Recent Advances in Nanostructured Inorganic Hole-Transporting Materials for Perovskite Solar Cells
Abstract
:1. Introduction
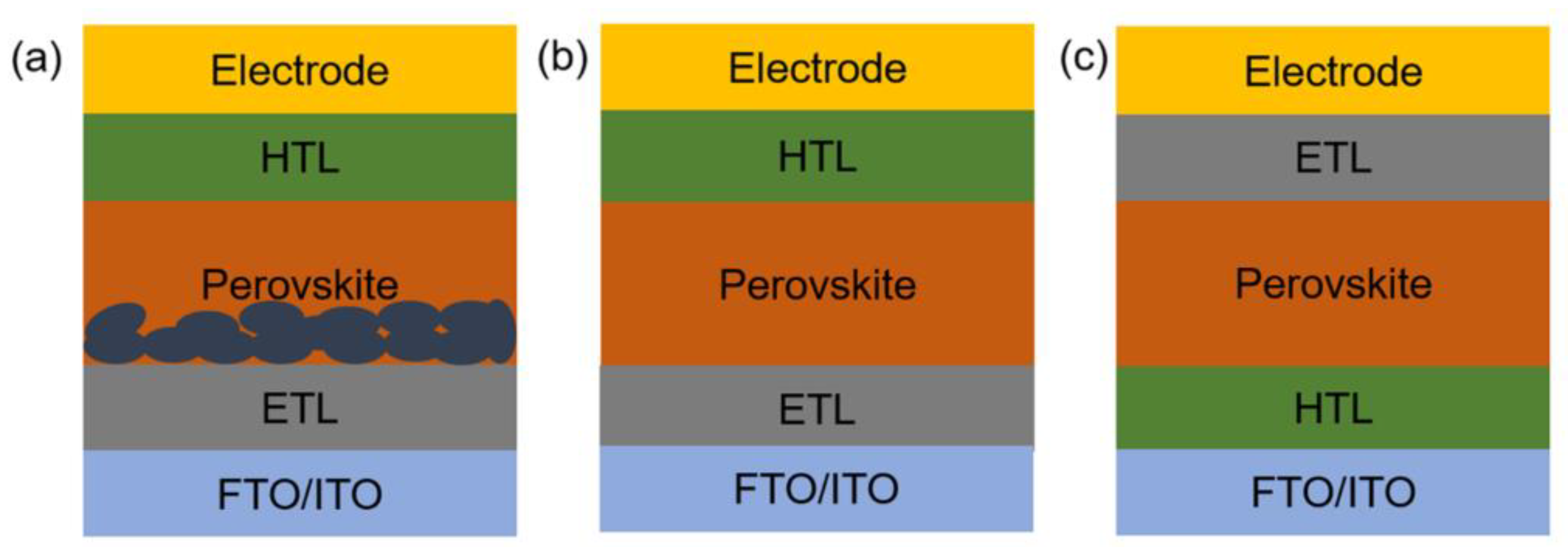
2. Current Research Status of HTLs in PSCs
2.1. The Crucial Roles of HTLs in PSCs
2.2. Advantages and Disadvantages of Organic HTLs
2.3. The Superiority of Inorganic HTLs and the Importance of Nanostructure Construction
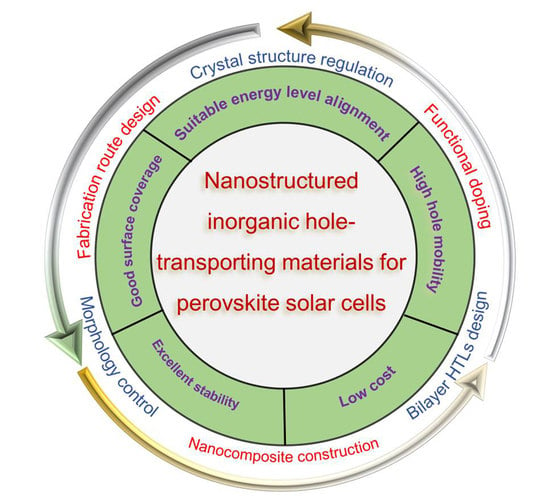
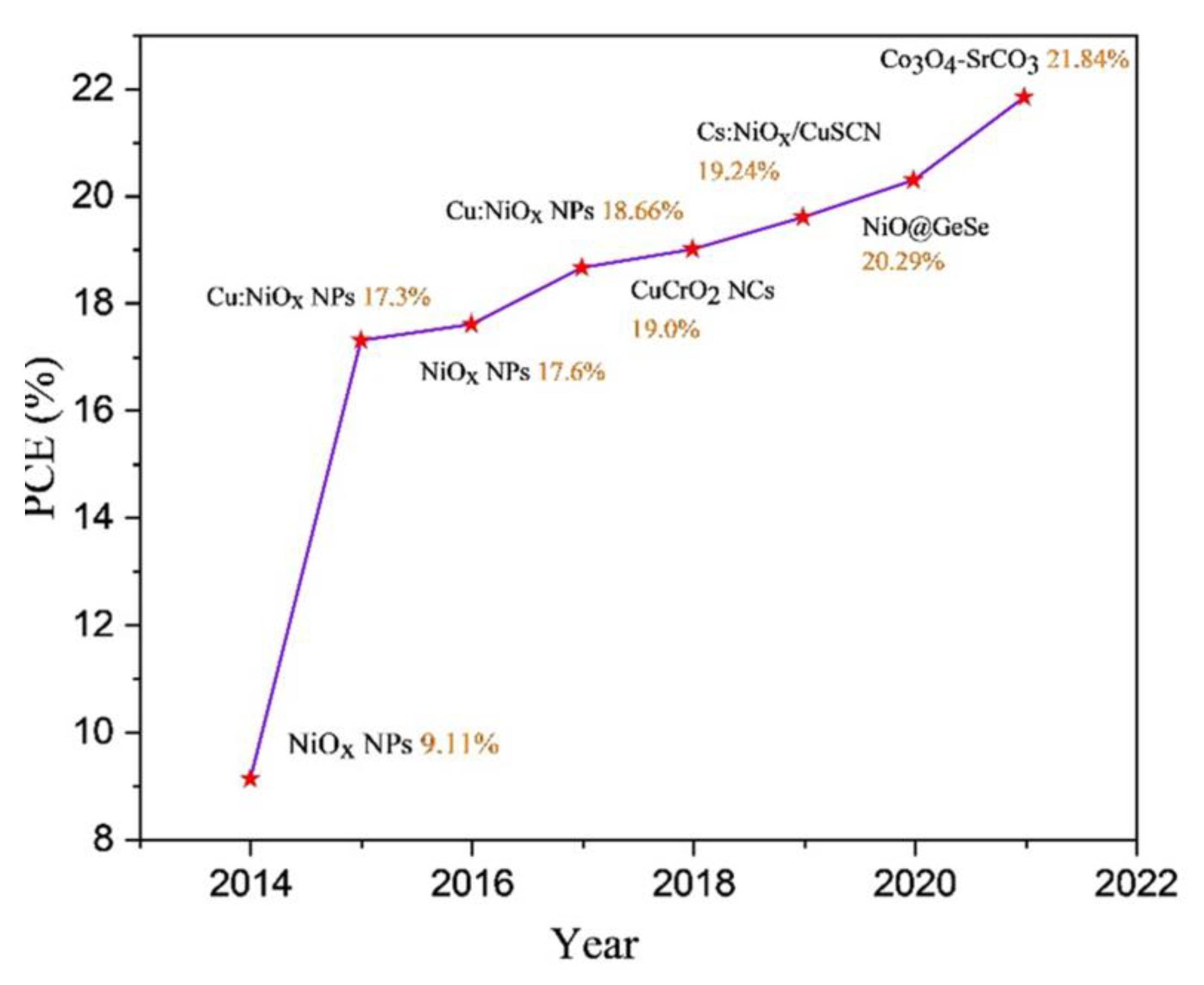
3. Advances in the Nanostructured Inorganic HTLs for PSCs
3.1. Fabrication Route Design
3.2. Functional/Selectively Doping
3.3. Morphology Control
3.4. Nanocomposite Construction
3.5. Crystal Structure Regulation and Bilayer HTL Design
4. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yun, S.; Qin, Y.; Uhl, A.R.; Vlachopoulos, N.; Yin, M.; Li, D.; Han, X.; Hagfeldt, A. New-generation integrated devices based on dye-sensitized and perovskite solar cells. Energy Environ. Sci. 2018, 11, 476–526. [Google Scholar] [CrossRef]
- Ramanujam, J.; Bishop, D.M.; Todorov, T.K.; Gunawan, O.; Rath, J.; Nekovei, R.; Artegiani, E.; Romeo, A. Flexible CIGS, CdTe and a-Si: H based thin film solar cells: A review. Prog. Mater. Sci. 2020, 110, 100619. [Google Scholar] [CrossRef]
- Xiang, H.; Liu, P.; Wang, W.; Ran, R.; Zhou, W.; Shao, Z. Towards highly stable and efficient planar perovskite solar cells: Materials development, defect control and interfacial engineering. Chem. Eng. J. 2021, 420, 127599. [Google Scholar] [CrossRef]
- Xiao, H.; Liu, P.; Wang, W.; Ran, R.; Zhou, W.; Shao, Z. Ruddlesden-Popper perovskite oxides for photocatalysis-based water splitting and wastewater treatment. Energy Fuels 2020, 34, 9208–9221. [Google Scholar] [CrossRef]
- Lang, F.; Gluba, M.A.; Albrecht, S.; Rappich, J.; Korte, L.; Rech, B.; Nickel, N.H. Perovskite solar cells with large-area CVD-graphene for tandem solar cells. J. Phys. Chem. Lett. 2015, 6, 2745–2750. [Google Scholar] [CrossRef] [Green Version]
- Han, X.; Liu, P.; Ran, R.; Wang, W.; Zhou, W.; Shao, Z. Non-metal fluorine doping in Ruddlesden-Popper perovskite oxide enables high-efficiency photocatalytic water splitting for hydrogen production. Mater. Today Energy 2022, 23, 100896. [Google Scholar] [CrossRef]
- Wang, S.; Liu, G.; Wang, L. Crystal facet engineering of photoelectrodes for photoelectrochemical water splitting. Chem. Rev. 2019, 119, 5192–5247. [Google Scholar]
- Fatima, H.; Azhar, M.R.; Khiadani, M.; Zhong, Y.; Wang, W.; Su, C.; Shao, Z. Prussian blue-conjugated ZnO nanoparticles for near-infrared light-responsive photocatalysis. Mater. Today Energy 2022, 23, 100895. [Google Scholar] [CrossRef]
- He, J.; Liu, P.; Ran, R.; Wang, W.; Zhou, W.; Shao, Z. Single-atom catalysts for high-efficiency photocatalytic and photoelectrochemical water splitting: Distinctive roles, unique fabrication methods and specific design strategies. J. Mater. Chem. A 2022, 10, 6835–6871. [Google Scholar] [CrossRef]
- Gong, J.; Li, C.; Wasielewski, M.R. Advances in solar energy conversion. Chem. Soc. Rev. 2019, 48, 1862–1864. [Google Scholar] [CrossRef]
- Yang, X.; Chen, Y.; Liu, P.; Xiang, H.; Wang, W.; Ran, R.; Zhou, W.; Shao, Z. Simultaneous power conversion efficiency and stability enhancement of Cs2AgBiBr6 lead-free inorganic perovskite solar cell through adopting a multifunctional dye interlayer. Adv. Funct. Mater. 2020, 30, 2001557. [Google Scholar] [CrossRef]
- Yu, Z.; Sun, L. Recent progress on hole-transporting materials for emerging organometal halide perovskite solar cells. Adv. Energy Mater. 2015, 5, 1500213. [Google Scholar] [CrossRef]
- Stolterfoht, M.; Caprioglio, P.; Wolff, C.M.; Márquez, J.A.; Nordmann, J.; Zhang, S.; Rothhardt, D.; Hörmann, U.; Amir, Y.; Redinger, A. The impact of energy alignment and interfacial recombination on the internal and external open-circuit voltage of perovskite solar cells. Energy Environ. Sci. 2019, 12, 2778–2788. [Google Scholar] [CrossRef] [Green Version]
- Xiang, H.; Liu, P.; Wang, W.; Ran, R.; Zhou, W.; Shao, Z. Sodium fluoride sacrificing layer concept enables high-efficiency and stable methylammonium lead iodide perovskite solar cells. J. Mater. Sci. Technol. 2022, 113, 138–146. [Google Scholar] [CrossRef]
- Zhou, H.; Chen, Q.; Li, G.; Luo, S.; Song, T.; Duan, H.-S.; Hong, Z.; You, J.; Yang, Y. Interface engineering of highly efficient perovskite solar cells. Science 2014, 345, 542–546. [Google Scholar] [CrossRef]
- Alkahtani, M.; Qasem, H.; Alenzi, S.M.; Alsofyani, N.; Alfahd, A.; Aljuwayr, A.; Hemmer, P.R. Electrodeposition of lithium-based upconversion nanoparticle thin films for efficient perovskite solar cells. Nanomaterials 2022, 12, 2115. [Google Scholar] [CrossRef]
- Liu, P.; Yang, X.; Chen, Y.; Xiang, H.; Wang, W.; Ran, R.; Zhou, W.; Shao, Z. Promoting the efficiency and stability of CsPbIBr2-based all-inorganic perovskite solar cells through a functional Cu2+ doping strategy. ACS Appl. Mater. Interfaces 2020, 12, 23984–23994. [Google Scholar] [CrossRef]
