Optimization of Technological Parameters of the Process of Forming Therapeutic Biopolymer Nanofilled Films
Abstract
:1. Introduction
- to build a mathematical model of the process of formation of therapeutic biopolymer nanofilled films;
- to study the influence of component composition and technological parameters of the process of formation of therapeutic biopolymer nanofilled films on their therapeutic and physical-mechanical properties;
- to choose the optimal component composition and technological parameters for forming therapeutic biopolymer nanofilled films, which provide the maximum therapeutic effect with sufficient vapor permeability, biodegradation duration, swelling, and elasticity;
- conduct a microscopic study of ZnO NPs and biopolymer nanofilled films;
- to study antimicrobial properties and biopolymer nanofilled films.
2. Materials and Methods
2.1. Research Materials and Equipment
2.2. Methods for Studying the Properties of Biopolymer Nanofilled Films
2.2.1. Elasticity
2.2.2. Vapor Permeability
2.2.3. Degradation Time
2.2.4. Isolation of ZnO NPs
2.2.5. Swelling
2.2.6. Antibacterial Activity
2.2.7. Observation of the Microstructure
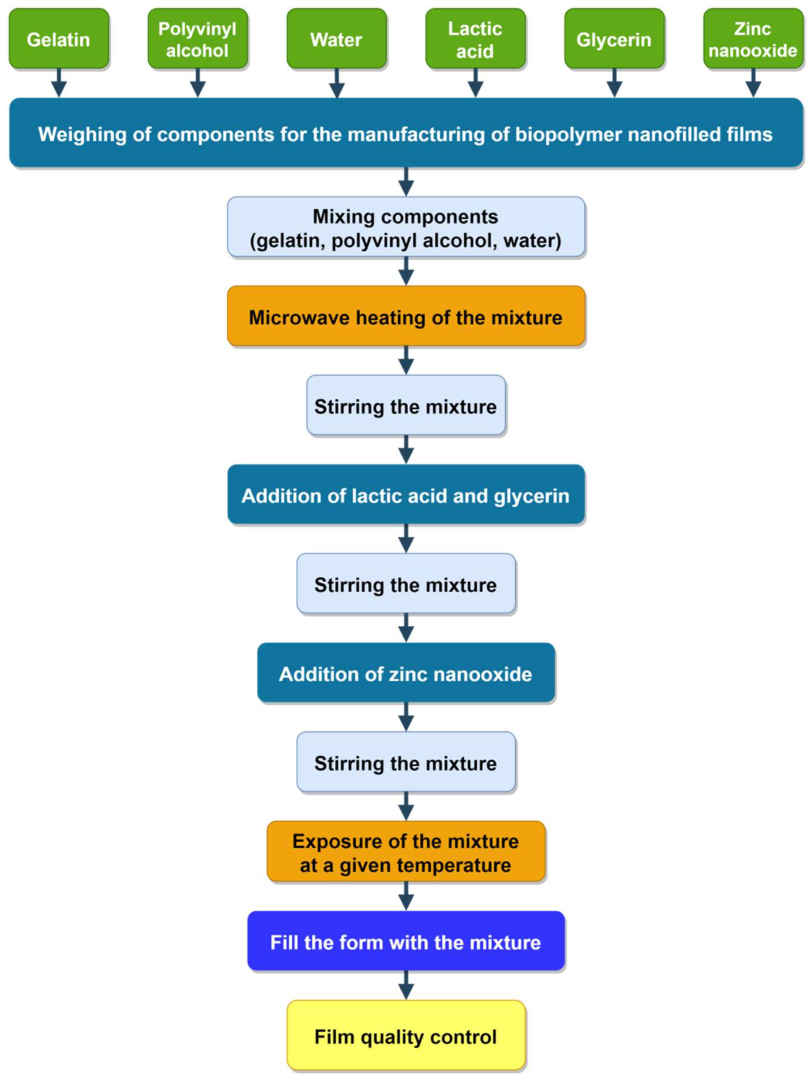
2.3. Methods of Planning Experimental Research
YV = f (C, I, t, T); YH = f (C, I, t, T); YE = f (C, I, t, T).
- (a)
- the total number of experiments significantly exceeds the number of regression coefficients;
