Research Progress on the Preparation and Applications of Laser-Induced Graphene Technology
Abstract
:1. Introduction
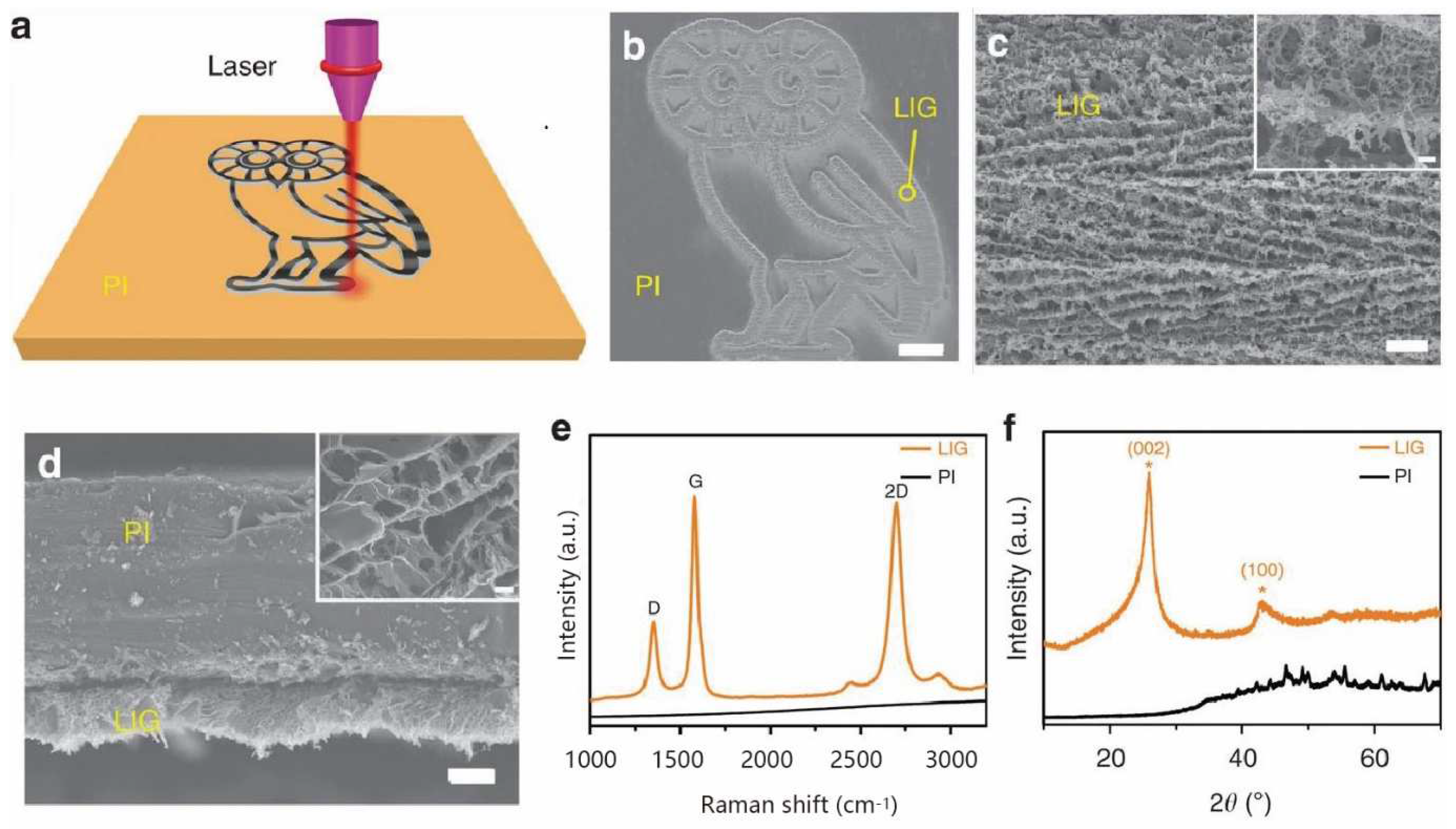
2. Process Control of LIG
2.1. Laser Processing Parameters

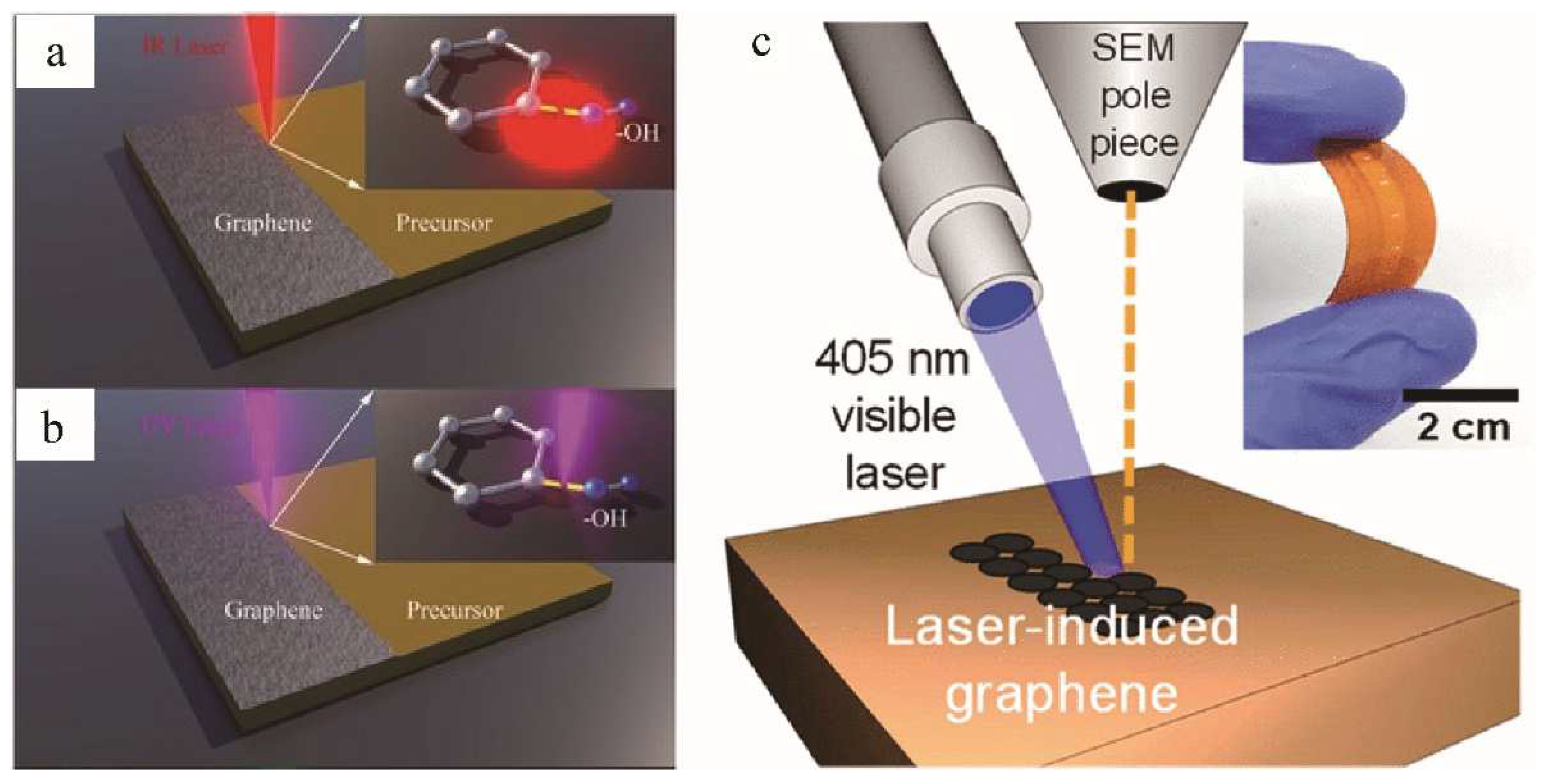
2.2. Laser Type
2.3. Precursor Materials
| Materials | Environment | Laser | Application | Refs. |
|---|---|---|---|---|
| PI | Ambient air | 10.6 µm CO2 laser | Micro supercapacitor\ Sensor\ Air filter\ Seawater desalination | [25,35,49,74,75] |
| Ambient air | 405nm visible laser | Sensor | [70] | |
| Ambient air | 405nm blue_violet laser | Micro supercapacitor | [56] | |
| O2/Air/Ar/H2/SF6/ | 10.6 µm CO2 laser | Micro supercapacitor\ Water treatment\ Air filter | [63] | |
| Paper | Ambient air | 10.6 µm CO2 laser | Sensor\ Micro supercapacitor | [46,58,76] |
| Cloth | Ambient air | 10.6 µm CO2 laser | Micro supercapacitor | [58] |
| Food | Ambient air | 10.6 µm CO2 laser | Micro supercapacitor | [58] |
| Xylan | Ambient air | 10.6 µm CO2 laser | Sensor | [76] |
| Pinewood | N2 | 1064nm nanosecond and picosecond laser | - | [77] |
| Wood | Ambient air | UV laser | Sensor\ Supercapacitor | [55] |
| Ar/H2 | 10.6 µm CO2 laser | Supercapacitor | [66] | |
| Leaves | Ambient air | UV laser | Sensor\ Supercapacitor | [38,55] |
| PSU | Ambient air | 10.6 µm CO2 laser | Water treatment\ Fuel battery | [78] |
| PEEK | Ambient air | 10.6 µm CO2 laser | - | [79] |
| SPEEK | Ambient air | 10.6 µm CO2 laser | Supercapacitor | [80] |
| PR | Ambient air | 405nm visible laser | Sensor\ Supercapacitor | [70] |
| Silk | Ambient air | Fiber laser | Sensor | [81] |
2.4. Doping and Process Atmosphere Control
3. Scale-up Production of LIG
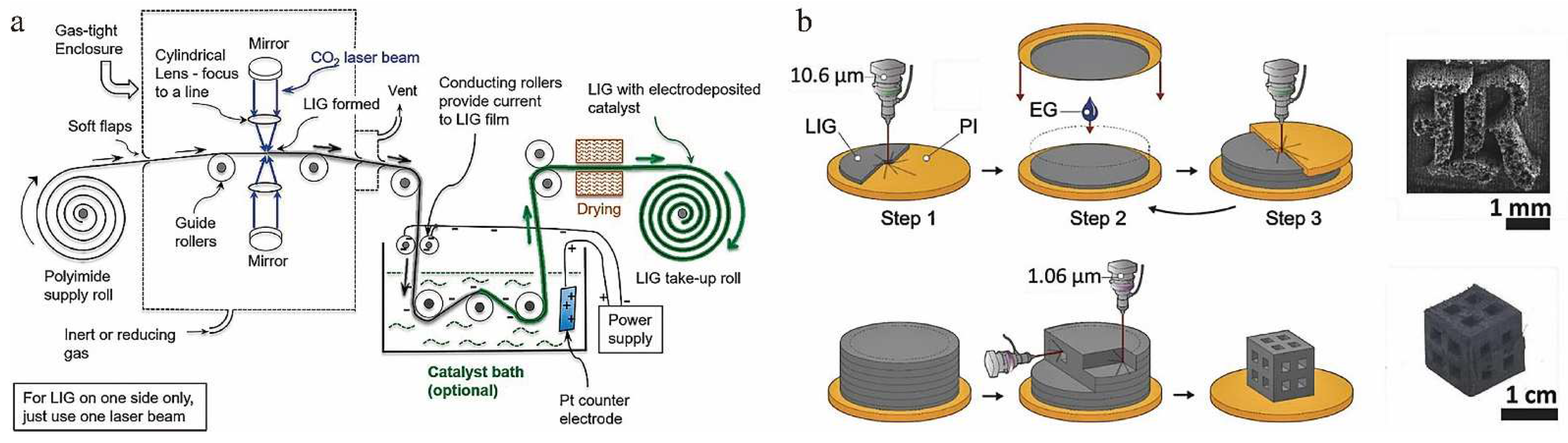
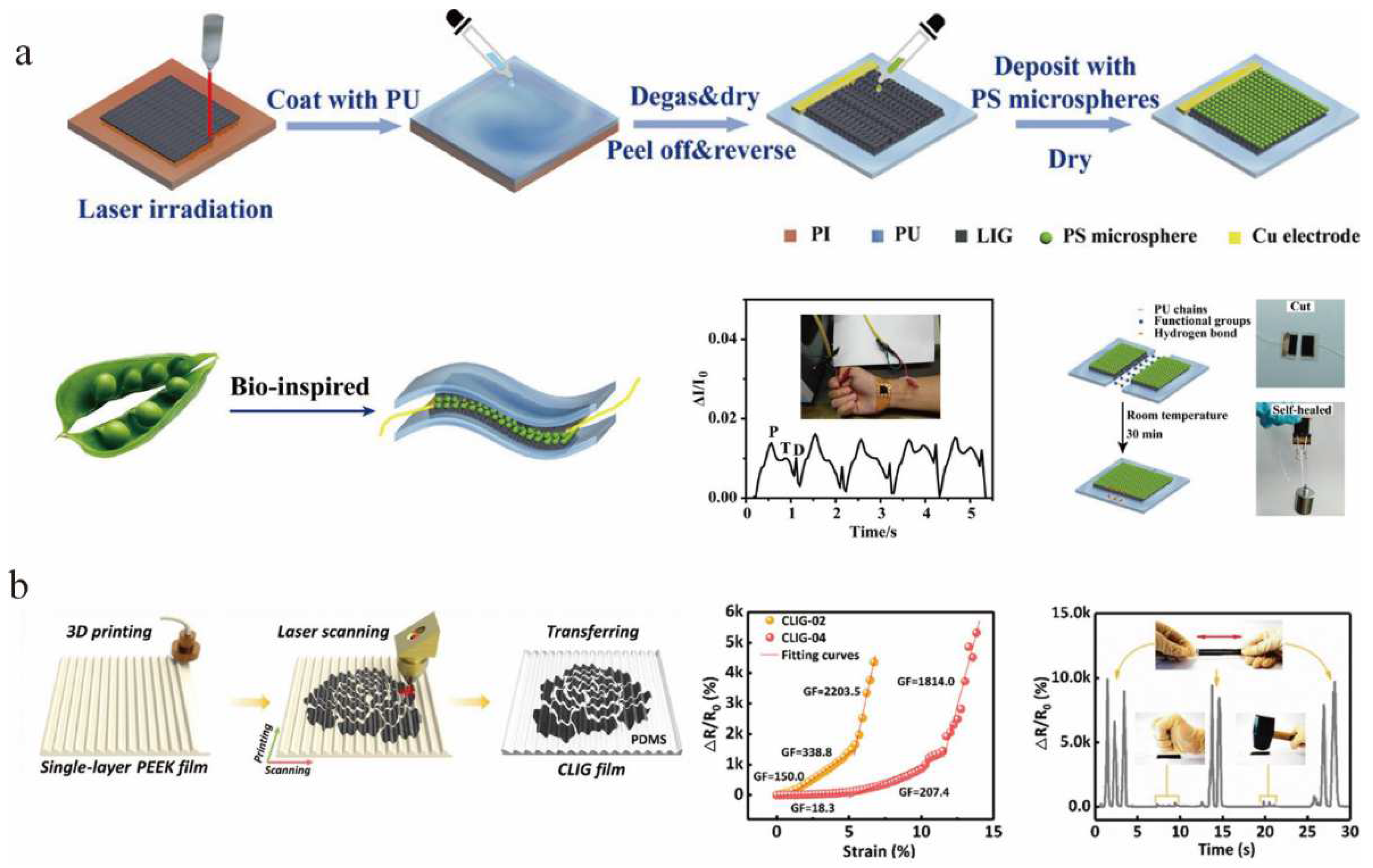
3.1. Roll-to-Roll Production of LIG
3.2. 3D Printing of LIG
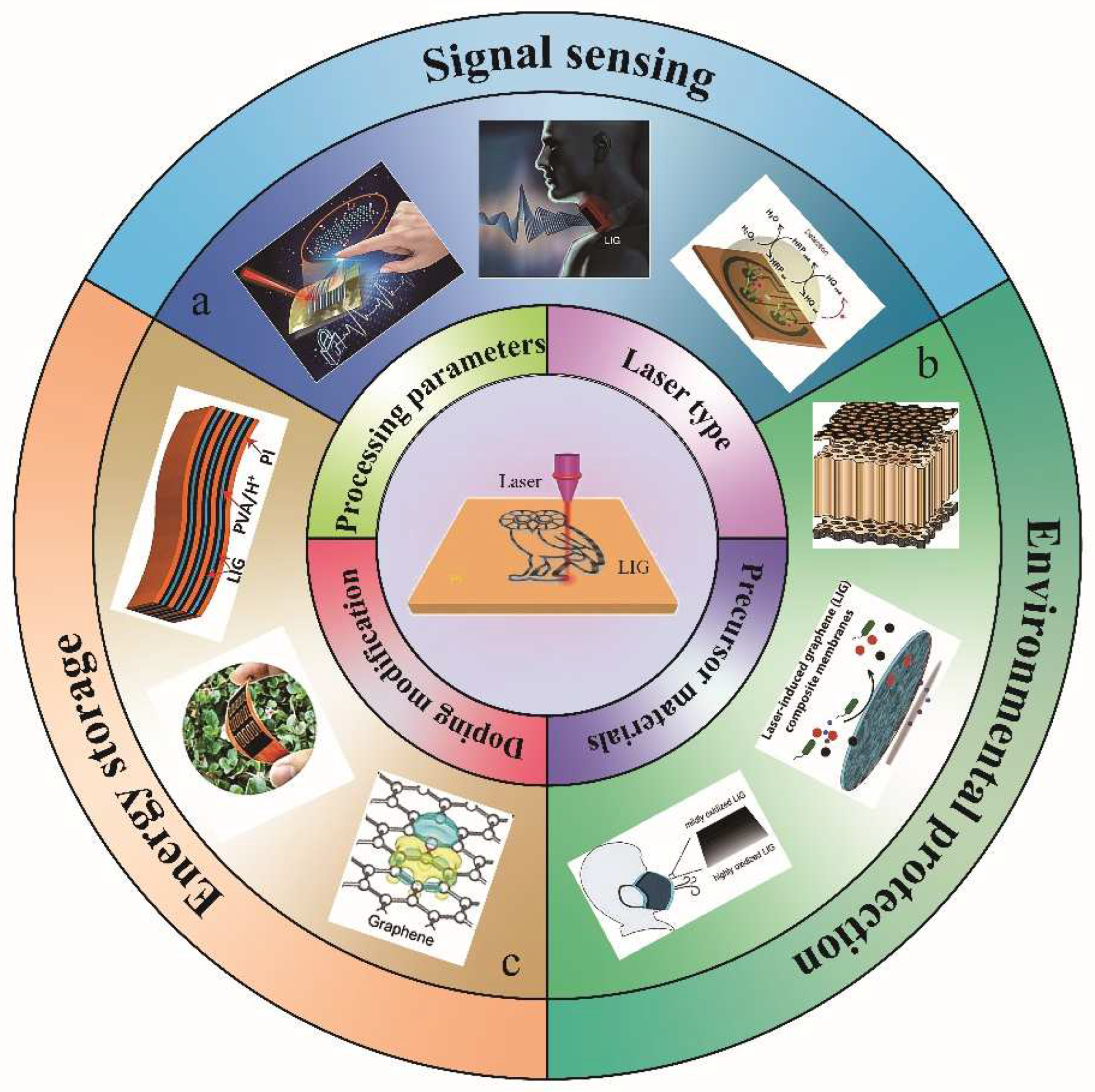
4. Applications of LIG