- Andreani, L.C.; Bozzola, A.; Kowalczewski, P.; Liscidini, M.; Redorici, L. Silicon solar cells: Toward the efficiency limits. Adv. Phys. 2019, 4, 1548305. [Google Scholar] [CrossRef] [Green Version]
- Ranjan, S.; Balaji, S.; Panella, R.A.; Ydstie, B.E. Silicon solar cell production. Comput. Chem. Eng. 2011, 35, 1439–1453. [Google Scholar] [CrossRef]
- Richards, B.S. Enhancing the performance of silicon solar cells via the application of passive luminescence conversion layers. Sol. Energy Mater. Sol. Cells 2006, 90, 2329–2337. [Google Scholar] [CrossRef]
- Schmager, R.; Gomard, G.; Richards, B.S.; Paetzold, U.W. Nanophotonic perovskite layers for enhanced current generation and mitigation of lead in perovskite solar cells. Sol. Energy Mater. Sol. Cells 2019, 192, 65–71. [Google Scholar] [CrossRef]
- Prochowicz, D.; Runjhun, R.; Tavakoli, M.M.; Yadav, P.; Saski, M.; Alanazi, A.Q.; Kubicki, D.J.; Kaszkur, Z.; Zakeeruddin, S.M.; Lewinski, J. Engineering of perovskite materials based on formamidinium and cesium hybridization for high-efficiency solar cells. Chem. Mater. 2019, 31, 1620–1627. [Google Scholar] [CrossRef]
- Yang, X.; Xie, A.; Xiang, H.; Wang, W.; Ran, R.; Zhou, W.; Shao, Z. First investigation of additive engineering for highly efficient Cs2AgBiBr6-based lead-free inorganic perovskite solar cells. Appl. Phys. Rev. 2021, 8, 041402. [Google Scholar] [CrossRef]
- Mei, A.; Li, X.; Liu, L.; Ku, Z.; Liu, T.; Rong, Y.; Xu, M.; Hu, M.; Chen, J.; Yang, Y. A hole-conductor-free, fully printable mesoscopic perovskite solar cell with high stability. Science 2014, 345, 295–298. [Google Scholar] [CrossRef]
- Yang, X.; Wang, W.; Ran, R.; Zhou, W.; Shao, Z. Recent advances in Cs2AgBiBr6-based halide double perovskites as lead-free and inorganic light absorbers for perovskite solar cells. Energy Fuels 2020, 34, 10513–10528. [Google Scholar] [CrossRef]
- Liu, P.; Chen, Y.; Xiang, H.; Yang, X.; Wang, W.; Ran, R.; Zhou, W.; Shao, Z. Benefitting from synergistic effect of anion and cation in antimony acetate for stable CH3NH3PbI3-based perovskite solar cell with efficiency beyond 21%. Small 2021, 17, 2102186. [Google Scholar] [CrossRef]
- Kojima, A.; Teshima, K.; Shirai, Y.; Miyasaka, T. Organometal halide perovskites as visible-light sensitizers for photovoltaic cells. J. Am Chem. Soc. 2009, 131, 6050–6051. [Google Scholar] [CrossRef]
- Jeong, J.; Kim, M.; Seo, J.; Lu, H.; Ahlawat, P.; Mishra, A.; Yang, Y.; Hope, M.A.; Eickemeyer, F.T.; Kim, M. Pseudo-halide anion engineering for α-FAPbI3 perovskite solar cells. Nature 2021, 592, 381–385. [Google Scholar] [CrossRef]
- Yelzhanova, Z.; Nigmetova, G.; Aidarkhanov, D.; Daniyar, B.; Baptayev, B.; Balanay, M.P.; Jumabekov, A.N.; Ng, A. A morphological study of solvothermally grown SnO2 nanostructures for application in perovskite solar cells. Nanomaterials 2022, 12, 1686. [Google Scholar] [CrossRef]
- Xiang, H.; Liu, P.; Ran, R.; Wang, W.; Zhou, W.; Shao, Z. Two-dimensional Dion-Jacobson halide perovskites as new-generation light absorbers for perovskite solar cells. Renew. Sust. Energy Rev. 2022, 166, 112614. [Google Scholar] [CrossRef]
- Jeon, N.J.; Noh, J.H.; Kim, Y.C.; Yang, W.S.; Ryu, S.; Seok, S.I. Solvent engineering for high-performance inorganic-organic hybrid perovskite solar cells. Nat. Mater. 2014, 13, 897–903. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wang, H.; Chen, W.; Jen, A.K.Y. CuGaO2: A promising inorganic hole-transporting material for highly efficient and stable perovskite solar cells. Adv. Mater. 2017, 29, 1604984. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Wang, W.; Liu, S.; Yang, H.; Shao, Z. Fundamental understanding of photocurrent hysteresis in perovskite solar cells. Adv. Energy. Mater. 2019, 9, 1803017. [Google Scholar] [CrossRef]
- Calió, L.; Kazim, S.; Grätzel, M.; Ahmad, S. Hole-transport materials for perovskite solar cells. Angew. Chem. Int. Ed. 2016, 55, 14522–14545. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Luo, X.; Yang, J.; Qiu, Q.; Xie, T.; Liang, T. Application of quantum dot interface modification layer in perovskite solar cells: Progress and perspectives. Nanomaterials 2022, 12, 2102. [Google Scholar] [CrossRef]
- Liu, P.; Han, N.; Wang, W.; Ran, R.; Zhou, W.; Shao, Z. High-quality Ruddlesden-Popper perovskite film formation for high-performance perovskite solar cells. Adv. Mater. 2021, 33, 2002582. [Google Scholar] [CrossRef]
- Zhao, D.; Wu, Y.; Tu, B.; Xing, G.; Li, H.; He, Z. Understanding the impact of Cu-In-Ga-S nanoparticles compactness on holes transfer of perovskite solar cells. Nanomaterials 2019, 9, 286. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Yang, X.; Liu, P.; Wang, W.; Ran, R.; Zhou, W.; Shao, Z. Improving moisture/thermal stability and efficiency of CH3NH3PbI3-based perovskite solar cells via gentle butyl acrylate additive strategy. Sol. RRL 2021, 5, 2000621. [Google Scholar] [CrossRef]