- (b)
- factors change on a small number of levels.
- points of complete or fractional factorial experiment N = 2n;
- “star” points (plan type “cross”) Nα = 2n;
- zero (central) points N0.
- when the ratio of the concentrations of the main components of the film, gelatin (G) to vinyl alcohol (V), are less than 1.2 is obtained very rapid degradation of the film, and at a ratio of more than 3.2 the film is characterised by high hardness and does not have good elastic properties;
- at the content of ZnO of less than 50 mg/L, the therapeutic (antimicrobial action) is not provided, and at the content of more than 230 mg/L, the therapeutic efficiency (antimicrobial action) of a film practically does not increase anymore;
- the exposure time in the furnace, t, min was chosen based on the need for complete (through) uniform heating of the mixture of film components in the crucible;
- the heating temperature T, °C of the mixture of film components were taken to ensure complete softening and dissolution of the components in water and ensure a uniform consistency of the film material.
3. Results
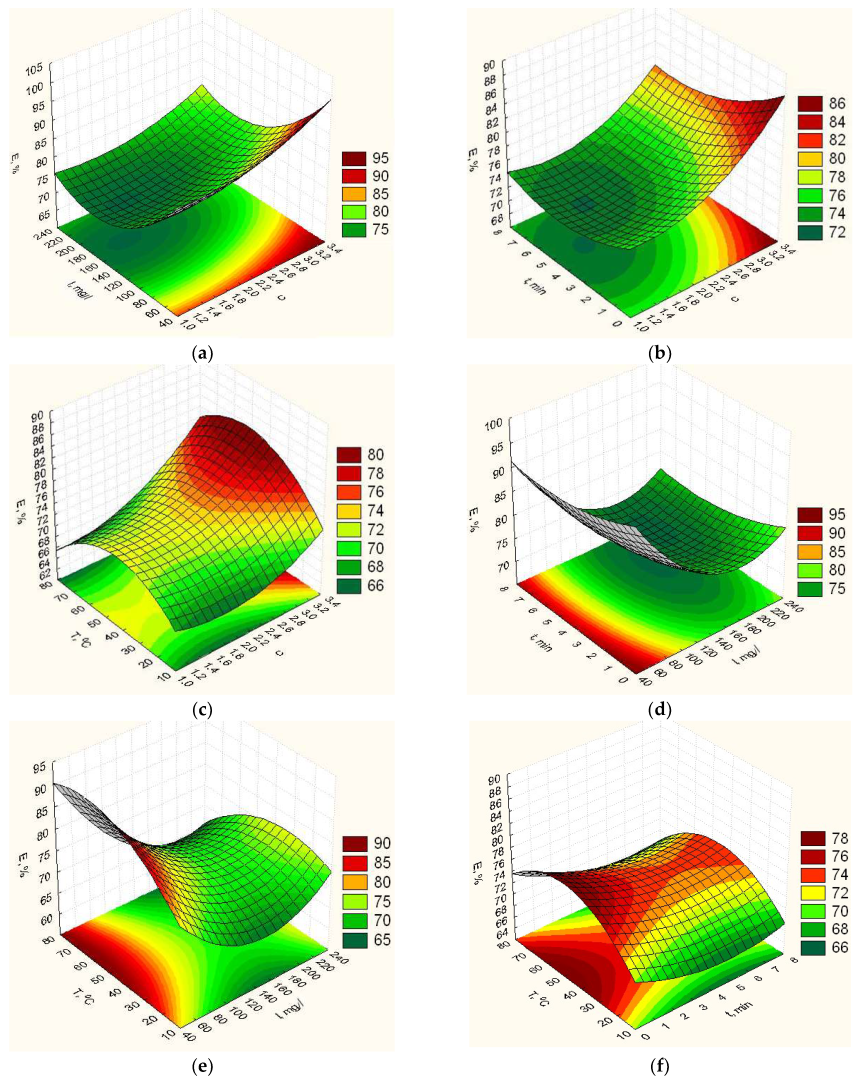
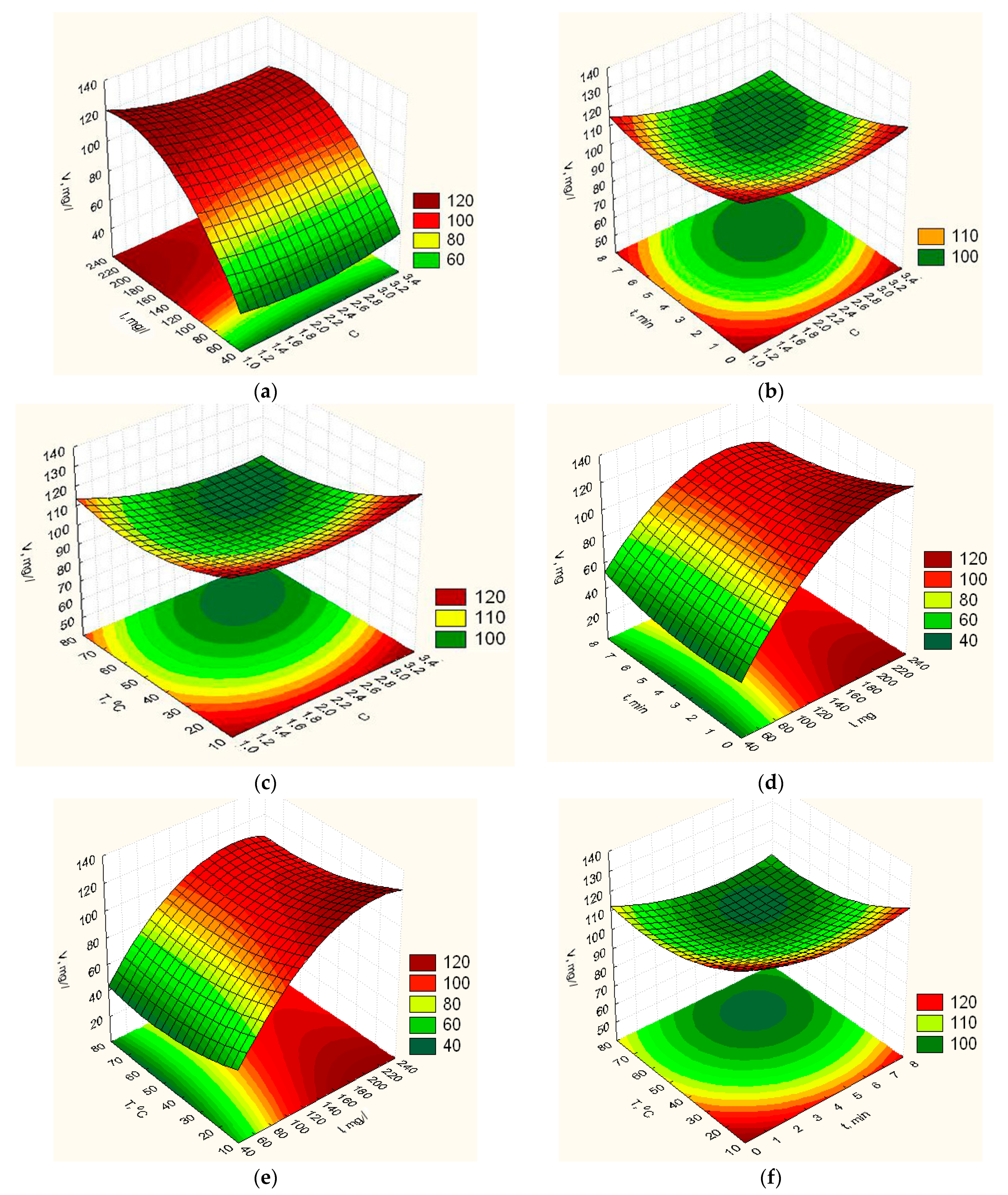
3.1. Optimization of Biopolymer Nanofilled Film Composition and Their Properties
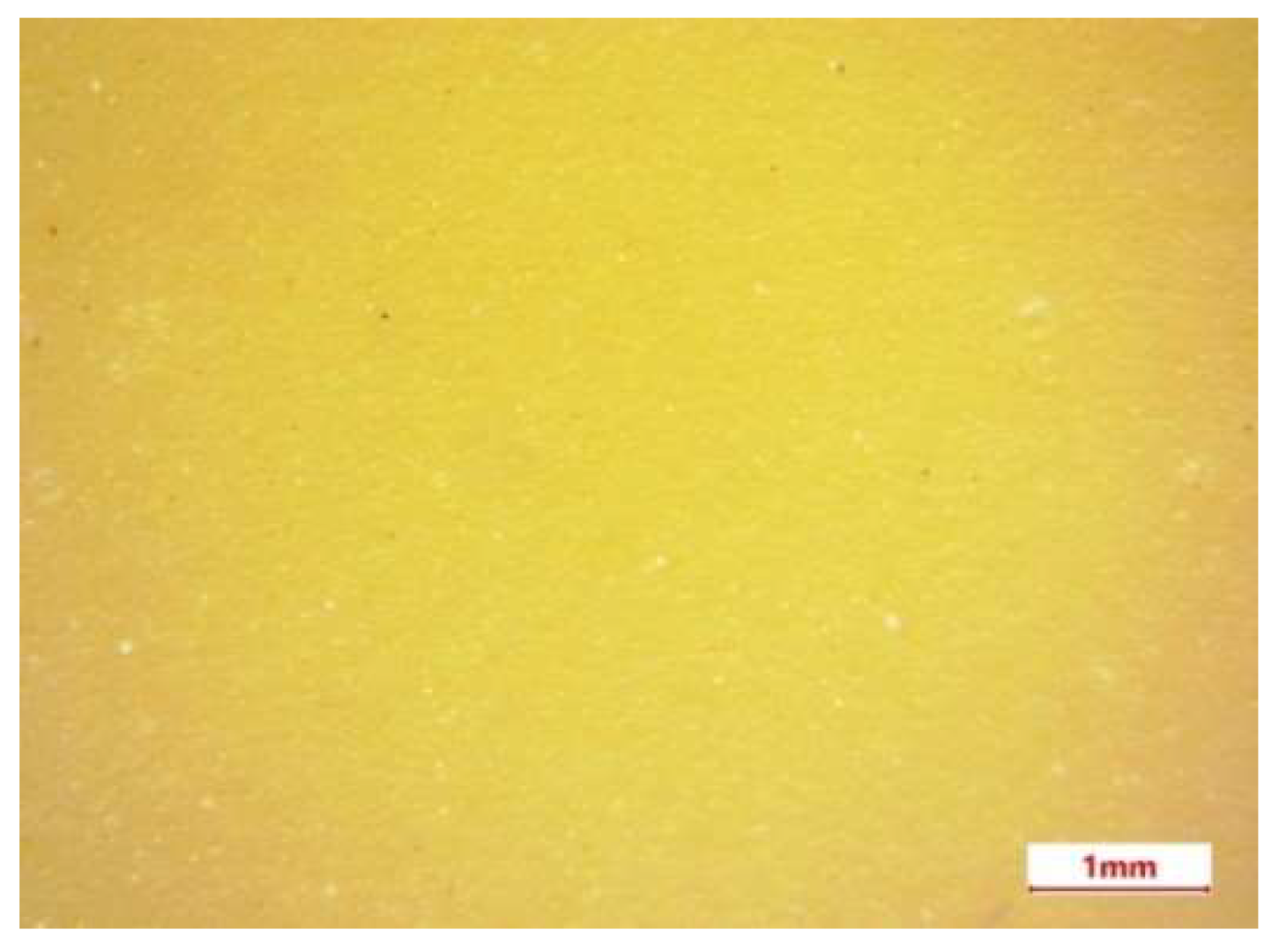
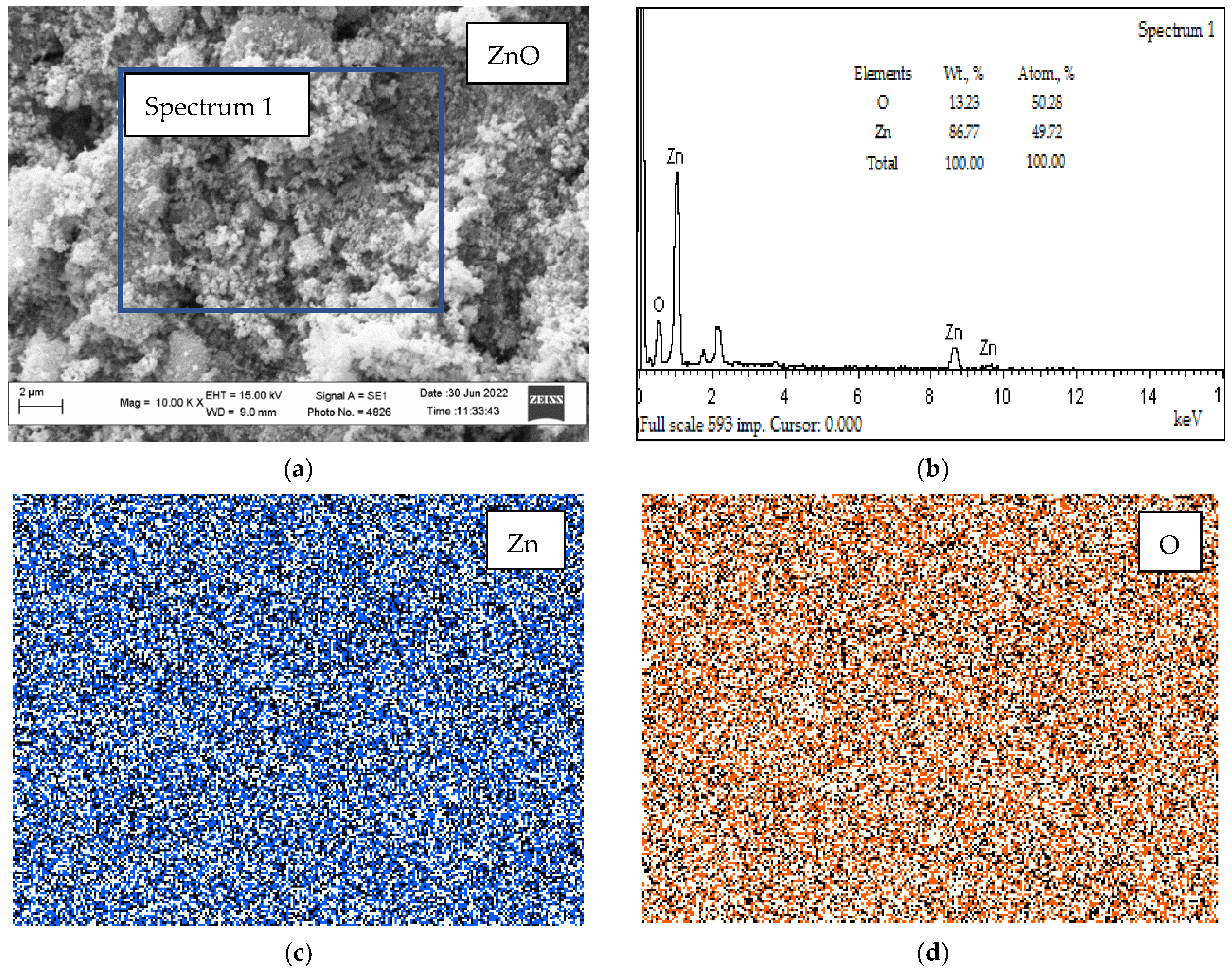
