Influence of Nanoparticles on Thermophysical Properties of Hybrid Nanofluids of Different Volume Fractions
Abstract
1. Introduction
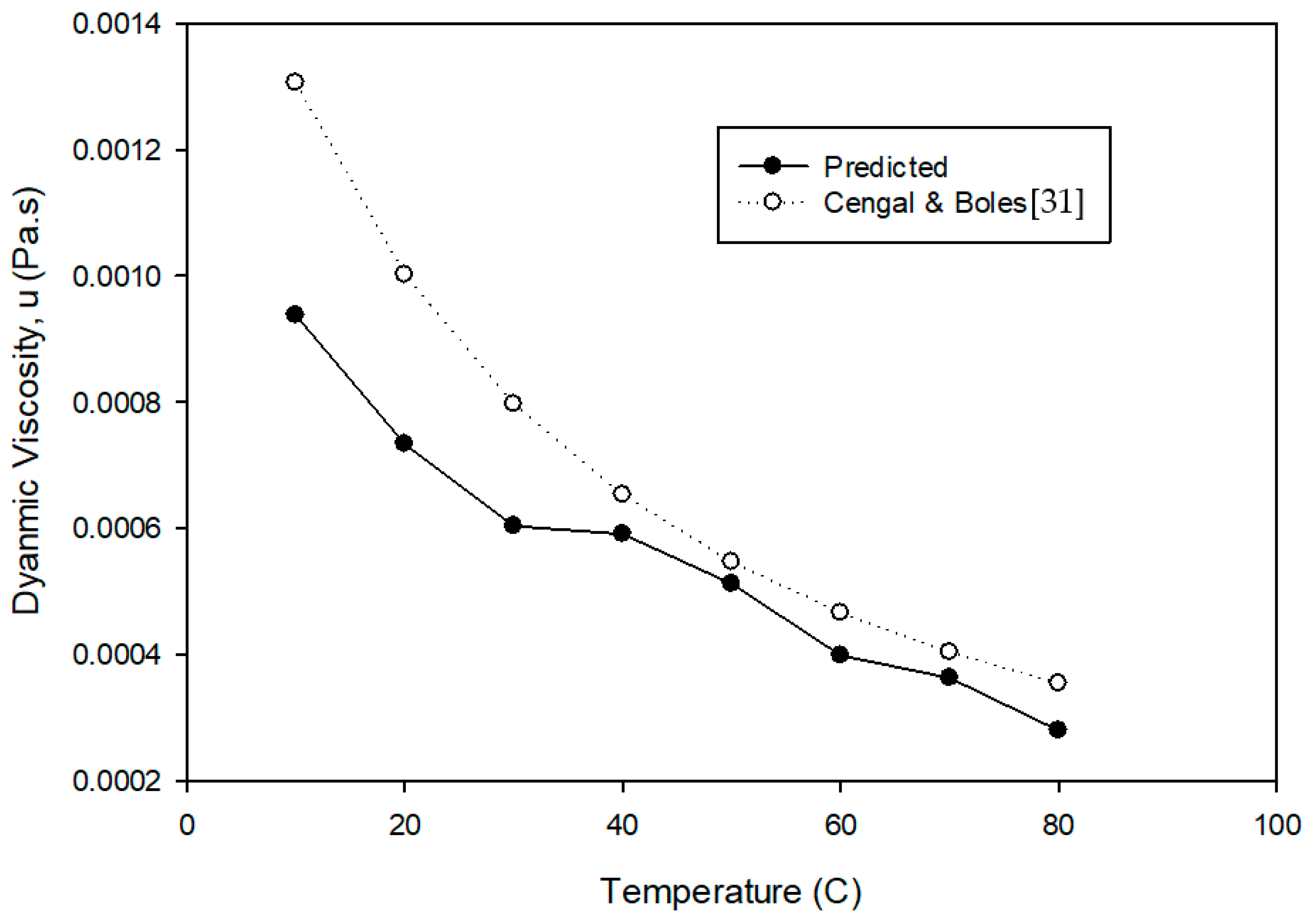
2. Dynamic Viscosity
