Photo- and Radiofrequency-Induced Heating of Photoluminescent Colloidal Carbon Dots
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of CDs
2.2. Instrumentation for Characterization
2.3. Photoinduced Heating
2.4. RF-Induced Heating
2.5. Fluorescence Measurements
3. Results and Discussion
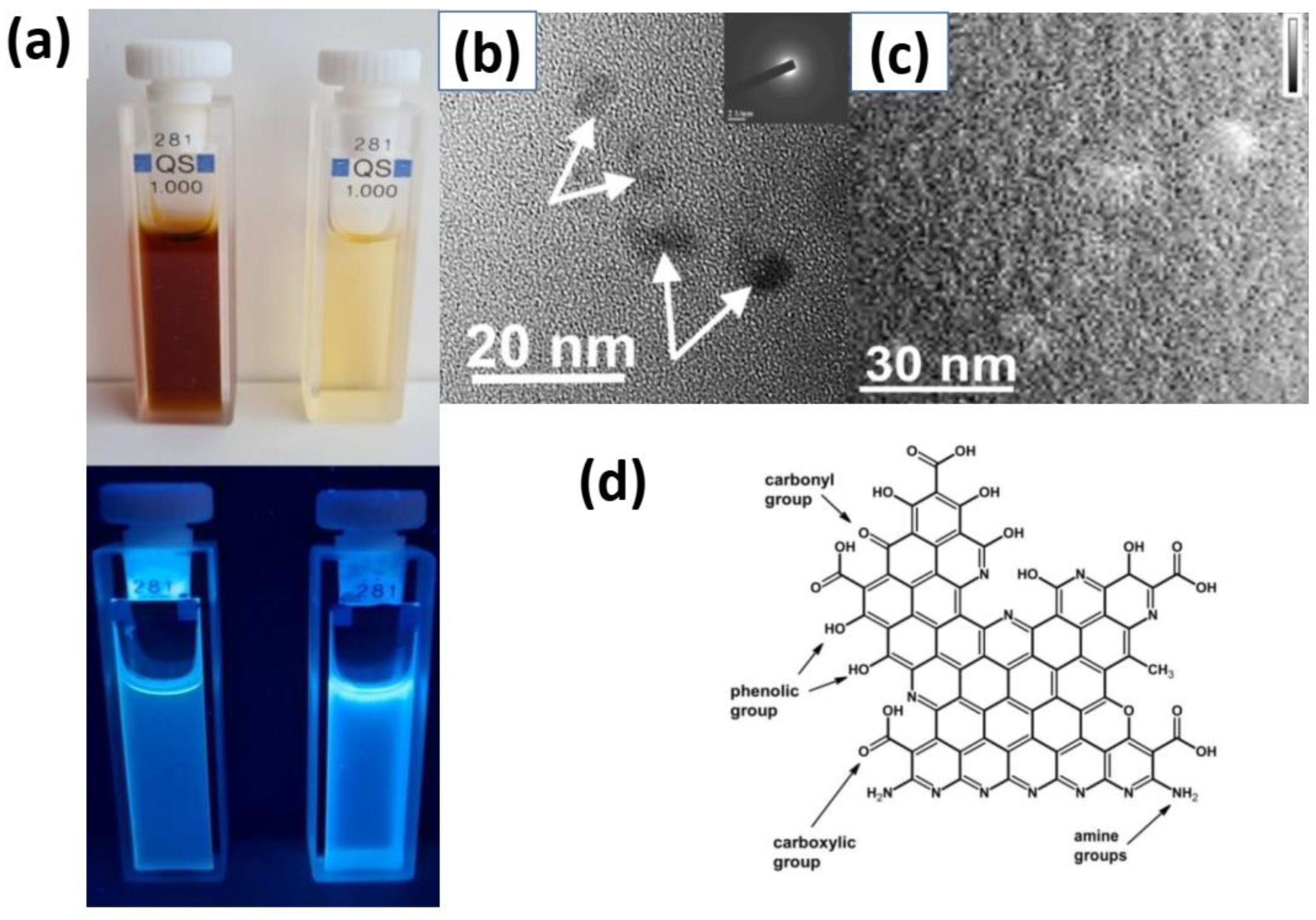
3.1. Structure of O, N-CDs
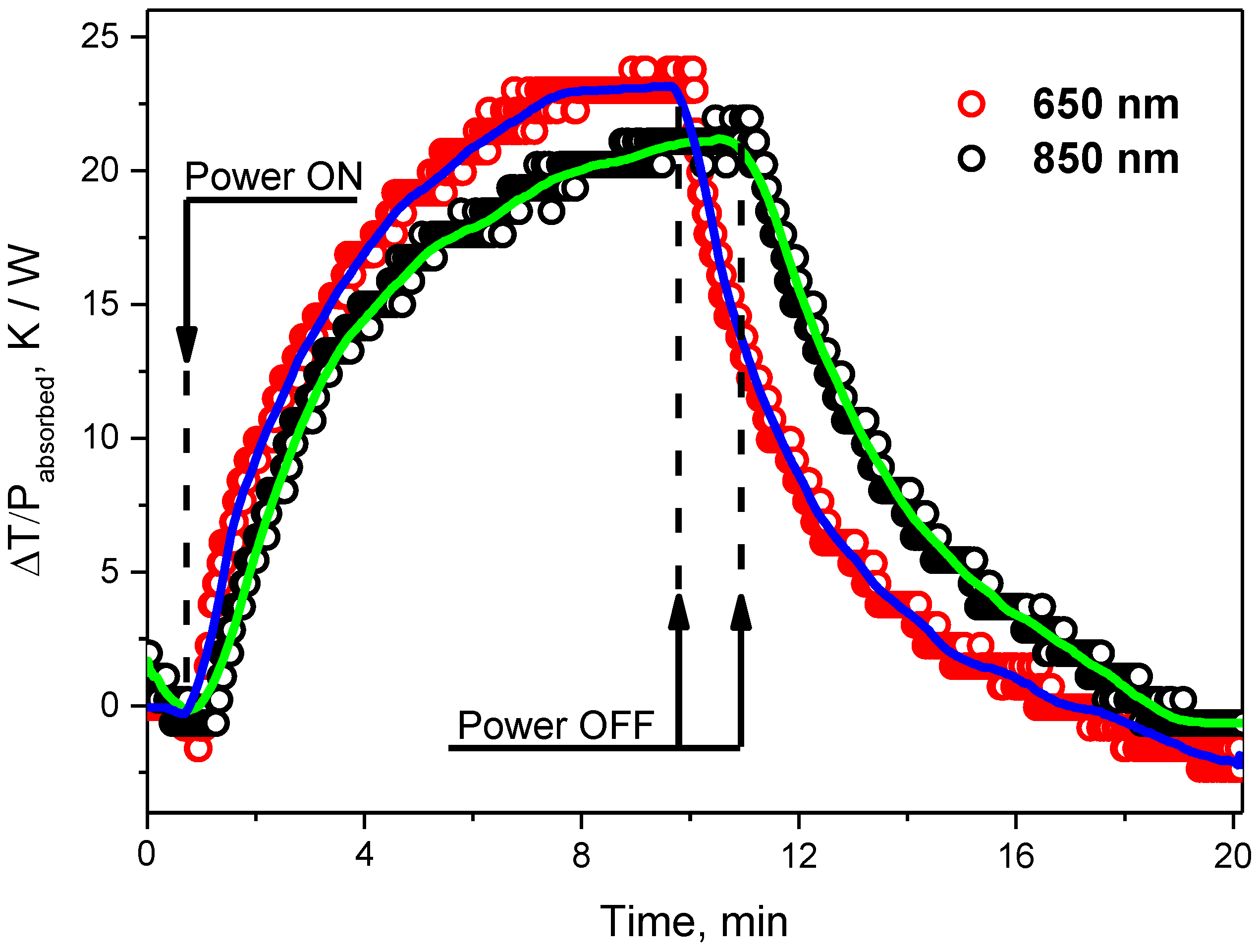
3.2. Photoinduced Heating of CDs-Based Colloids
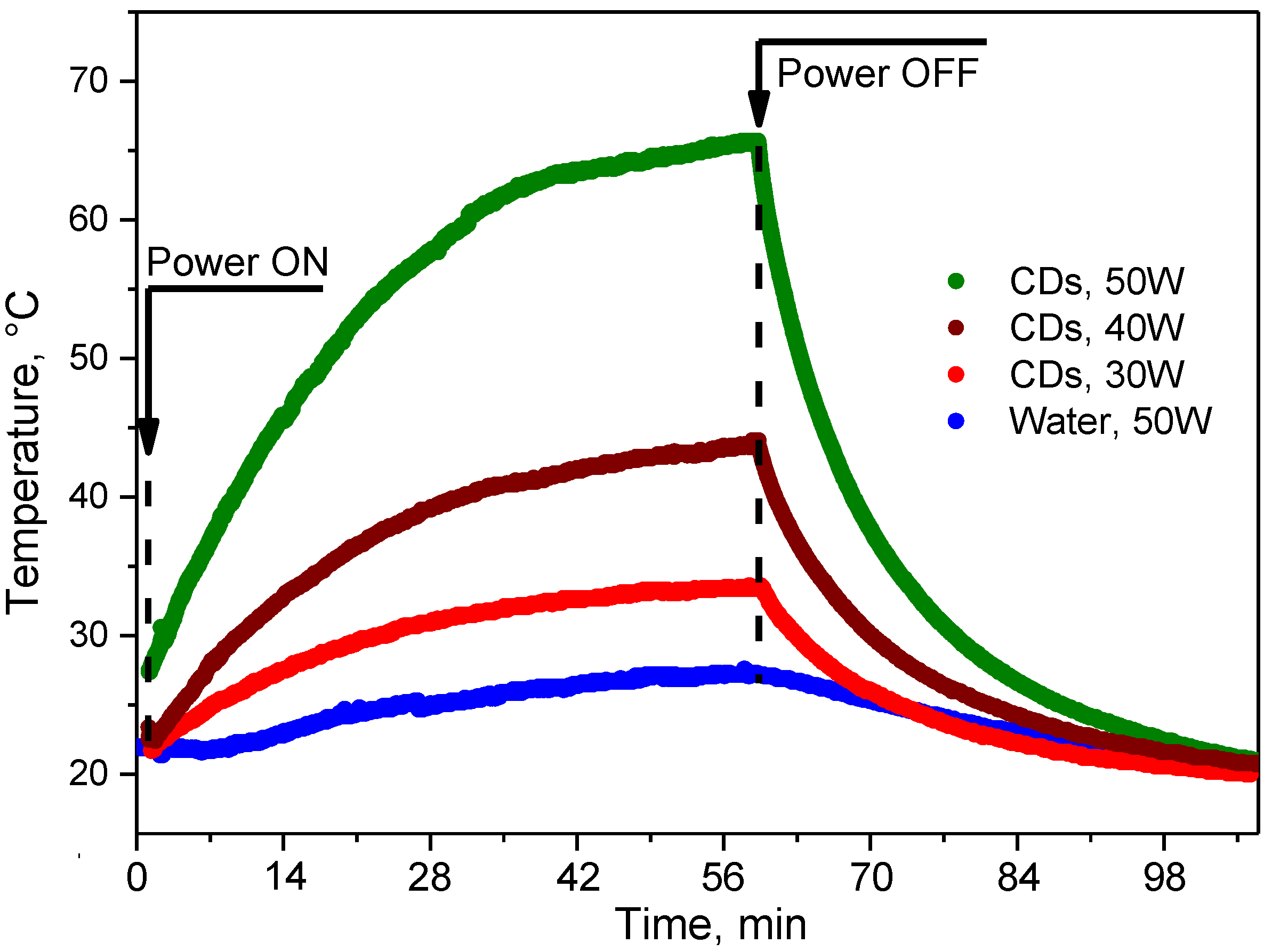
3.3. RF-Induced Heating of CDs-Based Colloids
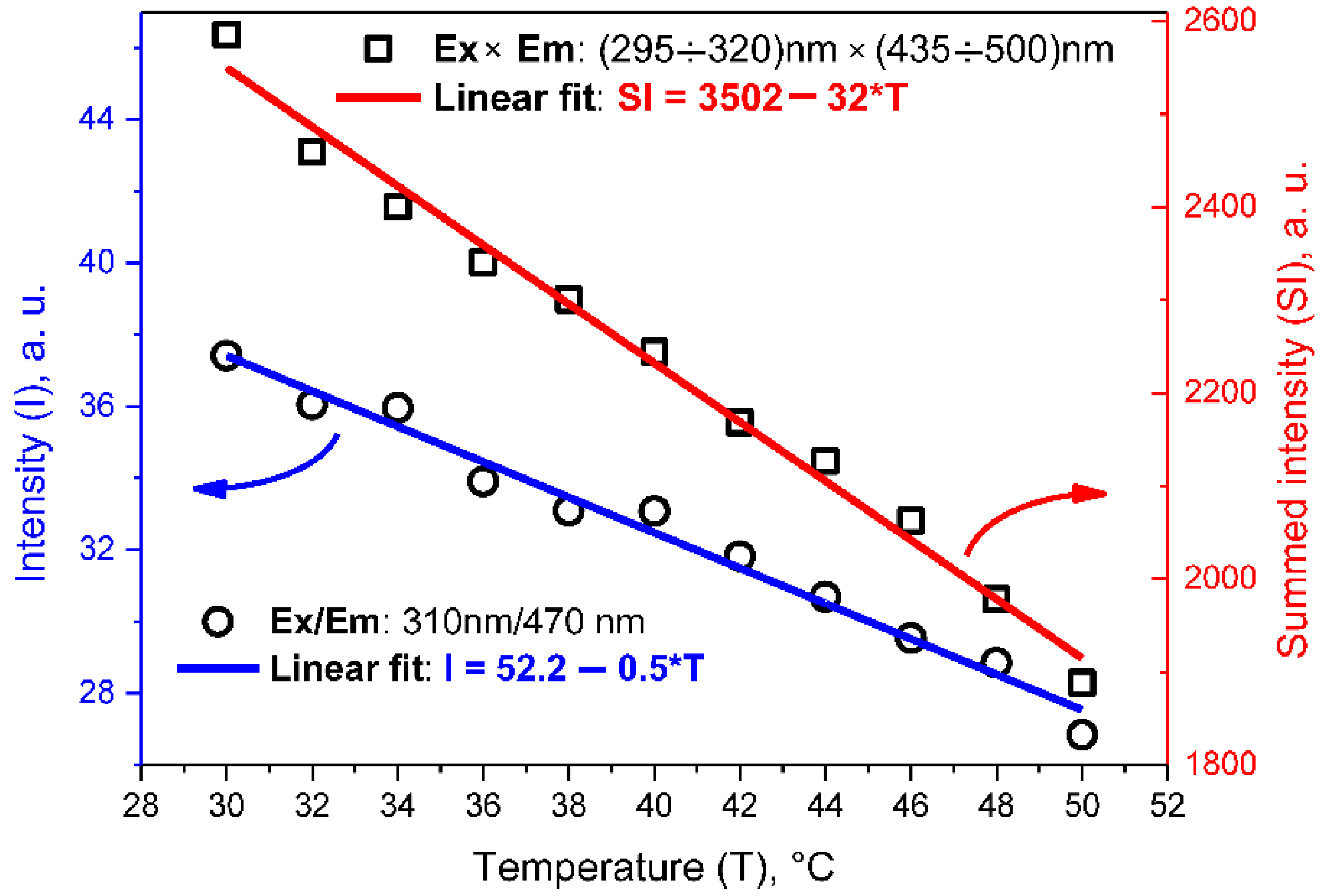
3.4. Thermometry with Fluorescent CDs-Based Colloids
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- del Rosal, B.; Ximendes, E.; Rocha, U.; Jaque, D. In vivo luminescence nanothermometry: From materials to applications. Adv. Opt. Mater. 2017, 5, 1600508. [Google Scholar] [CrossRef]
- Mintz, K.J.; Zhou, Y.; Leblanc, R.M. Recent development of carbon quantum dots regarding their optical properties, photoluminescence mechanism, and core structure. Nanoscale 2019, 11, 4634–4652. [Google Scholar] [CrossRef] [PubMed]
- Jorns, M.; Pappas, D.A. Review of fluorescent carbon dots, their synthesis, physical and chemical characteristics, and applications. Nanomaterials 2021, 11, 1448. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Wang, A.; Wang, R.; Liu, Z.; Sun, Y.; Shan, G.; Chen, Y.; Liu, Y. Carbon dots with molecular fluorescence and their application as a “turn-off” fluorescent probe for ferricyanide detection. Sci. Rep. 2019, 9, 10723. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Tang, K.; Yu, J.; Wang, H.; Tu, M.; Wang, X. Nitrogen and chlorine co-doped carbon dots as probe for sensing and imaging in biological samples. R. Soc. Open Sci. 2019, 6, 181557. [Google Scholar] [CrossRef] [Green Version]
- Jia, Q.; Zheng, X.; Ge, J.; Liu, W.; Ren, H.; Chen, S.; Wen, Y.; Zhang, H.; Wu, J.; Wang, P. Synthesis of carbon dots from Hypocrella bambusae for bimodel fluorescence/photoacoustic imaging-guided synergistic photodynamic/photothermal therapy of cancer. J. Colloid Interface Sci. 2018, 526, 302–311. [Google Scholar] [CrossRef]