3.2. Microscopic Studies
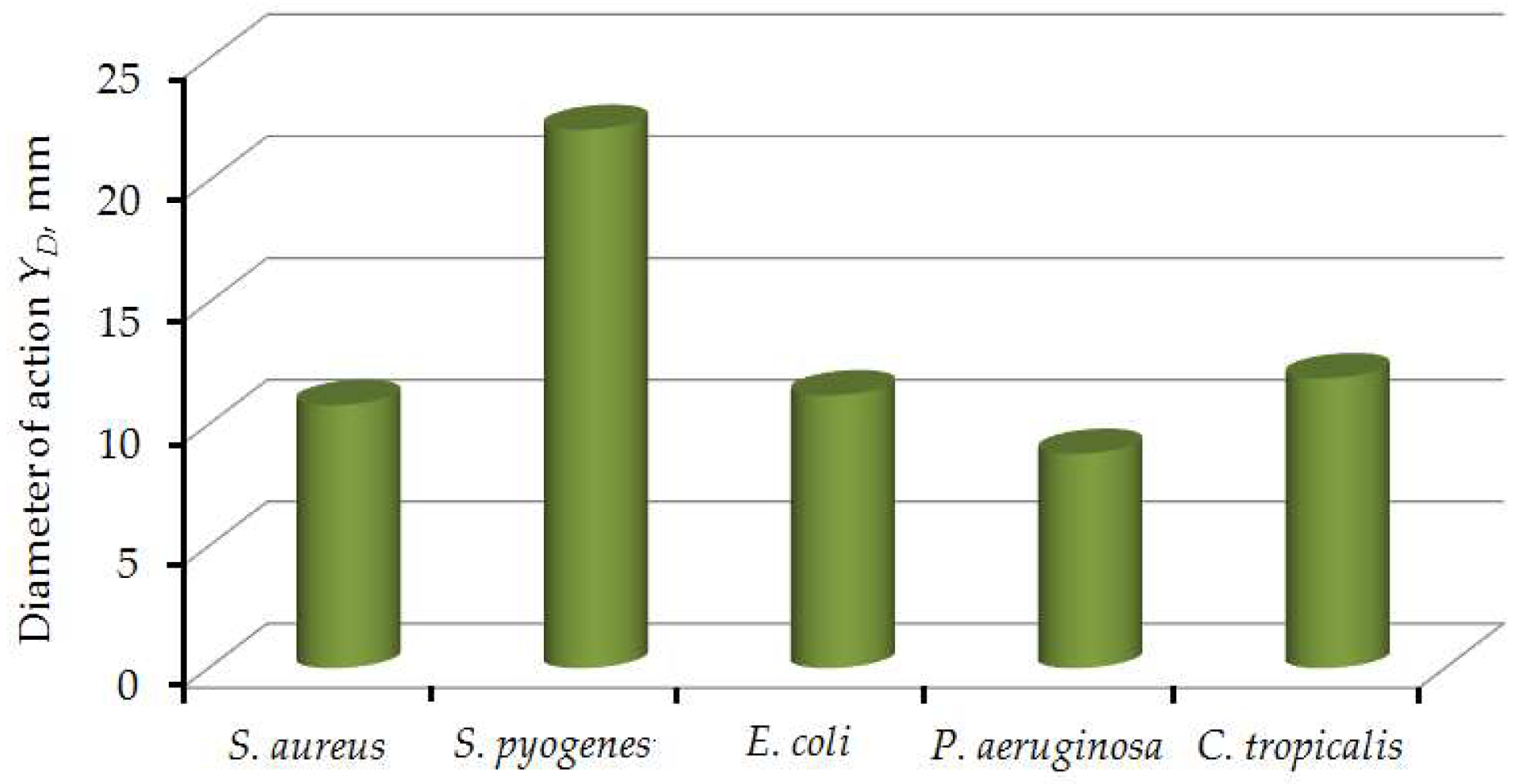
3.3. Antimicrobial Action
4. Discussion
5. Conclusions
- For the first time multifactor mathematical models of the second order for determination of physical-mechanical and medical properties are constructed (the elasticity YE, vapor permeability YP, degradation time Ytd, isolation of ZnO NPs YV and swelling YH) on the concentration ratio gelatin to vinyl alcohol C, the content of ZnO NPs I, exposure time in the furnace t and heating temperature T were built;
- Based on the obtained mathematical models, the values of the parameters of the process of formation biopolymer nanofilled films (component composition and technological parameters) were established to ensure optimal characteristics of the polymer film (the elasticity YE, vapor permeability YP, degradation time Ytd, isolation of ZnO NPs YV and swelling YH) (Table 2);
- It was substantiated that to ensure high therapeutic properties of the film, the final optimization must be carried out on the maximum isolation of ZnO NPs, which is provided for such optimal component composition and technological parameters of the process: concentration ratio C = 3.2, the content of ZnO NPs I = 178.3 mg/L, exposure time in the furnace t = 7.1 min and heating temperature T = 72.8 °C. And at the same time, high physical and mechanical indicators are provided.
