1. Introduction
In recent years, with the development of economic and social progress, human living standards have gradually improved, increasing attention has been paid to food safety, and requirements for food safety technology that are more stringent have been established. According to news reports and data, bacterial infections cause about 40% of all diseases and pose a huge threat to public health and the social economy [
1]. Today, common bacterial detection approaches include culture and colony counting methods [
2], enzyme-linked immunosorbent assay (ELISA) [
3], biosensor technology [
4], and adenosine triphosphate (ATP) and bioluminescence assay [
5].
Escherichia coli (
E. coli) is a common conditionally pathogenic bacterium. Under certain conditions, a part of
E. coli strains harboring virulence factors are considered to be pathogenic to humans, so they are often used as an indicator for monitoring the quality of food and drinking water. Currently, the approaches to detecting
E.coli have been divided into traditional detection methods and new modern detection methods. The traditional detection methods include the plate dilution method [
6], membrane filtration, fluorescence quenching [
7], and the use of quartz-crystal microbalance-based sensors [
8]. Although these methods have relatively high reliability and sensitivity, most of them still have some drawbacks. The plate dilution method, for example, was once regarded as a classic microbial detection method. However, this approach is comparatively complex because it requires the culturing and enriching of bacteria in the lab prior to testing. Thus, the detection cycle is longer, making real-time detection impossible. It is difficult to meet the current domestic and foreign requirements of bacteria detection in food. New modern detection methods include ATP bioluminescence technology [
9], magnetic-activated cell sorting (MACS) [
10], the biosensor detection method [
11], PCR detection technology [
12], the gene chip method [
13] and genome sequencing [
14]. Among these modern detection methods, such as immunoassays, which are being applied in industry, most modern methods cannot meet the demand for portable and on-site detection due to the high equipment cost and professional laboratory operation. At the same time, most of the traditional bacteria detection methods generally have disadvantages, such as being time-consuming, complicated operation, low signal-to-noise ratio and non-specific bacterial identification, and these shortcomings lead that not all traditional methods can meet industry needs in every context. Therefore, it is very important to develop a fast, portable and highly accurate bacterial detection system to ensure food safety and maintain public health and environmental sanitation.
The combination of immunomagnetic separation technology and ATP bioluminescence technology provides a way to detect bacteria. However, how to improve the accuracy and expand the detection limit is an important question we face.
Immunomagnetic separation technology is one of the research hotspots in the field of bacterial detection. Magnetic nanoprobes are an indispensable part of immunomagnetic separation technology, based on nanotechnology and immunology, using various magnetic nanoparticles coated with immunoactive substances for immunological or biological analysis. Fish et al. realized a method to detect the concentration of
Bacillus spores rapidly using immunomagnetic separation technology incorporating a chromatography technique in 2009 [
15]. Moreover, Kuang et al. used immunomagnetic separation combined with a fluorescent probe to detect
Salmonella with a sensitivity of 500 CFU/mL [
16]. In 2016, Mengxu et al. designed an electrochemical immunosensor to detect
E. coli and
Salmonella typhimurium in food based on immunomagnetic separation technology and screen-printed interdigitated microelectrodes [
17]. In recent years, immunomagnetic separation technology has developed rapidly and become a common detection method.
ATP bioluminescence technology is one of the most common microbiological detection methods, which can determine the total number of microorganisms in food. ATP is an important energy substance in cells that can store and provide energy. It is immanent in all cells and usually measured by the fluorescein–luciferase luminescence system. The wide adhibition of ATP bioluminescence technology in food detection and the medical industry is due to the creation and application of portable ATP Detectors. Murphy et al. used ATP bioluminescence technology to detect bacteria in liquid milk [
18]. Although this method was proven feasible by comparing the results from the standard plate count method, it is difficult to use on-site. In 2017, Zhang et al. utilized ATP bioluminescence technology to detect the number of
E. coli bacteria in food. The quantitative results demonstrated that their method was useful in detecting the bacteria with a sensitivity of 3.0 × 10
2 CFU mL
−1 [
19]. Xu et al. combined transparent graphene electrodes with ATP bioluminescence technology to improve the accuracy of
E. coli detection in food [
20], but sensitivity remained low.
According to the research, temperature change has an obvious influence on the bacteria-detecting process. Heat-treatment pathogen technology is used to adopt heating, thermal insulation, and cooling to determine the properties or reaction states of pathogens under different temperatures, which can be better used in the field of bacterial detection or bacterial inactivation. Today, in addition to inactivating bacteria at high temperatures, the pathogen heat-treatment technology can be used to enhance the signal strength for bacterial detection. In 2017, Lee et al. found that the amount of ATP released from bacterial cells, such as
Salmonella enteritidis,
E. coli O157:H7, and
Bacillus cereus, can be increased by subjecting the samples to heat treatment, which helps detect the number of bacteria colonies using ATP bioluminescence technology [
21].
Because photoelectric conversion is applied in this system, graphene, a new bioassay material, has attracted our attention because of its excellent light transmission and electrical conductivity. In 2004, Novoselov et al. prepared graphene films by mechanical exfoliation and discovered their unique electronic properties [
22]. Li et al. prepared graphene films by graphene chemical vapor deposition and developed a process to transfer graphene film to various substrates, making it possible to prepare transparent graphene electrodes [
23]. In 2010, Bae et al. produced transparent graphene electrodes using a roll-to-roll production method and wet chemicals [
24]. Afterwards, it was found that the optical transmittance of transparent graphene electrodes is 97.4% and that their conductance can be as low as 125 Ω
−1, which is better than the other conventional transparent electrodes, such as those fabricated from indium tin oxide. Moreover, graphene also has excellent thermal conductivity, of up to 5300 W/m · K, higher than that of carbon nanotubes and diamonds.
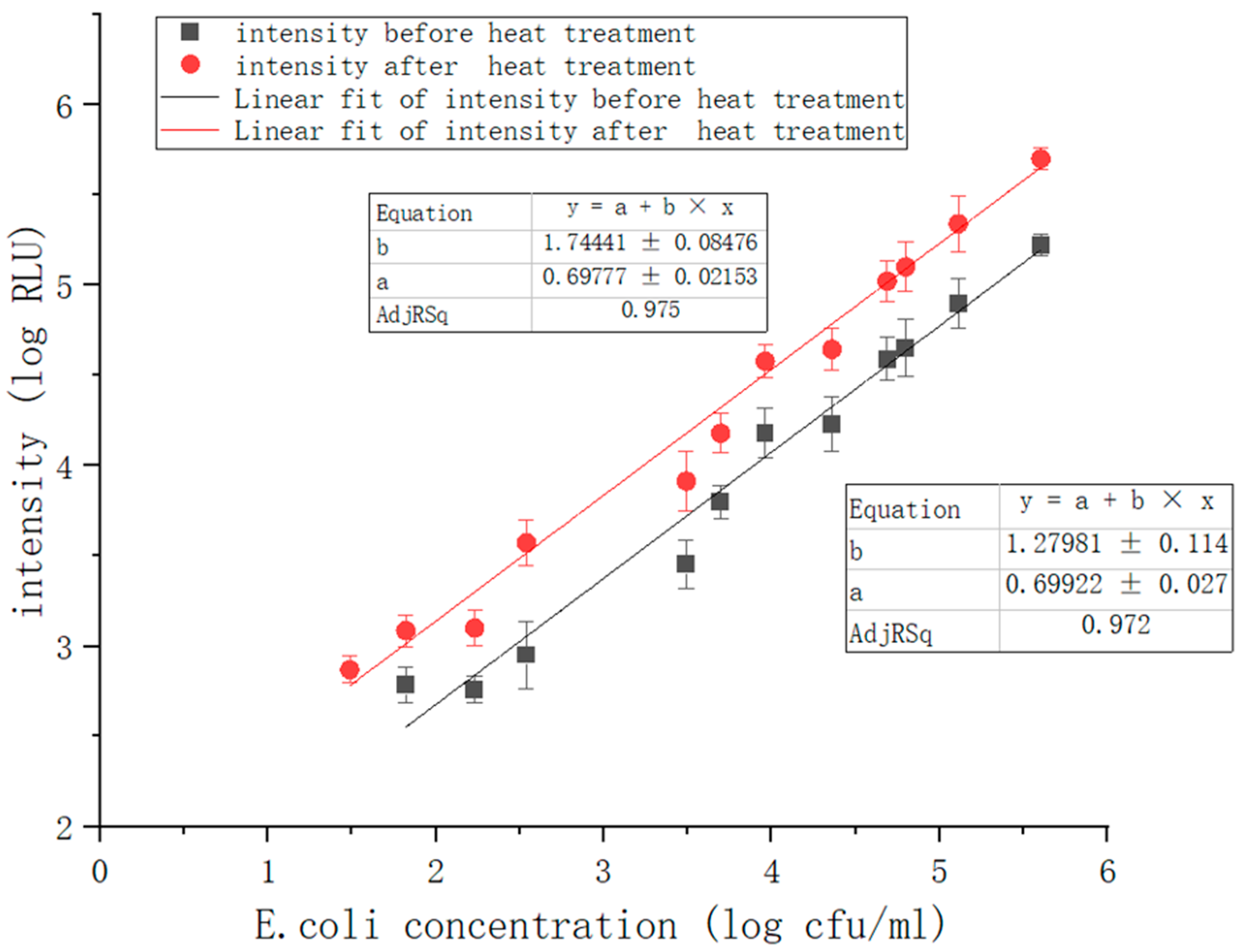
Based on the above studies, our final goal is to further enhance the accuracy and sensitivity of the ATP bioluminescence detection system and expand its detection limit. Therefore, we designed a rapid detection system based on immunomagnetic separation technology, ATP bioluminescence technology, and pathogen heat-treatment technology. In addition, the system utilizes electric field force to enrich ATP. The system uses the biotinylated
E. coli antibodies and streptavidin-modified magnetic nanoparticles to prepare the magnetic nanoprobes with an avidin-biotin link reaction. The detection tube is prepared by the chemical vapor deposition (CVD) of graphene [
25]. The probes capture the pathogens, and then to enhance the amount of ATP the target pathogens release, heat treatment is carried out on the samples before the target pathogen is lysed. Among them, thin-film heaters and resistance temperature detectors (RTDs), the main components of heat treatment, have been widely applied in sensor chips, biological chips, and microfluid chips [
26,
27]. The released ATP can be enriched by an electric field applied through transparent graphene electrodes because ATP is negatively charged under weakly alkaline conditions. The experiment showed that the number of bacterial colonies is proportional to the luminescence intensity. As the intensity of ATP bioluminescence attenuates over time, we add another light source to reduce the error and use photomultiplier tubes (PMT) for luminescence acquisition and photoelectrical signal conversion. We then carry out a series of optimizations in the subsequent signal processing of the system to meet the requirements of measurement accuracy and achieve quantitative detection. Compared to the previous system, the heat treatment module is added, and the ATP bioluminescence reaction chamber is improved. Because temperature change has a significant impact on the bacteria-detecting process, the addition of heat treatment modules can eliminate the influence of ambient temperature change on ATP bioluminescence reaction, increase the relative luminescence unit (RLU), enhance the luminescence signal, and improve the accuracy and sensitivity of detection results. The results show that under the optimal experimental conditions, the linear correlation coefficient of the system could reach 0.975, and the detection concentration of bacteria was in the range of 1.7 × 10
1–10
6 CFU/mL. The combination of these technologies not only improves detection results and makes the system more convenient but also shows good performance in field detection.