- Zhao, S.; Wu, S.; Jia, Q.; Huang, L.; Lan, M.; Wang, P.; Zhang, W. Lysosome-targetable carbon dots for highly efficient photothermal/photodynamic synergistic cancer therapy and photoacoustic/two-photon excited fluorescence imaging. Chem. Eng. J. 2020, 388, 124212. [Google Scholar] [CrossRef]
- Lan, M.; Guo, L.; Zhao, S.; Zhang, Z.; Jia, Q.; Yan, L.; Xia, J.; Zhang, H.; Wang, P.; Zhang, W. Carbon dots as multifunctional phototheranostic agents for photoacoustic/fluorescence imaging and photothermal/photodynamic synergistic cancer therapy. Adv. Ther. 2018, 1, 1800077. [Google Scholar] [CrossRef]
- Shi, W.; Han, Q.; Wu, J.; Ji, C.; Zhou, Y.; Li, S.; Gao, L.; Leblanc, R.M.; Peng, Z. Synthesis mechanisms, structural models, and photothermal therapy applications of top-down carbon dots from carbon powder, graphite, graphene, and carbon nanotubes. Int. J. Mol. Sci. 2022, 23, 1456. [Google Scholar] [CrossRef]
- Geng, B.; Yang, D.; Pan, D.; Wang, L.; Zheng, F.; Shen, W.; Zhang, C.; Li, X. NIR-responsive carbon dots for efficient photothermal cancer therapy at low power densities. Carbon 2018, 134, 153–162. [Google Scholar] [CrossRef]
- Ju, Y.; Bing Dong, B.; Yuc, J.; Hou, Y. Inherent multifunctional inorganic nanomaterials for imaging-guided cancer therapy. Nano Today 2019, 26, 108–122. [Google Scholar] [CrossRef]
- Thakur, M.; Kumawat, M.K.; Srivastava, R. Multifunctional graphene quantum dots for combined photothermal and photodynamic therapy coupled with cancer cell tracking applications. RSC Adv. 2017, 7, 5251–5261. [Google Scholar] [CrossRef] [Green Version]
- Yang, W.; Wei, B.; Yang, Z.; Sheng, L. Facile synthesis of novel carbon-dots/hemin nanoplatforms for synergistic photo-thermal and photo-dynamic therapies. J. Inorg. Biochem. 2019, 193, 166–172. [Google Scholar] [CrossRef] [PubMed]