- It was shown that the calculated values of the optimization parameters: YE, YP, Ytd, YH for the optimal values of the technological parameters of the film at which the maximumYV is provided differ by 17.2; 12.8; 21.3; 21.5% respectively.
- The results of SEM/EDS analysis of the microstructure of a section of biopolymer nanofilled films indicate the presence of a uniform distribution in the matrix of ZnO NPs, which have an antibacterial effect on pathogenic microorganisms.
- Research results showed high antimicrobial properties due to the release of ZnO NPs from the composition biopolymer nanofilled films.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Experiment Number | Levels of Factors | The Average Value of the Optimization Parameters | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| x0 | x1 | x2 | x3 | x4 | Yav | Elasticity YE, % | Vapour Permeability YP, g/(cm2 h) | Degradation Time Ytd, h | Isolation of NPs YV, mg/L | Swelling YH, % | |
| 1 | +1 | −1 | −1 | −1 | −1 | Yav01 | 75.9 | 461.0 | 36.9 | 77.0 | 327.2 |
| 2 | +1 | −1 | −1 | −1 | +1 | Yav02 | 81.8 | 456.8 | 46.8 | 75.2 | 321.6 |
| 3 | +1 | −1 | −1 | +1 | −1 | Yav03 | 77.9 | 427.4 | 42.8 | 80.6 | 310.1 |
| 4 | +1 | −1 | −1 | +1 | +1 | Yav04 | 79.0 | 380.2 | 47.6 | 77.0 | 288.3 |
| 5 | +1 | −1 | +1 | −1 | −1 | Yav05 | 73.4 | 466.2 | 43.7 | 127.4 | 294.7 |
| 6 | +1 | −1 | +1 | −1 | +1 | Yav06 | 69.1 | 414.0 | 48.2 | 121.1 | 291.7 |
| 7 | +1 | −1 | +1 | +1 | −1 | Yav07 | 71.2 | 427.9 | 47.5 | 118.4 | 281 |
| 8 | +1 | −1 | +1 | +1 | +1 | Yav08 | 67.6 | 363.3 | 49.7 | 112.1 | 268.3 |
| 9 | +1 | +1 | −1 | −1 | −1 | Yav09 | 83.9 | 489.3 | 45.2 | 79.7 | 365.6 |
| 10 | +1 | +1 | −1 | −1 | +1 | Yav10 | 88.1 | 480.3 | 53.1 | 72.5 | 332 |
| 11 | +1 | +1 | −1 | +1 | −1 | Yav11 | 80.4 | 416.9 | 47.0 | 79.7 | 338.4 |
| 12 | +1 | +1 | −1 | +1 | +1 | Yav12 | 85.2 | 395.5 | 52.6 | 71.6 | 295 |
| 13 | +1 | +1 | +1 | −1 | −1 | Yav13 | 76.3 | 459.9 | 47.3 | 124.7 | 347.9 |
| 14 | +1 | +1 | +1 | −1 | +1 | Yav14 | 75.6 | 433.6 | 52.5 | 113.0 | 323.4 |
| 15 | +1 | +1 | +1 | +1 | −1 | Yav15 | 74.0 | 413.4 | 49.8 | 112.1 | 330.3 |
| 16 | +1 | +1 | +1 | +1 | +1 | Yav16 | 74.1 | 372.8 | 52.8 | 101.3 | 296 |
| 17 | +1 | −1.4826 | 0 | 0 | 0 | Yav17 | 72.7 | 421.0 | 45.4 | 106.7 | 283.5 |
| 18 | +1 | +1.4826 | 0 | 0 | 0 | Yav18 | 79.2 | 437.5 | 51.5 | 102.2 | 326.3 |
| 19 | +1 | 0 | −1.4826 | 0 | 0 | Yav19 | 88.6 | 365.1 | 41.2 | 49.1 | 310.7 |
| 20 | +1 | 0 | +1.4826 | 0 | 0 | Yav20 | 74.3 | 350.4 | 44.3 | 108.5 | 302.4 |
| 21 | +1 | 0 | 0 | −1.4826 | 0 | Yav21 | 76.9 | 452.4 | 45.2 | 107.6 | 323.4 |
| 22 | +1 | 0 | 0 | +1.4826 | 0 | Yav22 | 73.1 | 355.6 | 47.7 | 100.3 | 285.4 |
| 23 | +1 | 0 | 0 | 0 | −1.4826 | Yav23 | 69.7 | 436 | 43.6 | 113.9 | 320.2 |
| 24 | +1 | 0 | 0 | 0 | +1.4826 | Yav24 | 70.0 | 386.9 | 51.2 | 95.9 | 285.5 |
| 25 | +1 | 0 | 0 | 0 | 0 | Yav25 | 73.3 | 394.6 | 44.4 | 99.5 | 288.4 |
| 26 | +1 | 0 | 0 | 0 | 0 | Yav26 | 73.3 | 394.6 | 44.4 | 99.5 | 288.5 |

References
- Bembenek, M. The Influence of the Use of Polymer Lining within the Roller Press Gravity Feeder on Briquette Quality. Polymers 2020, 12, 2489. [Google Scholar] [CrossRef]
- Prokopenko, D.; Shatskyi, I.; Vorobiov, M.; Ropyak, L. Cyclic deformation of separating tape in electromagnetic rolling pump. J. Phys. Conf. Ser. 2021, 1741, 012029. [Google Scholar] [CrossRef]
- Onysko, O.; Kopei, V.; Panchuk, V.; Medvid, I.; Lukan, T. Analytical study of kinematic rake angles of cutting edge of lathe tool for tapered thread manufacturing. In Lecture Notes in Mechanical Engineering; Springer: Cham, Switzerland, 2020; pp. 236–245. [Google Scholar] [CrossRef]
- Kopei, V.B.; Onysko, O.R.; Panchuk, V.G. Computerized system based on FreeCAD for geometric simulation of the oil and gas equipment thread turning. IOP Conf. Ser. Mater. Sci. Eng. 2019, 477, 012032. [Google Scholar] [CrossRef]
- Chuzhak, A.; Sulyma, V.; Ropyak, L.; Velychkovych, A.; Vytvytskyi, V. Mathematical Modelling of Destabilization Stress Factors of Stable-Elastic Fixation of Distal Trans- and Suprasyndesmotic Fibular Fractures. J. Healthc. Eng. 2021, 2021, 6607364. [Google Scholar] [CrossRef]
- Bazaluk, O.; Chuzhak, A.; Sulyma, V.; Velychkovych, A.; Ropyak, L.; Vytvytskyi, V.; Mykhailiuk, V.; Lozynskyi, V. Determining the Tightrope Tightening Force for Effective Fixation of the Tibiofibular Syndesmosis during Osteomeatal Synthesis of Fibula Injuries. Appl. Sci. 2022, 12, 4903. [Google Scholar] [CrossRef]
- Primo, J.d.O.; Horsth, D.F.; Correa, J.d.S.; Das, A.; Bittencourt, C.; Umek, P.; Buzanich, A.G.; Radtke, M.; Yusenko, K.V.; Zanette, C.; et al. Synthesis and Characterization of Ag/ZnO Nanoparticles for Bacteria Disinfection in Water. Nanomaterials 2022, 12, 1764. [Google Scholar] [CrossRef]
- Li, D.; Liu, H.; Shen, Y.; Wu, H.; Liu, F.; Wang, L.; Liu, Q.; Deng, B. Preparation of PI/PTFE-PAI Composite Nanofiber Aerogels with Hierarchical Structure and High-Filtration Efficiency. Nanomaterials 2020, 10, 1806. [Google Scholar] [CrossRef] [PubMed]
- Vaze, N.; Soorneedi, A.R.; Moore, M.D.; Demokritou, P. Inactivating SARS-CoV-2 Surrogates on Surfaces Using Engineered Water Nanostructures Incorporated with Nature Derived Antimicrobials. Nanomaterials 2022, 12, 1735. [Google Scholar] [CrossRef]
- Ghauri, Z.H.; Islam, A.; Qadir, M.A.; Gull, N.; Haider, B.; Khan, R.U.; Riaz, T. Development and evaluation of pH-sensitive biodegradable ternary blended hydrogel films (Chitosan/Guar gum/PVP) for drug delivery application. Sci. Rep. 2021, 11, 21255. [Google Scholar] [CrossRef]
- Kesharwani, P.; Bisht, A.; Alexander, A.; Dave, V.; Sharma, S. Biomedical applications of hydrogels in drug delivery system: An update. J. Drug Deliv. Sci. Technol. 2021, 66, 102914. [Google Scholar] [CrossRef]
- Valdivia, V.; Gimeno-Ferrero, R.; Pernia Leal, M.; Paggiaro, C.; Fernández-Romero, A.M.; González-Rodríguez, M.L.; Fernández, I. Biologically Relevant Micellar Nanocarrier Systems for Drug Encapsulation and Functionalization of Metallic Nanoparticles. Nanomaterials 2022, 12, 1753. [Google Scholar] [CrossRef] [PubMed]