2.1. Thermal Conductivity
2.2. Isobaric Specific Heat Capacity
2.3. Atomic and Molecular Set-Up
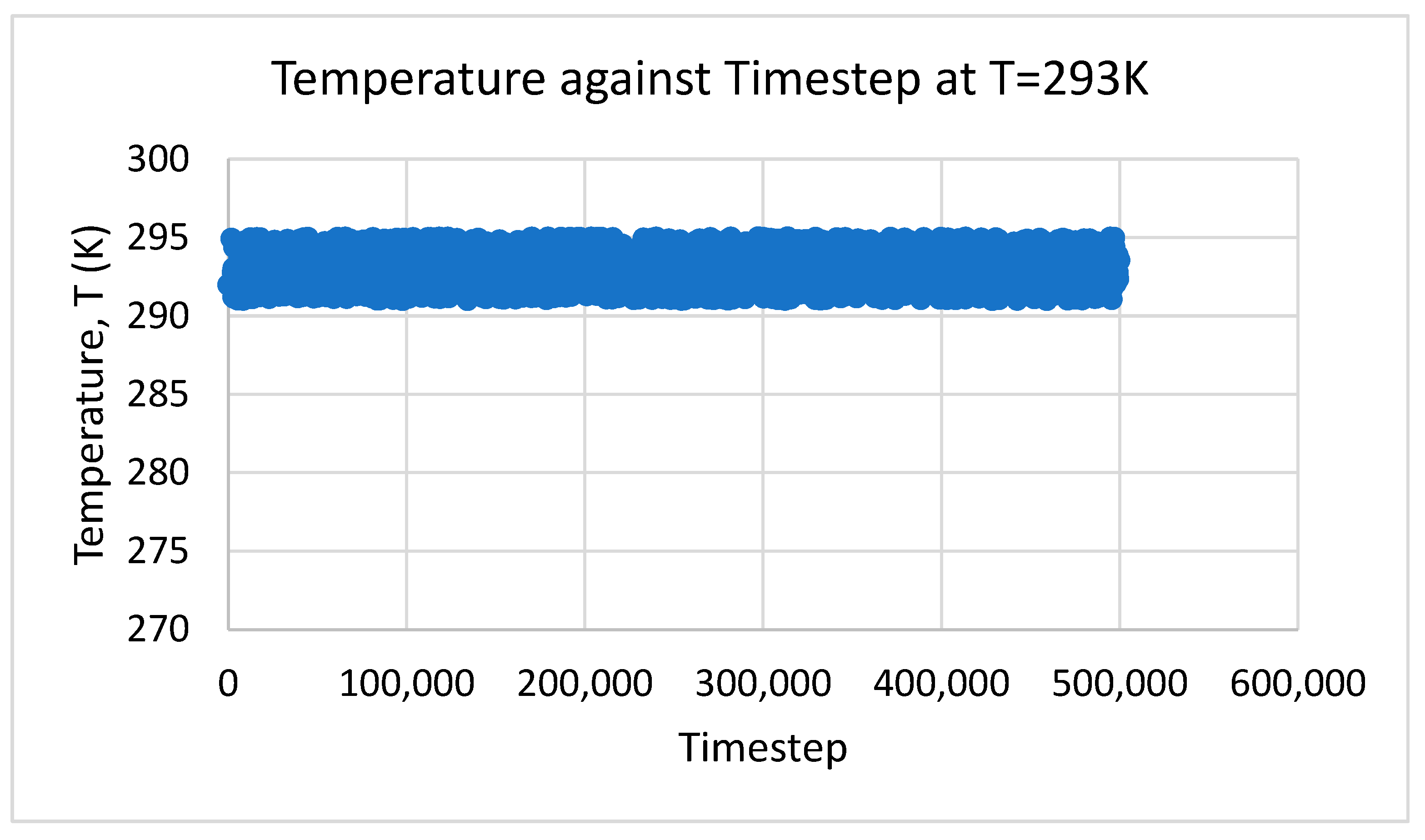
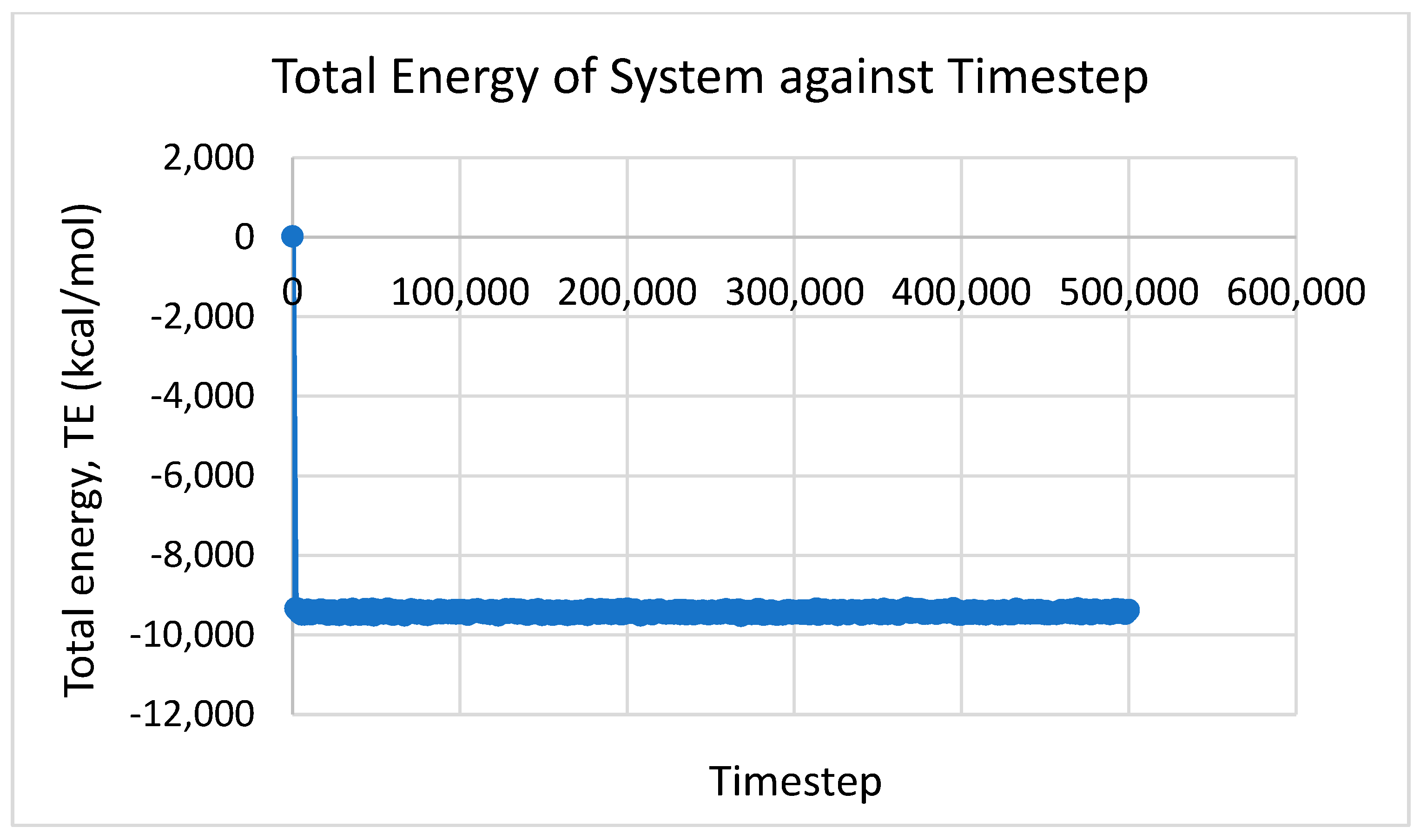