- Tamarov, K.P.; Osminkina, L.A.; Zinovyev, S.V.; Maximova, K.A.; Kargina, J.V.; Gongalsky, M.B.; Ryabchikov, Y.; Al-Kattan, A.; Sviridov, A.P.; Sentis, M.; et al. Radio frequency radiation-induced hyperthermia using Si nanoparticle-based sensitizers for mild cancer therapy. Sci. Rep. 2014, 4, 7034. [Google Scholar] [CrossRef]
- Liu, X.; Chen, H.-J.; Chen, X.; Alfadhl, Y.; Yu, J.; Wen, D. Radiofrequency heating of nanomaterials for cancer treatment: Progress, controversies, and future development. Appl. Phys. Rev. 2015, 2, 011103. [Google Scholar] [CrossRef]
- Gannon, C.J.; Cherukuri, P.; Yakobson, B.I.; Cognet, L.; Kanzius, J.S.; Kittrell, C.; Weisman, R.B.; Pasquali, M.; Schmidt, H.K.; Smalley, R.E.; et al. Carbon nanotube-enhanced thermal destruction of cancer cells in a noninvasive radiofrequency field. Cancer 2007, 110, 2654–2665. [Google Scholar] [CrossRef]
- Vashisth, A.; Upama, S.T.; Anas, M.; Oh, J.-H.; Patil, N.; Green, M.J. Radio frequency heating and material processing using carbon susceptors. Nanoscale Adv. 2021, 3, 5255–5264. [Google Scholar] [CrossRef]
- Yu, P.; Wen, X.; Toh, Y.-R.; Tang, J. Temperature-dependent fluorescence in carbon dots. J. Phys. Chem. C 2012, 116, 25552–25557. [Google Scholar] [CrossRef]
- Piñol, R.; Brites, C.D.S.; Silva, N.J.; Carlos, L.D.; Millán, A. nanoscale thermometry for hyperthermia applications. In Micro and Nano Technologies; Elsevier: Amsterdam, The Netherlands, 2019; pp. 139–172. [Google Scholar] [CrossRef]
- Mohammed, L.J.; Omer, K.M. Carbon dots as new generation materials for nanothermometer: Review. Nanoscale Res. Lett. 2020, 15, 182. [Google Scholar] [CrossRef]
- Mohammed, L.J.; Omer, K.M. Dual functional highly luminescence B, N co-doped carbon nanodots as nanothermometer and Fe3+/Fe2+ sensor. Sci. Rep. 2020, 10, 3028. [Google Scholar] [CrossRef] [Green Version]
- Lee, B.H.; McKinney, R.L.; Hasan, M.T.; Naumov, A.V. Graphene quantum dots as intracellular imaging-based temperature sensors. Materials 2021, 14, 616. [Google Scholar] [CrossRef]