- Pantus, A.V.; Rozhko, M.M.; Kutsyk, R.V.; Kozovyi, R.V.; Melnichuk, M.V. Antimicrobial activity of impregnated antibiotic matrix materials for bone tissue defect reconstruction. Arch. Balk. Med. Union 2020, 55, 22–30. [Google Scholar] [CrossRef]
- Lal, A.; Alam, M.K.; Ahmed, N.; Maqsood, A.; Al-Qaisi, R.K.; Shrivastava, D.; Alkhalaf, Z.A.; Alanazi, A.M.; Alshubrmi, H.R.; Sghaireen, M.G.; et al. Nano Drug Delivery Platforms for Dental Application: Infection Control and TMJ Management—A Review. Polymers 2021, 13, 4175. [Google Scholar] [CrossRef] [PubMed]
- Prots, H.; Rozhko, M.; Ozhogan, Z.; Hajoshko, O.; Nychyporchuk, H. Diagnostic Value Of Biochemical Markers Of Bone Remodeling For Predicting The Results Of Dental Implantation In Patients With Generalized Periodontitis. Georgian Med. News 2021, 316–317, 83–88. [Google Scholar]
- Motelica, L.; Popescu, A.; Răzvan, A.-G.; Oprea, O.; Truşcă, R.-D.; Vasile, B.-S.; Dumitru, F.; Holban, A.-M. Facile Use of ZnO Nanopowders to Protect Old Manual Paper Documents. Materials 2020, 13, 5452. [Google Scholar] [CrossRef]
- Motelica, L.; Ficai, D.; Oprea, O.; Ficai, A.; Trusca, R.-D.; Andronescu, E.; Holban, A.M. Biodegradable Alginate Films with ZnO Nanoparticles and Citronella Essential Oil—A Novel Antimicrobial Structure. Pharmaceutics 2021, 13, 1020. [Google Scholar] [CrossRef]
- Popadyuk, O.Y. Assessment of degradable and mechanical properties of nano-containing wound healing polymer materials. Novosti Khirurgii 2017, 25, 454–458. [Google Scholar] [CrossRef] [Green Version]
- Popadyuk, O.Y.; Malyshevska, O.S.; Ropyak, L.Y.; Vytvytskyi, V.S.; Droniak, M.M. Study of nano-containing biopolymer films therapeutic and physical-mechanical properties. Novosti Khirurgii 2019, 27, 16–25. [Google Scholar] [CrossRef]
- Kumar, R.; Umar, A.; Kumar, G.; Nalwa, H.S. Antimicrobial properties of ZnO nanomaterials: A review. Ceram. Int. 2017, 43, 3940–3961. [Google Scholar] [CrossRef]
- Bedian, L.; Villalba-Rodríguez, A.M.; Hernández-Vargas, G.; Parra-Saldivar, R.; Iqbal, H.M.N. Bio-based materials with novel characteristics for tissue engineering applications—A review. Int. J. Biol. Macromol. 2017, 98, 837–846. [Google Scholar] [CrossRef]
- Prasad, A. Bioabsorbable polymeric materials for biofilms and other biomedical applications: Recent Future Trends. Mater. Today Proc. 2021, 44, 2447–2453. [Google Scholar] [CrossRef]
- Song, X.; Liu, P.; Xiaohu Liu, X.; Wang, Y.; Wei, H.; Zhang, J.; Yu, L.; Yan, X.; He, Z. Dealing with MDR bacteria and biofilm in the post-antibiotic era: Application of antimicrobial peptides-based nano-formulation. Mater. Sci. Eng. C 2021, 128, 112318. [Google Scholar] [CrossRef] [PubMed]
- Kalia, V.C.; Singh Patel, S.K.; Shanmugam, R.; Lee, J.-K. Polyhydroxyalkanoates: Trends and advances toward biotechnological applications. Bioresour. Technol. 2021, 326, 124737. [Google Scholar] [CrossRef] [PubMed]
- Bulbuk, O.; Velychkovych, A.; Mazurenko, V.; Ropyak, L.; Pryhorovska, T. Analytical estimation of tooth strength, restored by direct or indirect restorations. Eng. Solid Mech. 2019, 7, 193–204. [Google Scholar] [CrossRef]
- Claaßen, C.; Dannecker, M.; Grübel, J.; Kotzampasi, M.E.; Tovar, G.E.M.; Stanzel, B.V.; Borchers, K. The choice of biopolymer is crucial to trigger angiogenesis with vascular endothelial growth factor releasing coatings. J. Mater. Sci. Mater. Med. 2020, 31, 93. [Google Scholar] [CrossRef]
- Ghosh, S.; Singh, B.P.; Webster, T.J. Nanoparticle-impregnated biopolymers as novel antimicrobial nanofilms. Biopolym. -Based Nano Film. 2021, 2021, 269–309. [Google Scholar] [CrossRef]
- Birk, S.E.; Boisen, A.; Nielsen, L.H. Polymeric nano- and microparticulate drug delivery systems for treatment of biofilms. Adv. Drug Deliv. Rev. 2021, 174, 30–52. [Google Scholar] [CrossRef]
- de Vargas, V.H.; Marczak, L.D.F.; Flôres, S.H.; Mercali, G.D. Advanced Technologies Applied to Enhance Properties and Structure of Films and Coatings: A Review. Food Bioprocess Technol. 2022, 15, 1224–1247. [Google Scholar] [CrossRef]
- Croitoru, A.-M.; Karaçelebi, Y.; Saatcioglu, E.; Altan, E.; Ulag, S.; Aydoğan, H.K.; Sahin, A.; Motelica, L.; Oprea, O.; Tihauan, B.-M.; et al. Electrically Triggered Drug Delivery from Novel Electrospun Poly(Lactic Acid)/Graphene Oxide/Quercetin Fibrous Scaffolds for Wound Dressing Applications. Pharmaceutics 2021, 13, 957. [Google Scholar] [CrossRef]
- Oprea, A.E.; Pandel, L.M.; Dumitrescu, A.M.; Andronescu, E.; Grumezescu, V.; Chifiriuc, M.C.; Mogoanta, L.; Balseanu, T.A.; Mogosanu, G.D.; Socol, G.; et al. Bioactive ZnO Coatings Deposited by MAPLE-An Appropriate Strategy to Produce Efficient Anti-Biofilm Surfaces. Molecules 2016, 21, 220. [Google Scholar] [CrossRef] [Green Version]
- Dincă, V.; Mocanu, A.; Isopencu, G.; Busuioc, C.; Brajnicov, S.; Vlad, A.; Icriverzi, M.; Roseanu, A.; Dinescu, M.; Stroescu, M.; et al. Biocompatible pure ZnO nanoparticles-3D bacterial cellulose biointerfaces with antibacterial properties. Arab. J. Chem. 2020, 13, 3521–3533. [Google Scholar] [CrossRef]
- Li, X.; Yang, X.; Deng, H.; Guo, Y.; Xue, J. Gelatin films incorporated with thymol nanoemulsions: Physical properties and antimicrobial activities. Int. J. Biol. Macromol. 2020, 150, 16–68. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.-Y.; Li, F.-L.; Zhang, Y.-W.; Gupta, R.K.; Patel, S.K.S.; Lee, J.-K. Recent Strategies for the Immobilization of Therapeutic Enzymes. Polymers 2022, 14, 1409. [Google Scholar] [CrossRef] [PubMed]
- Vasile, B.S.; Oprea, O.; Voicu, G.; Ficai, A.; Andronescu, E.; Teodorescu, A.; Holban, A. Synthesis and characterization of a novel controlled release zinc oxide/gentamicin-chitosan composite with potential applications in wounds care. Int. J. Pharm. 2014, 463, 161–169. [Google Scholar] [CrossRef]