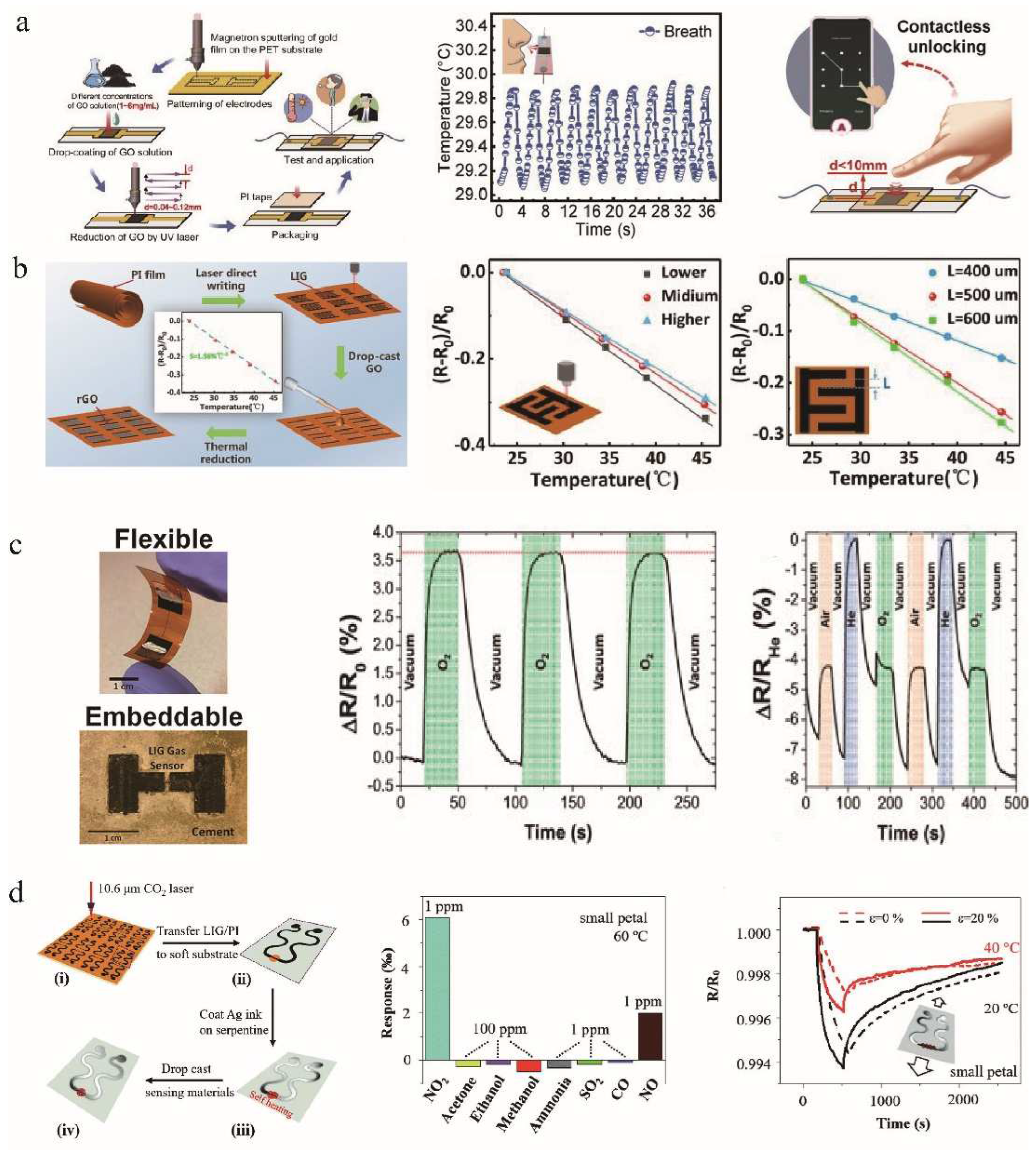
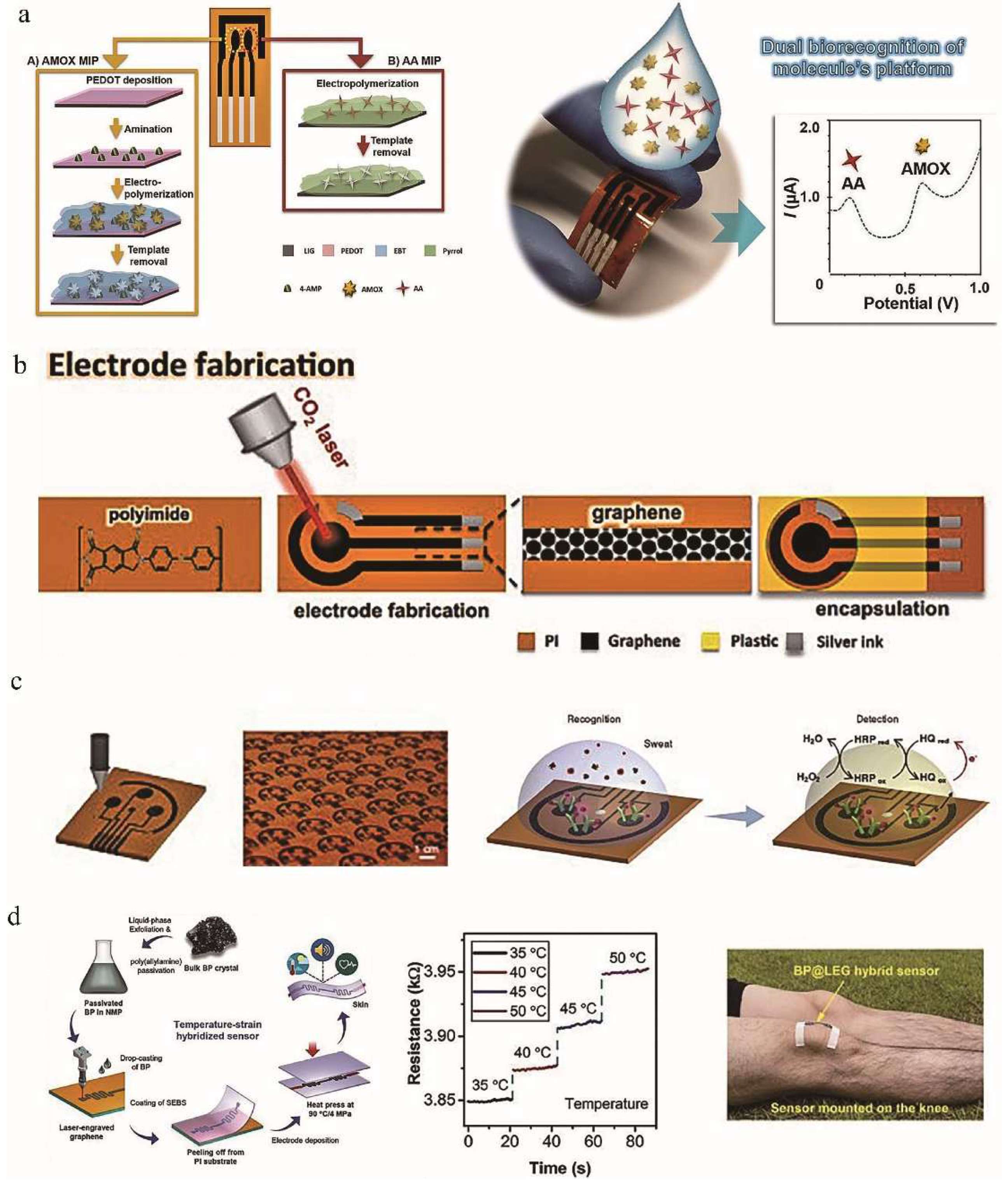
4.1. Signal Sensing
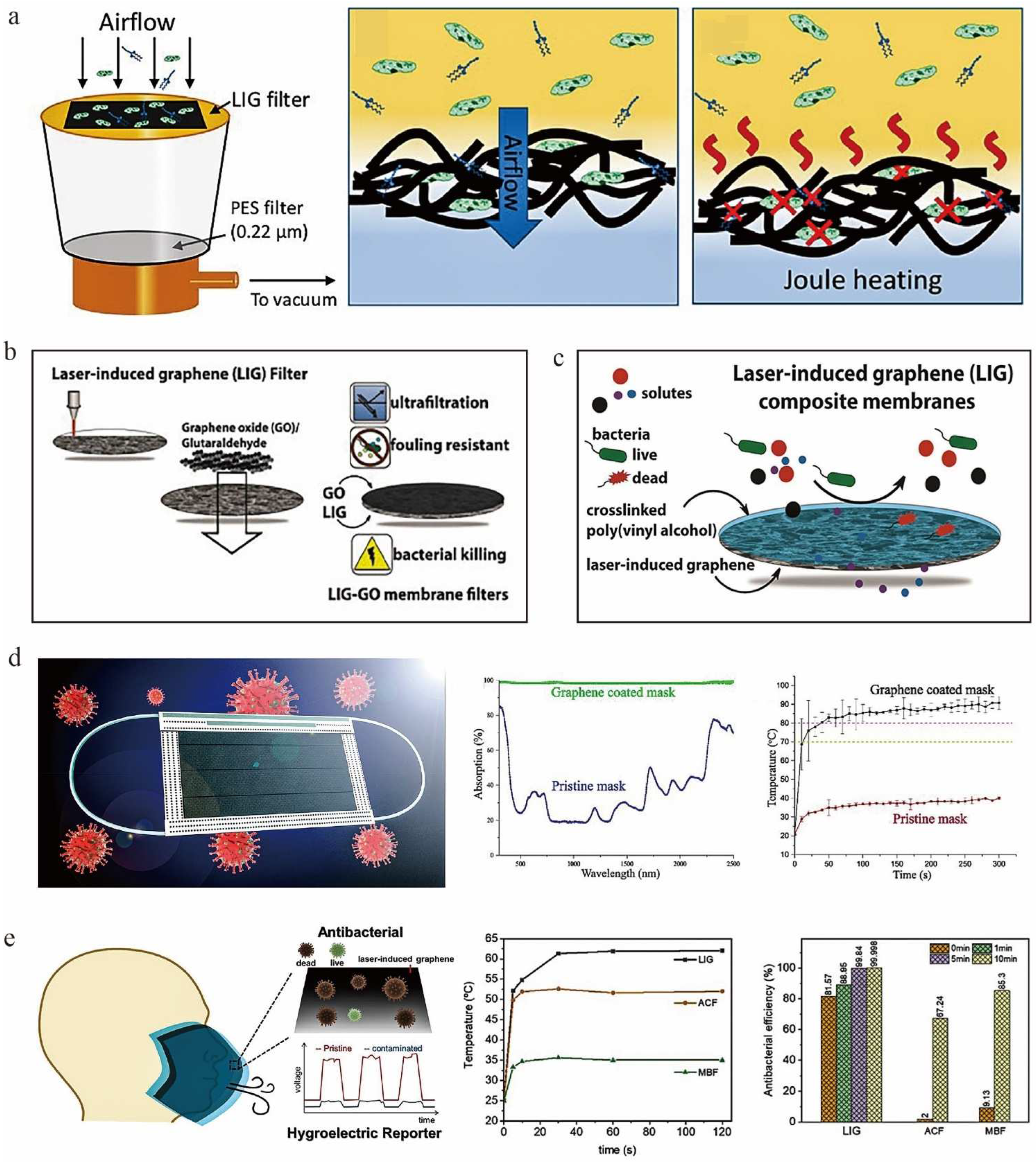
4.2. Environmental Protection
4.3. Energy Storage
5. Conclusions and Outlook
- (1)
- Although there have been many kinds of graphene preparation technologies, the large-scale, low-cost, environment-friendly, high-quality, and large-size macro-preparation technologies have not yet made substantial breakthroughs, making it difficult to meet the needs of industrial mass production. Exploring new preparation methods, determining more suitable laser parameters, and finding new precursor materials will provide new possibilities for the large-scale production of high-quality graphene;
- (2)
- Single-element doping and two-element co-doping have been studied to prepare LIG, which improves the electrochemical properties to a certain extent, and provides a new idea for multi-element co-doping. Therefore, researchers can further explore other new elements and develop multi-element co-doping to endow LIG with more excellent properties, and endow LIG with broader application prospects;
- (3)
- Scientists have developed a variety of sensors, such as pressure sensors, strain sensors, temperature sensors, biosensors, gas sensors, etc. From a practical point of view, these sensors have a single function and cannot acquire multiple stimuli at the same time. Studying multifunctional LIG-based sensors that can detect multiple stimuli is a fascinating direction;
- (4)
- When LIG is used in masks, its safety is a primary concern. A Canadian research institute has indicated that the use of masks containing graphene may cause the wearer to inhale graphene particles. To the best of the authors' knowledge, there are no relevant reports on the safety assessment of LIG-based masks for this issue so far. Addressing this issue is an important goal for ongoing research.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| LIG | Laser-induced graphene |