3. Results and Discussion
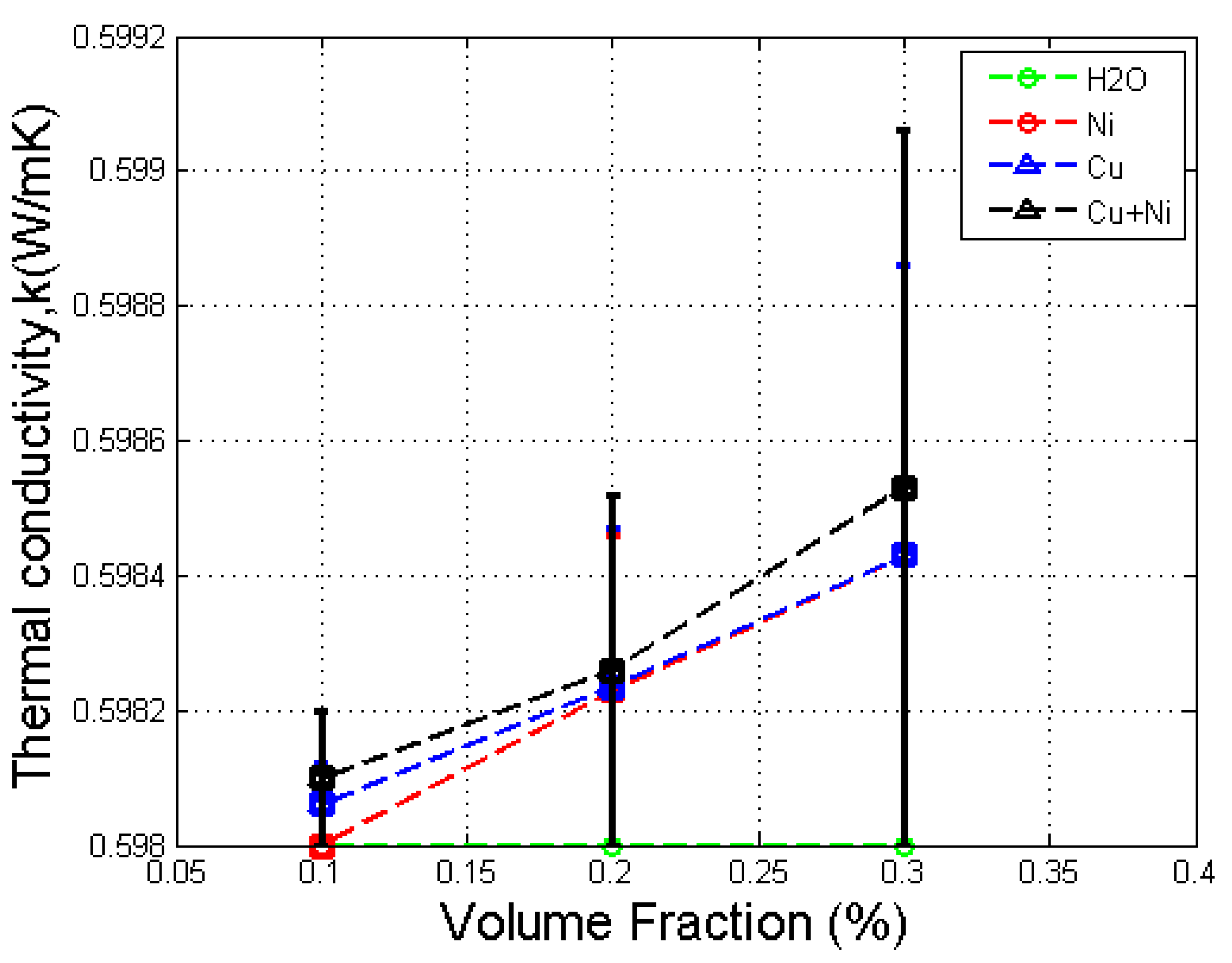
3.1. Effect of Volume Fraction in Thermal Conductivity
3.2. Effect of Volume Fraction in Specific Heat