2. Detection Principle
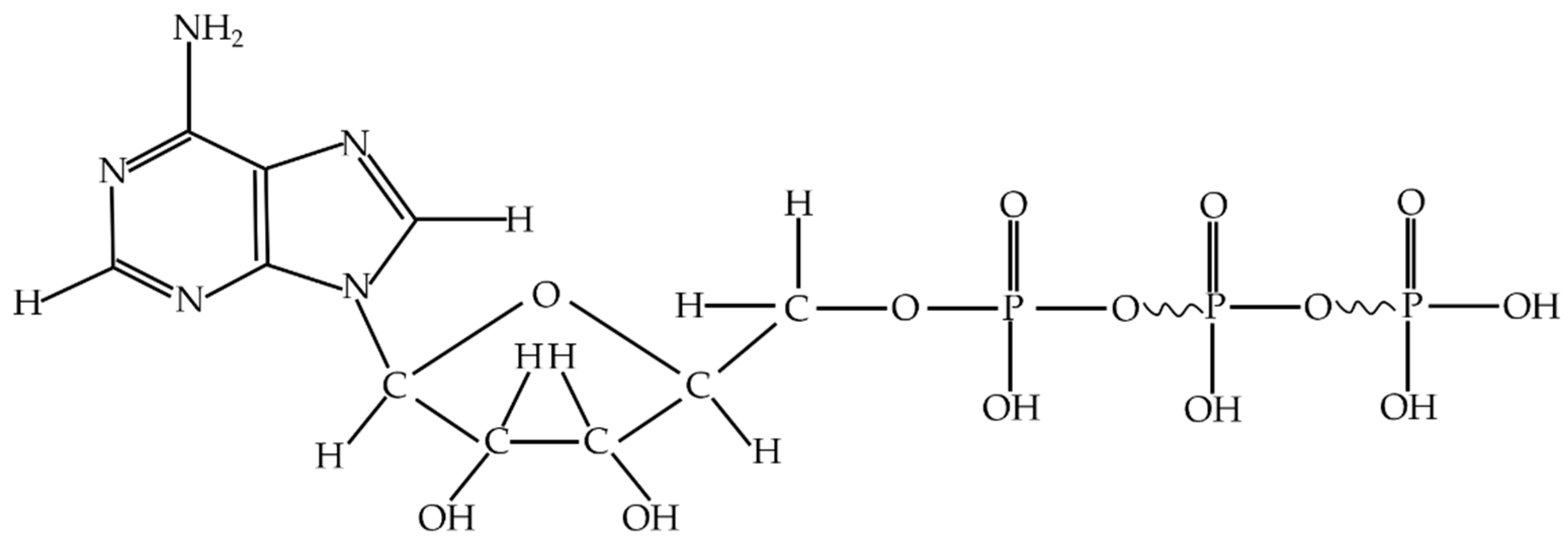
Adenine nucleoside triphosphate, abbreviated as adenosine triphosphate (ATP), is an energy-supplying cell substance. It exists in the cells of all kinds of organisms [
28]. ATP consists of an adenine molecule, a ribose molecule and nucleotides formed with three linked phosphate groups [
29]. ATP releases a large amount of energy when it hydrolyzes, the most direct energy source in living organisms.
Figure 1 shows the molecular constitution of adenosine triphosphate.
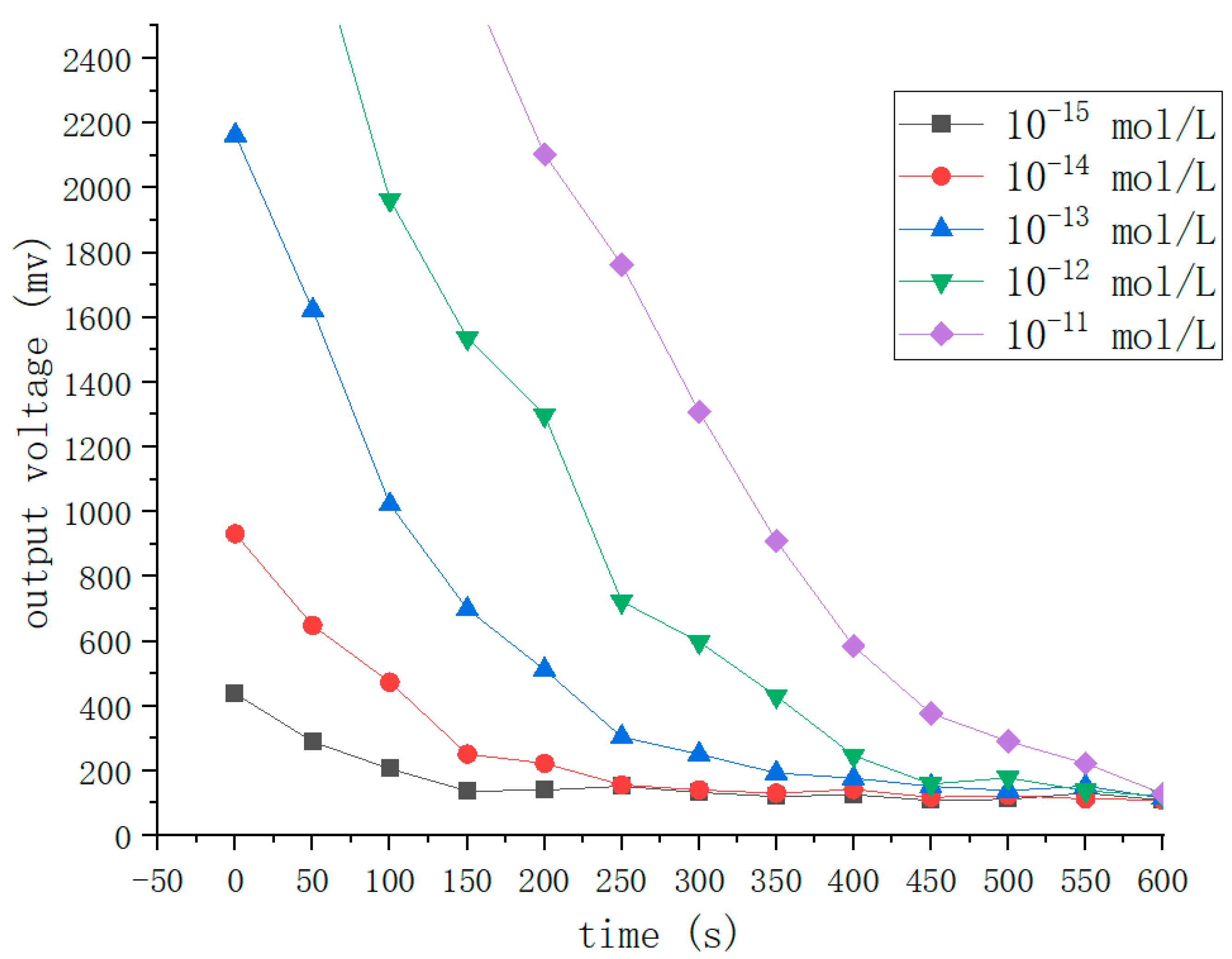
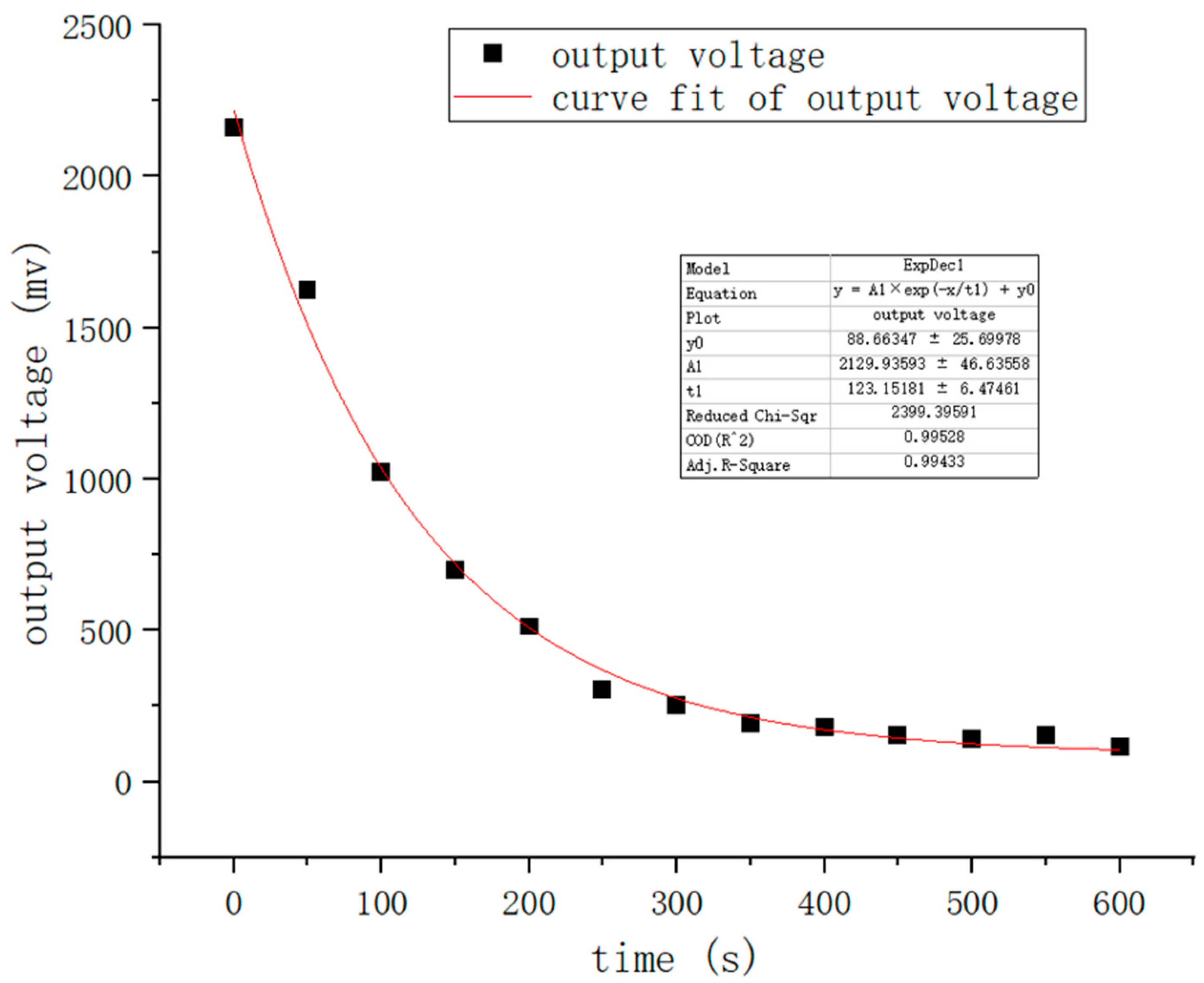
In general, the quantity of ATP is roughly the same in each bacterium, at approximately 1–2 fg per cell [
30]. Moreover, the mechanism, luciferin reacting with oxygen under the action of luciferase to produce bioluminescence, was discovered in 1947, and ATP can provide the energy for this reaction. According to our previous experiments, the experimental reagent’s luminescence intensity has a strong linear relationship with the concentration of ATP in a certain concentration range. The above findings provide theoretical bases for measuring the amount of bacteria utilizing ATP bioluminescence technology. In the experiments, we need to use a reagent group including an erase reagent, lysis reagent, luciferase, and fluorescein. The function of the erase reagent is to remove the effects of somatic cells and free ATP. The function of the lysis reagent is to lyse