| GO | Graphene oxide |
| LIGF | LIG fiber |
| LIGP | LIG paper |
| F-LIG | Fluorine-doped laser-induced graphene |
| DLW | Direct laser writing |
| PS | Polystyrene |
| MIP | Molecularly imprinted polymer |
| SSFL | Spatially shaped femtosecond laser |
| MSC | Micro-supercapacitor |
| MEA | Membrane-electrode assembly |
| RGO | Reduced graphene oxide |
| PI | Polyimide |
| UV laser | Ultraviolet laser |
| GP | Graphene paper |
| LIGC | LIG composite |
| PDMS | Polydimethylsiloxane |
| CLIG | Corrugated LIG |
| PVA | Polyvinyl alcohol |
| FsLIG | Femtosecond laser-induced graphene |
| SC | Supercapacitor |
| EBFC | Enzyme biofuel cell |
References
- Mayorov, A.S.; Gorbachev, R.V.; Morozov, S.V.; Britnell, L.; Jalil, R.; Ponomarenko, L.A.; Blake, P.; Novoselov, K.S.; Watanabe, K.; Taniguchi, T. Micrometer-scale ballistic transport in encapsulated graphene at room temperature. Nano Lett. 2011, 11, 2396–2399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nair, R.R.; Blake, P.; Grigorenko, A.N.; Novoselov, K.S.; Booth, T.J.; Stauber, T.; Peres, N.M.; Geim, A.K. Fine structure constant defines visual transparency of graphene. Science 2008, 320, 1308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balandin, A.A. Thermal properties of graphene and nanostructured carbon materials. Nat. Mater. 2011, 10, 569–581. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balandin, A.A.; Ghosh, S.; Bao, W.; Calizo, I.; Teweldebrhan, D.; Miao, F.; Lau, C.N. Superior thermal conductivity of single-layer graphene. Nano Lett. 2008, 8, 902–907. [Google Scholar] [CrossRef] [PubMed]
- Stoller, M.D.; Park, S.; Zhu, Y.; An, J.; Ruoff, R.S. Graphene-based ultracapacitors. Nano Lett. 2008, 8, 3498–3502. [Google Scholar] [CrossRef]