- Kalytchuk, S.; Poláková, K.; Wang, Y.; Froning, J.P.; Cepe, K.; Rogach, A.L.; Zbořil, R. Carbon dot nanothermometry: Intracellular photoluminescence lifetime thermal sensing. ACS Nano 2017, 11, 1432–1442. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Liu, Y.; Zhao, H.; Vomiero, A.; Li, R. Highly efficient ratiometric nanothermometers based on colloidal carbon quantum dots. J. Mater. Chem. B 2021, 9, 4111–4119. [Google Scholar] [CrossRef]
- Nguyen, V.; Yan, L.; Xu, H.; Yue, M. One-step synthesis of multi-emission carbon nanodots for ratiometric temperature sensing. Appl. Surf. Sci. 2018, 427, 1118–1123. [Google Scholar] [CrossRef]
- Sonsin, A.F.; Nascimento, S.M.S.; Albuquerque, I.M.B.; Silva, E.C.O.; Rocha, J.C.A.; Oliveira, R.S.; Barbosa, C.D.; Angeles, E.S.; Souza, S.T.; Fonseca, E.J.S. Temperature-dependence on the optical properties of chitosan carbon dots in the solid state. RSC Adv. 2021, 11, 2767–2773. [Google Scholar] [CrossRef] [PubMed]
- Ivanov, I.I.; Zaderko, A.N.; Lysenko, V.; Clopeau, T.; Lisnyak, V.V.; Skryshevsky, V.A. Photoluminescent recognition of strong alcoholic beverages with carbon nanoparticles. ACS Omega 2021, 6, 18802–18810. [Google Scholar] [CrossRef]
- Lisnyak, V.V.; Zaderko, A.N.; Mariychuk, R.; Lysenko, V.; Boldyrieva, O.Y.; Skryshevsky, V.A.; Mussabek, G.; Taurbayev, Y.; Zhylkybayeva, N.; Tananiko, O.Y. Preparation and characterization of F-, O-, and N-containing carbon nanoparticles for pH sensing. Appl. Nanosci. 2022, 12, 795–803. [Google Scholar] [CrossRef]
- Moran, C.H.; Wainerdi, S.M.; Cherukuri, T.K.; Kittrell, C.; Wiley, B.J.; Nicholas, N.W.; Curley, S.A.; Kanzius, J.S.; Cherukuri, P. Size-dependent joule heating of gold nanoparticles using capacitively coupled radiofrequency fields. Nano Res. 2009, 2, 400–405. [Google Scholar] [CrossRef] [Green Version]
- Grigoriev, A.A.; Grigoryeva, M.S.; Kargina, Y.V.; Kharin, A.Y.; Zavestovskaya, I.N.; Kanavin, A.P.; Timoshenko, V.Y. Radiofrequency heating of nanoparticles for biomedical applications. Bull. Lebedev Phys. Inst. 2021, 48, 170–174. [Google Scholar] [CrossRef]