E. coli and release ATP. Luciferase and fluorescein provide raw materials for the bioluminescence process and react in the presence of Mg
2 + and O
2. In addition, we subject the captured
E. coli pathogen to heating during detection, which can increase the amount of ATP the pathogen releases and enhance detection sensitivity. In our previous study, we found that the reagent’s concentration affected the luminescence intensity. Therefore, we subjected the reagent concentration to 30–40 mg/L to stabilize the peak luminescence intensity. When the ATP concentration is less than 10
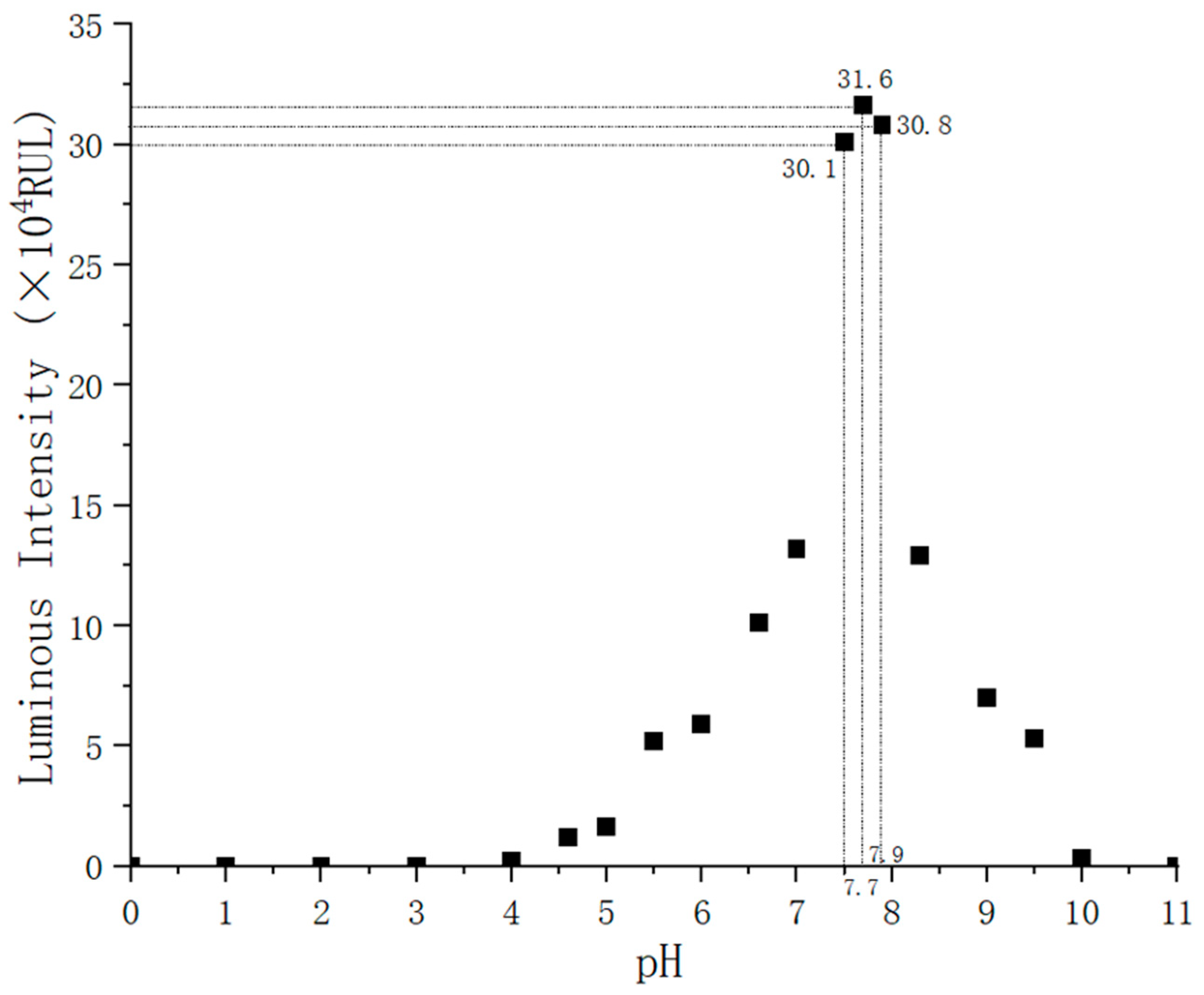
−7 mol/L, we observe a strong linear relationship between the ATP content and light intensity when the other reactants are sufficient in the reaction process. Because the solution’s pH affects luciferase activity, it will directly cause the emitting light to deviate from its wavelength peak. The results of experimental data show that a stable peak wavelength of light is acquired when the pH is between 7.5 and 7.8. In this process, almost all of the energy from ATP is converted into light energy. The amount of
E. coli cells can be measured indirectly using Equation (1), where
represents luminous energy.