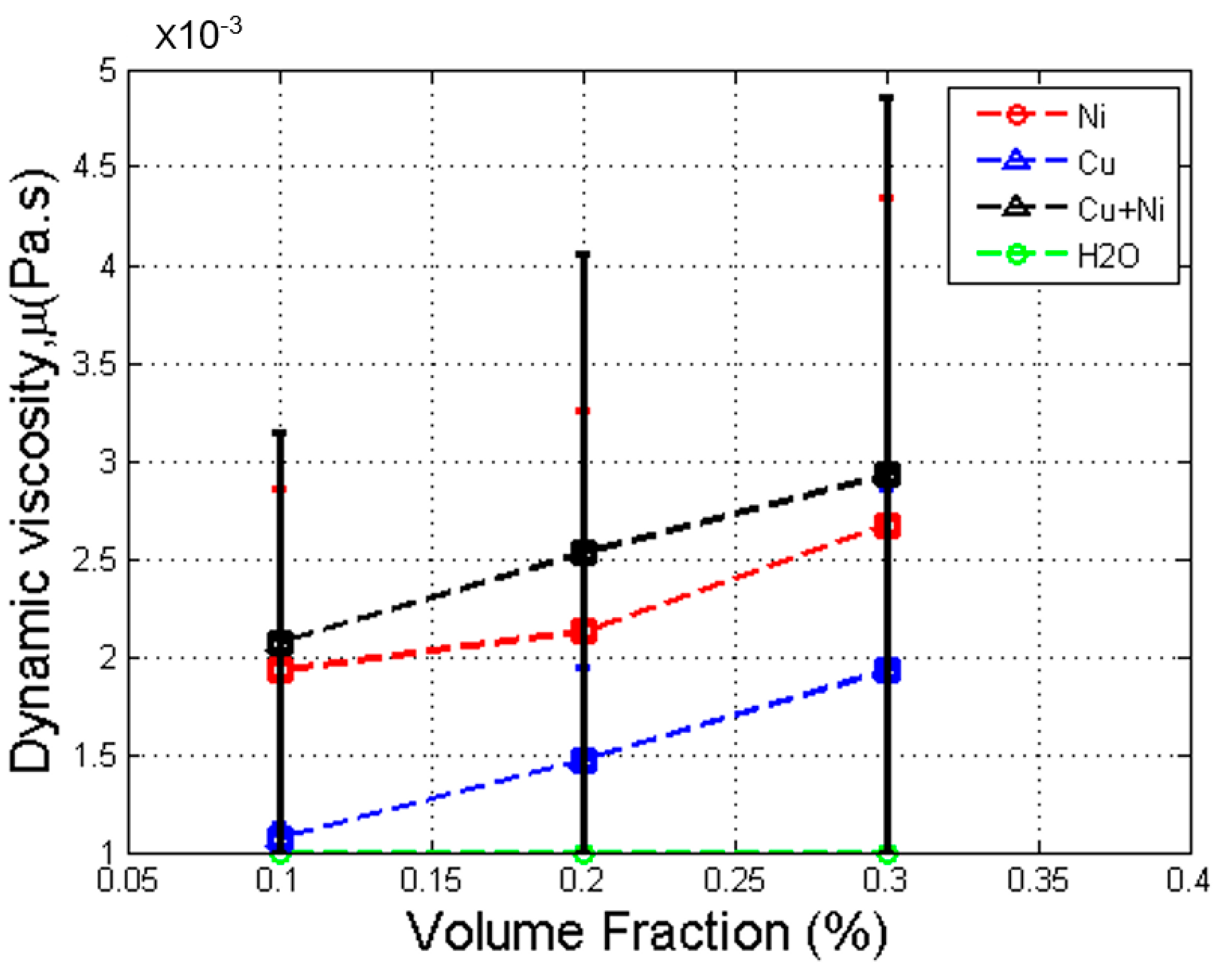
3.3. Effect of Volume Fraction in Dynamic Viscosity
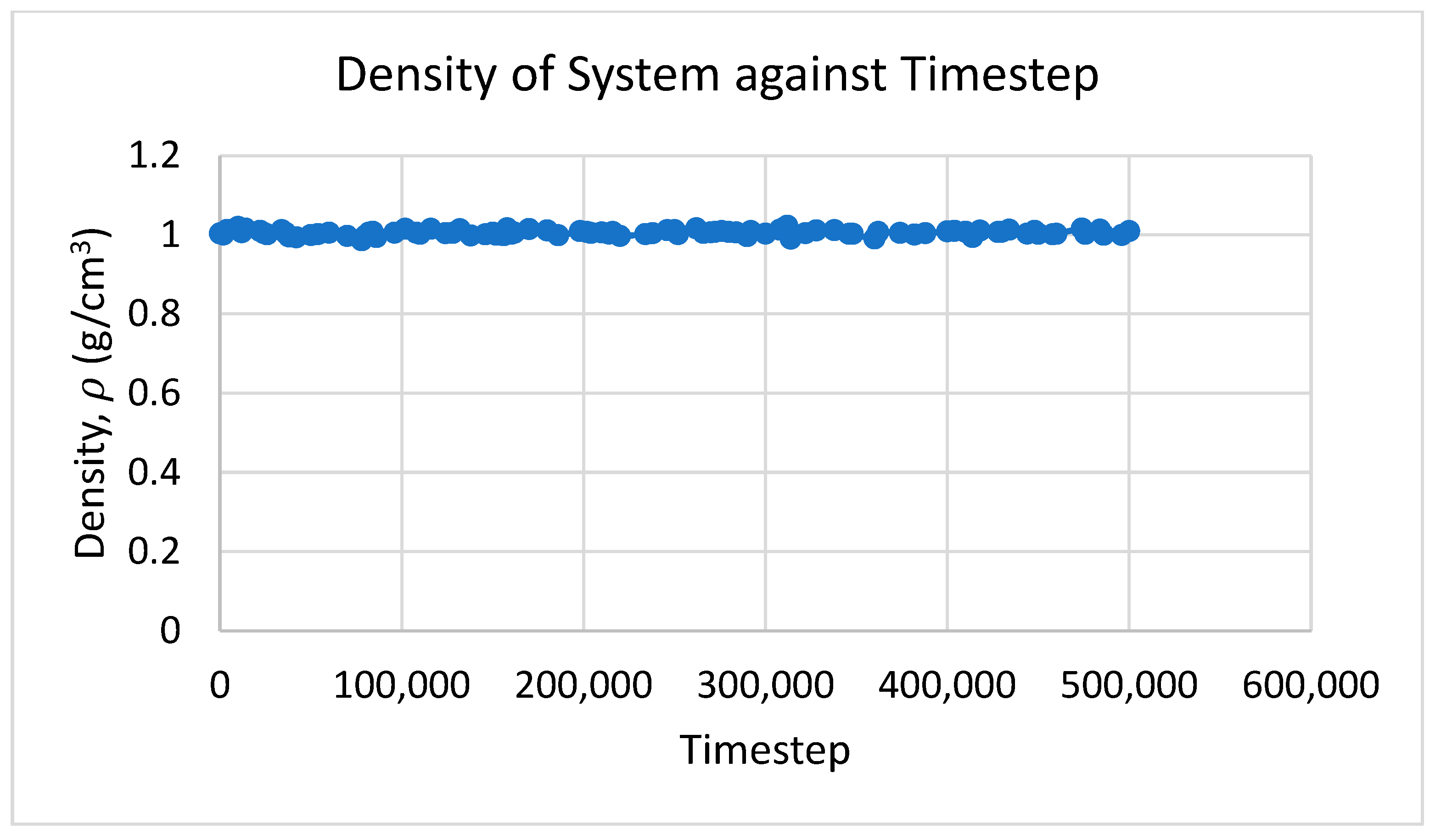
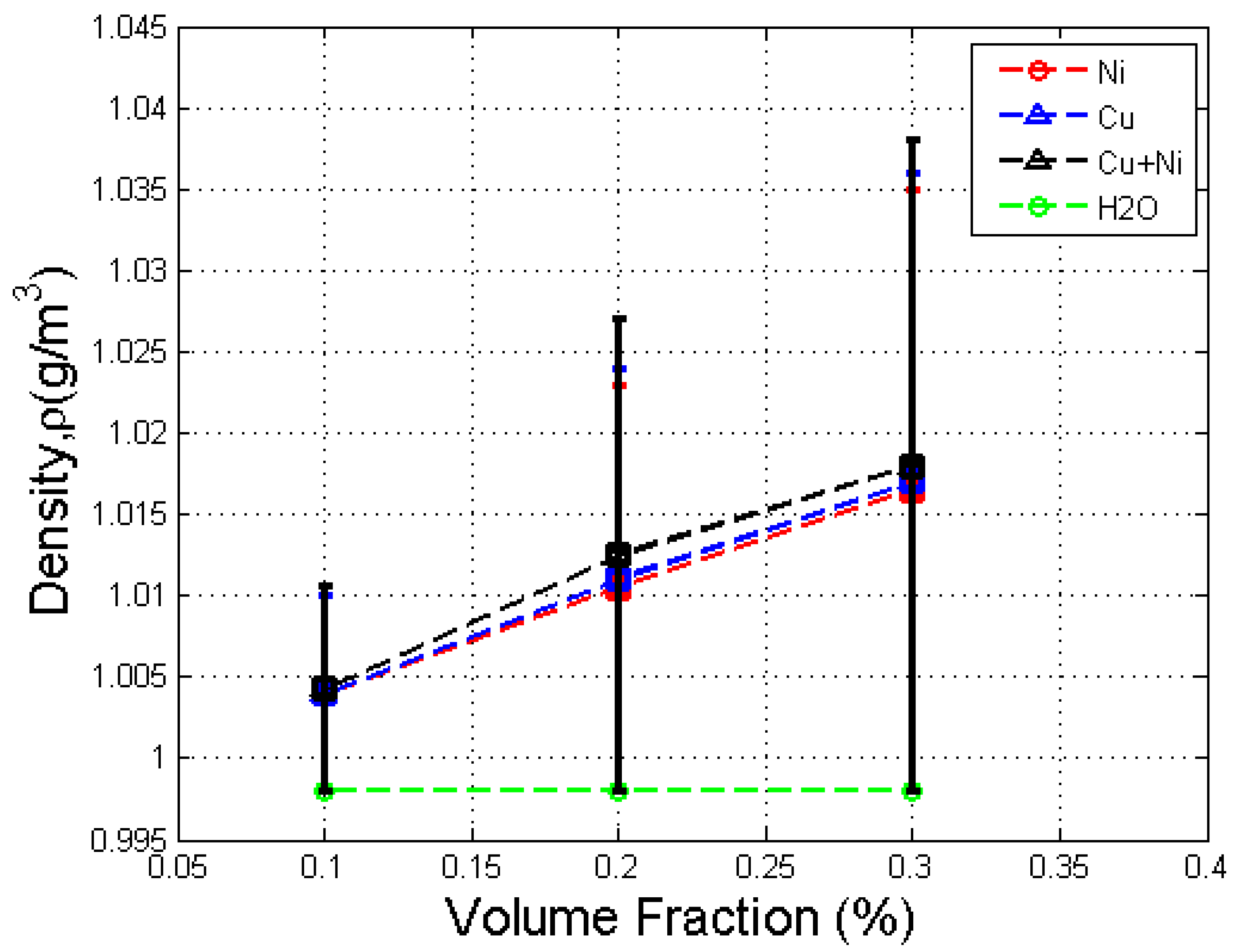
3.4. Effect of Volume Fraction on Density
4. Conclusions
- ➢
- The thermal conductivity of the Cu–Ni hybrid nanofluid increased with volume fraction and was larger than that of the base fluid and both mono nanofluids.