- Atabaev, T.S.; Sayatova, S.; Molkenova, A.; Taniguchi, I. Nitrogen-doped carbon nanoparticles for potential temperature sensing applications. Sens. Bio-Sens. Res. 2019, 22, 100253. [Google Scholar] [CrossRef]
- Guo, Z.; Luo, J.; Zhu, Z.; Sun, Z.; Zhang, X.; Wu, Z.-C.; Mo, F.; Guan, A. A facile synthesis of high-efficient N,S co-doped carbon dots for temperature sensing application. Dyes Pigments 2020, 173, 107952. [Google Scholar] [CrossRef]
- Wang, C.; Xu, Z.; Cheng, H.; Lin, H.; Humphrey, M.G.; Zhang, C. A hydrothermal route to water-stable luminescent carbon dots as nanosensors for pH and temperature. Carbon 2015, 82, 87–95. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, Y.; Yan, Z.; Chen, J. Simultaneous determination of temperature and erlotinib by novel carbon-based sensitive nanoparticles. Sens. Actuators B Chem. 2018, 255, 986–994. [Google Scholar] [CrossRef]
- Yang, J.-M.; Yang, H.; Lin, L. Quantum dot nano thermometers reveal heterogeneous local thermogenesis in living cells. ACS Nano 2011, 5, 5067–5071. [Google Scholar] [CrossRef]
- Cui, X.; Wang, Y.; Liu, J.; Yang, Q.; Zhang, B.; Gao, Y.; Zhang, Y.; Lu, G. Dual functional N- and S-co-doped carbon dots as the sensor for temperature and Fe3+ ions. Sens. Actuators B Chem. 2017, 242, 1272–1280. [Google Scholar] [CrossRef]
- Lucchese, M.; Stavale, F.; Ferreira, E.H.M.; Vilani, C.; Moutinho, M.V.O.; Capaz, R.B.; Achete, C.A.; Jorio, A. Quantifying ion-induced defects and Raman relaxation length in graphene. Carbon 2010, 48, 1592–1597. [Google Scholar] [CrossRef]
- Bokobza, L.; Bruneel, J.-L.; Couzi, M. Raman spectra of carbon-based materials (from graphite to carbon black) and of some silicone composites. C-J. Carbon Res. 2015, 1, 77–94. [Google Scholar] [CrossRef] [Green Version]