- Geim, A.K. Graphene: Status and prospects. Science 2009, 324, 1530–1534. [Google Scholar] [CrossRef] [Green Version]
- Reina, A.; Jia, X.; Ho, J.; Nezich, D.; Son, H.; Bulovic, V.; Dresselhaus, M.S.; Kong, J. Large area, few-layer graphene films on arbitrary substrates by chemical vapor deposition. Nano Lett. 2009, 9, 30–35. [Google Scholar] [CrossRef]
- Olenych, I.B.; Aksimentyeva, O.I.; Monastyrskii, L.S.; Horbenko, Y.Y.; Partyka, M.V.; Luchechko, A.P.; Yarytska, L.I. Effect of graphene oxide on the properties of porous silicon. Nanoscale Res. Lett. 2016, 11, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Zhang, Y.; Zhi, C.; Wang, X.; Tang, D.; Xu, Y.; Weng, Q.; Jiang, X.; Mitome, M.; Golberg, D. Three-dimensional strutted graphene grown by substrate-free sugar blowing for high-power-density supercapacitors. Nat. Commun. 2013, 4, 1–8. [Google Scholar] [CrossRef]
- Kataria, S.; Wagner, S.; Ruhkopf, J.; Gahoi, A.; Pandey, H.; Bornemann, R.; Vaziri, S.; Smith, A.D.; Ostling, M.; Lemme, M.C. Chemical vapor deposited graphene: From synthesis to applications. Phys. Status Solidi 2014, 211, 2439–2449. [Google Scholar] [CrossRef]
- Trusovas, R.; Račiukaitis, G.; Niaura, G.; Barkauskas, J.; Valušis, G.; Pauliukaite, R. Recent advances in laser utilization in the chemical modification of graphene oxide and its applications. Adv. Opt. Mater. 2016, 4, 37–65. [Google Scholar] [CrossRef]
- Hummers Jr, W.S.; Offeman, R.E. Preparation of graphitic oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Lomeda, J.R.; Doyle, C.D.; Kosynkin, D.V.; Hwang, W.-F.; Tour, J.M. Diazonium functionalization of surfactant-wrapped chemically converted graphene sheets. J. Am. Chem. Soc. 2008, 130, 16201–16206. [Google Scholar] [CrossRef] [PubMed]
- Tung, V.C.; Allen, M.J.; Yang, Y.; Kaner, R.B. High-throughput solution processing of large-scale graphene. Nat. Nanotechnol. 2009, 4, 25–29. [Google Scholar] [CrossRef] [PubMed]