- ➢
- The specific heat of the Cu–Ni hybrid nanofluid decreased with volume fraction and was lower than that of the base fluid but higher than that of both mono nanofluids.
- ➢
- The viscosity of the Cu–Ni hybrid nanofluid increased with volume fraction and was higher than that of the base fluid and both mono nanofluids.
- ➢
- The density of the Cu–Ni hybrid nanofluid increased with volume fraction and was higher than that of the base fluid and the Ni nanofluid but lower than that of the Cu nanofluid.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Iijima, M.; Kamiya, H. Surface modification for improving the stability of nanoparticles in liquid media. KONA Poweder Part. J. 2009, 27, 119–129. [Google Scholar] [CrossRef]
- Choi, S.U.S.; Eastman, J.A. Enhancing thermal conductivity of fluids with nanoparticles. In Proceedings of the International Mechanical Engineering Congress and Exposition, San Francisco, CA, USA, 12–17 November 1995. [Google Scholar]
- Tiwari, A.K.; Gosh, P.; Sarkar, J. Performance comparison of the plate heat exchanger using different nanofluids. Exp. Therm. Fluid Sci. 2013, 49, 141–151. [Google Scholar] [CrossRef]
- Zhao, C.; Chen, Y.K.; Ren, G. A study of tribological properties of water-based ceria nanofluids. Tribol. Trans. 2013, 56, 275–283. [Google Scholar] [CrossRef]
- Hayat, T.; Nadeem, S. Heat transfer enhancement with Ag–CuO/water hybrid nanofluid. Results Phys. 2017, 7, 2317–2324. [Google Scholar] [CrossRef]
- Wilk, J.; Smusz, R.; Grosicki, S. Thermophysical properties of water based Cu nanofluid used in special type of coil heat exchanger. Appl. Therm. Eng. 2017, 127, 933–943. [Google Scholar] [CrossRef]
- Mostafizur, R.M.; Saidur, R.; Abdul Aziz, A.R.; Bhuiyan, M.H.U. Investigation on stability and density of methanol based TiO2 nanofluids. IOP Conf. Ser. Mater. Sci. Eng. 2015, 88, 012057. [Google Scholar] [CrossRef]
- Fadhilah, S.A.; Hidayat, I.; Hilwa, M.Z.; Faizah, H.N.; Marhamah, R.S. Thermophysical properties of Copper/Water nanofluid for automotive cooling system–Mathematical modeling. J. Mech. Eng. Technol. 2013, 5, 27–39. [Google Scholar]
- Sundar, L.S.; Singh, M.K.; Bidkin, I.; Sousa, A.C.M. Experimental investigations in heat transfer and friction factor of magnetic Ni nanofluid flowing in a tube. Int. J. Heat Mass Transf. 2014, 70, 224–234. [Google Scholar] [CrossRef]
- Rajabpour, A.; Akizi, F.Y.; Heyhat, M.M.; Gordiz, K. Molecular dynamics simulation of the specific heat capacity of water-Cu nanofluids. Int. Nano Lett. 2013, 3, 1–6. [Google Scholar] [CrossRef]
- Shahrul, I.; Mahbubul, I.M.; Khaleduzzaman, S.S.; Saidur, R.; Sabri, M.F.M. A comparative review on the specific heat of nanofluids for energy perspective. Renew. Sustain. Energy Rev. 2014, 38, 88–98. [Google Scholar] [CrossRef]