- Cintra, E.P.; Córdoba de Torresi, S.I.; Errien, N.; Louarn, G. Determination of the formation of ladder structure in poly(5-amino-1-naphthol) by resonant Raman and XPS characterization. Macromolecules 2003, 36, 6, 2079–2084. [Google Scholar] [CrossRef]
- Mochalin, V.; Osswald, S.; Gogotsi, Y. Contribution of functional groups to the Raman spectrum of nanodiamond powders. Chem. Mater. 2009, 21, 273–279. [Google Scholar] [CrossRef]
- Santos, V.O.; Leite, I.R.; Brolo, A.G.; Rubim, J.C. The electrochemical reduction of CO2 on a copper electrode in 1-n-butyl-3-methyl imidazolium tetrafluoroborate (BMI. BF4) monitored by surface-enhanced Raman scattering (SERS). J. Raman Spectrosc. 2016, 47, 674–680. [Google Scholar] [CrossRef]
- Matsoso, B.J.; Ranganathan, K.; Mutuma, B.K.; Lerotholi, T.; Jones, G.; Coville, N.J. Time-dependent evolution of the nitrogen configurations in N-doped graphene films. RSC Adv. 2016, 6, 106914–106920. [Google Scholar] [CrossRef] [Green Version]
- Menges, F. Spectragryph Optical Spectroscopy Software, Version 1.2.14. Available online: http://www.effemm2.de/spectragryph/ (accessed on 15 May 2022).
- Wojdyr, M. A Curve Fitting and Data Analysis Program, Version 1.3.1. Available online: https://fityk.nieto.pl/ (accessed on 15 May 2022).
- Sun, Y.-P.; Zhou, B.; Lin, Y.; Wang, W.; Fernando, S.; Pathak, P.; Meziani, M.J.; Harruff, B.A.; Wang, X.; Wang, H.; et al. Quantum-sized carbon dots for bright and colorful photoluminescence. J. Am. Chem. Soc. 2006, 128, 7756–7757. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Wang, Q.; Shen, G.; Zhang, C.; Li, C.; Ji, W.; Wang, C.; Cui, D. A multifunctional ribonuclease A-conjugated carbon dot cluster nanosystem for synchronous cancer imaging and therapy. Nanoscale Res. Lett. 2014, 9, 397. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pan, D.; Zhang, J.; Li, Z.; Wu, C.; Yan, X.; Wu, M. Observation of pH-, solvent-, spin-, and excitation-dependent blue photoluminescence from carbon nanoparticles. Chem. Commun. 2010, 46, 3681–3683. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.; Travas-Sejdic, J. Simple aqueous solution route to luminescent carbogenic dots from carbohydrates. Chem. Mater. 2009, 21, 5563–5565. [Google Scholar] [CrossRef]
