrand, E. Droplet evaporation study applied to DNA chip manufacturing. Langmuir 2005, 21, 9130–9136. [Google Scholar] [CrossRef] [PubMed]
- Dufresne, E.R.; Corwin, E.I.; Greenblatt, N.; Ashmore, J.; Wang, D.; Dinsmore, A.D.; Cheng, J.; Xie, X.; Hutchinson, J.W.; Weitz, D.A. Flow and fracture in drying nanoparticle suspensions. Phys. Rev. Lett. 2003, 91, 224501. [Google Scholar] [CrossRef]
- Sarkar, A.; Tirumkudulu, M.S. Asymptotic analysis of stresses near a crack tip in a two-dimensional colloidal packing saturated with liquid. Phys. Rev. E 2011, 83, 051401. [Google Scholar] [CrossRef] [PubMed]
- Kasyap, T.; Koch, D.L.; Wu, M. Bacterial collective motion near the contact line of an evaporating sessile drop. Phys. Fluids 2014, 26, 111703. [Google Scholar] [CrossRef]
- Blossey, R.; Bosio, A. Contact line deposits on cDNA microarrays: A “twin-spot effect”. Langmuir 2002, 18, 2952–2954. [Google Scholar] [CrossRef]
- Hu, J.-B.; Chen, Y.-C.; Urban, P.L. Coffee-ring effects in laser desorption/ionization mass spectrometry. Anal. Chim. Acta 2013, 766, 77–82. [Google Scholar] [CrossRef] [PubMed]
- Lai, Y.-H.; Cai, Y.-H.; Lee, H.; Ou, Y.-M.; Hsiao, C.-H.; Tsao, C.-W.; Chang, H.-T.; Wang, Y.-S. Reducing spatial heterogeneity of MALDI samples with Marangoni flows during sample preparation. J. Am. Soc. Mass Spectrom. 2016, 27, 1314–1321. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Hu, A.; Bai, S.; Ma, Y.; Su, Q. Surface-enhanced Raman spectra of medicines with large-scale self-assembled silver nanoparticle films based on the modified coffee ring effect. Nanoscale Res. Lett. 2014, 9, 87. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Deng, Y.; Zhu, X.-Y.; Kienlen, T.; Guo, A. Transport at the air/water interface is the reason for rings in protein microarrays. J. Am. Chem. Soc. 2006, 128, 2768–2769. [Google Scholar] [CrossRef] [PubMed]
- Inanlu, M.J.; Shojaan, B.; Farhadi, J.; Bazargan, V. Effect of Particle Concentration on Surfactant-Induced Alteration of the Contact Line Deposition in Evaporating Sessile Droplets. Langmuir 2021, 37, 2658–2666. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Bai, L.; Yang, J.; Gu, H.; Zhong, Q.; Xie, Z.; Gu, Z. Self-assembled colloidal arrays for structural color. Nanoscale Adv. 2019, 1, 1672–1685. [Google Scholar] [CrossRef]
- Fosso, N.; Frau, E.; Schintke, S. Structuring of magnetic nanoparticle fluids for decorative and functional printing and coating. In Proceedings of the 2018 20th International Conference on Transparent Optical Networks (ICTON), Bucharest, Romania, 1–5 July 2018; pp. 1–4. [Google Scholar] [CrossRef]
- Hirohata, A.; Yamada, K.; Nakatani, Y.; Prejbeanu, I.-L.; Diény, B.; Pirro, P.; Hillebrands, B. Review on spintronics: Principles and device applications. J. Magn. Magn. Mater. 2020, 509, 166711. [Google Scholar] [CrossRef]
- Li, H.; Friend, J.R.; Yeo, L.Y. Microfluidic colloidal island formation and erasure induced by surface acoustic wave radiation. Phys. Rev. Lett. 2008, 101, 084502. [Google Scholar] [CrossRef] [PubMed]
- Kajiya, T.; Kobayashi, W.; Okuzono, T.; Doi, M. Controlling profiles of polymer dots by switching between evaporation and condensation. Langmuir 2010, 26, 10429–10432. [Google Scholar] [CrossRef] [PubMed]
- Sliz, R.; Czajkowski, J.; Fabritius, T. Taming the coffee ring effect: Enhanced thermal control as a method for thin-film nanopatterning. Langmuir 2020, 36, 9562–9570. [Google Scholar] [CrossRef] [PubMed]
- Soltman, D.; Subramanian, V. Inkjet-printed line morphologies and temperature control of the coffee ring effect. Langmuir 2008, 24, 2224–2231. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-H.; Park, S.-B.; Kim, J.H.; Zin, W.-C. Polymer transports inside evaporating water droplets at various substrate temperatures. J. Phys. Chem. C 2011, 115, 15375–15383. [Google Scholar] [CrossRef]
- Pierce, S.M.; Chan, K.B.; Zhu, H. Residual patterns of alkyl polyoxyethylene surfactant droplets after water evaporation. J. Agric. Food Chem. 2008, 56, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Attinger, D.; Moore, C.; Donaldson, A.; Jafari, A.; Stone, H.A. Fluid dynamics topics in bloodstain pattern analysis: Comparative review and research opportunities. Forensic Sci. Int. 2013, 231, 375–396. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.; A. M., T.; Cho, G.; Lee, J. Control of the Drying Patterns for Complex Colloidal Solutions and Their Applications. Nanomaterials 2022, 12, 2600. https://doi.org/10.3390/nano12152600
Lee S, A. M. T, Cho G, Lee J. Control of the Drying Patterns for Complex Colloidal Solutions and Their Applications. Nanomaterials. 2022; 12(15):2600. https://doi.org/10.3390/nano12152600
Chicago/Turabian StyleLee, Saebom, Tiara A. M., Gyoujin Cho, and Jinkee Lee. 2022. "Control of the Drying Patterns for Complex Colloidal Solutions and Their Applications" Nanomaterials 12, no. 15: 2600. https://doi.org/10.3390/nano12152600
APA StyleLee, S., A. M., T., Cho, G., & Lee, J. (2022). Control of the Drying Patterns for Complex Colloidal Solutions and Their Applications. Nanomaterials, 12(15), 2600. https://doi.org/10.3390/nano12152600