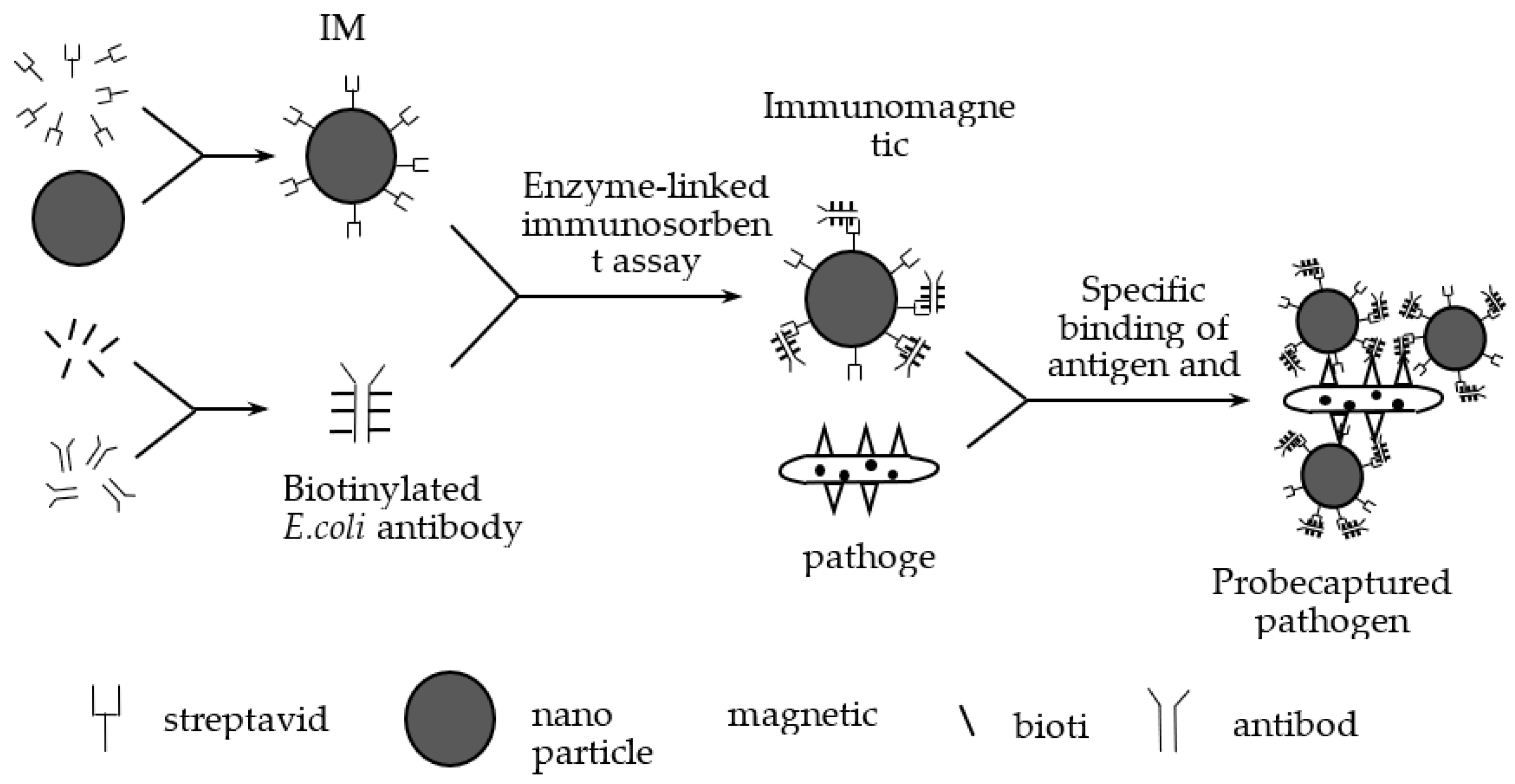
A nanoprobe is a nanoscale biosensor that can detect single living cells, which have the characteristics of nanoscale size and real-time monitoring and causes little damage to cells [
31]. The immunomagnetic beads (IMB) separation technique is a new immunological technique that combines the high specificity of immunological response with the unique magnetic responsiveness of magnetic beads. It is a kind of immunological detection method with strong specificity and high sensitivity. IBMs are magnetic beads wrapped with monoclonal antibodies, which have the strengths of high speed, high efficiency and simple operation. Therefore, they have a wide range of applications in the enrichment and separation of bacteria [
32]. Compared to the traditional methods, IBMs do not have the disadvantages of time-consuming techniques and a pre-enrichment requirement. Magnetic nanoparticles can be collected by a magnetic field, and this property can be used to combine bacteria with magnetic nanoparticles for specific separation and to capture target bacteria.
In our previous study, we designed medium-size IBMs (120–200 nm) to capture
E. coli [
19]. First, we utilized streptavidin to modify IMBs to obtain magnetic beads whose surface is modified with streptavidin. Then we mixed the
E. coli-specific antibodies and biotin to get biotinylated
E. coli antibodies. We prepared immunomagnetic nanoprobes by combining magnetic beads modified with streptavidin with biotinylated
E. coli antibodies by enzyme-linked immunosorbent assay [
33,
34]. We used immunomagnetic nanoprobes to capture
E. coli by antigen-antibody reaction, and we prepared the IMB/antibody–
Escherichia coli-immune compounds. An antibody is an immunoglobulin produced by plasma cells differentiated from B cells in response to the stimulation of antigenic substances, and antibodies can specifically bind to the corresponding antigen. In our experiments, we studied
E. coli O157:H7 and used
E. coli O157:H7 monoclonal antibodies to capture them. These antibodies bound specifically to the somatic antigen (O antigen) and the flagellar antigen (H antigen) of
E. coli O157:H7, respectively, to achieve the effect of capturing the bacterium.
Figure 2 shows the preparation and capture processes. To enrich and isolate the magnetic immune compounds, we designed a magnetic field at the two polar ends of the samples.
Graphene is a material with a mono-layered two-dimensional hexagonal lattice structure [
35]. Due to graphene’s excellent properties, such as good electrical conductivity [
36], light transmission, excellent mechanical properties, and electron mobility at room temperature, graphene and its chemically modified derivatives can be used as graphene transparent electrodes. Moreover, graphene has high thermal conductivity, which allows us to apply it for heat and cooling.
In previous studies, we found that temperature has a significant impact on the amount of ATP bacterial cells release. Therefore, in this study, we found a method that improves the sensitivity by increasing the amount of ATP that
E. coli releases by subjecting the samples to heat treatment [
21]. Here, we designed a temperature control module accurately. A heat treatment unit comprises three parts: a thermal cycler, reaction chambers, and a temperature detection and signal feedback web. The thermal cycler comprises a heating component, a cooling component and a thermal signal controller. However, the great difficulties of designing the thermal cycle structure lie in the accurate control of temperature and excellent thermal uniformity around the reaction chamber.
To solve those problems, we designed a thin-film heater. The thin-film heater, which is formed by depositing polycrystalline or metallic materials on the substrate surfaces [
37,
38], is very popular because of its advantages, such as low power consumption, high operation speed, and strong temperature control performance, and it is crucial for the portability of bacterial detection equipment. To meet space and environmental needs, we designed a flexible polyimide thin-film heater that mainly comprises external insulation material and internal heating wire. We chose polyimide as external insulation material because it has high-temperature resistance, high insulation and excellent mechanical tractility properties. Commonly, the materials of the heating wire used in thin-film heaters are divided into Fe-Cr-Al alloy and nickel-chromium alloy. Here, we selected nickel-chromium alloy as the material for the thin-film heater because it has excellent thermoelectric properties. It is difficult to deform at high temperatures, and it has the advantageous qualities of having a long service life, being non-magnetic, having strong corrosion resistance, etc.