- De, B.; Karak, N. A green and facile approach for the synthesis of water soluble fluorescent carbon dots from banana juice. RSC Adv. 2013, 3, 8286–8290. [Google Scholar] [CrossRef]
- Zhou, Y.; Desserre, A.; Sharma, S.K.; Li, S.; Marksberry, M.H.; Chusuei, C.C.; Blackwelder, P.L.; Leblanc, R.M. Gel-like carbon dots: Characterization and their potential applications. Chemphyschem 2017, 18, 890–897. [Google Scholar] [CrossRef]
- Zhou, Y.; Liyanage, P.Y.; Devadoss, D.; Rios Guevara, L.R.; Cheng, L.; Graham, R.M.; Chand, H.S.; Al-Youbi, A.O.; Bashammakh, A.S.; El-Shahawi, M.S.; et al. Nontoxic amphiphilic carbon dots as promising drug nanocarriers across the blood–brain barrier and inhibitors of β-amyloid. Nanoscale 2019, 11, 22387–22397. [Google Scholar] [CrossRef]
- Wang, H.; Sun, P.; Cong, S.; Wu, J.; Gao, L.; Wang, Y.; Dai, X.; Zou, G. Nitrogen-doped carbon dots for “green” quantum dot solar cells. Nanoscale Res. Lett. 2016, 11, 27. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Zhang, Y.; Wang, Y.; Kalytchuk, S.; Kershaw, S.V.; Wang, Y.; Wang, P.; Zhang, T.; Zhao, Y.; Zhang, H.; et al. Color-switchable electroluminescence of carbon dot light-emitting diodes. ACS Nano 2013, 7, 11234–11241. [Google Scholar] [CrossRef]
- Zheng, B.; Chen, Y.; Li, P.; Wang, Z.; Cao, B.; Qi, F.; Liu, J.; Qiu, Z.; Zhang, W. Ultrafast ammonia-driven, microwave-assisted synthesis of nitrogen-doped graphene quantum dots and their optical properties. Nanophotonics 2017, 6, 259–267. [Google Scholar] [CrossRef]
- Tang, L.; Ji, R.; Li, X.; Bai, G.; Liu, C.P.; Hao, J.; Lin, J.; Jiang, H.; Teng, K.S.; Yang, Z.; et al. Deep ultraviolet to near-infrared emission and photoresponse in layered n-doped graphene quantum dots. ACS Nano 2014, 8, 6312–6320. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Ji, R.; Li, X.; Teng, K.S.; Lau, S.P. Energy-level structure of nitrogen-doped graphene quantum dots. J. Mater. Chem. C 2013, 1, 4908–4915. [Google Scholar] [CrossRef]