- Jheng, B.R.; Chiu, P.T.; Yang, S.H.; Tong, Y.L. Using ZnCo2O4 nanoparticles as the hole transport layer to improve long term stability of perovskite solar cells. Sci. Rep. 2022, 12, 2921. [Google Scholar] [CrossRef]
- Liu, P.; Xiang, H.; Wang, W.; Ran, R.; Zhou, W.; Shao, Z. A bilateral cyano molecule serving as an effective additive enables high-efficiency and stable perovskite solar cells. J. Energy. Chem. 2021, 62, 243–251. [Google Scholar] [CrossRef]
- Singh, R.; Singh, P.K.; Bhattacharya, B.; Rhee, H.-W. Review of current progress in inorganic hole-transport materials for perovskite solar cells. Appl. Mater. Today 2019, 14, 175–200. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, L.; Zhang, Y.; Gao, H.; Yan, H. Large-area perovskite solar cells–a review of recent progress and issues. RSC Adv. 2018, 8, 10489–10508. [Google Scholar] [CrossRef] [Green Version]
- Xing, G.; Mathews, N.; Sun, S.; Lim, S.S.; Lam, Y.M.; Grätzel, M.; Mhaisalkar, S.; Sum, T.C. Long-range balanced electron-and hole-transport lengths in organic-inorganic CH3NH3PbI3. Science 2013, 342, 344–347. [Google Scholar] [CrossRef]
- Chen, T.; He, R.; Zhang, F.; Hao, X.; Xuan, Z.; Wang, Y.; Wang, W.; Zhao, D.; Zhang, J.; Wu, L. GABr post-treatment for high-performance MAPbI3 solar cells on rigid glass and flexible substrate. Nanomaterials 2021, 11, 750. [Google Scholar] [CrossRef]
- Zhu, H.; Liu, Y.; Eickemeyer, F.T.; Pan, L.; Ren, D.; Ruiz-Preciado, M.A.; Carlsen, B.; Yang, B.; Dong, X.; Wang, Z. Tailored amphiphilic molecular mitigators for stable perovskite solar cells with 23.5% efficiency. Adv. Mater. 2020, 32, 1907757. [Google Scholar] [CrossRef]
- Ecker, B.; Nolasco, J.C.; Pallarés, J.; Marsal, L.F.; Posdorfer, J.; Parisi, J.; von Hauff, E. Degradation effects related to the hole transport layer in organic solar cells. Adv. Funct. Mater. 2011, 21, 2705–2711. [Google Scholar] [CrossRef]
- Tsai, H.; Nie, W.; Blancon, J.-C.; Stoumpos, C.C.; Asadpour, R.; Harutyunyan, B.; Neukirch, A.J.; Verduzco, R.; Crochet, J.J.; Tretiak, S. High-efficiency two-dimensional Ruddlesden-Popper perovskite solar cells. Nature 2016, 536, 312–316. [Google Scholar] [CrossRef]
- Jung, M.C.; Qi, Y. Dopant interdiffusion effects in n-i-p structured Spiro-OMeTAD hole transport layer of organometal halide perovskite solar cells. Org. Electron. 2016, 31, 71–76. [Google Scholar] [CrossRef]
- Wang, S.; Yuan, W.; Meng, Y.S. Spectrum-dependent Spiro-OMeTAD oxidization mechanism in perovskite solar cells. ACS Appl. Mater. Interfaces 2015, 7, 24791–24798. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, Q.; Duan, H.-S.; Zhou, H.; Yang, Y.M.; Chen, H.; Luo, S.; Song, T.-B.; Dou, L.; Hong, Z. A dopant-free organic hole transport material for efficient planar heterojunction perovskite solar cells. J. Mater. Chem. A 2015, 3, 11940–11947. [Google Scholar] [CrossRef]
- Liu, J.; Wu, Y.; Qin, C.; Yang, X.; Yasuda, T.; Islam, A.; Zhang, K.; Peng, W.; Chen, W.; Han, L. A dopant-free hole-transporting material for efficient and stable perovskite solar cells. Energy Environ. Sci. 2014, 7, 2963–2967. [Google Scholar] [CrossRef]
- Meng, L.; You, J.; Guo, T.-F.; Yang, Y. Recent advances in the inverted planar structure of perovskite solar cells. Acc. Chem. Res. 2016, 49, 155–165. [Google Scholar] [CrossRef] [PubMed]
- Chiang, C.-H.; Chen, C.-C.; Nazeeruddin, M.K.; Wu, C.-G. A newly developed lithium cobalt oxide super hydrophilic film for large area, thermally stable and highly efficient inverted perovskite solar cells. J. Mater. Chem. A 2018, 6, 13751–13760. [Google Scholar] [CrossRef]
- Shariatinia, Z. Recent progress in development of diverse kinds of hole transport materials for the perovskite solar cells: A review. Renew. Sust. Energy Rev. 2020, 119, 109608. [Google Scholar] [CrossRef]
- Chen, W.; Wu, Y.; Yue, Y.; Liu, J.; Zhang, W.; Yang, X.; Chen, H.; Bi, E.; Ashraful, I.; Grätzel, M. Efficient and stable large-area perovskite solar cells with inorganic charge extraction layers. Science 2015, 350, 944–948. [Google Scholar] [CrossRef] [Green Version]
- Islam, M.A.; Sarkar, D.K.; Shahinuzzaman, M.; Wahab, Y.A.; Khandaker, M.U.; Tamam, N.; Sulieman, A.; Amin, N.; Akhtaruzzaman, M. Green synthesis of lead sulphide nanoparticles for high-efficiency perovskite solar cell applications. Nanomaterials 2022, 12, 1933. [Google Scholar] [CrossRef]
- Kung, P.K.; Li, M.H.; Lin, P.Y.; Chiang, Y.H.; Chan, C.R.; Guo, T.F.; Chen, P. A Review of inorganic hole transport materials for perovskite solar cells. Adv. Mater. Interfaces 2018, 5, 1800882. [Google Scholar] [CrossRef]
- Mashreghi, A.; Maleki, K.; Moradzadeh, M. Two-phase synthesized Cu2ZnSnS4 nanoparticles as inorganic hole-transporting material of paintable carbon-based perovskite solar cells. Sol. Energy 2020, 201, 547–554. [Google Scholar] [CrossRef]
- Ye, X.; Wen, Z.; Zhang, R.; Ling, H.; Xia, J.; Lu, X. High-performance and stable inverted perovskite solar cells using low-temperature solution-processed CuNbOx hole transport layer. J. Power Sources 2021, 483, 229194. [Google Scholar] [CrossRef]