A temperature detector is an essential part of the temperature detection and signal feedback unit. It is used not only to detect the temperature of the heater and the reaction chamber but also to feed the temperature signal to the MCU. The heating or cooling element is run by the thermal signal controller to maintain a stable temperature. We used the resistance temperature detector (RTD) in our system because of its high accuracy, high stability, and wide temperature-control range. In general, metallic materials are used for RTDs; temperature monitoring relies on the relationship between the resistance and the temperature [
39,
40,
41]. Platinum, copper and nickel are commonly used to manufacture RTDs, for they have the advantages of a high-temperature coefficient and quick response to temperature changes. Moreover, they are easily manufactured into refined coils. Among them, platinum, with its high stability, does not react physically or chemically in a certain temperature range. Platinum-resistance temperature detectors have a wide measuring range. In addition, they not only have better linearity than thermocouples and thermistors, but they are also more accurate and stable temperature sensors than any we have obtained up to now.
3. Testing Prototype Design
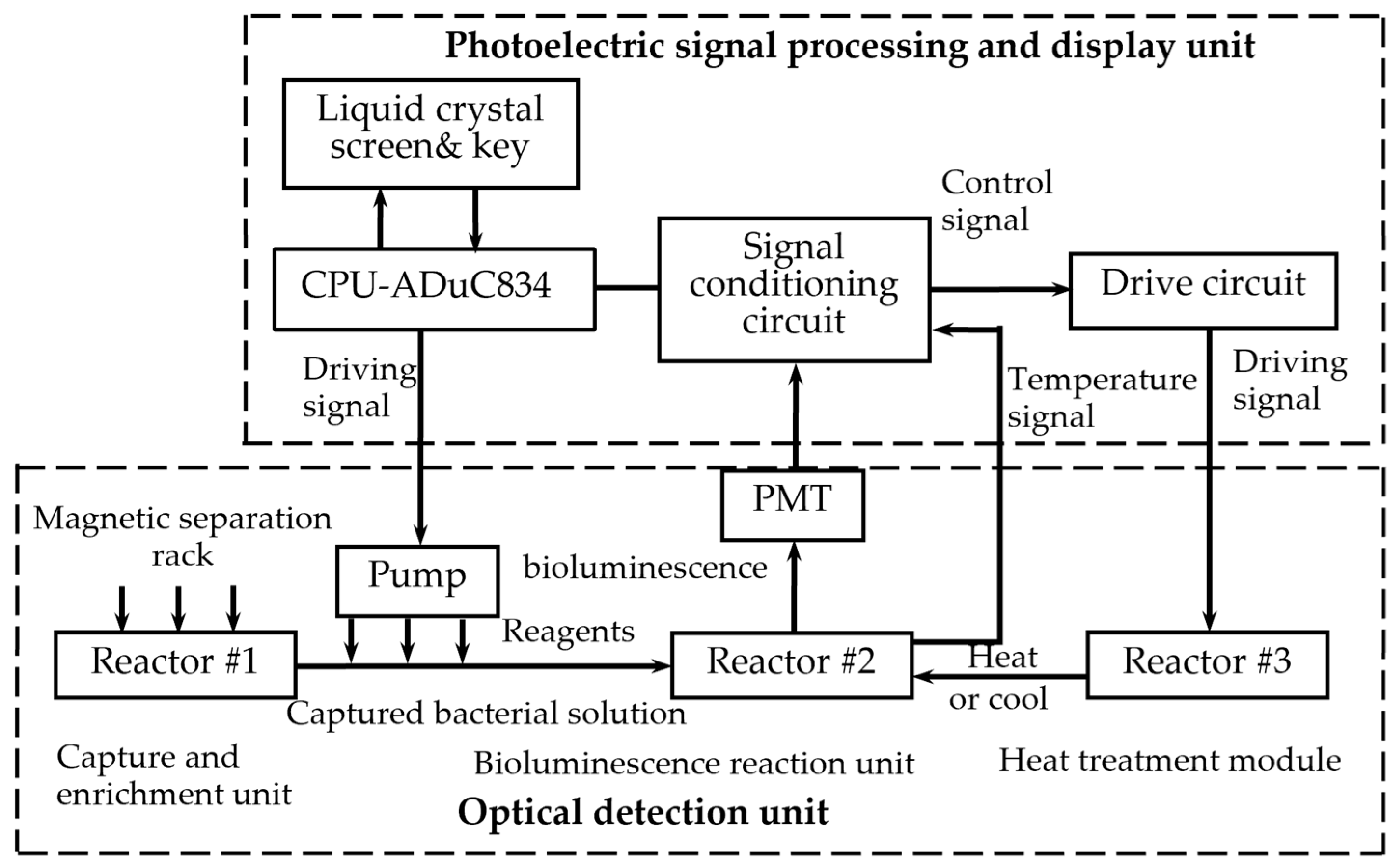
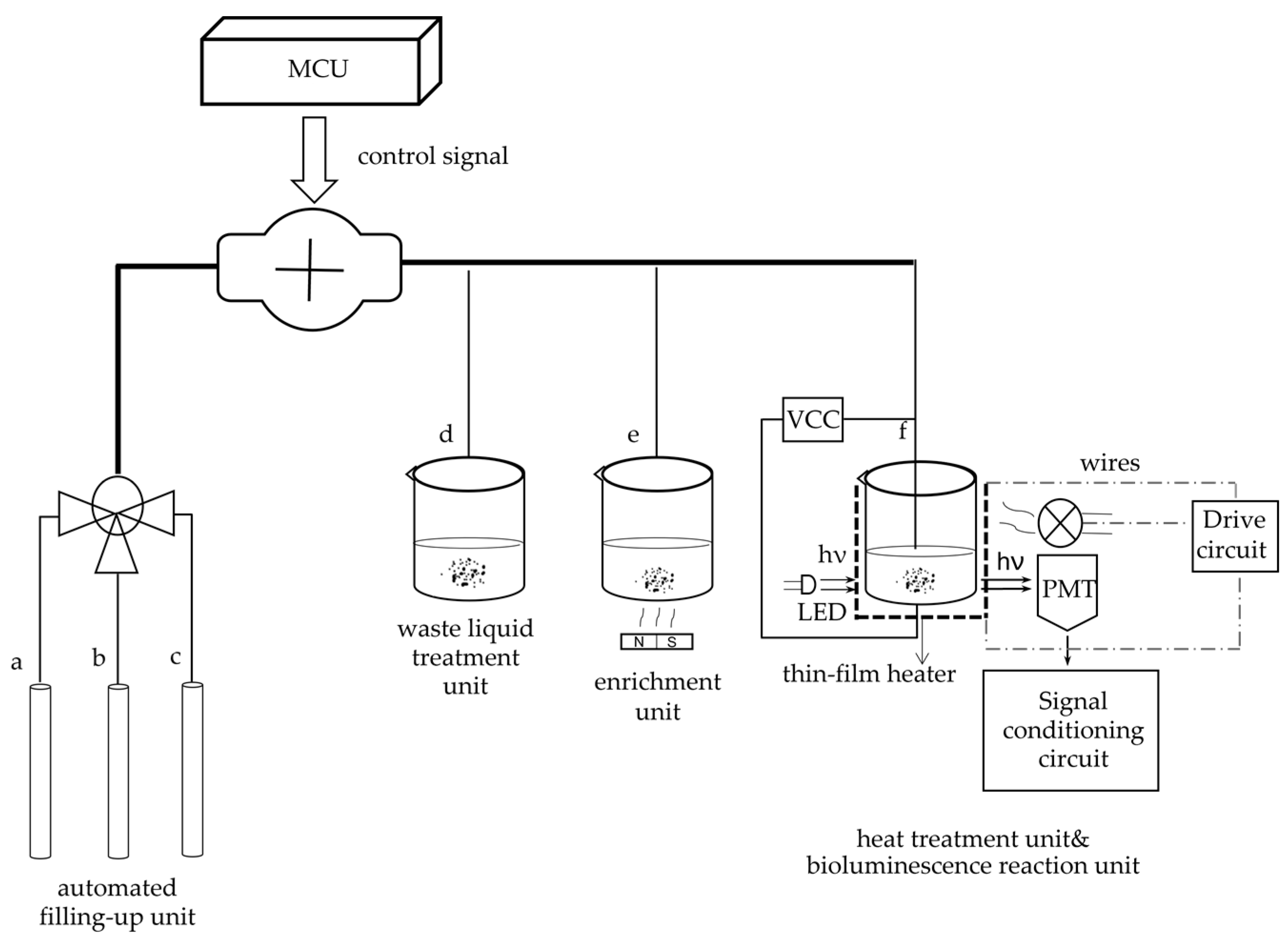
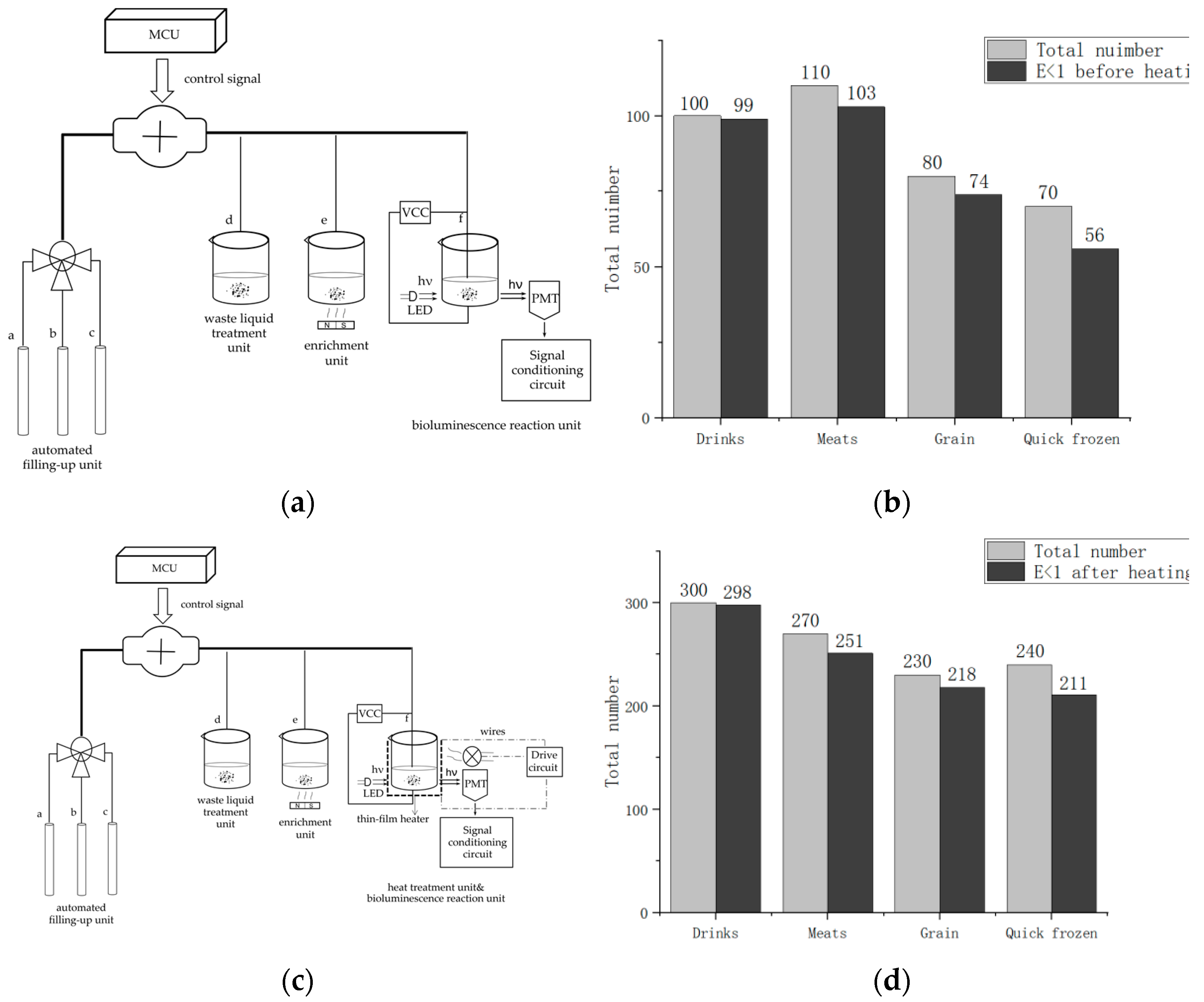
The system primarily includes two parts. One is the optical detection unit, and the other is the photoelectric signal processing and display unit, as
Figure 3 shows.
The optical detection unit comprises four components. Reactor #1 is a capture and enrichment unit, reactor #2 is a bioluminescence reaction unit, and reactor #3 is a heat treatment module. Additionally, the unit contains an automatic filling-up unit.
Reactor #1 is the capture and enrichment unit, which is intended to use our designed immunomagnetic nanoprobes to capture
E. coli pathogens and enrich bacteria by magnetic separation rack, as shown in
Figure 3. The details of
E. coli O157: H7 cultured are as follows.
E. coli O157: H7 standard strains were added to the modified
E. coli medium (37 °C, 24 h) and then homogenized in 300 μL sterile saline. After centrifugation at 5000 rpm, the precipitated supernatant was discarded and placed in saline suspension again. Finally, the original bacterial solution was obtained at a concentration of 10
7–10
8 CFU/mL. Multiple gradient concentrations of the samples were obtained by diluting the original bacterial solution. The samples were divided into two parts, one was for the conventional culture method, and the other was for detection by our method. In the following, we will describe the technical details. First, 50 µL of the immunomagnetic nanoprobes solution is added to the
E. coli solution and wait 10 min for the probe to capture the bacteria. Due to utilizing an enzyme-linked immunosorbent assay to bind
E. coli antibodies to the immunomagnetic nanoprobes, the probe can capture
E. coli through antigen-antibody reaction. Second, after the
E. coli is captured, magnetic separation is carried out. And under the action of the magnetic separation rack, the IMB/antibody–Escherichia coli-immune compounds are adsorbed onto the bottom of the test tube. Then, the supernatant is discarded after magnetic separation. Last but not least, to obtain the captured
E. coli solution, the enriched samples are washed three times with 150 mL of cleaning solution (10-mmol L-1 PBS, 0.05% Tween-20).
Reactor #2 is the bioluminescence reaction unit, which consists of a special detection tube and a photomultiplier (PMT). The detection tube is characterized by a graphite layer attached to the bottom. The heat treatment module (Reactor #3) is a temperature control device implemented using a heating component, a cooling component and a thermal signal controller. They cooperate to process the captured
E. coli solution. First, the solution used for the ATP bioluminescence testing is subjected to heat treatment to increase the amount of ATP released from
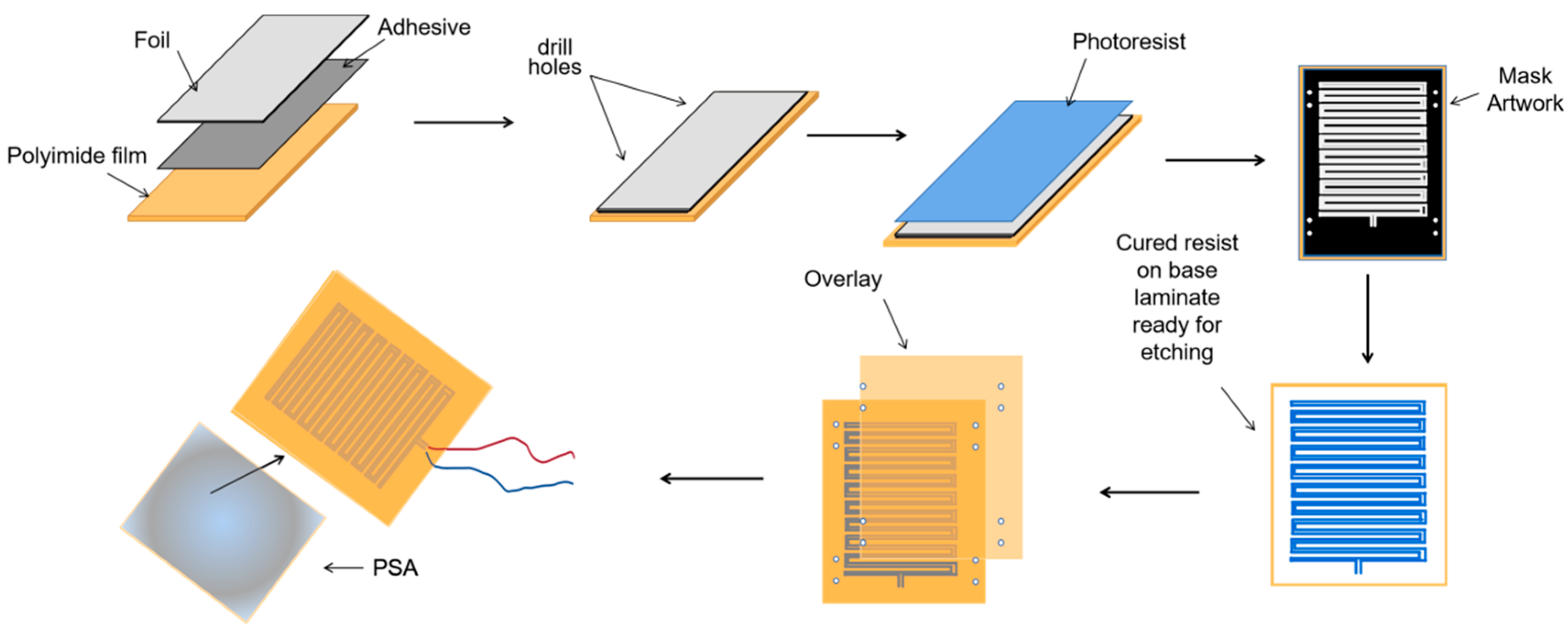
E. coli. We designed a kind of flexible thin-film heater as the heating component in the temperature control device, and its design was as shown in