- Mewada, A.; Pandey, S.; Shinde, S.; Mishra, N.; Oza, G.; Thakur, M.; Sharon, M.; Sharon, M. Green synthesis of biocompatible carbon dots using aqueous extract of Trapa bispinosa peel. Mater. Sci. Eng. C 2013, 33, 2914–2917. [Google Scholar] [CrossRef]
- Huang, G.; Chen, X.; Wang, C.; Zheng, H.; Huang, Z.; Chen, D.; Xie, H. Photoluminescent carbon dots derived from sugarcane molasses: Synthesis, properties, and applications. RSC Adv. 2017, 7, 47840–47847. [Google Scholar] [CrossRef] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mussabek, G.; Zhylkybayeva, N.; Lysenko, I.; Lishchuk, P.O.; Baktygerey, S.; Yermukhamed, D.; Taurbayev, Y.; Sadykov, G.; Zaderko, A.N.; Skryshevsky, V.A.; et al. Photo- and Radiofrequency-Induced Heating of Photoluminescent Colloidal Carbon Dots. Nanomaterials 2022, 12, 2426. https://doi.org/10.3390/nano12142426
Mussabek G, Zhylkybayeva N, Lysenko I, Lishchuk PO, Baktygerey S, Yermukhamed D, Taurbayev Y, Sadykov G, Zaderko AN, Skryshevsky VA, et al. Photo- and Radiofrequency-Induced Heating of Photoluminescent Colloidal Carbon Dots. Nanomaterials. 2022; 12(14):2426. https://doi.org/10.3390/nano12142426
Chicago/Turabian StyleMussabek, Gauhar, Nazym Zhylkybayeva, Ivan Lysenko, Pavlo O. Lishchuk, Saule Baktygerey, Dana Yermukhamed, Yerzhan Taurbayev, Gani Sadykov, Alexander N. Zaderko, Valeriy A. Skryshevsky, and et al. 2022. "Photo- and Radiofrequency-Induced Heating of Photoluminescent Colloidal Carbon Dots" Nanomaterials 12, no. 14: 2426. https://doi.org/10.3390/nano12142426
APA StyleMussabek, G., Zhylkybayeva, N., Lysenko, I., Lishchuk, P. O., Baktygerey, S., Yermukhamed, D., Taurbayev, Y., Sadykov, G., Zaderko, A. N., Skryshevsky, V. A., Lisnyak, V. V., & Lysenko, V. (2022). Photo- and Radiofrequency-Induced Heating of Photoluminescent Colloidal Carbon Dots. Nanomaterials, 12(14), 2426. https://doi.org/10.3390/nano12142426