- Sadasivuni, K.K.; Kafy, A.; Kim, H.-C.; Ko, H.-U.; Mun, S.; Kim, J. Reduced graphene oxide filled cellulose films for flexible temperature sensor application. Synth. Met. 2015, 206, 154–161. [Google Scholar] [CrossRef]
- Voiry, D.; Yang, J.; Kupferberg, J.; Fullon, R.; Lee, C.; Jeong, H.Y.; Shin, H.S.; Chhowalla, M. High-quality graphene via microwave reduction of solution-exfoliated graphene oxide. Science 2016, 353, 1413–1416. [Google Scholar] [CrossRef] [Green Version]
- Yi, M.; Shen, Z. A review on mechanical exfoliation for the scalable production of graphene. J. Mater. Chem. A 2015, 3, 11700–11715. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric field effect in atomically thin carbon films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef] [Green Version]
- Hass, J.; De Heer, W.; Conrad, E. The growth and morphology of epitaxial multilayer graphene. J. Phys. Condens. Matte 2008, 20, 323202. [Google Scholar] [CrossRef]
- Stankovich, S.; Dikin, D.A.; Dommett, G.H.; Kohlhaas, K.M.; Zimney, E.J.; Stach, E.A.; Piner, R.D.; Nguyen, S.T.; Ruoff, R.S. Graphene-based composite materials. Nature 2006, 442, 282–286. [Google Scholar] [CrossRef]
- Stankovich, S.; Dikin, D.A.; Piner, R.D.; Kohlhaas, K.A.; Kleinhammes, A.; Jia, Y.; Wu, Y.; Nguyen, S.T.; Ruoff, R.S. Synthesis of graphene-based nanosheets via chemical reduction of exfoliated graphite oxide. Carbon 2007, 45, 1558–1565. [Google Scholar] [CrossRef]
- Chen, R.; Luo, T.; Geng, D.; Shen, Z.; Zhou, W. Facile fabrication of a fast-response flexible temperature sensor via laser reduced graphene oxide for contactless human-machine interface. Carbon 2022, 187, 35–46. [Google Scholar] [CrossRef]
- Tao, Y.; Varghese, B.; Jaiswal, M.; Wang, S.; Zhang, Z.; Oezyilmaz, B.; Loh, K.P.; Tok, E.S.; Sow, C.H. Localized insulator-conductor transformation of graphene oxide thin films via focused laser beam irradiation. Appl. Phys. A 2012, 106, 523–531. [Google Scholar] [CrossRef]