Figure 4. We used the polyimide film as the substrate material (thickness 0.15 mm). Nickel-chromium alloy foil was selected as the resistance circuit material (thickness 0.05 mm). Nickel-chromium alloy foil was laminated onto the substrate (polyimide film). This step was achieved using a thin thermosetting adhesive layer with excellent bonding properties when bonded to both materials. After lamination was stabilized, the substrate was drilled and positioned so that each layer within the heater remained aligned during a period of manufacturing. Next, the photoresist was smeared evenly on the panel (nickel-chromium alloy foil). After the photoresist was attached well, we placed the advanced photomask tool on the resist. The mask, which was generated based on a CAD design tool, was the final design template of the heating element, including the applicable element width required to produce the correct resistive heater. Then, the exposure was carried out. In this process, the resist was exposed to ultraviolet light to cure for use as a chemical etching resist. Next, the uncured resist was removed to expose the foil for etching and foil removal. At the same time, the cured resist can protect the pattern of the heater element. The panel was then chemically etched, stripped, and cleaned to chemically remove the foil not protected by the resistor and to retain the heating element pattern on the panel. The next step was to place a top overlay on the panel and laminate it. The top layer, the polyimide film (thickness 0.15 mm), provided access to the heating elements, including connecting wires and the openings for assemblies. Finally, after the heating film was removed from the panel, the wire was connected to the heating film, and the high-performance pressure-sensitive adhesive was coated on the back of the heating film. We adhered the heating film to the inner wall of the container that holds the test tube, and the heating film was wrapped tightly on the outer wall of the test tube for heating. Resistance temperature detector (RTD) PT100′s connector was fixated in the reserved position between the thin-film heater and the test tube for monitoring and feedback on the temperature of the test tube. Both the thin-film heater and RTD were controlled by the external drive circuit and the thermal signal controller. The thin-film heater was controlled using proportional-integral-derivative (PID) closed-loop controllers, which were implemented on CPU-ADUC834. CPU-ADUC834 is a microcontroller that has two independent ADCs [
42]. In addition, the thin film heater was driven by a double MOS parallel active output circuit. A PT100 resistance temperature detector (RTD) was connected with a MAX31865 chip to convert the voltage signal corresponding to test tube temperature into a digital signal. The MAX31865 chip was then connected with an ADUC834 to control the drive circuit according to the collected temperature. Because the optimal temperature for the bioluminescence reaction is 24.5 °C, we heated the tube to 50 °C for 5 min and then let it cool to 24.5 °C for 20 s for the next bioluminescence reaction. The cooling component was implemented by a low-power fan, which was also controlled by the ADUC834.
ATP bioluminescence reaction occurred in the detection tube in the fluorescent reaction unit (Reactor #2). We connected the graphene electrode to the upper electrode through a steady current source, creating a weak electric field in the detection tube. The studies show that graphene has good optical transmittance, so the PMT, which is used for light trapping and photoelectric conversion, can be placed on the side of the test tube. To avoid the interference of external light as much as possible to ensure the accuracy of detection, the entire bioluminescence reaction and light capture and detection by PMT were performed in a completely closed environment. The procedure for this part of the assay is as follows: 30 μL of enriched E. coli suspension was added to the special test tube with a graphene electrode attached to the bottom. The solution in the tube is heat-treated for 5 min using the flexible thin-film heater we designed and then cooled to the optimum ATP bioluminescence reaction