- Spirescu, V.A.; Șuhan, R.; Niculescu, A.-G.; Grumezescu, V.; Negut, I.; Holban, A.M.; Oprea, O.-C.; Bîrcă, A.C.; Vasile, B.Ș.; Grumezescu, A.M.; et al. Biofilm-Resistant Nanocoatings Based on ZnO Nanoparticles and Linalool. Nanomaterials 2021, 11, 2564. [Google Scholar] [CrossRef] [PubMed]
- Naskar, A.; Jana, B.; Kim, H.G.; Kwac, L.K. Effect of Ag2O on cell viability of ZnO nanoparticle synthesized by low temperature solution synthesis process. Biointerface Res. Appl. Chem. 2019, 9, 4011–4014. [Google Scholar] [CrossRef]
- Rayyif, S.M.I.; Mohammed, H.B.; Curuțiu, C.; Bîrcă, A.C.; Grumezescu, A.M.; Vasile, B.Ș.; Dițu, L.M.; Lazăr, V.; Chifiriuc, M.C.; Mihăescu, G.; et al. ZnO Nanoparticles-Modified Dressings to Inhibit Wound Pathogens. Materials 2021, 14, 3084. [Google Scholar] [CrossRef]
- Padmavathy, N.; Vijayaraghavan, R. Interaction of ZnO Nanoparticles with Microbes—A Physio and Biochemical Assay. J. Biomed. Nanotechnol. 2011, 7, 813–822. [Google Scholar] [CrossRef]
- Dutta, R.K.; Nenavathu, B.P.; Gangishetty, M.K.; Reddy, A.V.R. Antibacterial effect of chronic exposure of low concentration ZnO nanoparticles on E. coli. J. Environ. Sci. Health Part A 2013, 48, 871–878. [Google Scholar] [CrossRef]
- Razzaq, A.; Khan, Z.U.; Saeed, A.; Shah, K.A.; Khan, N.U.; Menaa, B.; Iqbal, H.; Menaa, F. Development of Cephradine-Loaded Gelatin/Polyvinyl Alcohol Electrospun Nanofibers for Effective Diabetic Wound Healing: In-Vitro and In-Vivo Assessments. Pharmaceutics 2021, 13, 349. [Google Scholar] [CrossRef]
- Spirescu, V.A.; Chircov, C.; Grumezescu, A.M.; Andronescu, E. Polymeric Nanoparticles for Antimicrobial Therapies: An up-to-date Overview. Polymers 2021, 13, 724. [Google Scholar] [CrossRef]
- Liu, D.; Ma, X.; Ji, Y.; Chen, R.; Zhou, S.; Yao, H.; Zhang, Z.; Ye, M.; Xu, Z.; Du, M. Bioresponsive nanotherapy for preventing dental caries by inhibiting multispecies cariogenic biofilms. Bioact. Mater. 2021, 14, 1–14. [Google Scholar] [CrossRef]
- Burda, I.; Barbezat, M.; Brunner, A.J. The effect of nano- and micron-scale filler modified epoxy matrix on glass-fiber reinforced polymer insulator component behavior. Proc. Inst. Mech. Eng. Part L J. Mater. Des. Appl. 2021, 235, 1287–1301. [Google Scholar] [CrossRef]
- Buketov, A.V.; Dolgov, N.A.; Sapronov, A.A.; Nigalatii, V.D. Adhesive Pull and Shear Strength of Epoxy Nanocomposite Coatings Filled with Ultradispersed Diamond. Strength Mater. 2018, 50, 425–431. [Google Scholar] [CrossRef]
- Vasyliev, M.O.; Mordyuk, B.M.; Sidorenko, S.I.; Voloshko, S.M.; Burmak, A.P.; Kindrachuk, M.V. Synthesis of deformation-induced nanocomposites on aluminium D16 alloy surface by ultrasonic impact treatment. Metallofiz. I Noveishie Tekhnologii 2016, 38, 545–563. [Google Scholar] [CrossRef] [Green Version]
- Tarelnyk, V.; Konoplianchenko, I.; Gaponova, O.; Antoszewski, B.; Kundera, C.; Martsynkovskyy, V.; Dovzhyk, M.; Dumanchuk, M.; Vasilenko, O. Application of Multicomponent Wear-Resistant Nanostructures Formed by Electrospark Allowing for Protecting Surfaces of Compression Joints Parts. Springer Proc. Phys. 2020, 240, 195–209. [Google Scholar] [CrossRef]
- Bazaluk, O.; Velychkovych, A.; Ropyak, L.; Pashechko, M.; Pryhorovska, T.; Lozynskyi, V. Influence of Heavy Weight Drill Pipe Material and Drill Bit Manufacturing Errors on Stress State of Steel Blades. Energies 2021, 14, 4198. [Google Scholar] [CrossRef]
- Krupnik, L.; Yelemessov, K.; Beisenov, B.; Baskanbayeva, D. Substantiation and process design to manufacture polymer-concrete transfer cases for mining machines. Min. Miner. Depos. 2020, 14, 103–109. [Google Scholar] [CrossRef]
- Velychkovych, A.; Ropyak, L.; Dubei, O. Strength Analysis of a Two-Layer PETF-Concrete Column with Allowance for Contact Interaction between Layers. Adv. Mater. Sci. Eng. 2021, 2021, 4517657. [Google Scholar] [CrossRef]
- Kurnosov, S.; Ropyak, L.; Velychkovych, A.; Pryhorovska, T.; Vytvytskyi, V. Mechanical—Insulating Method of Household and Industrial Waste Utilization. In Lecture Notes in Networks and Systems; Springer: Cham, Switzerland, 2021; Volume 233, pp. 431–441. [Google Scholar] [CrossRef]
- Qiu, J.; Liu, X. Nanostructured Biomaterials for Tissue Repair and Anti-Infection. Nanomaterials 2022, 12, 1573. [Google Scholar] [CrossRef]
- Ahmad, M.N.; Ishak, M.R.; Mohammad Taha, M.; Mustapha, F.; Leman, Z.; Anak Lukista, D.D.; Irianto; Ghazali, I. Application of Taguchi Method to Optimize the Parameter of Fused Deposition Modeling (FDM) Using Oil Palm Fiber Reinforced Thermoplastic Composites. Polymers 2022, 14, 2140. [Google Scholar] [CrossRef] [PubMed]
- Bembenek, M.; Kowalski, Ł.; Pawlik, J.; Bajda, S. Research on the Influence of the Load Direction and the Cross-Section Shape on the Young’s Modulus of Elements Produced by the Fused Deposition Modeling Method. J. Mater. Eng. Perform. 2022; In print. [Google Scholar] [CrossRef]
- Aulin, V.; Lysenko, S.; Lyashuk, O.; Hrinkiv, A.; Velykodnyi, D.; Vovk, Y.; Holub, D.; Chernai, A. Wear resistance increase of samples tribomating in oil composite with geo modifier КgМf-1. Tribol. Ind. 2019, 41, 156–165. [Google Scholar] [CrossRef]
- Frohn-Sörensen, P.; Geueke, M.; Engel, B.; Löffler, B.; Bickendorf, P.; Asimi, A.; Bergweiler, G.; Schuh, G. Design for 3D Printed Tools: Mechanical Material Properties for Direct Polymer Additive Tooling. Polymers 2022, 14, 1694. [Google Scholar] [CrossRef]
- Pasichnyk, V.; Kryvenko, M.; Burburska, S.; Haluzynskyi, O. Design and Engineering Assurance for the Customized Implants Production Using Additive Technologies. In Lecture Notes in Mechanical Engineering; Springer: Cham, Switzerland, 2021; Volume 1, pp. 81–94. [Google Scholar] [CrossRef]
- Berganza, E.; Apte, G.; Vasantham, S.K.; Nguyen, T.-H.; Hirtz, M. Integration of Biofunctional Molecules into 3D-Printed Polymeric Micro-/Nanostructures. Polymers 2022, 14, 1327. [Google Scholar] [CrossRef]
- Agarwal, S. Major factors affecting the characteristics of starch based biopolymer films. Eur. Polym. J. 2021, 160, 110788. [Google Scholar] [CrossRef]