- Zhu, Z.; Bai, Y.; Zhang, T.; Liu, Z.; Long, X.; Wei, Z.; Wang, Z.; Zhang, L.; Wang, J.; Yan, F.; et al. High-performance hole-extraction layer of sol-gel-processed NiO nanocrystals for inverted planar perovskite solar cells. Angew. Chem. Int. Ed. 2014, 53, 12571–12575. [Google Scholar]
- Park, J.H.; Seo, J.; Park, S.; Shin, S.S.; Kim, Y.C.; Jeon, N.J.; Shin, H.W.; Ahn, T.K.; Noh, J.H.; Yoon, S.C. Efficient CH3NH3PbI3 perovskite solar cells employing nanostructured p-type NiO electrode formed by a pulsed laser deposition. Adv. Mater. 2015, 27, 4013–4019. [Google Scholar] [CrossRef]
- Zhang, H.; Cheng, J.; Lin, F.; He, H.; Mao, J.; Wong, K.S.; Jen, A.K.; Choy, W.C. Pinhole-free and surface-nanostructured NiOx film by room-temperature solution process for high-performance flexible perovskite solar cells with good stability and reproducibility. ACS Nano 2016, 10, 1503–1511. [Google Scholar] [CrossRef]
- He, Q.; Yao, K.; Wang, X.; Xia, X.; Leng, S.; Li, F. Room-temperature and solution-processable cu-doped nickel oxide nanoparticles for efficient hole-transport layers of flexible large-area perovskite solar cells. ACS Appl. Mater. Interfaces 2017, 9, 41887–41897. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, H.; Zhu, H.; Chueh, C.C.; Chen, W.; Yang, S.; Jen, A.K.Y. Low-temperature solution-processed CuCrO2 hole-transporting layer for efficient and photostable perovskite solar cells. Adv. Energy Mater. 2018, 8, 1702762. [Google Scholar] [CrossRef]
- Mali, S.S.; Patil, J.V.; Kim, H.; Luque, R.; Hong, C.K. Highly efficient thermally stable perovskite solar cells via Cs:NiOx/CuSCN double-inorganic hole extraction layer interface engineering. Mater. Today 2019, 26, 8–18. [Google Scholar] [CrossRef]
- Mohammadi, M.H.; Fathi, D.; Eskandari, M. NiO@GeSe core-shell nano-rod array as a new hole transfer layer in perovskite solar cells: A numerical study. Sol. Energy 2020, 204, 200–207. [Google Scholar] [CrossRef]
- Ge, B.; Zhou, Z.R.; Wu, X.F.; Zheng, L.R.; Dai, S.; Chen, A.P.; Hou, Y.; Yang, H.G.; Yang, S. Self-organized Co3O4-SrCO3 percolative composites enabling nanosized hole transport pathways for perovskite solar cells. Adv. Funct. Mater. 2021, 31, 2106121. [Google Scholar] [CrossRef]
- Islam, M.B.; Yanagida, M.; Shirai, Y.; Nabetani, Y.; Miyano, K. NiOx hole transport layer for perovskite solar cells with improved stability and reproducibility. ACS Omega 2017, 2, 2291–2299. [Google Scholar] [CrossRef] [Green Version]
- Xi, Q.; Gao, G.; Zhou, H.; Zhao, Y.; Wu, C.; Wang, L.; Guo, P.; Xu, J. Highly efficient inverted solar cells based on perovskite grown nanostructures mediated by CuSCN. Nanoscale 2017, 9, 6136–6144. [Google Scholar] [CrossRef]
- Igbari, F.; Li, M.; Hu, Y.; Wang, Z.-K.; Liao, L.-S. A room-temperature CuAlO2 hole interfacial layer for efficient and stable planar perovskite solar cells. J. Mater. Chem. A 2016, 4, 1326–1335. [Google Scholar] [CrossRef]
- Motta, C.; El-Mellouhi, F.; Sanvito, S. Charge carrier mobility in hybrid halide perovskites. Sci. Rep. 2015, 5, 12746. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-S.; Lee, C.-R.; Im, J.-H.; Lee, K.-B.; Moehl, T.; Marchioro, A.; Moon, S.-J.; Humphry-Baker, R.; Yum, J.-H.; Moser, J.E. Lead iodide perovskite sensitized all-solid-state submicron thin film mesoscopic solar cell with efficiency exceeding 9%. Sci. Rep. 2012, 2, 591. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miyata, A.; Mitioglu, A.; Plochocka, P.; Portugall, O.; Wang, J.T.-W.; Stranks, S.D.; Snaith, H.J.; Nicholas, R.J. Direct measurement of the exciton binding energy and effective masses for charge carriers in organic-inorganic tri-halide perovskites. Nat. Phys. 2015, 11, 582–587. [Google Scholar] [CrossRef] [Green Version]
- Zhao, D.; Sexton, M.; Park, H.Y.; Baure, G.; Nino, J.C.; So, F. High-efficiency solution-processed planar perovskite solar cells with a polymer hole transport layer. Adv. Energy Mater. 2015, 5, 1401855. [Google Scholar] [CrossRef]
- Bag, A.; Radhakrishnan, R.; Nekovei, R.; Jeyakumar, R. Effect of absorber layer, hole transport layer thicknesses, and its doping density on the performance of perovskite solar cells by device simulation. Sol. Energy 2020, 196, 177–182. [Google Scholar] [CrossRef]
- Yu, Z.; Sun, L. Inorganic hole-transporting materials for perovskite solar cells. Small Methods 2018, 2, 1700280. [Google Scholar] [CrossRef]
- Yin, X.; Guo, Y.; Xie, H.; Que, W.; Kong, L.B. Nickel oxide as efficient hole transport materials for perovskite solar cells. Sol. RRL 2019, 3, 1900001. [Google Scholar] [CrossRef]
- Cavinato, L.M.; Fresta, E.; Ferrara, S.; Costa, R.D. Merging biology and photovoltaics: How nature helps sun-catching. Adv. Energy Mater. 2021, 11, 2100520. [Google Scholar] [CrossRef]
- Zhao, P.; Kim, B.J.; Jung, H.S. Passivation in perovskite solar cells: A review. Mater. Today Energy 2018, 7, 267–286. [Google Scholar] [CrossRef]