- Wan, Z.; Streed, E.W.; Lobino, M.; Wang, S.; Sang, R.T.; Cole, I.S.; Thiel, D.V.; Li, Q. Laser-Reduced Graphene: Synthesis, Properties, and Applications. Adv. Mater. Technol. 2018, 3, 1700315. [Google Scholar] [CrossRef]
- Lin, J.; Peng, Z.; Liu, Y.; Ruiz-Zepeda, F.; Ye, R.; Samuel, E.L.; Yacaman, M.J.; Yakobson, B.I.; Tour, J.M. Laser-induced porous graphene films from commercial polymers. Nat. Commun. 2014, 5, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Long, J.; Zhou, S.; Shi, D.; Huang, Y.; Chen, X.; Gao, J.; Zhao, N.; Wong, C.P. UV Laser-Induced Polyimide-to-Graphene Conversion: Modeling, Fabrication, and Application. Small Methods 2019, 3, 1900208. [Google Scholar] [CrossRef]
- Dong, Y.; Rismiller, S.C.; Lin, J. Molecular dynamic simulation of layered graphene clusters formation from polyimides under extreme conditions. Carbon 2016, 104, 47–55. [Google Scholar] [CrossRef] [Green Version]
- Vashisth, A.; Kowalik, M.; Gerringer, J.C.; Ashraf, C.; Van Duin, A.C.; Green, M.J. ReaxFF simulations of laser-induced graphene (LIG) formation for multifunctional polymer nanocomposites. ACS Appl. Nano Mater. 2020, 3, 1881–1890. [Google Scholar] [CrossRef]
- Khandelwal, M.; Van Tran, C.; Lee, J.; In, J.B. Nitrogen and boron co-doped densified laser-induced graphene for supercapacitor applications. Chem. Eng. J. 2022, 428, 131119. [Google Scholar] [CrossRef]
- Wang, X.; Sun, G.; Routh, P.; Kim, D.-H.; Huang, W.; Chen, P. Heteroatom-doped graphene materials: Syntheses, properties and applications. Chem. Soc. Rev. 2014, 43, 7067–7098. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Zhao, Y.; Li, X.; Jiang, L.; Qu, L. Laser-Based Growth and Treatment of Graphene for Advanced Photo-and Electro-Related Device Applications. Adv. Funct. Mater. 2022, 2203164. [Google Scholar] [CrossRef]