- Olmedo, M.M.; Iglesias Godino, F.J.; Liétora, P.F.; Corpas Iglesias, F.A.; Gómez de Salazar Caso de los Cobos, J.M. Corrosion and fracture analysis in screws of dental implants prostheses. New coatings. Eng. Fail. Anal. 2017, 82, 657–665. [Google Scholar] [CrossRef]
- Wang, Q.; Eltit, F.; Wang, R. Corrosion of Orthopedic Implants. Reference Module in Biomedical Sciences. Encycl. Biomed. Eng. 2019, 65–85. [Google Scholar] [CrossRef]
- Velychkovych, A.S.; Andrusyak, A.V.; Pryhorovska, T.O.; Ropyak, L.Y. Analytical model of oil pipeline overground transitions, laid in mountain areas. Oil Gas Sci. Technol. 2019, 74, 65. [Google Scholar] [CrossRef] [Green Version]
- Bazaluk, O.; Slabyi, O.; Vekeryk, V.; Velychkovych, A.; Ropyak, L.; Lozynskyi, V. A Technology of Hydrocarbon Fluid Production Intensification by Productive Stratum Drainage Zone Reaming. Energies 2021, 14, 3514. [Google Scholar] [CrossRef]
- Silva, P.; Oliveira, S.H.; Vinhas, G.M.; Carvalho, L.J.; Baraúna, O.S.; Urtiga Filho, S.L.; Lima, M.A.G.A. Tetrakis hydroxymethyl phosphonium sulfate (THPS) with biopolymer as strategy for the control of microbiologically influenced corrosion in a dynamic system. Chem. Eng. Process.-Process Intensif. 2021, 160, 108272. [Google Scholar] [CrossRef]
- Korniy, S.A.; Zin, I.M.; Tymus, M.B.; Khlopyk, O.P.; Holovchuk, M.Y. Steel Corrosion Inhibition by Microbial Polysaccharide and Tartrate Mixture. J. Bio Tribo Corros. 2022, 8, 6. [Google Scholar] [CrossRef]
- Saakiyan, L.S.; Efremov, A.P.; Ropyak, L.Y.; Gorbatskii, A.V. A method of microelectrochemical investigations. Sov. Mater. Sci. 1987, 23, 267–269. [Google Scholar] [CrossRef]
- Asadauskas, S.J.; Griguceviien, A.; Leinartas, K.; Brainskien, D. Application of three-electrode electrolytic cell to evaluate thin films of vegetable and mineral oils. Tribol. Int. 2011, 44, 557–564. [Google Scholar] [CrossRef]
- Joshi, J.; Homburg, S.V.; Ehrmann, A. Atomic Force Microscopy (AFM) on Biopolymers and Hydrogels for Biotechnological Applications--Possibilities and Limits. Polymers 2022, 14, 1267. [Google Scholar] [CrossRef]
- Antonyuk, V.S.; Bilokin’, S.O.; Bondarenko, M.O.; Bondarenko, Y.Y.; Kovalenko, Y.I. Formation of wear-resistant coatings on silicon probes for atomic force microscopy by thermal vacuum evaporation. J. Superhard Mater. 2015, 37, 112–119. [Google Scholar] [CrossRef]
- Bulbuk, O.; Bulbuk, O.; Rozhko, M. A New Cavity Classification LOV/DD: An Original Hypothesis. Dent. Hypotheses 2020, 11, 24–27. [Google Scholar] [CrossRef]
- Shatskyi, I.; Kurtash, I. Strength of plate with the filled crack under multiparameter loading. Procedia Struct. Integr. 2018, 13, 1482–1487. [Google Scholar] [CrossRef]
- Belmas, I.; Kogut, P.; Kolosov, D.; Samusia, V.; Onyshchenko, S. Rigidity of elastic shell of rubber-cable belt during displacement of cables relatively to drum. E3S Web Conf. 2019, 109, 00005. [Google Scholar] [CrossRef]
- Prades, L.; Fabbri, S.; Dorado, A.D.; Gamisans, X.; Stoodley, P.; Picioreanu, C. Computational and experimental investigation of biofilm disruption dynamics induced by high-velocity gas jet impingement. ASM J. Mbio 2020, 11, e02813–e02819. [Google Scholar] [CrossRef] [Green Version]
- Shihab, T.; Prysyazhnyuk, P.; Semyanyk, I.; Anrusyshyn, R.; Ivanov, O.; Troshchuk, L. Thermodynamic Approach to the Development and Selection of Hardfacing Materials in Energy Industry. Manag. Syst. Prod. Eng. 2020, 28, 84–89. [Google Scholar] [CrossRef]
- Melnick, O.B.; Soolshenko, V.K.; Levchuk, K.H. Thermodynamic prediction of phase composition of transition metals high-entropy alloys. Metallofiz. Noveishie Tekhnol. 2020, 42, 1387–1400. [Google Scholar] [CrossRef]
- Levchuk, K.H.; Radchenko, T.M.; Tatarenko, V.A. High-temperature entropy effects in the tetragonality of the ordering interstitial-substitutional solution based on the body-centred tetragonal metal. Metallofiz. Noveishie Tekhnol. 2021, 43, 1–26. [Google Scholar] [CrossRef]
- Prysyazhnyuk, P.; Bishchak, R.; Korniy, S.; Panchuk, M.; Kaspruk, V. Virtual crystal approximation study of the complex refractory carbides based on Ti-Nb-Mo-V-C system with CASTEP computer code. In Proceedings of the 1st International Workshop on Information Technologies: Theoretical and Applied Problems 2021, Ternopil, Ukraine, 16–18 November 2021; Volume 3039, pp. 300–305. [Google Scholar]
- Prysyazhnyuk, P.; Shlapak, L.; Semyanyk, I.; Kotsyubynsky, V.; Troshchuk, L.; Korniy, S.; Artym, V. Analysis of the effects of alloying with Si and Cr on the properties of manganese austenite based on AB INITIO modelling. East. Eur. J. Enterp. Technol. 2020, 6, 28–36. [Google Scholar] [CrossRef]
- Myronyuk, O.; Dudko, V.; Baklan, D.; Melnyk, L. Study of structure influence on wear resistance of hierarchial superhydrophobic coatings. East. Eur. J. Enterp. Technol. 2017, 3, 44–49. [Google Scholar] [CrossRef] [Green Version]
- Horn, H.; Lackner, S. Modeling of biofilm systems: A review. Adv. Biochem. Eng. Biotechnol. 2014, 146, 53–76. [Google Scholar] [CrossRef]
- Rakhmanova, A.; Kalybekkyzy, S.; Soltabayev, B.; Bissenbay, A.; Kassenova, N.; Bakenov, Z.; Mentbayeva, A. Application of Response Surface Methodology for Optimization of Nanosized Zinc Oxide Synthesis Conditions by Electrospinning Technique. Nanomaterials 2022, 12, 1733. [Google Scholar] [CrossRef]
- Krasoń, J.; Debska, B.; Lichołai, L. Designing Cement Mortars Modified with Cork and Rubber Waste Using Theory of the Experiment. J. Ecol. Eng. 2019, 20, 121–130. [Google Scholar] [CrossRef]
- Rakhmanova, A.; Wang, T.; Xing, G.; Ma, L.; Hong, Y.; Lu, Y.; Xin, L.; Xin, W.; Zhu, Q.; Lü, X. Isolation and identification of microorganisms in Kazakhstan koumiss and their application in preparing cow-milk koumiss. J. Dairy Sci. 2021, 104, 151–166. [Google Scholar] [CrossRef]
- Popadyuk, O.Y.; Nichitaylo, M.Y.; Henik, S.M.; Melnik, M.V.; Melnyk, D.O. Biodegradable Polymer Film “Biodep-Nano”. Ukraine Patent 110594, 10 October 2016. [Google Scholar]