- Tumen-Ulzii, G.; Matsushima, T.; Adachi, C. Mini-review on efficiency and stability of perovskite solar cells with Spiro-OMeTAD hole transport layer: Recent progress and perspectives. Energy Fuels 2021, 35, 18915–18927. [Google Scholar] [CrossRef]
- Saianand, G.; Sonar, P.; Wilson, G.J.; Gopalan, A.I.; Roy, V.A.; Unni, G.E.; Qiao, Q. Current advancements on charge selective contact interfacial layers and electrodes in flexible hybrid perovskite photovoltaics. J. Energy Chem. 2021, 54, 151–173. [Google Scholar] [CrossRef]
- Nazari, P.; Ansari, F.; Abdollahi, B.; Ahmadi, V.; Payandeh, M.; Salavati-Niasari, M. Physicochemical interface engineering of CuI/Cu as advanced potential hole-transporting materials/metal contact couples in hysteresis-free ultralow-cost and large-area perovskite solar cells. J. Phys. Chem. C. 2017, 121, 21935–21944. [Google Scholar] [CrossRef]
- Zhang, L.; Zhou, X.; Xie, J.; Hu, B.; Liu, P.; Chen, S.; Bae, S.-H.; Kim, J.; Dai, S.; Xu, B. Learning from hole-transporting polymers in regular perovskite solar cells to construct efficient conjugated polyelectrolytes for inverted devices. Chem. Eng. J. 2021, 420, 129735. [Google Scholar] [CrossRef]
- Wang, C.; Hu, J.; Li, C.; Qiu, S.; Liu, X.; Zeng, L.; Liu, C.; Mai, Y.; Guo, F. Spiro-linked molecular hole-transport materials for highly efficient inverted perovskite solar cells. Sol. RRL 2020, 4, 1900389. [Google Scholar] [CrossRef]
- Boyd, C.C.; Shallcross, R.C.; Moot, T.; Kerner, R.; Bertoluzzi, L.; Onno, A.; Kavadiya, S.; Chosy, C.; Wolf, E.J.; Werner, J.; et al. Overcoming redox reactions at perovskite-nickel oxide interfaces to boost voltages in perovskite solar cells. Joule 2020, 4, 1759–1775. [Google Scholar] [CrossRef]
- Zhang, J.; Long, J.; Huang, Z.; Yang, J.; Li, X.; Dai, R.; Chen, Y. Obstructing interfacial reaction between NiOx and perovskite to enable efficient and stable inverted perovskite solar cells. Chem. Eng. J. 2021, 426, 131357. [Google Scholar] [CrossRef]
- Lin, C.H.; Hu, L.; Guan, X.; Kim, J.; Huang, C.Y.; Huang, J.K.; Wu, T. Electrode engineering in halide perovskite electronics: Plenty of room at the interfaces. Adv. Mater. 2022, 34, 2108616. [Google Scholar] [CrossRef]
- Mangalam, J.; Rath, T.; Weber, S.; Kunert, B.; Dimopoulos, T.; Fian, A.; Trimmel, G. Modification of NiOx hole transport layers with 4-bromobenzylphosphonic acid and its influence on the performance of lead halide perovskite solar cells. J. Mater. Sci. Mater. Electron. 2019, 30, 9602–9611. [Google Scholar] [CrossRef] [Green Version]
- Omrani, M.; Keshavarzi, R.; Abdi-Jalebi, M.; Gao, P. Impacts of plasmonic nanoparticles incorporation and interface energy alignment for highly efficient carbon-based perovskite solar cells. Sci. Rep. 2022, 12, 5367. [Google Scholar] [CrossRef]
- Li, B.; Rui, Y.; Xu, J.; Wang, Y.; Yang, J.; Zhang, Q.; Müller-Buschbaum, P. Solution-processed p-type nanocrystalline CoO films for inverted mixed perovskite solar cells. J. Colloid. Interf. Sci. 2020, 573, 78–86. [Google Scholar] [CrossRef]
- Ansari, F.; Salavati-Niasari, M.; Amiri, O.; Mir, N.; Abdollahi Nejand, B.; Ahmadi, V. Magnetite as inorganic hole transport material for lead halide perovskite-based solar cells with enhanced stability. Ind. Eng. Chem. Res. 2020, 59, 743–750. [Google Scholar] [CrossRef]
- Franckevičius, M.; Mishra, A.; Kreuzer, F.; Luo, J.; Zakeeruddin, S.M.; Grätzel, M. A dopant-free spirobi [cyclopenta [2, 1-b: 3, 4-b′] dithiophene] based hole-transport material for efficient perovskite solar cells. Mater. Horiz. 2015, 2, 613–618. [Google Scholar] [CrossRef]
- Ahn, N.; Son, D.-Y.; Jang, I.-H.; Kang, S.M.; Choi, M.; Park, N.-G. Highly reproducible perovskite solar cells with average efficiency of 18.3% and best efficiency of 19.7% fabricated via Lewis base adduct of lead (II) iodide. J. Am. Chem. Soc. 2015, 137, 8696–8699. [Google Scholar] [CrossRef]
- Chen, Y.; Yang, X.; Wang, W.; Ran, R.; Zhou, W.; Shao, Z. Tuning the A-site cation deficiency of La0.8Sr0.2FeO3−δ perovskite oxides for high-efficiency triiodide reduction reaction in dye-sensitized solar cells. Energy Fuels 2020, 34, 11322–11329. [Google Scholar] [CrossRef]
- Ren, G.; Han, W.; Deng, Y.; Wu, W.; Li, Z.; Guo, J.; Bao, H.; Liu, C.; Guo, W. Strategies of modifying spiro-OMeTAD materials for perovskite solar cells: A review. J. Mater. Chem. A 2021, 9, 4589–4625. [Google Scholar] [CrossRef]
- Li, Y.; Li, Z.; Liu, F.; Wei, J. Defects and passivation in perovskite solar cells. Surf. Innov. 2022, 10, 3–20. [Google Scholar] [CrossRef]
- Jeon, N.J.; Lee, H.G.; Kim, Y.C.; Seo, J.; Noh, J.H.; Lee, J.; Seok, S.I. O-Methoxy substituents in Spiro-OMeTAD for efficient inorganic-organic hybrid perovskite solar cells. J. Am. Chem. Soc. 2014, 136, 7837–7840. [Google Scholar] [CrossRef]
- Hu, Z.; Fu, W.; Yan, L.; Miao, J.; Yu, H.; He, Y.; Huang, W. Effects of heteroatom substitution in spiro-bifluorene hole transport materials. Chem. Sci. 2016, 7, 5007–5012. [Google Scholar] [CrossRef] [Green Version]
