Visible-Light-Active Black TiO2 Nanoparticles with Efficient Photocatalytic Performance for Degradation of Pharmaceuticals
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of Black TiO2 Nanoparticles
2.2. Photocatalysts Characterization
2.3. Photocatalytic Experiments
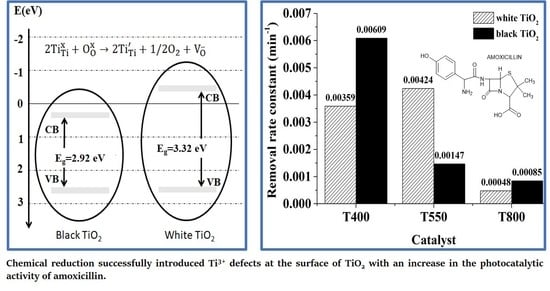
3. Results and Discussion
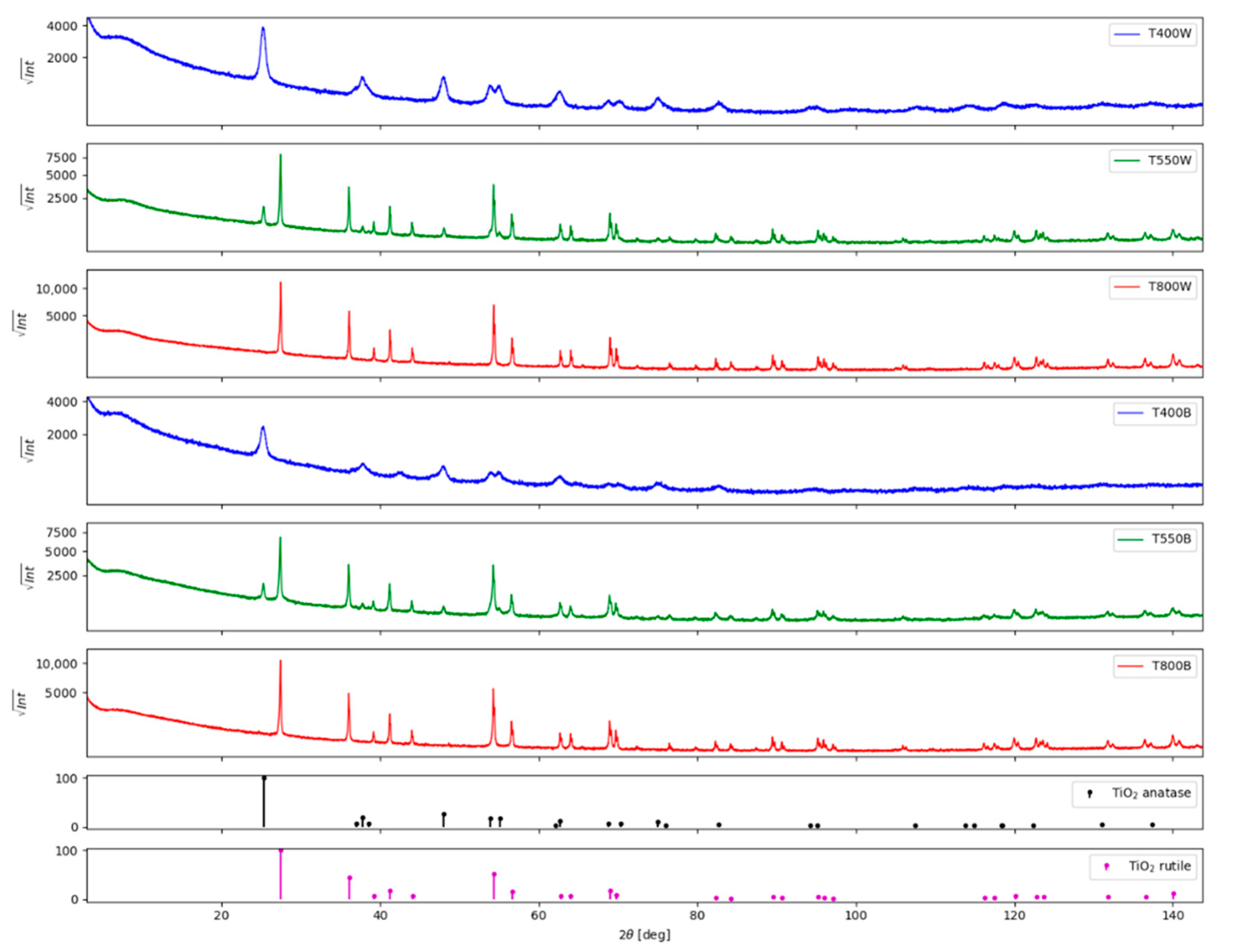
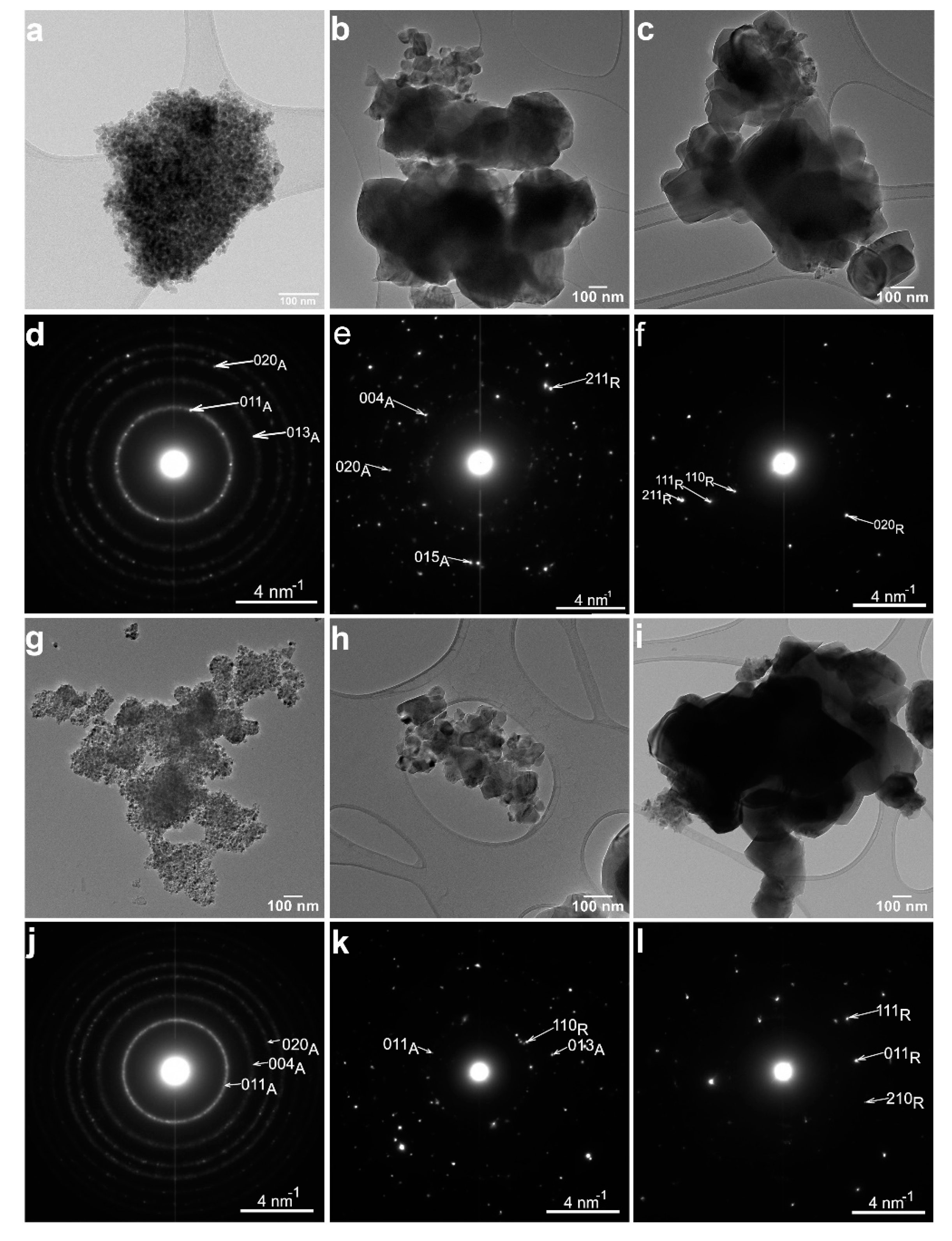
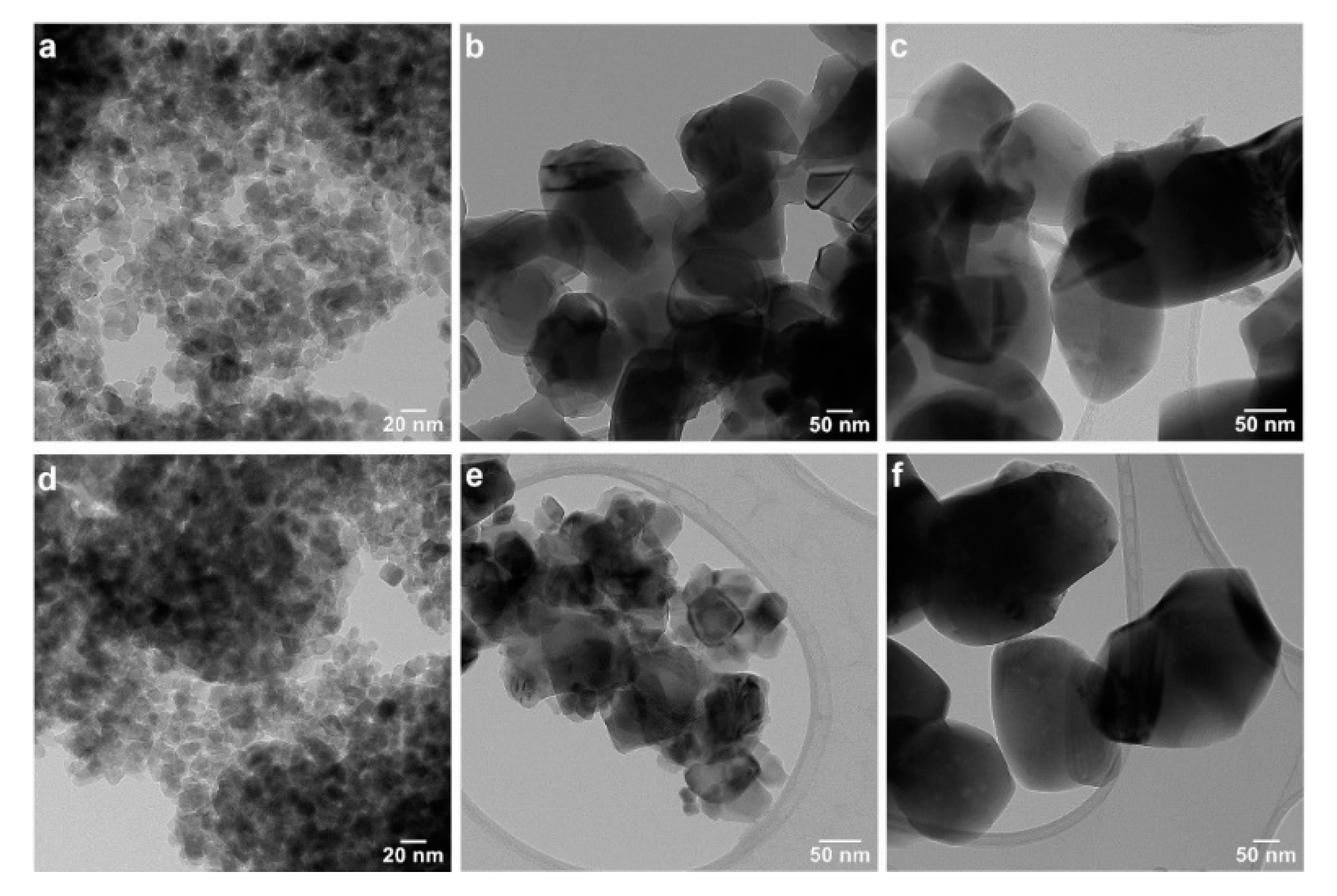
3.1. Structural and Morphological Characterization
3.1.1. X-ray Diffraction
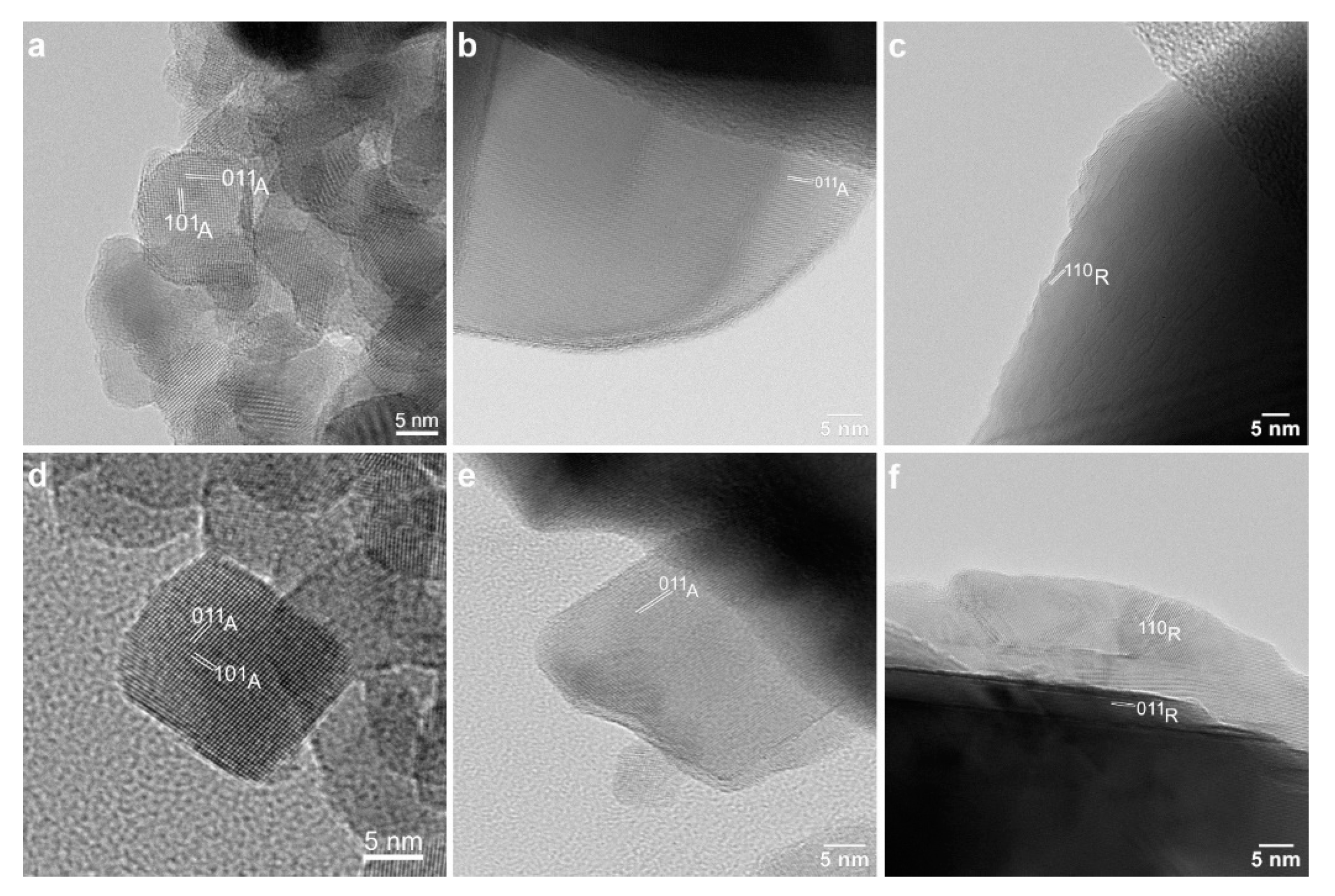
3.1.2. Transmission Electron Microscopy
3.1.3. Particle Size Distribution
3.1.4. Brunauer–Emmett–Teller (BET) Investigations
3.2. Spectroscopic Characterization
3.2.1. Optical Properties
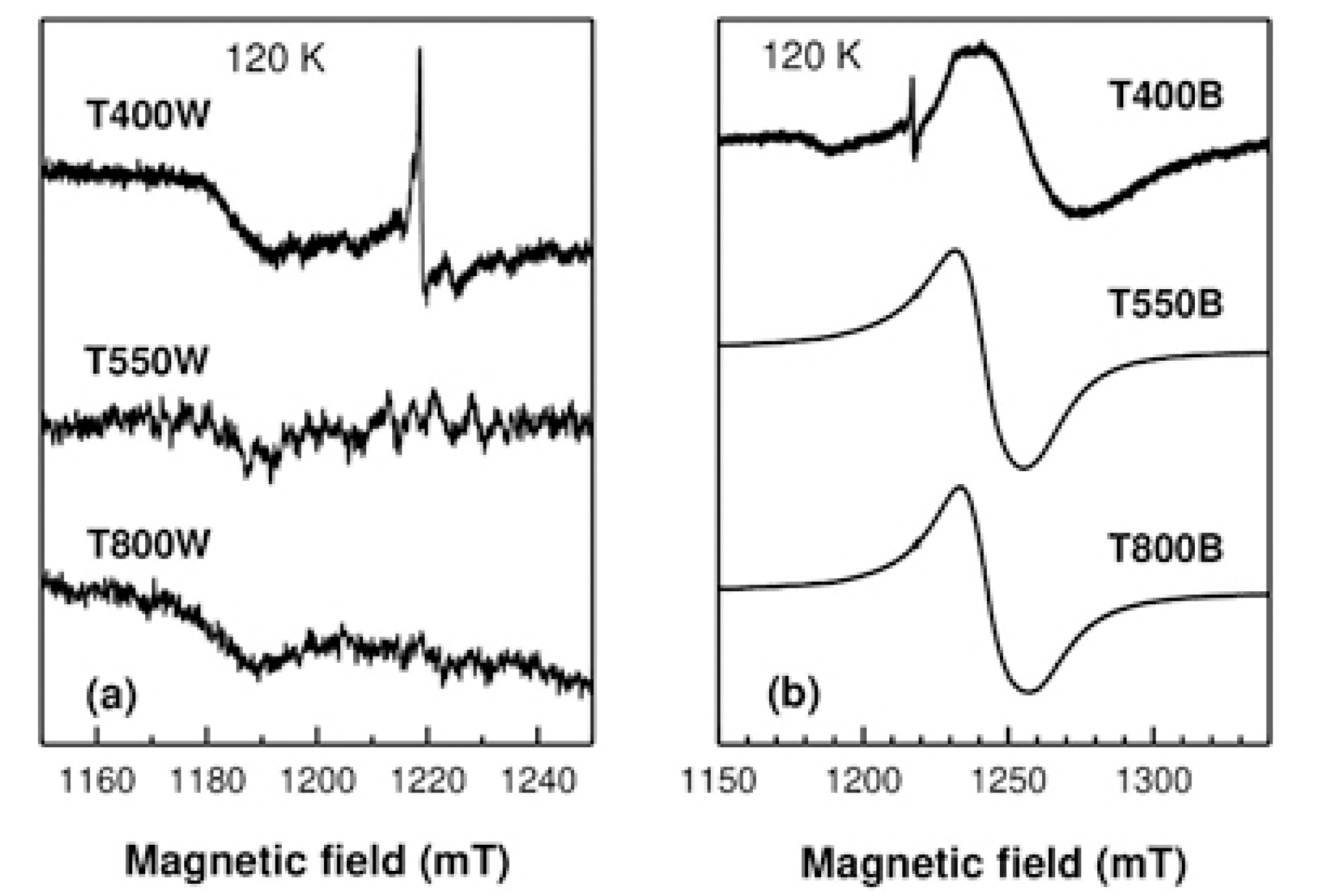
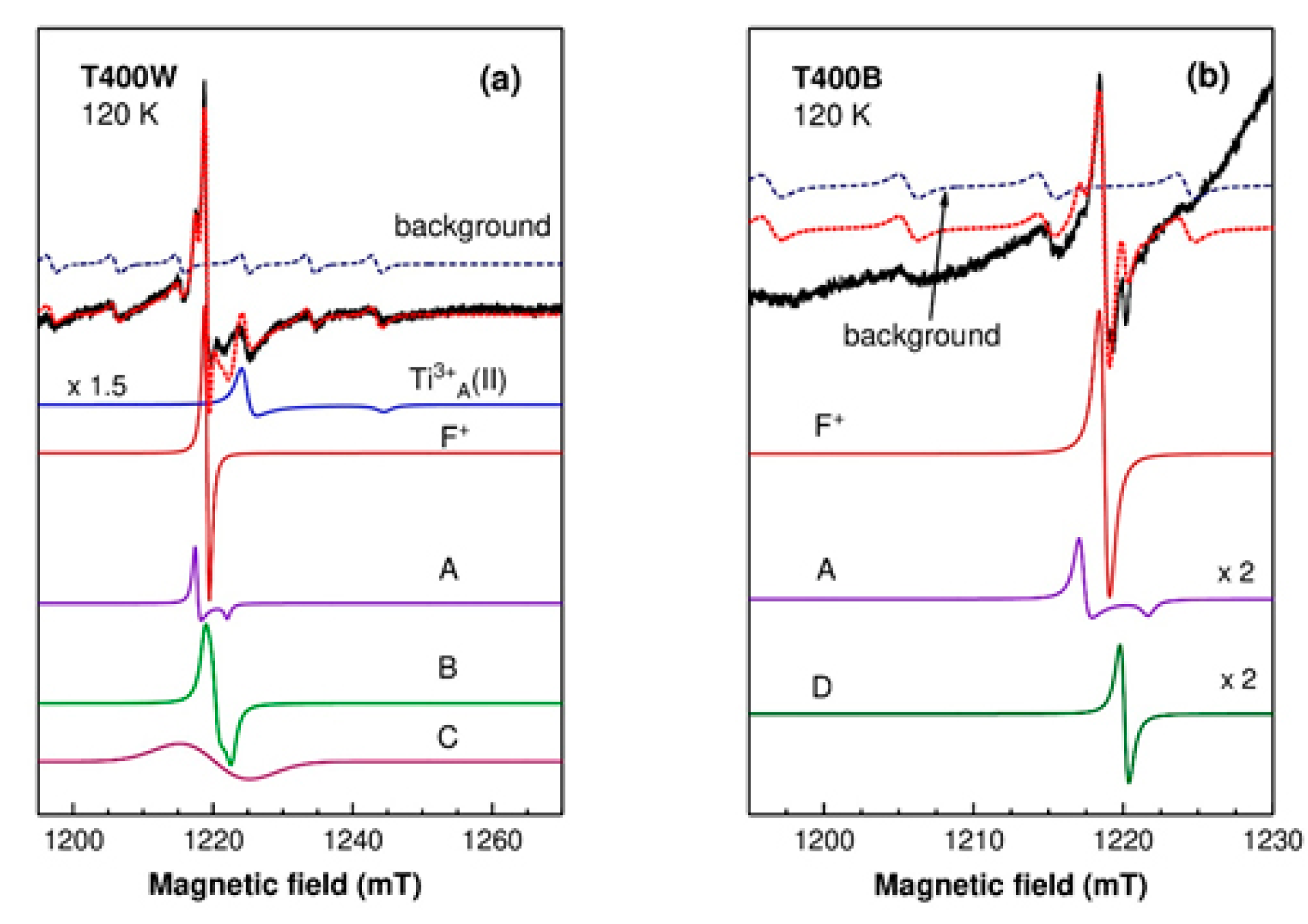
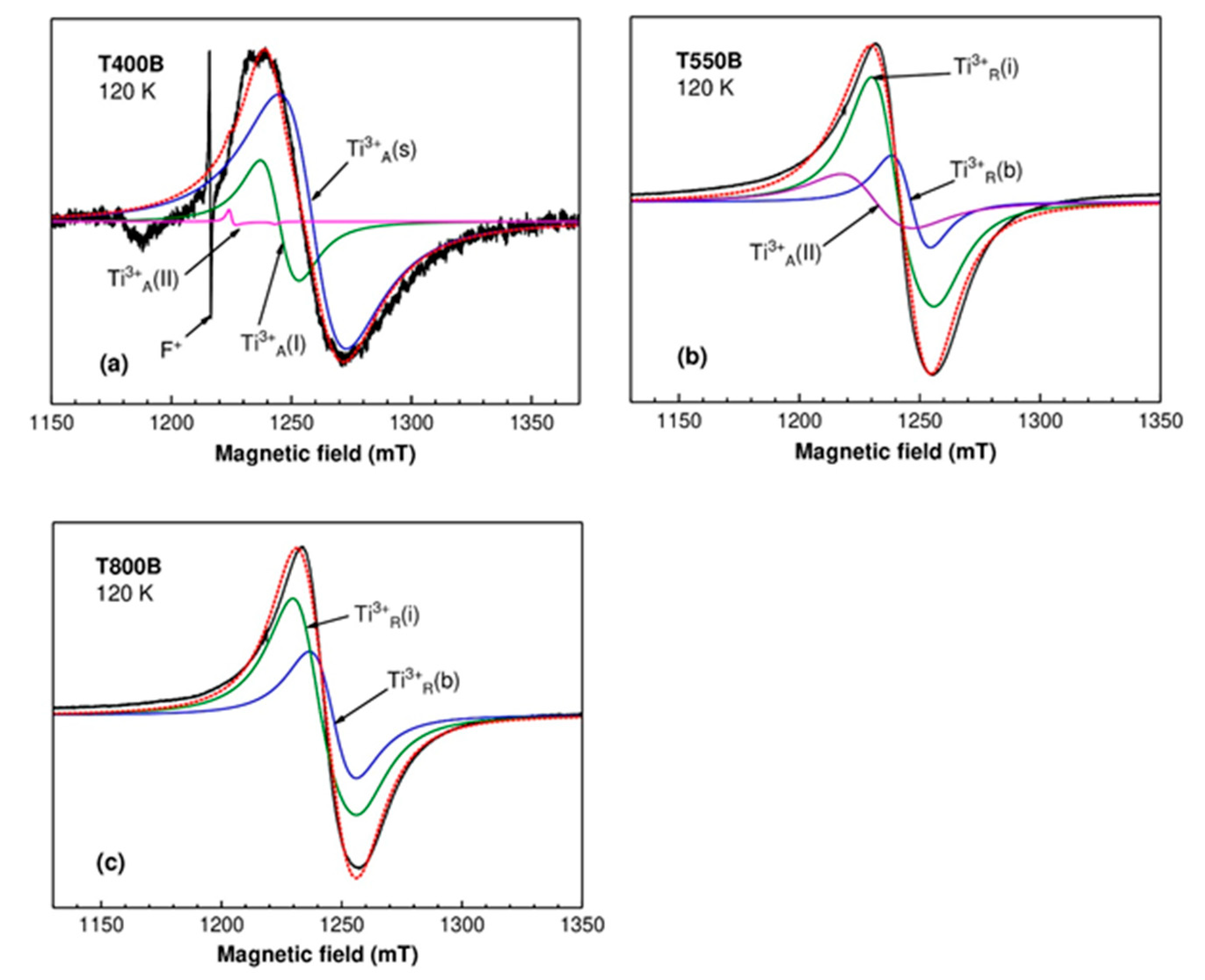
3.2.2. Electron Paramagnetic Resonance
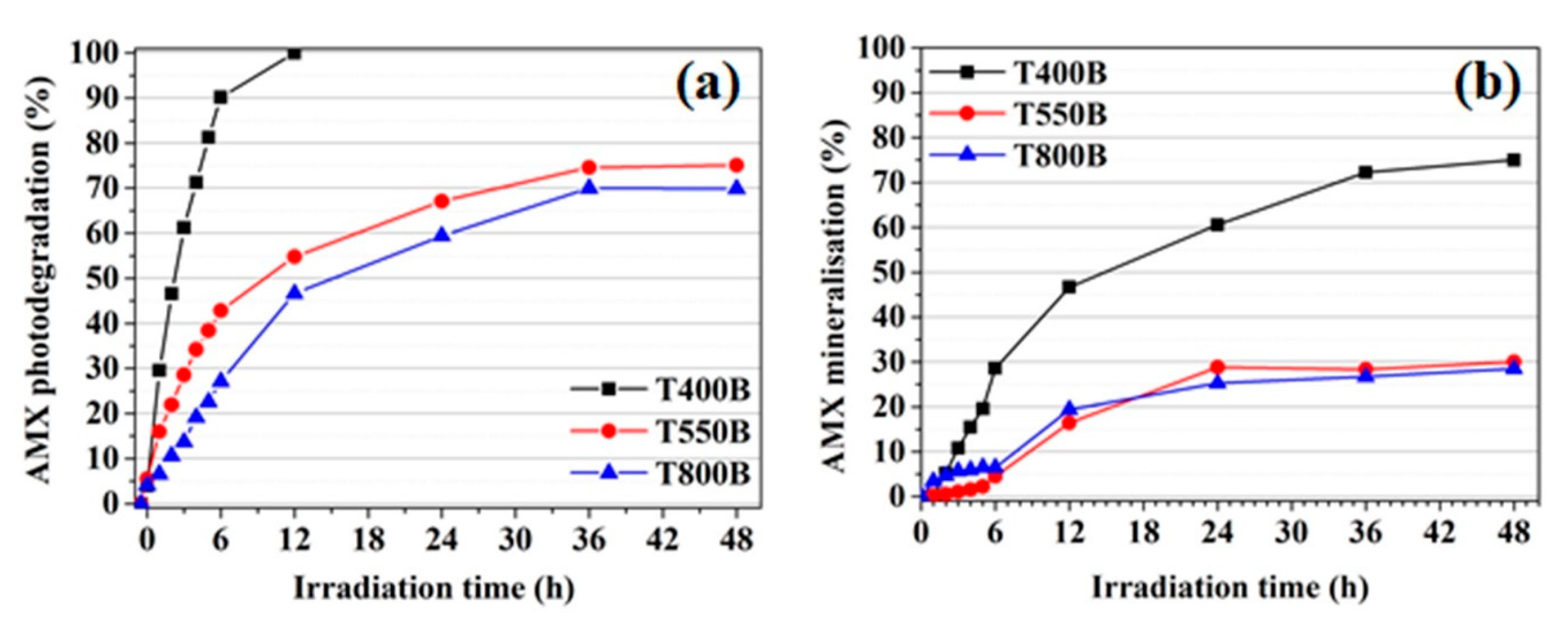
3.3. Photodegradation and Mineralization of Amoxicillin
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hasija, V.; Raizada, P.; Hosseini-Bandegharaei, A.; Thakur, V.K.; Van Le, Q.; Nguyen, V.H.; Singh, P. A Strategy to Develop Efficient Ag3PO4-based Photocatalytic Materials Toward Water Splitting: Perspectives and Challenges. ChemCatChem 2021, 13, 2965–2987. [Google Scholar] [CrossRef]
- Loeb, S.K.; Alvarez, P.J.J.; Brame, J.A.; Cates, E.L.; Choi, W.; Crittenden, J.; Dionysiou, D.D.; Li, Q.; Li-Puma, G.; Quan, X.; et al. The Technology Horizon for Photocatalytic Water Treatment: Sunrise or Sunset? Environ. Sci. Technol. 2019, 53, 2937–2947. [Google Scholar] [CrossRef] [PubMed]
- Ren, G.; Han, H.; Wang, Y.; Liu, S.; Zhao, J.; Meng, X.; Li, Z. Recent Advances of Photocatalytic Application in Water Treatment: A Review. Nanomaterials 2021, 11, 1804. [Google Scholar] [CrossRef]