temperature using the cooling fan. Then 270 μL of the detection reagent was accurately injected with a peristaltic pump. The lysis agent in the detection reagent can lyse E. coli and release ATP. Meanwhile, the graphene electrode is energized by a built-in current source to create an electric field outside the test tube. Moreover, the experiments indicate that the luminescence from the ATP bioluminescence reaction is stronger under weak alkaline conditions compared to other acid-base conditions, and ATP has a negative charge under weak alkaline conditions. ATP will be enriched at the bottom of the test tube under the action of the electric field. The electric field also can enhance the activity of ATPASE and accelerate the oxidation of luciferase, thus enhancing the stability and accuracy of the system. After adding the detection reagent, the energy for the bioluminescence reaction is provided by ATP, and the luminescence emitted from the ATP bioluminescence reaction is captured by the PMT. The system automatically reads the optical signal after 60 s. And an internal calibration and compensation unit was used to detect and compensate for light attenuation, which will be described in the following. It is essential to add a filtering circuit in the design to reduce external noise interference due to the unavoidable system noise and weak input signals.
For portability and automation, we added an automated filling-up unit to the system, which is controlled by a microcontroller and enables the system to automatically select the appropriate reagents and quickly fill the required dose of reagents as required, as shown in
Figure 5. In this unit, a single reagent hose line is represented by a thin line, and multiple reagent line hoses are represented by thick lines. For the tubes used in each unit, we choose the hoses characterized by high elasticity, low adhesiveness and low permeability. The different types of reagents for system selection are stored in different containers. Erase reagent, lysis reagent (50 mmol/LTris-HCl, 150 mmol/L NaCl, 0.02% NaN
3, 100 μg/mL PMSF, 1 μg/mL Aprotinin, 1% Triton X-100) and bioluminescent reaction reagent (luciferase/luciferin reagent substrate, from Beijing YPH Biotechnology Co., Ltd., Beijing, China) is stored in three tubes a, b and c, respectively. Using a peristaltic pump in the automatic filling device, the required reagents are added to each unit via hoses to complete the test.
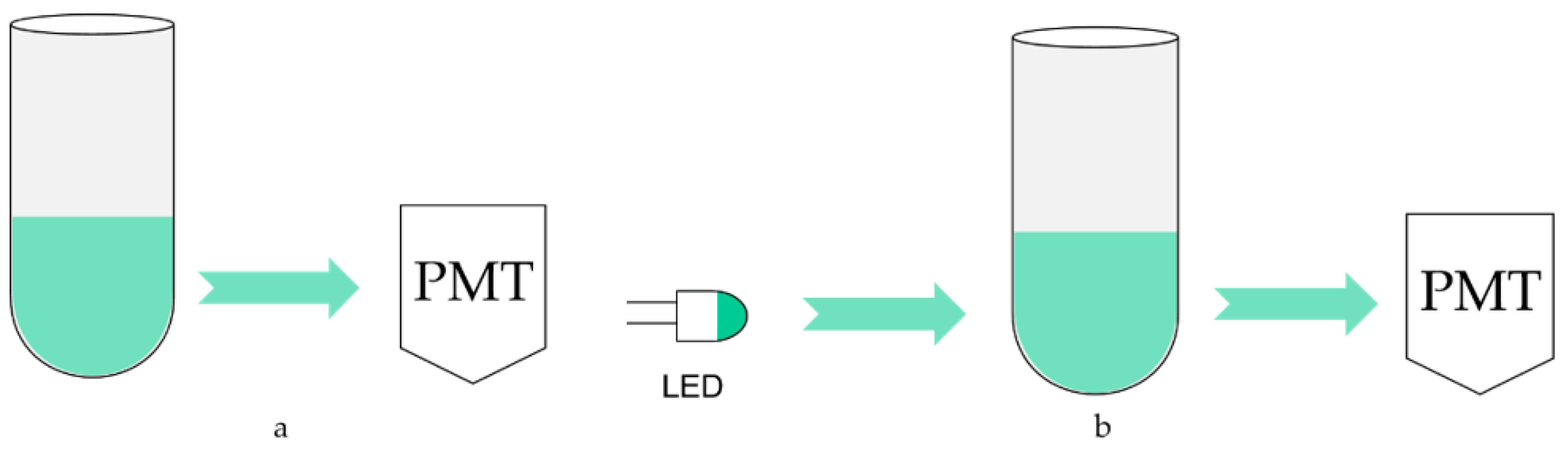
Although the test is carried out in a closed device, some external light still enters the reactor through cracks and transparent tube refraction, which forms scatting light. The light emitted by ATP luminescence is also absorbed by the solution because the ATP luminescence reaction takes place in the solution. These two points may affect the testing results, so the optical calibration method is adopted for compensation, and its schematic diagram is shown in
Figure 6.
Figure 6a is the schematic diagram without using the optical calibration method. The sample and detection reagent react to release bioluminescence in an optical reaction cell, which is directly detected by photomultiplier tubes. Therefore, it is inevitable to be affected by the absorption of luminescence and scatting light. To solve this problem, a light-emitting diode (LED) is installed on the opposite side of the photomultiplier tube, as shown in
Figure 6b. The luminescence wavelength of the LED is required to be consistent with ATP bioluminescence, whose peak wavelength is 562 nm. And under the control of a constant current source, make its luminescence as stable as possible. When no detection reagent is added, the photomultiplier tube first detects and records the original light intensity of the LED and then turns it off. The approaches to close include power supply and shutter. After adding the detection reagent and bioluminescence detection, the LED is turned on again, and its luminescence is detected by a photomultiplier tube after passing through the optical reaction cell. Because the absorption and scattering of the light attenuate it, the detected bioluminescence signal can be corrected by the degree of attenuation to achieve the calibration function.