- Wu, F.; Yan, K.; Wu, H.; Niu, B.; Liu, Z.; Li, Y.; Zuo, L.; Chen, H. Tuning interfacial chemical interaction for high-performance perovskite solar cell with PEDOT:PSS as hole transporting layer. J. Mater. Chem. A 2021, 9, 14920–14927. [Google Scholar] [CrossRef]
- Shi, H.; Liu, C.; Jiang, Q.; Xu, J. Effective approaches to improve the electrical conductivity of PEDOT: PSS: A review. Adv. Electron. Mater. 2015, 1, 1500017. [Google Scholar] [CrossRef]
- Cheng, N.; Liu, Z.; Yu, Z.; Li, W.; Zhao, Z.; Xiao, Z.; Lei, B.; Sun, S.; Zi, W. High performance inverted perovskite solar cells using PEDOT:PSS/KCl hybrid hole transporting layer. Org. Electron. 2021, 98, 106298. [Google Scholar] [CrossRef]
- Li, P.; Mohamed, M.I.O.; Xu, C.; Wang, X.; Tang, X. Electrical property modified hole transport layer (PEDOT:PSS) enhance the efficiency of perovskite solar cells: Hybrid co-solvent post-treatment. Org. Electron. 2020, 78, 105582. [Google Scholar] [CrossRef]
- Hu, W.; Xu, C.Y.; Niu, L.B.; Elseman, A.M.; Wang, G.; Liu, D.B.; Yao, Y.Q.; Liao, L.P.; Zhou, G.D.; Song, Q.L. High open-circuit voltage of 1.134 V for inverted planar perovskite solar cells with sodium citrate-doped PEDOT:PSS as a hole transport layer. ACS Appl. Mater. Interfaces 2019, 11, 22021–22027. [Google Scholar] [CrossRef] [PubMed]
- Neophytou, M.; Griffiths, J.; Fraser, J.; Kirkus, M.; Chen, H.; Nielsen, C.B.; McCulloch, I. High mobility, hole transport materials for highly efficient PEDOT:PSS replacement in inverted perovskite solar cells. J. Mater. Chem. C 2017, 5, 4940–4945. [Google Scholar] [CrossRef] [Green Version]
- Anrango-Camacho, C.; Pavón-Ipiales, K.; Frontana-Uribe, B.A.; Palma-Cando, A. Recent advances in hole-transporting layers for organic solar cells. Nanomaterials 2022, 12, 443. [Google Scholar] [CrossRef]
- Patel, V.D.; Gupta, D. Solution-processed metal-oxide based hole transport layers for organic and perovskite solar cell: A review. Mater. Today Commun. 2022, 31, 103664. [Google Scholar] [CrossRef]
- Pandey, J.; Hua, B.; Ng, W.; Yang, Y.; van der Veen, K.; Chen, J.; Geels, N.J.; Luo, J.-L.; Rothenberg, G.; Yan, N. Developing hierarchically porous MnOx/NC hybrid nanorods for oxygen reduction and evolution catalysis. Green Chem. 2017, 19, 2793–2797. [Google Scholar] [CrossRef]
- Wang, K.-C.; Jeng, J.-Y.; Shen, P.-S.; Chang, Y.-C.; Diau, E.W.-G.; Tsai, C.-H.; Chao, T.-Y.; Hsu, H.-C.; Lin, P.-Y.; Chen, P. P-type mesoscopic nickel oxide/organometallic perovskite heterojunction solar cells. Sci. Rep. 2014, 4, 4756. [Google Scholar] [CrossRef] [Green Version]
- Wang, K.-C.; Shen, P.-S.; Li, M.-H.; Chen, S.; Lin, M.-W.; Chen, P.; Guo, T.-F. Low-temperature sputtered nickel oxide compact thin film as effective electron blocking layer for mesoscopic NiO/CH3NH3PbI3 perovskite heterojunction solar cells. ACS Appl. Mater. Interfaces 2014, 6, 11851–11858. [Google Scholar] [CrossRef]
- Xiang, W.; Pan, J.; Chen, Q. In situ formation of NiOx interlayer for efficient n-i-p inorganic perovskite solar cells. ACS Appl. Energy Mater. 2020, 3, 5977–5983. [Google Scholar] [CrossRef]
- Papadas, I.T.; Ioakeimidis, A.; Armatas, G.S.; Choulis, S.A. Low-temperature combustion synthesis of a spinel NiCo2O4 hole transport layer for perovskite photovoltaics. Adv. Sci. 2018, 5, 1701029. [Google Scholar] [CrossRef]
- Ouyang, D.; Chen, C.; Huang, Z.; Zhu, L.; Yan, Y.; Choy, W.C.H. Hybrid 3D nanostructure-based hole transport layer for highly efficient inverted perovskite solar cells. ACS Appl. Mater. Interfaces 2021, 13, 16611–16619. [Google Scholar] [CrossRef]
- Li, Z. Stable perovskite solar cells based on WO3 nanocrystals as hole transport layer. Chem. Lett. 2015, 44, 1140–1141. [Google Scholar] [CrossRef]
- Yao, K.; Li, F.; He, Q.; Wang, X.; Jiang, Y.; Huang, H.; Jen, A.K.Y. A copper-doped nickel oxide bilayer for enhancing efficiency and stability of hysteresis-free inverted mesoporous perovskite solar cells. Nano Energy 2017, 40, 155–162. [Google Scholar] [CrossRef]
- Tien, C.-H.; Chen, L.-C.; Lee, K.-L. Ultra-thin and high transparent Cu2ZnSnSe4/NiOx double-layered inorganic hole-transporting layer for inverted structure CH3NH3PbI3 perovskite solar cells. J. Alloy Compd. 2021, 873, 159804. [Google Scholar] [CrossRef]
- Tang, J.; Jiao, D.; Zhang, L.; Zhang, X.; Xu, X.; Yao, C.; Wu, J.; Lan, Z. High-performance inverted planar perovskite solar cells based on efficient hole-transporting layers from well-crystalline NiO nanocrystals. Sol. Energy 2018, 161, 100–108. [Google Scholar] [CrossRef]
- Tong, X.; Li, F.; Jin, J.; Guo, X.; Li, J.; Yan, F.; Zhao, X.-Z.; Tai, Q. Solution-processed NiOx nanoparticles with a wide pH window as an efficient hole transport material for high performance tin-based perovskite solar cells. J. Phys. D Appl. Phys. 2021, 54, 144002. [Google Scholar] [CrossRef]
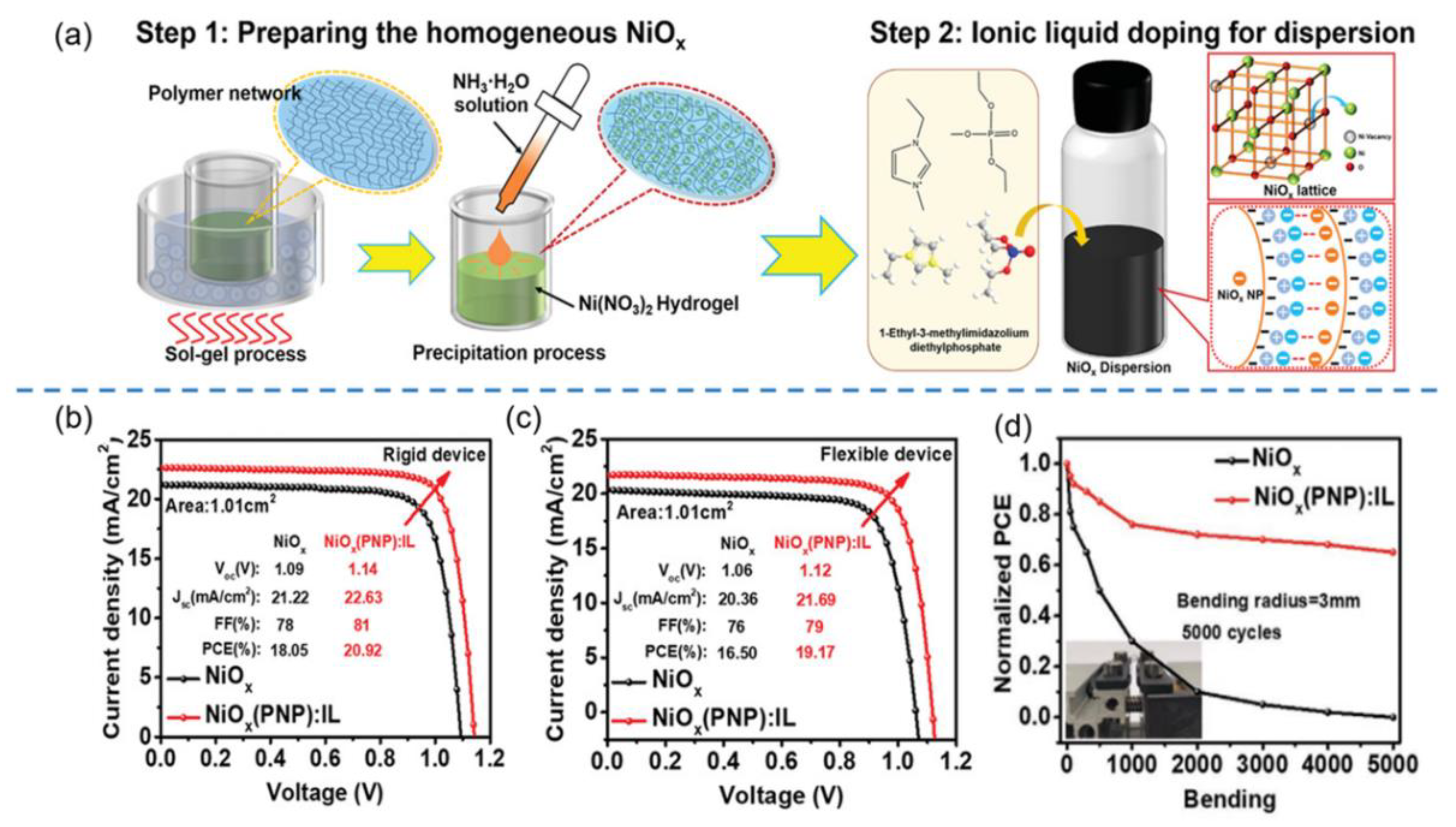