- Peng, Y.; Zhao, W.; Ni, F.; Yu, W.; Liu, X. Forest-like Laser-Induced Graphene Film with Ultrahigh Solar Energy Utilization Efficiency. ACS Nano 2021, 15, 19490–19502. [Google Scholar] [CrossRef] [PubMed]
- Tao, L.-Q.; Tian, H.; Liu, Y.; Ju, Z.-Y.; Pang, Y.; Chen, Y.-Q.; Wang, D.-Y.; Tian, X.-G.; Yan, J.-C.; Deng, N.-Q. An intelligent artificial throat with sound-sensing ability based on laser induced graphene. Nat. Commun. 2017, 8, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Torrente-Rodríguez, R.M.; Tu, J.; Yang, Y.; Min, J.; Wang, M.; Song, Y.; Yu, Y.; Xu, C.; Ye, C.; IsHak, W.W. Investigation of cortisol dynamics in human sweat using a graphene-based wireless mHealth system. Matter 2020, 2, 921–937. [Google Scholar] [CrossRef]
- Huang, L.; Ling, L.; Su, J.; Song, Y.; Wang, Z.; Tang, B.Z.; Westerhoff, P.; Ye, R. Laser-engineered graphene on wood enables efficient antibacterial, anti-salt-fouling, and lipophilic-matter-rejection solar evaporation. ACS Appl. Mater. Interfaces 2020, 12, 51864–51872. [Google Scholar] [CrossRef]
- Huang, L.; Xu, S.; Wang, Z.; Xue, K.; Su, J.; Song, Y.; Chen, S.; Zhu, C.; Tang, B.Z.; Ye, R. Self-reporting and photothermally enhanced rapid bacterial killing on a laser-induced graphene mask. ACS Nano 2020, 14, 12045–12053. [Google Scholar] [CrossRef] [PubMed]
- Thakur, A.K.; Singh, S.P.; Kleinberg, M.N.; Gupta, A.; Arnusch, C.J. Laser-induced graphene–PVA composites as robust electrically conductive water treatment membranes. ACS Appl. Mater. Interfaces 2019, 11, 10914–10921. [Google Scholar] [CrossRef] [PubMed]
- Le, T.S.D.; Lee, Y.A.; Nam, H.K.; Jang, K.Y.; Yang, D.; Kim, B.; Yim, K.; Kim, S.W.; Yoon, H.; Kim, Y.J. Green Flexible Graphene-Inorganic-Hybrid Micro-Supercapacitors Made of Fallen Leaves Enabled by Ultrafast Laser Pulses. Adv. Funct. Mater. 2021, 2107768. [Google Scholar] [CrossRef]