- Bazaluk, O.; Dubei, O.; Ropyak, L.; Shovkoplias, M.; Pryhorovska, T.; Lozynskyi, V. Strategy of compatible use of jet and plunger pump with chrome parts in oil well. Energies 2022, 15, 83. [Google Scholar] [CrossRef]
- Kusyi, Y.M.; Kuk, A.M. Investigation of the technological damageability of castings at the stage of design and technological preparation of the machine Life Cycle. J. Phys. Conf. Ser. 2020, 1426, 012034. [Google Scholar] [CrossRef]
- Vach, W. Regression Models as a Tool in Medical Research, 1st ed.; Chapman and Hall/CRCition: New York, NY, USA, 2013; p. 496. ISBN 9781466517486. [Google Scholar]
- Batool, M.; Khurshid, S.; Qureshi, Z.; Daoush, W.M. Adsorption, antimicrobial and wound healing activities of biosynthesised zinc oxide nanoparticles. Chem. Pap. 2021, 75, 893–907. [Google Scholar] [CrossRef]
- Thanasrisuebwong, P.; Jones, J.R.; Eiamboonsert, S.; Ruangsawasdi, N.; Jirajariyavej, B.; Naruphontjirakul, P. Zinc-Containing Sol–Gel Glass Nanoparticles to Deliver Therapeutic Ions. Nanomaterials 2022, 12, 1691. [Google Scholar] [CrossRef]
- Vicentini, D.S.; Smania, A., Jr.; Laranjeira, M.C.M. Chitosan/poly (vinyl alcohol) films containing ZnO nanoparticles and plasticizers. Mater. Sci. Eng. C 2010, 30, 503–508. [Google Scholar] [CrossRef]
- Chursin, V.I.; Lyubimtseva, E.S. Effect of Polysaccharide Constituents on the Properties of Biopolymer Composites and Films Based on Them. Fibre. Chem. 2020, 51, 362–367. [Google Scholar] [CrossRef]
- Stoncius, A.; Liascukiene, I.; Jankauskas, S.; Asadauskas, S.J. Volatiles from thin film degradation of bio-based, synthetic and mineral basestocks. Ind. Lubr. Tribol. 2013, 65, 209–215. [Google Scholar] [CrossRef]
- Leja, K.; Lewandowicz, G. Polymer biodegradation and biodegradable polymers—A review. Pol. J. Environ. Stud. 2010, 19, 255–266. [Google Scholar]
- Pires, J.R.A.; Souza, V.G.L.; Fuciños, P.; Pastrana, L.; Fernando, A.L. Methodologies to Assess the Biodegradability of Bio-Based Polymers—Current Knowledge and Existing Gaps. Polymers 2022, 14, 1359. [Google Scholar] [CrossRef]
- Hauser, M.; Nowack, B. Probabilistic modelling of nanobiomaterial release from medical applications into the environment. Environ. Int. 2021, 146, 106184. [Google Scholar] [CrossRef] [PubMed]
- Coll, C.; Notter, D.; Gottschalk, F.; Sun, T.; Som, C.; Nowack, B. Probabilistic environmental risk assessment of five nanomaterials (nano-TiO2, nano-Ag, nano-ZnO, CNT, and fullerenes). Nanotoxicology 2016, 10, 436–444. [Google Scholar] [CrossRef]
- Giaveri, S.; Schmitt, A.M.; Roset Julià, L.; Scamarcio, V.; Murello, A.; Cheng, S.; Menin, L.; Ortiz, D.; Patiny, L.; Bolisetty, S.; et al. Nature-Inspired Circular-Economy Recycling for Proteins: Proof of Concept. Adv. Mater. 2021, 33, 2104581. [Google Scholar] [CrossRef] [PubMed]



| Levels of Factors | Coded Values | Natural Values | ||||||
|---|---|---|---|---|---|---|---|---|
| x1 | x2 | x3 | x4 | Concentration Ratio (G/V), C | The Content of ZnO NPs, I, mg/L | Exposure Time in the Furnace, t, min | Heating Tempe- rature, T, °C | |
| The main level | 0 | 0 | 0 | 0 | 2.2 | 140 | 4.0 | 46 |
| Interval of variation | 1 | 1 | 1 | 1 | 0.7 | 60 | 2.0 | 18 |
| The upper level | +1 | +1 | +1 | +1 | 2.9 | 200 | 6.0 | 64 |
| The lower level | −1 | −1 | −1 | −1 | 1.5 | 80 | 2.0 | 28 |
| Star points (+) | +1.4826 | +1.4826 | +1.4826 | +1.4826 | 3.2 | 230 | 7.0 | 73 |
| Star points (–) | −1.4826 | −1.4826 | −1.4826 | −1.4826 | 1.2 | 50 | 1.0 | 19 |
| Optimization Parameters | Technological Parameters | ||||
|---|---|---|---|---|---|
| Name | Optimal Value | Concentration Ratio (G/V), C | The Content of ZnO NPs, I, mg/L | Exposure Time in the Furnace, t, min | Heating Temperature, T, °C |
| Minimum Values | |||||
| 1.2 | 50.0 | 1.0 | 19.0 | ||
| Optimal Values | |||||
| Elasticity YE, % | 87.6 | 1.6 | 172.5 | 5.0 | 40.6 |
| Vapour permeability YP, g/(cm2 h) | 407.4 | 2.2 | 117.7 | 7.0 | 59.3 |
| Degradation time Ytd, h | 44.4 | 1.9 | 227.9 | 3.2 | 32.2 |
| Isolation of ZnO NPs YV, mg/L | 114.4 | 3.2 | 178.3 | 7.1 | 72.8 |
| Swelling YH, % | 327.4 | 1.4 | 185.7 | 6.1 | 58.2 |
| – | Maximum Values | ||||
| 3.2 | 230.0 | 7.0 | 73.0 | ||
| Optimization Parameters | Technological Parameters | Optimization Parameters Deviation, % | ||||
|---|---|---|---|---|---|---|
| Name | Optimal Value | Concentra- tion Ratio (G/V), C | The Content of ZnO NPs, I, mg/L | Exposure Time in the Furnace, t, min | Heating Temperature, T, °C | |
| Optimal Values | ||||||
| 3.2 | 178.3 | 7.1 | 72.8 | |||
| The Values of the Optimization Parameters are Calculated | ||||||
| Elasticity YE, % | 87.6 | 105.8 | 17.2 | |||
| Vapor permeability YP, g/(cm2 h) | 407.4 | 467.3 | 12.8 | |||
| Degradation time Ytd, h | 44.4 | 56.4 | 21.3 | |||
| Isolation of ZnO NPs YV, mg/L | 114.4 | 114.4 | 0 | |||
| Swelling YH, % | 327.4 | 417.1 | 21.5 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bembenek, M.; Popadyuk, O.; Shihab, T.; Ropyak, L.; Uhryński, A.; Vytvytskyi, V.; Bulbuk, O. Optimization of Technological Parameters of the Process of Forming Therapeutic Biopolymer Nanofilled Films. Nanomaterials 2022, 12, 2413. https://doi.org/10.3390/nano12142413
Bembenek M, Popadyuk O, Shihab T, Ropyak L, Uhryński A, Vytvytskyi V, Bulbuk O. Optimization of Technological Parameters of the Process of Forming Therapeutic Biopolymer Nanofilled Films. Nanomaterials. 2022; 12(14):2413. https://doi.org/10.3390/nano12142413
Chicago/Turabian StyleBembenek, Michał, Oleg Popadyuk, Thaer Shihab, Liubomyr Ropyak, Andrzej Uhryński, Vasyl Vytvytskyi, and Oleksandr Bulbuk. 2022. "Optimization of Technological Parameters of the Process of Forming Therapeutic Biopolymer Nanofilled Films" Nanomaterials 12, no. 14: 2413. https://doi.org/10.3390/nano12142413
APA StyleBembenek, M., Popadyuk, O., Shihab, T., Ropyak, L., Uhryński, A., Vytvytskyi, V., & Bulbuk, O. (2022). Optimization of Technological Parameters of the Process of Forming Therapeutic Biopolymer Nanofilled Films. Nanomaterials, 12(14), 2413. https://doi.org/10.3390/nano12142413