- Zhang, S.; Wang, H.; Duan, X.; Rao, L.; Gong, C.; Fan, B.; Xing, Z.; Meng, X.; Xie, B.; Hu, X. Printable and homogeneous NiOx hole transport layers prepared by a polymer-network gel method for large-area and flexible perovskite solar cells. Adv. Funct. Mater. 2021, 31, 2106495. [Google Scholar] [CrossRef]
- Chen, H.; Peng, Z.; Xu, K.; Wei, Q.; Yu, D.; Han, C.; Li, H.; Ning, Z. Band alignment towards high-efficiency NiOx-based Sn-Pb mixed perovskite solar cells. Sci. China Mater. 2020, 64, 537–546. [Google Scholar] [CrossRef]
- Wang, Q.; Chueh, C.C.; Zhao, T.; Cheng, J.; Eslamian, M.; Choy, W.C.; Jen, A.K.Y. Effects of self-assembled monolayer modification of nickel oxide nanoparticles layer on the performance and application of inverted perovskite solar cells. ChemSusChem 2017, 10, 3794–3803. [Google Scholar] [CrossRef] [Green Version]
- Kaneko, R.; Chowdhury, T.H.; Wu, G.; Kayesh, M.E.; Kazaoui, S.; Sugawa, K.; Lee, J.-J.; Noda, T.; Islam, A.; Otsuki, J. Cobalt-doped nickel oxide nanoparticles as efficient hole transport materials for low-temperature processed perovskite solar cells. Sol. Energy 2019, 181, 243–250. [Google Scholar] [CrossRef]
- Chandrasekhar, P.S.; Seo, Y.-H.; Noh, Y.-J.; Na, S.-I. Room temperature solution-processed Fe doped NiOx as a novel hole transport layer for high efficient perovskite solar cells. Appl. Surf. Sci. 2019, 481, 588–596. [Google Scholar] [CrossRef]
- Chen, W.; Wu, Y.; Fan, J.; Djurišić, A.B.; Liu, F.; Tam, H.W.; Ng, A.; Surya, C.; Chan, W.K.; Wang, D.; et al. Understanding the doping effect on NiO: Toward high-performance inverted perovskite solar cells. Adv. Energy Mater. 2018, 8, 1703519. [Google Scholar] [CrossRef]
- Bao, H.; Du, M.; Wang, H.; Wang, K.; Zuo, X.; Liu, F.; Liu, L.; Eder, D.; Cherevan, A.; Wang, S.; et al. Samarium-doped nickel oxide for superior inverted perovskite solar cells: Insight into doping effect for electronic applications. Adv. Funct. Mater. 2021, 31, 2102452. [Google Scholar] [CrossRef]
- Thakur, U.K.; Kumar, P.; Gusarov, S.; Kobryn, A.E.; Riddell, S.; Goswami, A.; Alam, K.M.; Savela, S.; Kar, P.; Thundat, T. Consistently high Voc values in p-i-n type perovskite solar cells using Ni3+-Doped NiO nanomesh as the hole transporting layer. ACS Appl. Mater. Interfaces 2020, 12, 11467–11478. [Google Scholar] [CrossRef] [PubMed]
- Mohamadkhani, F.; Heidariramsheh, M.; Javadpour, S.; Ghavaminia, E.; Mahdavi, S.M.; Taghavinia, N. Sb2S3 and Cu3SbS4 nanocrystals as inorganic hole transporting materials in perovskite solar cells. Sol. Energy 2021, 223, 106–112. [Google Scholar] [CrossRef]
- Khorasani, A.; Marandi, M.; Khosroshahi, R.; Malekshahi Byranvand, M.; Dehghani, M.; Iraji Zad, A.; Tajabadi, F.; Taghavinia, N. Optimization of CuIn1−xGaxS2 nanoparticles and their application in the hole-transporting layer of highly efficient and stable mixed-halide perovskite solar cells. ACS Appl. Mater. Interfaces 2019, 11, 30838–30845. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zhang, M.; Xu, X.; Cai, F.; Yuan, H.; Bu, L.; Li, W.; Zhu, A.; Zhao, Z.; Wang, M.; et al. NiO nanosheets as efficient top hole transporters for carbon counter electrode based perovskite solar cells. J. Mater. Chem. A 2015, 3, 24121–24127. [Google Scholar] [CrossRef]
- Remya, R.; Gayathri, P.T.G.; Deb, B. Studies on solution-processed tungsten oxide nanostructures for efficient hole transport in the inverted polymer solar cells. Mater. Chem. Phys. 2020, 255, 123584. [Google Scholar] [CrossRef]
- Ramachandran, K.; Jeganathan, C.; Prabhakaran, R.; Wakisaka, M.; Paruthimal Kalaignan, G.; Karuppuchamy, S. High performing air stable inverted perovskite solar cells using nanostructured CuSCN thin film as hole transport material. Sol. Energy Mater. Sol. Cells 2021, 231, 111116. [Google Scholar] [CrossRef]
- Ramachandran, K.; Jeganathan, C.; Karuppuchamy, S. Surfactant assisted electrochemical growth of ultra-thin CuSCN nanowires for inverted perovskite solar cell applications. Org. Electron. 2021, 95, 106214. [Google Scholar] [CrossRef]
- Li, Z.; Yin, X.; Song, L.; Chen, W.H.; Du, P.; Li, N.; Xiong, J. NiCo2O4 arrays with a tailored morphology as hole transport layers of perovskite solar cells. Dalton Trans. 2021, 50, 5845–5852. [Google Scholar] [CrossRef]
- Cao, J.; Wu, B.; Peng, J.; Feng, X.; Li, C.; Tang, Y. Copper-copper iodide hybrid nanostructure as hole transport material for efficient and stable inverted perovskite solar cells. Sci. China Chem. 2019, 62, 363–369. [Google Scholar] [CrossRef]
- Lee, B.; Yun, A.J.; Kim, J.; Gil, B.; Shin, B.; Park, B. Aminosilane-modified CuGaO2 nanoparticles incorporated with CuSCN as a hole-transport layer for efficient and stable perovskite solar cells. Adv. Mater. Interfaces 2019, 6, 1901372. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.; Lee, Y.; Gil, B.; Yun, A.J.; Kim, J.; Woo, H.; Park, K.; Park, B. A Cu2O-CuSCN nanocomposite as a hole-transport material of perovskite solar cells for enhanced carrier transport and suppressed interfacial degradation. ACS Appl. Energy Mater. 2020, 3, 7572–7579. [Google Scholar] [CrossRef]