- Kumar, M.P.; Jagannathan, R.; Ravichandran, S. Photoelectrochemical System for Unassisted High-Efficiency Water-Splitting Reactions Using N-Doped TiO2 Nanotubes. Energy Fuels 2020, 34, 9030–9036. [Google Scholar] [CrossRef]
- Serpone, N.; Emeline, A.V. Semiconductor Photocatalysis—Past, Present, and Future Outlook. J. Phys. Chem. Lett. 2012, 3, 673–677. [Google Scholar] [CrossRef]
- Uyguner Demirel, C.S.; Birben, N.C.; Bekbolet, M. A comprehensive review on the use of second generation TiO2 photocatalysts: Microorganism inactivation. Chemosphere 2018, 211, 420–448. [Google Scholar] [CrossRef]
- Serpone, N.; Emeline, A.V.; Kuznetsov, V.N.; Ryabchuk, V.K. Second Generation Visible-Light-Active Photocatalysts: Preparation, Optical Properties, and Consequences of Dopants on the Band Gap Energy of TiO2. In Environmentally Benign Photocatalysts; Springer: New York, NY, USA, 2010; pp. 35–111. ISBN 9780387484440. [Google Scholar]
- Ansari, S.A.; Cho, M.H. Highly Visible Light Responsive, Narrow Band gap TiO2 Nanoparticles Modified by Elemental Red Phosphorus for Photocatalysis and Photoelectrochemical Applications. Sci. Rep. 2016, 6, 25405. [Google Scholar] [CrossRef]
- Choi, H.; Khan, S.; Choi, J.; Dinh, D.T.T.; Lee, S.Y.; Paik, U.; Cho, S.H.; Kim, S. Synergetic control of band gap and structural transformation for optimizing TiO2 photocatalysts. Appl. Catal. B Environ. 2017, 210, 513–521. [Google Scholar] [CrossRef]
- Liu, J.; Zhao, Y.; Shi, L.; Yuan, S.; Fang, J.; Wang, Z.; Zhang, M. Solvothermal synthesis of crystalline phase and shape controlled Sn4+-Doped TiO2 nanocrystals: Effects of reaction solvent. ACS Appl. Mater. Interfaces 2011, 3, 1261–1268. [Google Scholar] [CrossRef]
- Khurana, C.; Pandey, O.P.; Chudasama, B. Synthesis of visible light-responsive cobalt-doped TiO2 nanoparticles with tunable optical band gap. J. Sol-Gel Sci. Technol. 2015, 75, 424–435. [Google Scholar] [CrossRef]