- Pak, B.C.; Cho, Y.I. Hydrodynamic and heat transfer study of dispersed fluids with submicron metallic oxide particles. Exp. Heat Transf. 2007, 11, 151–170. [Google Scholar] [CrossRef]
- Chaupis, J.E.R.; Oliveira, G.A.; Filho, E.P.B. Numerical analysis of nanofluids flowing in a straight pipe. In Proceedings of the 21st Brazillian Congress of Mechanical Engineering, Natal, Brazil, 24–28 October 2011. [Google Scholar]
- Heris, S.Z.; Nole, S.H.; Talaii, E.; Sargolzaei, J. Numerical Investigation of Al2O3/water Nanofluid laminar convective heat transfer through triangular ducts. Nanoscale Res. Lett. 2011, 6, 179. [Google Scholar] [CrossRef] [PubMed]
- Takabi, B.; Salehi, S. Augmentation of the heat transfer performance of a sinusoidal corrugated enclosure by employing hybrid nanofluid. Adv. Mech. Eng. 2014, 6, 147059. [Google Scholar]
- Sundar, L.S.; Singh, M.K.; Sousa, A.C.M. Enhanced heat transfer and friction factor of MWCNT–Fe3O4/water hybrid nanofluids. Int. Commun. Heat Mass Transf. 2014, 52, 73–83. [Google Scholar] [CrossRef]
- Sundar, L.S.; Ramana, E.V.; Graca, M.P.F.; Singh, M.K.; Sousa, A.C.M. Nanodiamond-Fe3O4 nanofluids: Preparation and measurement of viscosity, electrical and thermal conductivities. Int. Commun. Heat Mass Transf. 2016, 73, 62–74. [Google Scholar] [CrossRef]
- Sarkar, J.; Ghosh, P.; Adil, A. A review on hybrid nanofluids: Recent research, development and applications. Renew. Sustain. Energy Rev. 2015, 43, 164–177. [Google Scholar] [CrossRef]
- Gao, Y.; Xi, Y.; Yang, Z.; Sasmito, A.P.; Mujumdar, A.S.; Wang, L. Experimental investigation of specific heat of aqueous graphene oxide Al2O3 hybrid nanofluid. Therm. Sci. 2021, 25, 515–525. [Google Scholar] [CrossRef]
- Suresh, S.; Venkitaraj, K.P.; Selvakumar, P.; Chandrasekar, M. Effect of Al2O3–Cu/water hybrid nanofluid in heat transfer. Exp. Therm. Fluid Sci. 2012, 38, 54–60. [Google Scholar] [CrossRef]
- Babar, H.; Sajid, M.U.; Ali, H. Viscosity of hybrid nanofluids: A critical review. Therm. Sci. 2019, 23, 1713–1754. [Google Scholar] [CrossRef]
- Wang, H.; Chen, X. Numerical simulation of heat transfer and flow of Al2O3-water nanofluid in microchannel heat sink with cantor fractal structure based on genetic algorithm. Anal. Chim. Acta 2022, 1221, 339927. [Google Scholar] [CrossRef]
- Kirova, E.M.; Norman, G.E. Viscosity calculations at molecular dynamics simulations. J. Phys. Conf. Ser. Mater. Sci. Eng. 2015, 653, 012106. [Google Scholar] [CrossRef]
- Alkhwaji, A.; Elbahloul, S.; Abdullah, M.Z.; Abu Bakar, K.F.B. Selected water properties from molecular dynamics for engineering purposes. Mol. Liq. 2021, 324, 114703. [Google Scholar] [CrossRef]
- Alkhwaji, A.; Elbahloul, S.; Abu Bakar, K.F.B.; Abdullah, M.Z. The comparison between water models in predicting water thermal and dynamic properties from molecular dynamics. Int. J. Sci. Technol. Res. 2020, 9, 511–516. [Google Scholar]