- Ramachandran, K.; Jeganathan, C.; Subbian, K. One-step electrodeposition of CuSCN/CuI nanocomposite and its hole transport-ability in inverted planar perovskite solar cells. Nanotechnology 2021, 32, 325402. [Google Scholar] [CrossRef]
- Hazeghi, F.; Mozaffari, S.; Ghorashi, S.M.B. Metal organic framework-derived core-shell CuO@NiO nanosphares as hole transport material in perovskite solar cell. J. Solid State Electrochem. 2020, 24, 1427–1438. [Google Scholar] [CrossRef]
- Heidariramsheh, M.; Mirhosseini, M.; Abdizadeh, K.; Mahdavi, S.M.; Taghavinia, N. Evaluating Cu2SnS3 nanoparticle layers as hole-transporting materials in perovskite solar cells. ACS Appl. Energy Mater. 2021, 4, 5560–5573. [Google Scholar] [CrossRef]
- Ramachandran, K.; Jeganathan, C.; Karuppuchamy, S. Electrodeposition of nanostructured bilayer CuI@CuSCN as hole transport material for highly efficient inverted perovskite solar cells. J. Alloy Compd. 2021, 881, 160530. [Google Scholar] [CrossRef]
- Ramachandran, K.; Jeganathan, C.; Kalaignan, G.P.; Karuppuchamy, S. Nanostructured bilayer CuSCN@CuI thin films as efficient inorganic hole transport material for inverted perovskite solar cells. Ceram. Int. 2021, 47, 17883–17894. [Google Scholar] [CrossRef]
- Seo, S.; Park, I.J.; Kim, M.; Lee, S.; Bae, C.; Jung, H.S.; Park, N.-G.; Kim, J.Y.; Shin, H. An ultra-thin, un-doped NiO hole transporting layer of highly efficient (16.4%) organic-inorganic hybrid perovskite solar cells. Nanoscale 2016, 8, 11403–11412. [Google Scholar] [CrossRef]
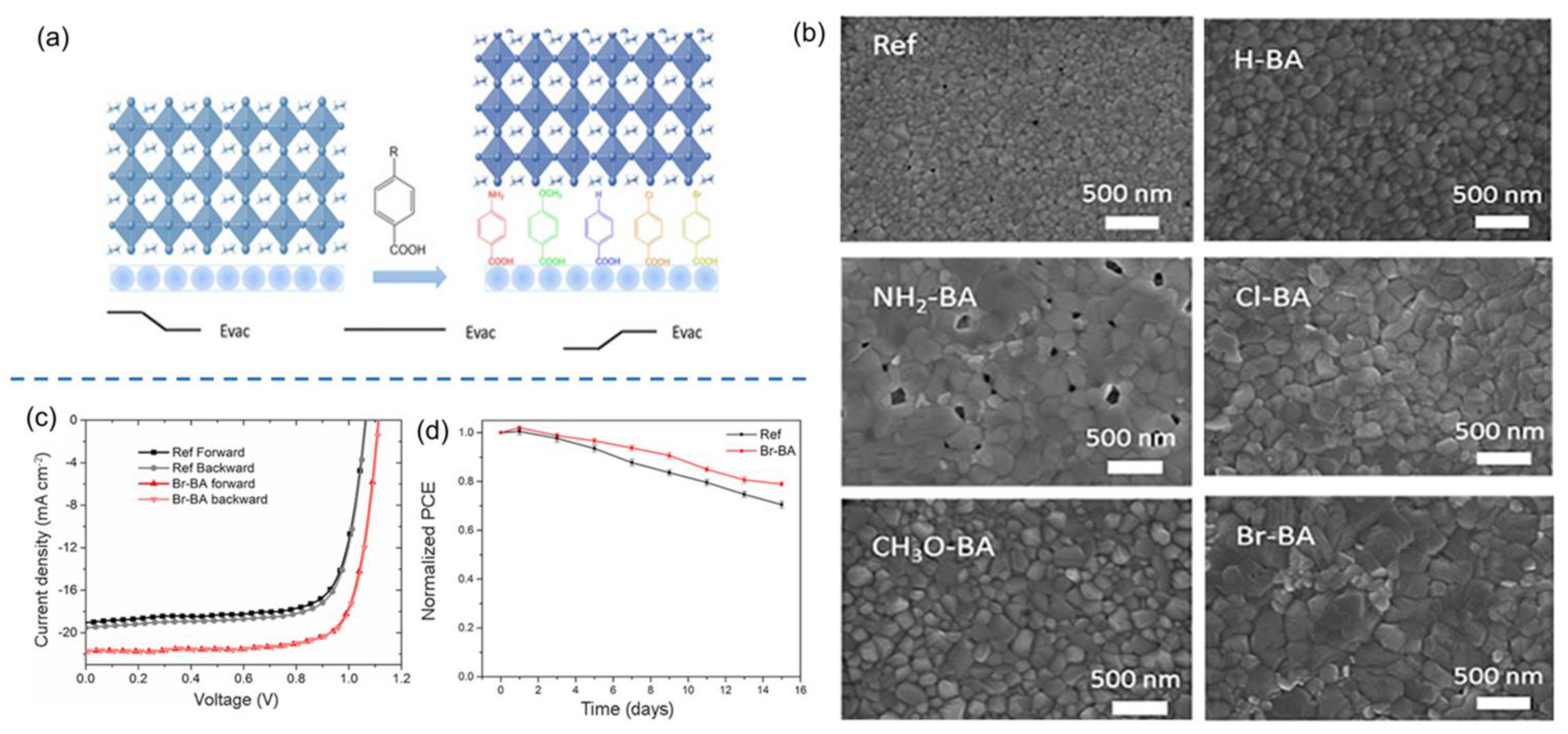
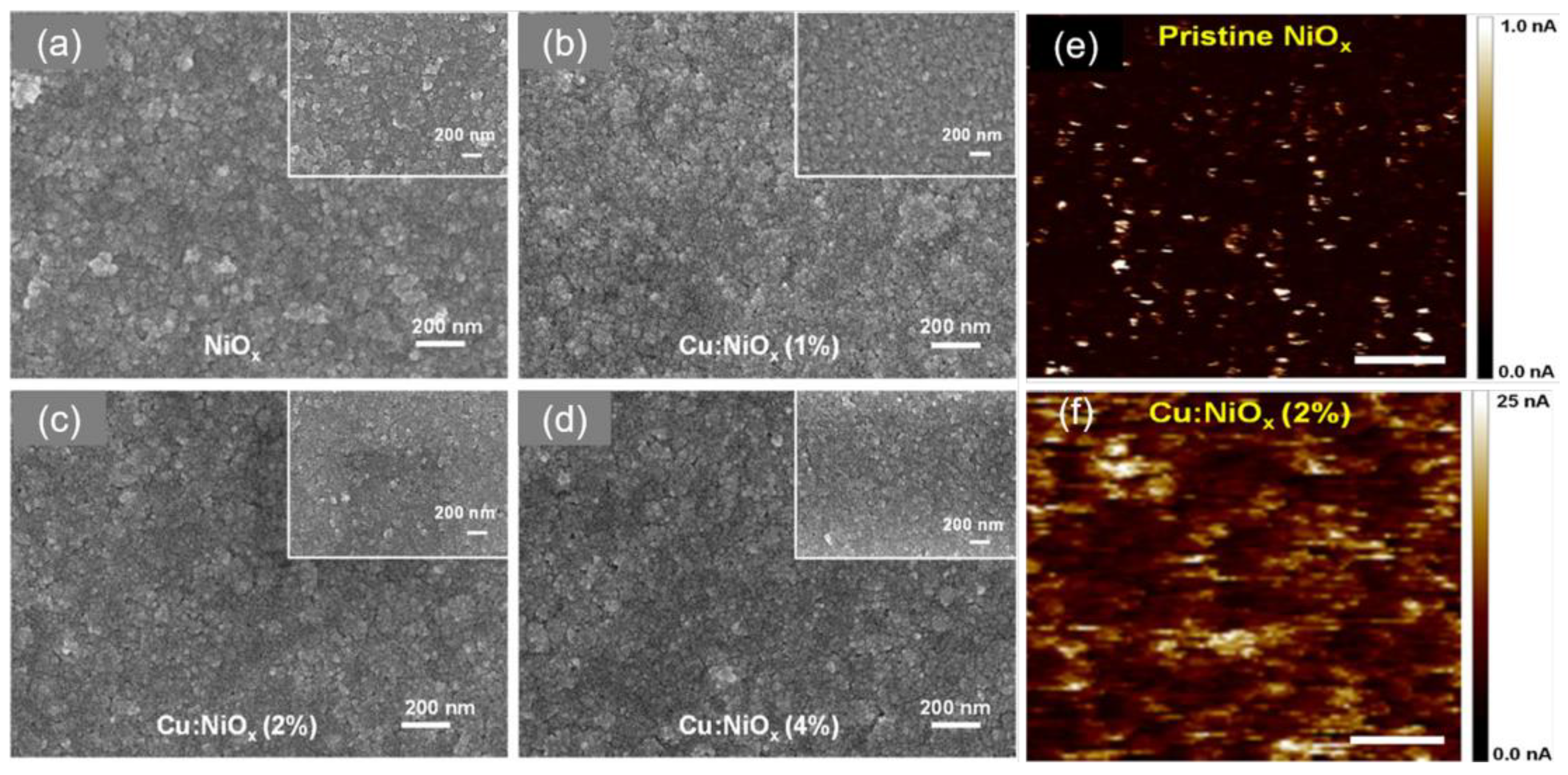
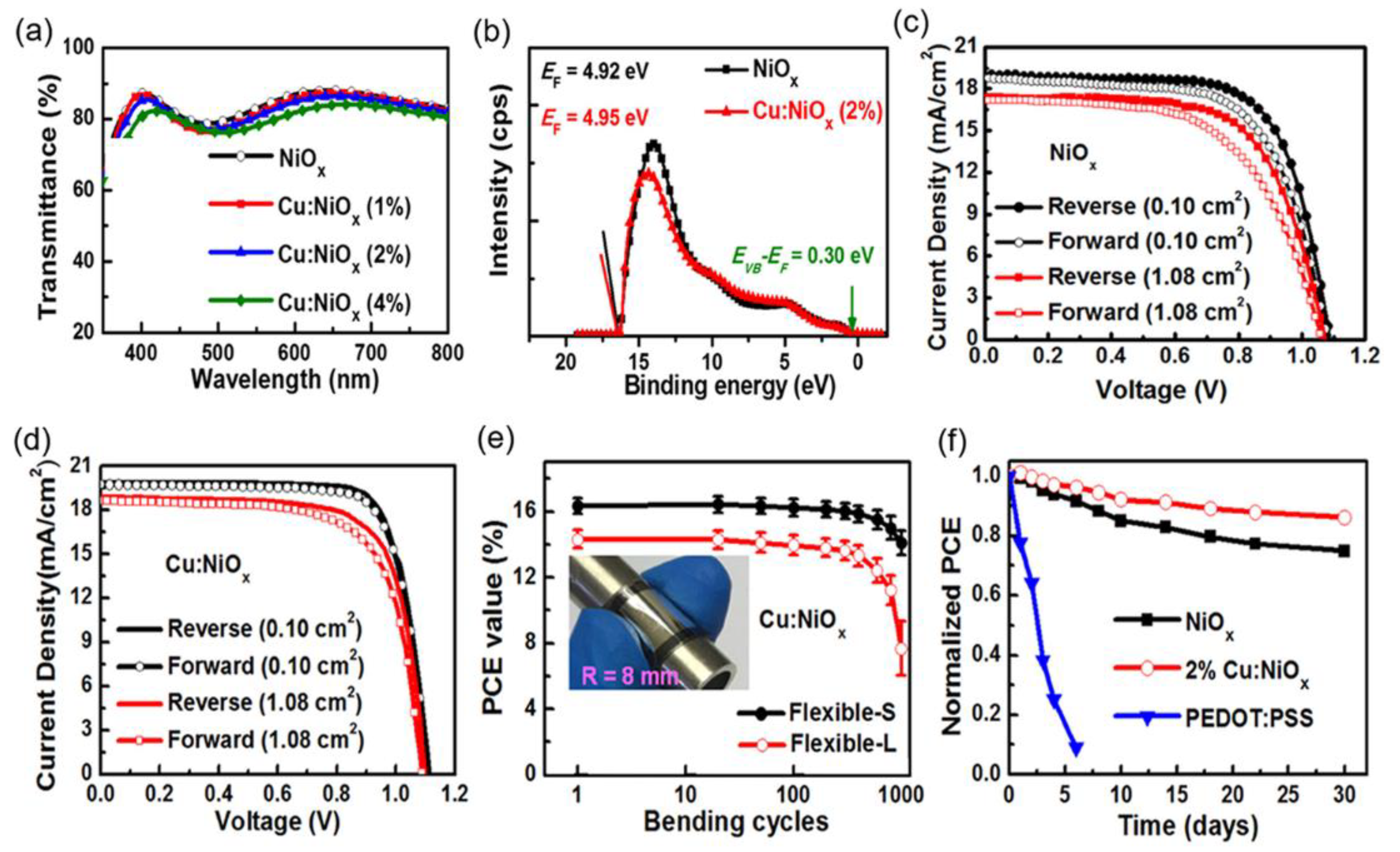

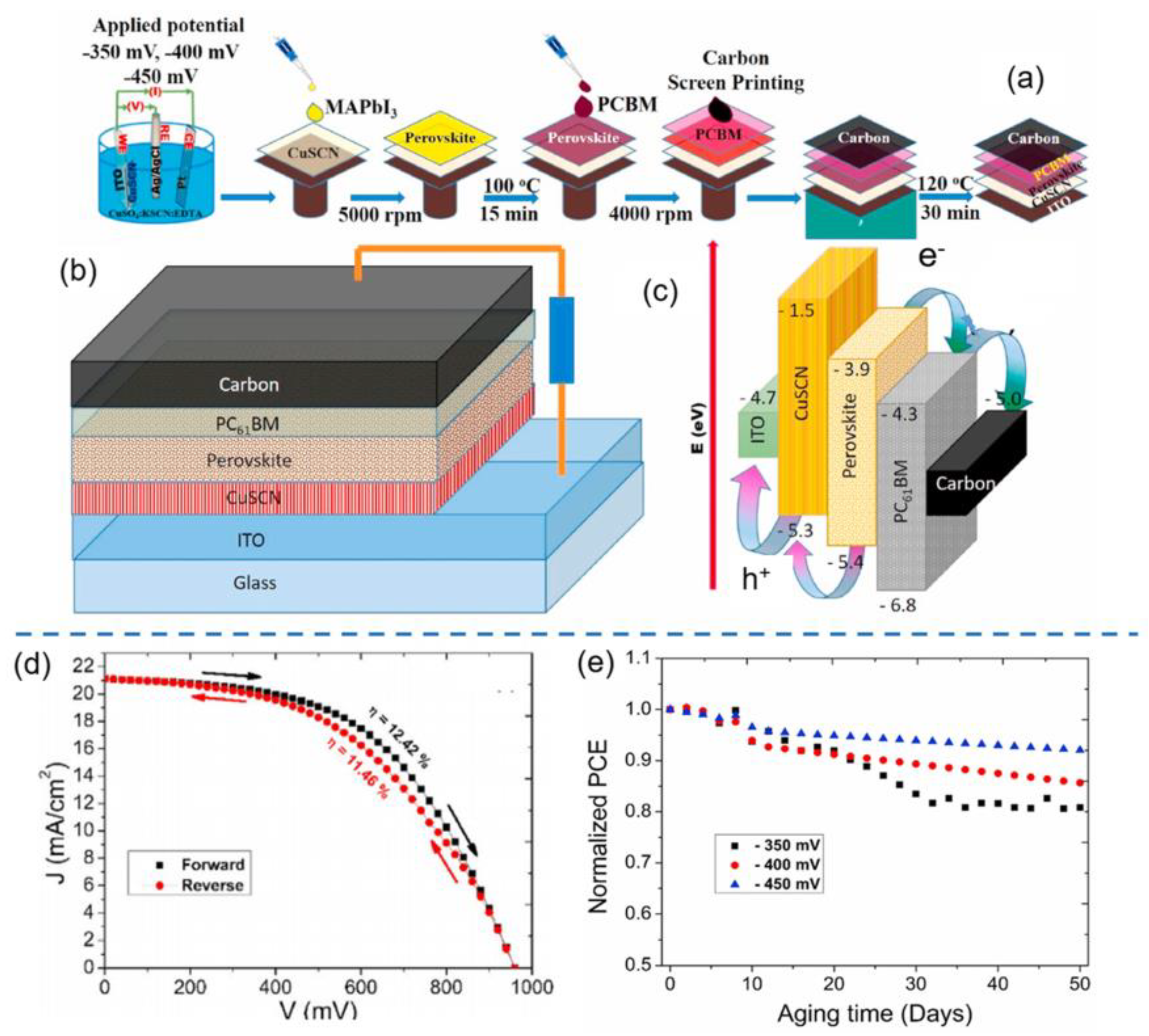
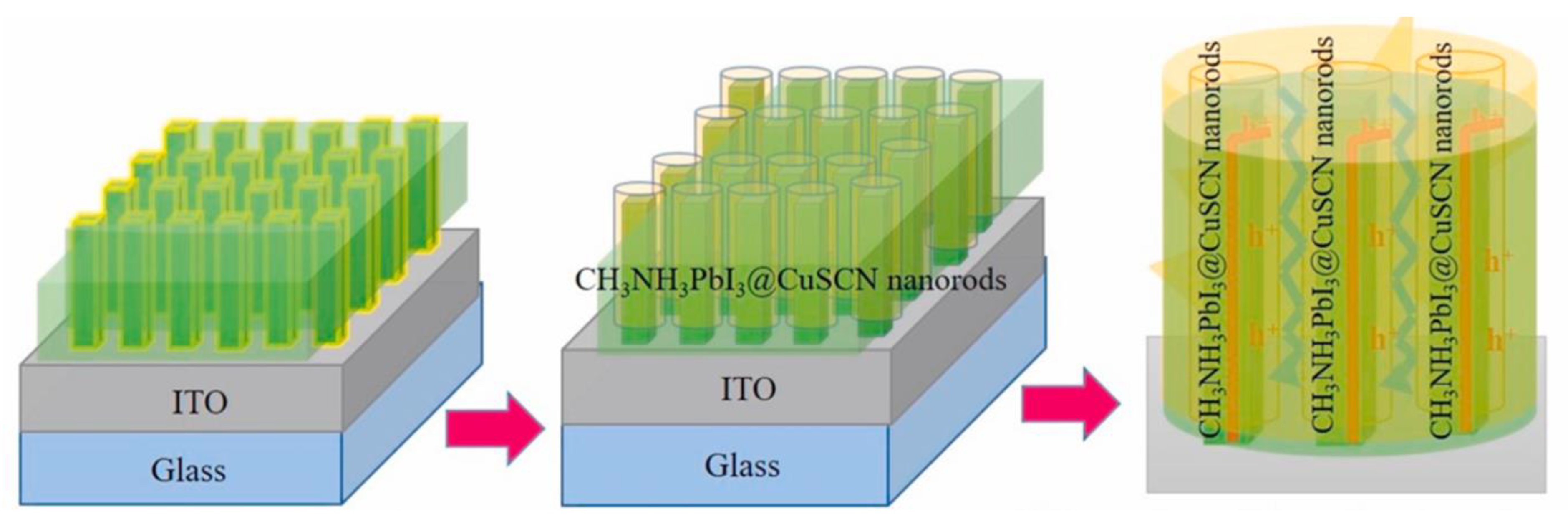
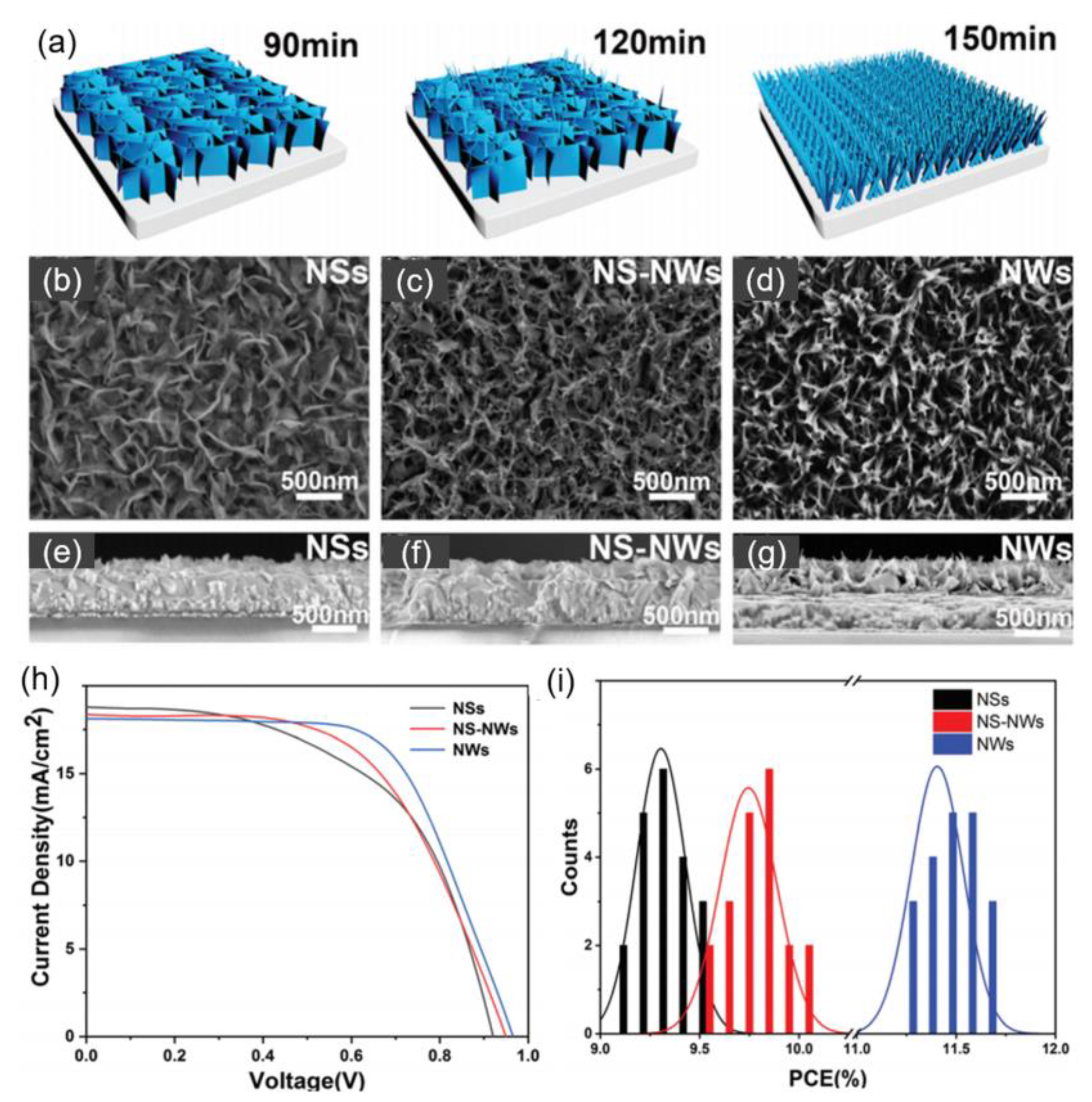
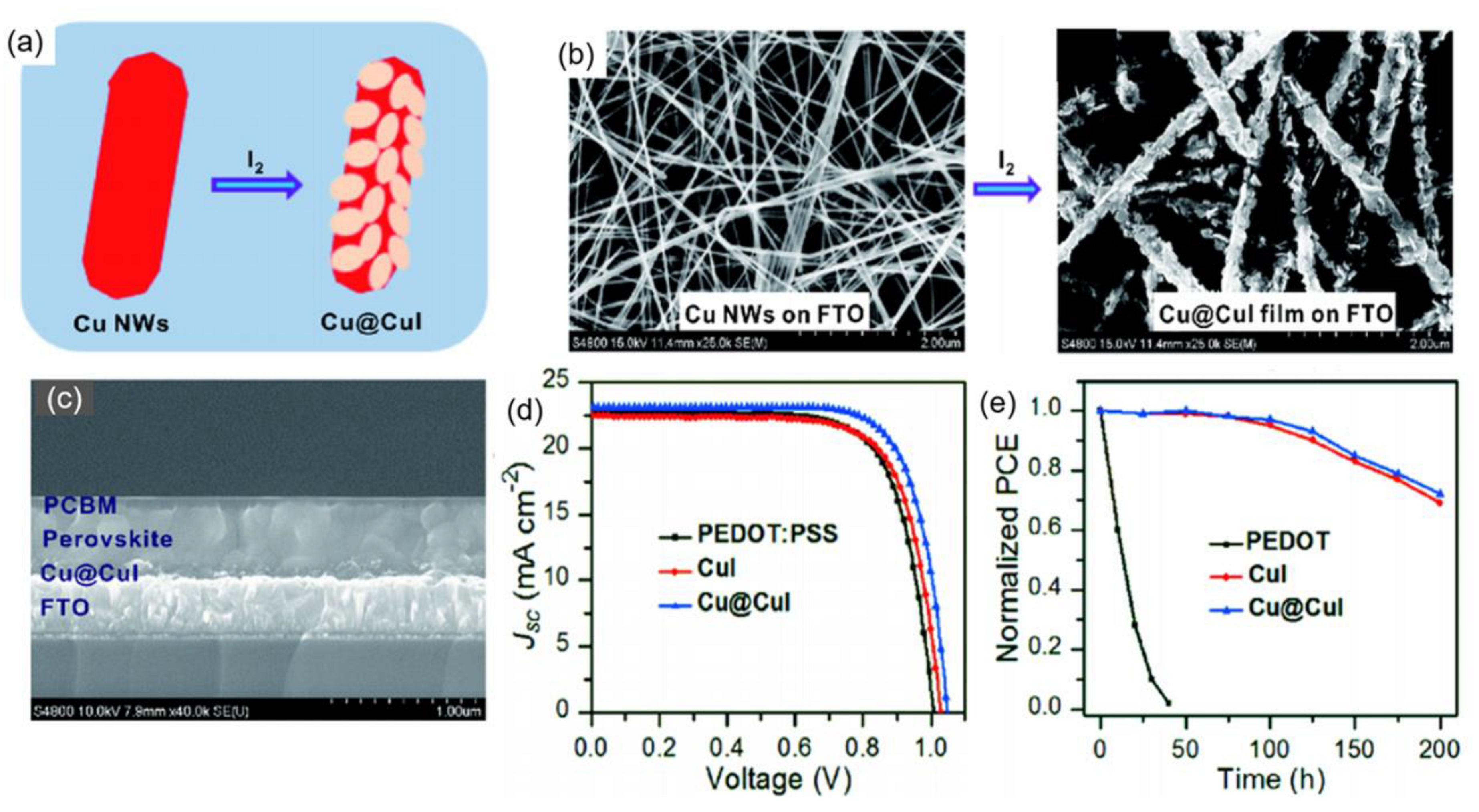
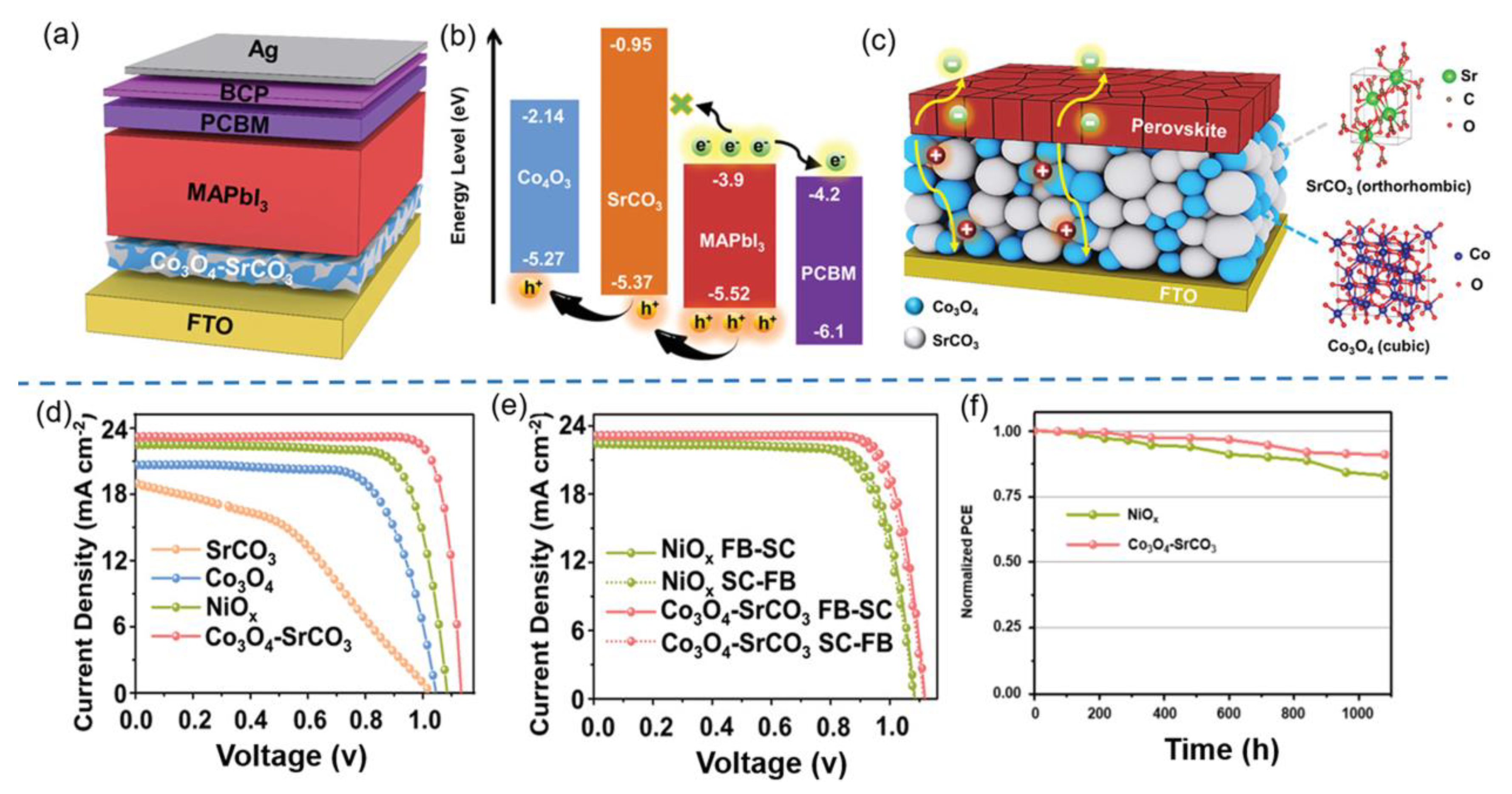


| HTL | Cell Configuration | Hole Mobility (cm2 V−1 s−1) | PCE (%) | Ref |
|---|---|---|---|---|
| Co-NiO NPs | ITO/Co-NiO NPs/MAPbI3/PCBM/Ag | / | 14.50 | [121] |
| Fe-NiOx NPs | ITO/Fe-NiOx NPs/MAPbI3/PCBM/BCP/Ag | 6.22 | 17.57 | [122] |
| Ni3+-NiO NSs | FTO/Ni3+-NiO NSs/FA0.83MA0.17PbI2.49Br0.51/PCBM/ZnO/Al | / | 17.75 | [117] |
| Cu-NiOx NPs | ITO/Cu-NiOx NPs/MAPbI3/PCBM/BCP/Ag | 1.05 × 10−2 | 18.58 | [63] |
| Cu:NiO NPs | ITO/Cu:NiO NPs/MAPbI3/C60/BCP/Ag | 2.53 | 20.15 | [123] |
| Sm:NiOx | ITO/Sm:NiOx/FA0.92Cs0.08PbI3 | / | 20.71 | [124] |
| Cu3SbS4 NCs | FTO/c-TiO2/m-TiO2/Cs0.0475FA0.7885MA0.1615PbI2.49Br0.51/Cu3SbS4 NCs/Au | / | 13.00 | [126] |
| CuIn0.5Ga0.5S2 NPs | FTO/TiO2/Cs0.0475FA0.7885MA0.1615 PbI2.49 Br0.51/CuIn0.5Ga0.5S2 NPs/Au | 0.4 × 10−3 | 15.58 | [127] |
| HTL | Cell Configuration | Ambient Stability (PCE Retention Ratio) | PCE (%) | Ref |
|---|---|---|---|---|
| CuSCN NRs | ITO/CuSCN NRs/MAPbI3/PCBM/Carbon | 80% after 1200 h | 12.42 | [130] |
| CuSCN hexagonal prisms | ITO/CuSCN hexagonal prisms/MAPbI3/C60/Bphen/Ag | / | 11.40 | [69] |
| CuSCN NWs | ITO/CuSCN NWs/MAPbI3/PC61BM/Carbon | ~100% after 1272 h | 16.99 | [131] |
| NiCo2O4 NWs | ITO/NiCo2O4 NWs/MAPbI3/PC61BM/Ag | 80% after 240 h | 11.58 | [132] |
| WO3 NSs | ITO/Nafion modified-ZnO/MAPbI3/P3HT:PCBM/WO3 NSs/Ag | ~100% after 240 h | 7.76 | [129] |
| HTL | Cell Configuration | Ambient Stability (PCE Retention Ratio) | PCE (%) | Ref |
|---|---|---|---|---|
| Cu@CuI | FTO/Cu@CuI/CsFAMAPb(BrI)3 | 70% after 200 h | 18.8 | [133] |
| CuCaO2–CuSCN | ITO/SnO2/Cs0.0475FA0.7885MA0.1615PbI2.49 Br0.51/CuCaO2-CuSCN/Au | 80% after 400 h | 16.70 | [134] |
| Cu2O–CuSCN | ITO/SnO2/Cs0.0475FA0.7885MA0.1615PbI2.49 Br0.51/Cu2O-CuSCN/Au | >90% after 720 h | 19.20 | [135] |
| CuSCN/CuI | ITO/CuSCN/CuI/MAPbI3/PCBM/Carbon | 95% after 1400 h | 18.82 | [136] |
| NiO@GeSe | ITO/TiO2/NiO@GeSe/MAPbI3/CuSCN/Au | / | 20.29 | [66] |
| Co3O4–SrCO3 | FTO/Co3O4-SrCO3/MAPbI3/PCBM/BCP/Ag | ~100% after 300 h | 21.84 | [67] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, D.; Xiang, H.; Ran, R.; Wang, W.; Zhou, W.; Shao, Z. Recent Advances in Nanostructured Inorganic Hole-Transporting Materials for Perovskite Solar Cells. Nanomaterials 2022, 12, 2592. https://doi.org/10.3390/nano12152592
Huang D, Xiang H, Ran R, Wang W, Zhou W, Shao Z. Recent Advances in Nanostructured Inorganic Hole-Transporting Materials for Perovskite Solar Cells. Nanomaterials. 2022; 12(15):2592. https://doi.org/10.3390/nano12152592
Chicago/Turabian StyleHuang, Dingyan, Huimin Xiang, Ran Ran, Wei Wang, Wei Zhou, and Zongping Shao. 2022. "Recent Advances in Nanostructured Inorganic Hole-Transporting Materials for Perovskite Solar Cells" Nanomaterials 12, no. 15: 2592. https://doi.org/10.3390/nano12152592
APA StyleHuang, D., Xiang, H., Ran, R., Wang, W., Zhou, W., & Shao, Z. (2022). Recent Advances in Nanostructured Inorganic Hole-Transporting Materials for Perovskite Solar Cells. Nanomaterials, 12(15), 2592. https://doi.org/10.3390/nano12152592