- Hu, H.; Lin, Y.; Hu, Y.H. Phase role of white TiO2 precursor in its reduction to black TiO2. Phys. Lett. A 2019, 383, 2978–2982. [Google Scholar] [CrossRef]
- Tian, M.; Mahjouri-Samani, M.; Eres, G.; Sachan, R.; Yoon, M.; Chisholm, M.F.; Wang, K.; Puretzky, A.A.; Rouleau, C.M.; Geohegan, D.B.; et al. Structure and Formation Mechanism of Black TiO2 Nanoparticles. ACS Nano 2015, 9, 10482–10488. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Wu, P.; Wu, J.; Hou, J.; Bai, H.; Liu, Z. Effective protect of oxygen vacancies in carbon layer coated black TiO2−x/CNNS hetero-junction photocatalyst. Chem. Eng. J. 2019, 359, 58–68. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, A.Y.; Si, Y.; Pei, D.N.; Yu, H.Q. Photochemical Protection of Reactive Sites on Defective TiO2-x Surface for Electrochemical Water Treatment. Environ. Sci. Technol. 2019, 53, 7641–7652. [Google Scholar] [CrossRef]
- Yu, P.; Zhang, J.Z. Some interesting properties of black hydrogen-treated TiO2 nanowires and their potential application in solar energy conversion. Sci. China-Chem. 2015, 58, 1810–1815. [Google Scholar] [CrossRef]
- Wang, D.; Liu, J.; Bao, X.; Qing, C.; Zhu, T.; Wang, H.-E. Macro/Mesoporous Carbon/Defective TiO2 Composite as a Functional Host for Lithium–Sulfur Batteries. ACS Appl. Energy Mater. 2022, 5, 2573–2579. [Google Scholar] [CrossRef]
- del Prado, F.; Andersen, H.F.; Taeño, M.; Mæhlen, J.P.; Ramírez-Castellanos, J.; Maestre, D.; Karazhanov, S.; Cremades, A. Comparative study of the implementation of tin and titanium oxide nanoparticles as electrodes materials in Li-ion batteries. Sci. Rep. 2020, 10, 5503. [Google Scholar] [CrossRef]
- Sun, H.; Xie, D.; Song, Z.; Liang, C.; Xu, L.; Qu, X.; Yao, Y.; Li, D.; Zhai, H.; Zheng, K.; et al. Interface Defects Passivation and Conductivity Improvement in Planar Perovskite Solar Cells Using Na2S-Doped Compact TiO2 Electron Transport Layers. ACS Appl. Mater. Interfaces 2020, 12, 22853–22861. [Google Scholar] [CrossRef] [PubMed]