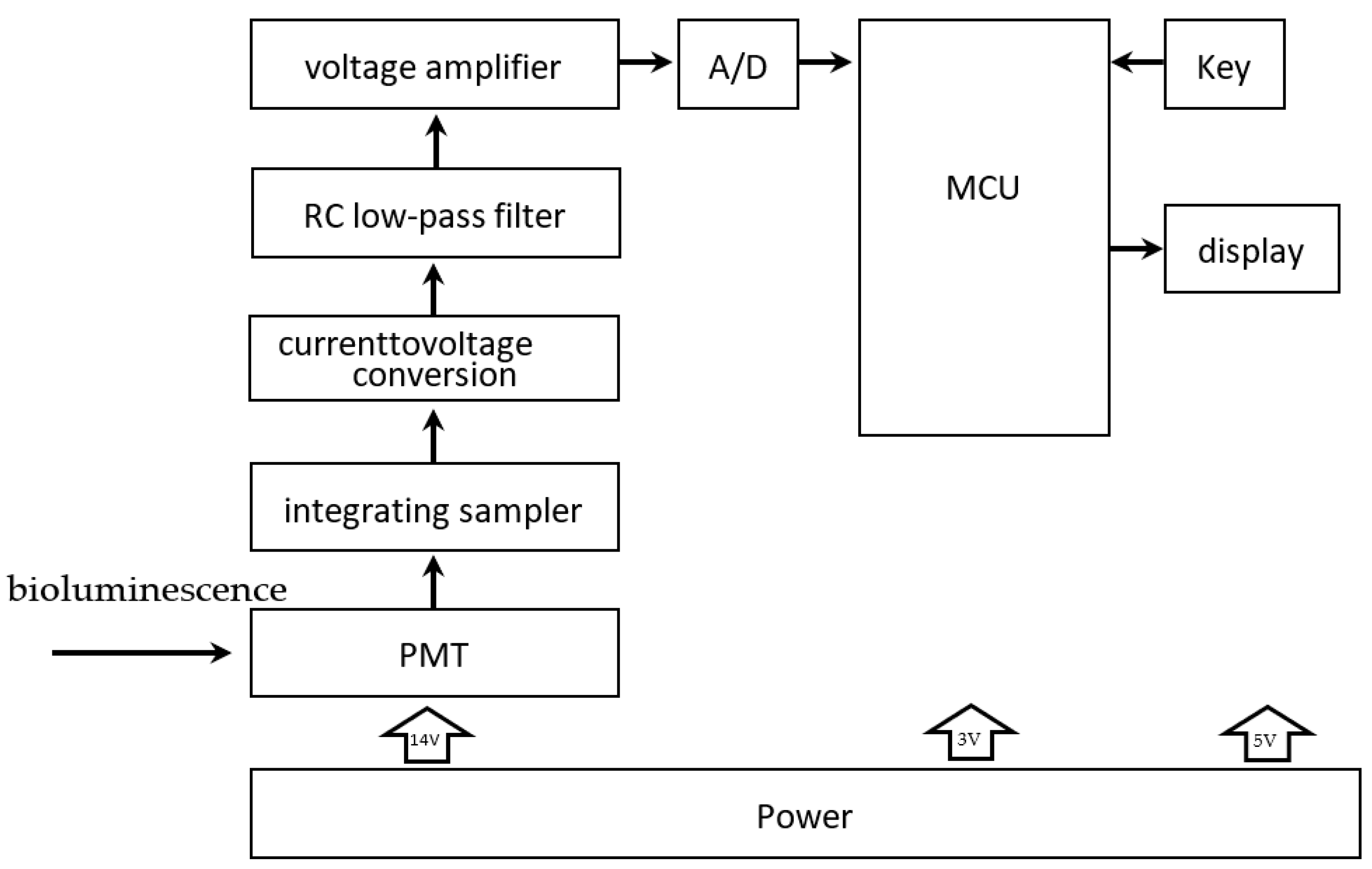
After processing, the bioluminescence reaction is carried out in a special detector tube in reactor #2, with graphene electrodes attached to the bottom. The tube is chosen to use a high-transmittance glass test tube to improve the light transmission. The PMT is responsible for collecting the light signal, obtaining the relative luminous intensity, and completing the photoelectric conversion. As shown in
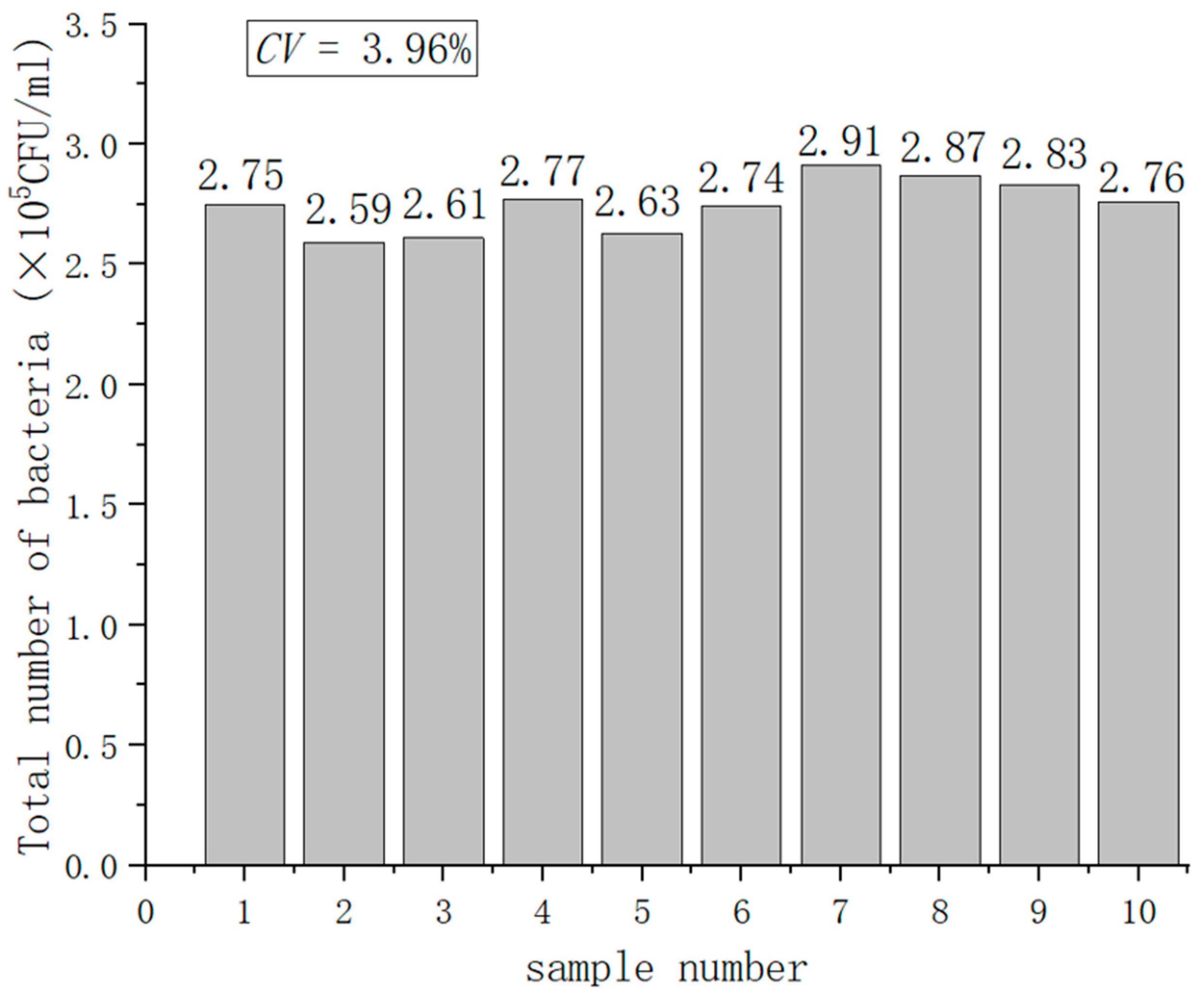
Figure 7, current signals derived from photoelectric conversion are turned into analog voltage signals by an integrated operational amplifier circuit and become digital signals after analog-to-digital conversion. Then they are sent to a microcontroller for processing. A complete inspection process is controlled in 30 min. Moreover, to automate and improve the system’s efficiency, we designed an automatic cleaning function, which can be automatically cleaned between two inspection processes. The waste liquid obtained after cleaning is delivered to the waste liquid pool. This function can reduce the interference between two consecutive assays.