- Berendsen, H.J.C.; Grigera, J.R.; Straatsma, T.P. The missing term in effective pair potentials. Phys. Chem. 1987, 91, 6269–6271. [Google Scholar] [CrossRef]
- Jones, J.E. On the Determination of Molecular Fields. Proc. R. Soc. A-Math. Phys. Eng. Sci. 1924, 106, 441–462. [Google Scholar]
- Allen, M.P.; Tildesley, D.J. Computer Simulation of Liquids; Oxford Science Publications: Oxford, UK, 1989. [Google Scholar]
- Kang, H.; Zhang, Y.; Yang, M. Molecular dynamics simulation of thermal conductivity of Cu-Ar nanofluid using EAM potential for Cu-Cu interactions. Appl. Phys. A 2011, 103, 1001–1008. [Google Scholar] [CrossRef]
- Sahu, R.K.; Somashekhar, S.H.; Manivannan, P.V. Investigation on copper nanofluid obtained through micro electrical discharge machining for dispersion stability and thermal conductivity. Procedia Eng. 2013, 64, 946–955. [Google Scholar] [CrossRef][Green Version]
- Cengal, Y.A.; Boles, M.A. Thermodynamics-An Engineering Approach, 4th ed.; McGrawhill: New York, NY, USA, 2002. [Google Scholar]
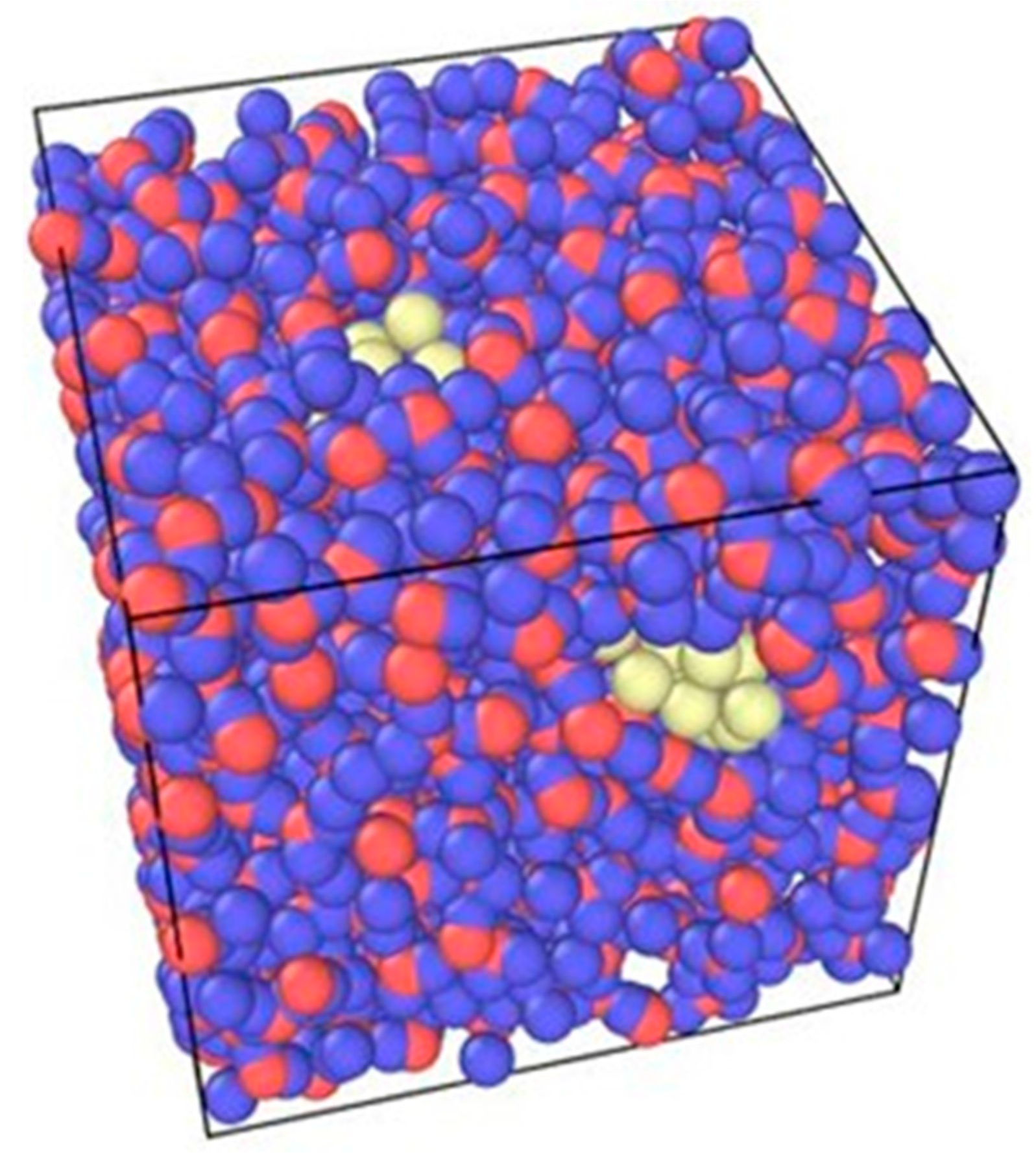

| Type of Particle | Molar Mass (g/mol) |
|---|---|
| Hydrogen (H) | 1.008 |
| Oxygen (O) | 15.9994 |
| Copper (Cu) | 63.55 |
| Nickel (Ni) | 58.6934 |
| Interactions | Name of EAM Potential File |
|---|---|
| Cu-Cu | Cu_zhou.eam.alloy |
| Ni-Ni | NiAlH_jea.eam.alloy |
| Cross-Interactions | Interaction Strength, ε (kcal/mol) | Interaction Length Scale, σ (A) |
|---|---|---|
| Cu-H2O | 0.80 | 1.60 |
| Ni-H2O | 0.80 | 1.30 |
| Cu-Ni | 1.00 | 0.70 |
| Fluid | [28] | |
|---|---|---|
| Water | 0.99656 | 0.9982 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdullah, M.Z.; Yu, K.H.; Loh, H.Y.; Kamarudin, R.; Gunnasegaran, P.; Alkhwaji, A. Influence of Nanoparticles on Thermophysical Properties of Hybrid Nanofluids of Different Volume Fractions. Nanomaterials 2022, 12, 2570. https://doi.org/10.3390/nano12152570
Abdullah MZ, Yu KH, Loh HY, Kamarudin R, Gunnasegaran P, Alkhwaji A. Influence of Nanoparticles on Thermophysical Properties of Hybrid Nanofluids of Different Volume Fractions. Nanomaterials. 2022; 12(15):2570. https://doi.org/10.3390/nano12152570
Chicago/Turabian StyleAbdullah, Mohd Zulkifly, Kok Hwa Yu, Hao Yuan Loh, Roslan Kamarudin, Prem Gunnasegaran, and Abdusalam Alkhwaji. 2022. "Influence of Nanoparticles on Thermophysical Properties of Hybrid Nanofluids of Different Volume Fractions" Nanomaterials 12, no. 15: 2570. https://doi.org/10.3390/nano12152570
APA StyleAbdullah, M. Z., Yu, K. H., Loh, H. Y., Kamarudin, R., Gunnasegaran, P., & Alkhwaji, A. (2022). Influence of Nanoparticles on Thermophysical Properties of Hybrid Nanofluids of Different Volume Fractions. Nanomaterials, 12(15), 2570. https://doi.org/10.3390/nano12152570