- Ullattil, S.G.; Narendranath, S.B.; Pillai, S.C.; Periyat, P. Black TiO2 Nanomaterials: A Review of Recent Advances. Chem. Eng. J. 2018, 343, 708–736. [Google Scholar] [CrossRef]
- Liang, Y.; Huang, G.; Xin, X.; Yao, Y.; Li, Y.; Yin, J.; Li, X.; Wu, Y.; Gao, S. Black titanium dioxide nanomaterials for photocatalytic removal of pollutants: A review. J. Mater. Sci. Technol. 2022, 112, 239–262. [Google Scholar] [CrossRef]
- Fu, F.Y.; Shown, I.; Li, C.S.; Raghunath, P.; Lin, T.Y.; Billo, T.; Wu, H.L.; Wu, C.I.; Chung, P.W.; Lin, M.C.; et al. KSCN-induced Interfacial Dipole in Black TiO2 for Enhanced Photocatalytic CO2 Reduction. ACS Appl. Mater. Interfaces 2019, 11, 25186–25194. [Google Scholar] [CrossRef] [PubMed]
- Naldoni, A.; Altomare, M.; Zoppellaro, G.; Liu, N.; Kment, Š.; Zbořil, R.; Schmuki, P. Photocatalysis with reduced TiO2: From Black TiO2 to cocatalyst-free hydrogen production. ACS Catal. 2019, 9, 345–364. [Google Scholar] [CrossRef] [Green Version]
- Naldoni, A.; Allieta, M.; Santangelo, S.; Marelli, M.; Fabbri, F.; Cappelli, S.; Bianchi, C.L.; Psaro, R.; Dal Santo, V. Effect of Nature and Location of Defects on Bandgap Narrowing in Black TiO2 Nanoparticles. J. Am. Chem. Soc. 2012, 134, 7600–7603. [Google Scholar] [CrossRef] [PubMed]
- Nam, I.; Son, H.; Choi, Y. Colorimetric oxygen sensor based on nano-sized black TiO2 catalysts: DFT modeling and experiments. Mol. Catal. 2018, 459, 16–20. [Google Scholar] [CrossRef]
- Li, C.F.; Guo, X.; Shang, Q.H.; Yan, X.; Ren, C.; Lang, W.Z.; Guo, Y.J. Defective TiO2 for Propane Dehydrogenation. Ind. Eng. Chem. Res. 2020, 59, 4377–4387. [Google Scholar] [CrossRef]
- Katal, R.; Salehi, M.; Davood Abadi Farahani, M.H.; Masudy-Panah, S.; Ong, S.L.; Hu, J. Preparation of a New Type of Black TiO2 under a Vacuum Atmosphere for Sunlight Photocatalysis. ACS Appl. Mater. Interfaces 2018, 10, 35316–35326. [Google Scholar] [CrossRef] [PubMed]
- Zuñiga-Ibarra, V.A.; Shaji, S.; Krishnan, B.; Johny, J.; Sharma Kanakkillam, S.; Avellaneda, D.A.; Martinez, J.A.A.; Das Roy, T.K.; Ramos-Delgado, N.A. Synthesis and characterization of black TiO2 nanoparticles by pulsed laser irradiation in liquid. Appl. Surf. Sci. 2019, 483, 156–164. [Google Scholar] [CrossRef]
- Zhu, S.; Yu, Z.; Zhang, L.; Watanabe, S. Solution Plasma-Synthesized Black TiO2 Nanoparticles for Solar–Thermal Water Evaporation. ACS Appl. Nano Mater. 2021, 4, 3940–3948. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, J.; Cai, Z.; Huang, H.; Huang, T.; Wang, P.; Wang, X. Facile and large-scale synthesis of defective black TiO2−x (B) nanosheets for efficient visible-light-driven photocatalytic hydrogen evolution. Catalysts 2019, 9, 1048. [Google Scholar] [CrossRef] [Green Version]
- Andronic, L.; Enesca, A. Black TiO2 Synthesis by Chemical Reduction Methods for Photocatalysis Applications. Front. Chem. 2020, 8, 565489. [Google Scholar] [CrossRef]
- Lim, J.; Kim, S.H.; Aymerich Armengol, R.; Kasian, O.; Choi, P.P.; Stephenson, L.T.; Gault, B.; Scheu, C. Atomic-Scale Mapping of Impurities in Partially Reduced Hollow TiO2 Nanowires. Angew. Chem. 2020, 59, 5651–5655. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andronic, L.; Isac, L.; Miralles-Cuevas, S.; Visa, M.; Oller, I.; Duta, A.; Malato, S. Pilot-plant evaluation of TiO2 and TiO2-based hybrid photocatalysts for solar treatment of polluted water. J. Hazard. Mater. 2016, 320, 469–478. [Google Scholar] [CrossRef] [PubMed]
- Andronic, L.; Enesca, A.; Cazan, C.; Visa, M. TiO2–active carbon composites for wastewater photocatalysis. J. Sol-Gel Sci. Technol. 2014, 71, 396–405. [Google Scholar] [CrossRef]
- Andronic, L.; Lelis, M.; Enesca, A.; Karazhanov, S. Photocatalytic activity of defective black-titanium oxide photocatalysts towards pesticide degradation under UV/VIS irradiation. Surf. Interfaces 2022, 32, 102123. [Google Scholar] [CrossRef]
- Coelho, A.A. TOPAS and TOPAS-Academic: An optimization program integrating computer algebra and crystallographic objects written in C++. J. Appl. Crystallogr. 2018, 51, 210–218. [Google Scholar] [CrossRef] [Green Version]
- Stoll, S.; Schweiger, A. EasySpin, a comprehensive software package for spectral simulation and analysis in EPR. J. Magn. Reson. 2006, 178, 42–55. [Google Scholar] [CrossRef]
- Grecu, M.N.; Macovei, D.; Ghica, D.; Logofatu, C.; Valsan, S.; Apostol, N.G.; Lungu, G.A.; Negrea, R.F.; Piticescu, R.R. Co environment and magnetic defects in anatase CoxTi1−xO2 nanopowders. Appl. Phys. Lett. 2013, 102, 161909. [Google Scholar] [CrossRef]
- Mudalige, T.; Qu, H.; Van Haute, D.; Ansar, S.M.; Paredes, A.; Ingle, T. Chapter 11—Characterization of Nanomaterials: Tools and Challenges. In Micro and Nano Technologies; López Rubio, A., Fabra Rovira, M.J., Martínez Sanz, M., Gómez-Mascaraque, L.G., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 313–353. ISBN 978-0-12-814130-4. [Google Scholar]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef] [Green Version]
- Tolea, F.; Grecu, M.N.; Kuncser, V.; Constantinescu, S.G.; Ghica, D. On the role of Fe ions on magnetic properties of doped TiO2 nanoparticles. Appl. Phys. Lett. 2015, 106, 142404. [Google Scholar] [CrossRef]
- D’Arienzo, M.; Carbajo, J.; Bahamonde, A.; Crippa, M.; Polizzi, S.; Scotti, R.; Wahba, L.; Morazzoni, F. Photogenerated Defects in Shape-Controlled TiO2 Anatase Nanocrystals: A Probe To Evaluate the Role of Crystal Facets in Photocatalytic Processes. J. Am. Chem. Soc. 2011, 133, 17652–17661. [Google Scholar] [CrossRef]
- Chiesa, M.; Giamello, E. Carbon Dioxide Activation by Surface Excess Electrons: An EPR Study of the CO2− Radical Ion Adsorbed on the Surface of MgO. Eur. J. Chem. 2007, 13, 1261–1267. [Google Scholar] [CrossRef] [PubMed]
- Grecu, M.N.; Constantinescu, S.; Tărăbăşanu-Mihăilă, D.; Ghica, D.; Bibicu, I. Spin dynamics in Fe-doped TiO2 anatase nanoparticles. Phys. Status Solidi 2011, 248, 2927–2931. [Google Scholar] [CrossRef]
- Berger, T.; Sterrer, M.; Diwald, O.; Knözinger, E.; Panayotov, D.; Thompson, T.L.; Yates, J.T. Light-induced charge separation in anatase TiO2 particles. J. Phys. Chem. B 2005, 109, 6061–6068. [Google Scholar] [CrossRef] [PubMed]
- Chiesa, M.; Paganini, M.C.; Livraghi, S.; Giamello, E. Charge trapping in TiO2 polymorphs as seen by Electron Paramagnetic Resonance spectroscopy. Phys. Chem. Chem. Phys. 2013, 15, 9435. [Google Scholar] [CrossRef]
- Livraghi, S.; Rolando, M.; Maurelli, S.; Chiesa, M.; Paganini, M.C.; Giamello, E. Nature of reduced states in titanium dioxide as monitored by electron paramagnetic resonance II: Rutile and brookite cases. J. Phys. Chem. C 2014, 118, 22141–22148. [Google Scholar] [CrossRef] [Green Version]
- Ma, R.; Chen, T. Checking the Synergetic Effect between Anatase and Rutile. J. Phys. Chem. C 2019, 123, 19479–19485. [Google Scholar] [CrossRef]

| Samples | SBET (m2/g) | Average Pore Volume (cm3/g) | Average Pore Diameter (nm) 1 | Average Particle Size (nm) 2 | Average Aggregate Particle Size (nm) 3 |
|---|---|---|---|---|---|
| TiO2 sol-gel | 266.9 | 0.2352 | 4.58 | 22.48 | - |
| T400W | 89.08 | 0.1631 | 7.49 | 67.35 | 1911 |
| T400B | 90.21 | 0.1460 | 6.32 | 66.5 | 333.3 |
| T550W | 5.64 | 0.0095 | 13.15 | 1062 | 828.8 |
| T550B | 7.099 | 0.0113 | 14.94 | 845 | 376.5 |
| T800W | 2.41 | 0.0011 | 2.15 | 2484 | 457.7 |
| T800B | 3.86 | 0.0058 | 8.38 | 1552 | 401.9 |
| Centers | Assignment | g Values | Reference |
|---|---|---|---|
| Ti3+A(I) | Ti3+ in bulk anatase | gx = gy = 1.9640 gz = 1.9495 | [45] |
| Ti3+A(II) | Ti3+ in bulk anatase | gx = gy = 1.992 gz = 1.962 | [45] [46] |
| Ti3+A(s) | Ti3+ in disordered environment (surface) in anatase | g = 1.940 ± 0.004 | This work |
| Ti3+R(b) | Ti3+ in regular cation site in bulk rutile | gx = 1.969 gy = 1.960 gz = 1.949 | [46] |
| Ti3+R(i) | Ti3+ in interstitial site in bulk rutile | gx = 1.9787 gy = 1.9750 gz = 1.9424 | [46] |
| Ti3+R(s) | Ti3+ in surface or subsurface sites (dehydrated/hydrated surface) in rutile | gx = 1.970/1.973 gy = 1.961 gz = 1.948 | [47] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andronic, L.; Ghica, D.; Stefan, M.; Mihalcea, C.G.; Vlaicu, A.-M.; Karazhanov, S. Visible-Light-Active Black TiO2 Nanoparticles with Efficient Photocatalytic Performance for Degradation of Pharmaceuticals. Nanomaterials 2022, 12, 2563. https://doi.org/10.3390/nano12152563
Andronic L, Ghica D, Stefan M, Mihalcea CG, Vlaicu A-M, Karazhanov S. Visible-Light-Active Black TiO2 Nanoparticles with Efficient Photocatalytic Performance for Degradation of Pharmaceuticals. Nanomaterials. 2022; 12(15):2563. https://doi.org/10.3390/nano12152563
Chicago/Turabian StyleAndronic, Luminita, Daniela Ghica, Mariana Stefan, Catalina Gabriela Mihalcea, Aurel-Mihai Vlaicu, and Smagul Karazhanov. 2022. "Visible-Light-Active Black TiO2 Nanoparticles with Efficient Photocatalytic Performance for Degradation of Pharmaceuticals" Nanomaterials 12, no. 15: 2563. https://doi.org/10.3390/nano12152563
APA StyleAndronic, L., Ghica, D., Stefan, M., Mihalcea, C. G., Vlaicu, A.-M., & Karazhanov, S. (2022). Visible-Light-Active Black TiO2 Nanoparticles with Efficient Photocatalytic Performance for Degradation of Pharmaceuticals. Nanomaterials, 12(15), 2563. https://doi.org/10.3390/nano12152563