- Peng, Z.; Lin, J.; Ye, R.; Samuel, E.L.; Tour, J.M. Flexible and stackable laser-induced graphene supercapacitors. ACS Appl. Mater. Interfaces 2015, 7, 3414–3419. [Google Scholar] [CrossRef]
- Yi, J.; Chen, J.; Yang, Z.; Dai, Y.; Li, W.; Cui, J.; Ciucci, F.; Lu, Z.; Yang, C. Facile patterning of laser-induced graphene with tailored li nucleation kinetics for stable lithium-metal batteries. Adv. Energy Mater. 2019, 9, 1901796. [Google Scholar] [CrossRef]
- Duy, L.X.; Peng, Z.; Li, Y.; Zhang, J.; Ji, Y.; Tour, J.M. Laser-induced graphene fibers. Carbon 2018, 126, 472–479. [Google Scholar] [CrossRef]
- Sopronyi, M.; Sima, F.; Vaulot, C.; Delmotte, L.; Bahouka, A.; Matei Ghimbeu, C. Direct synthesis of graphitic mesoporous carbon from green phenolic resins exposed to subsequent UV and IR laser irradiations. Sci. Rep. 2016, 6, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.F.; Qin, W.M.; Huang, X.A. Characterization and thermal degradation of polyimides derived from ODPA and several alicyclic-containing diamines. Polym. Eng. Sci. 2008, 48, 1151–1156. [Google Scholar] [CrossRef]
- Kononenko, T.; Pimenov, S.; Kononenko, V.; Zavedeev, E.; Konov, V.; Dumitru, G.; Romano, V. Laser-induced spallation in diamond-like carbon films. Appl. Phys. A 2004, 79, 543–549. [Google Scholar] [CrossRef]
- Chen, Y.; Long, J.; Xie, B.; Kuang, Y.; Chen, X.; Hou, M.; Gao, J.; Liu, H.; He, Y.; Wong, C.-P. One-Step Ultraviolet Laser-Induced Fluorine-Doped Graphene Achieving Superhydrophobic Properties and Its Application in Deicing. ACS Appl. Mater. Interfaces 2022. [Google Scholar] [CrossRef]
- Kulyk, B.; Silva, B.F.; Carvalho, A.F.; Silvestre, S.; Fernandes, A.J.; Martins, R.; Fortunato, E.; Costa, F.M. Laser-induced graphene from paper for mechanical sensing. ACS Appl. Mater. Interfaces 2021, 13, 10210–10221. [Google Scholar] [CrossRef]
- Lamberti, A.; Perrucci, F.; Caprioli, M.; Serrapede, M.; Fontana, M.; Bianco, S.; Ferrero, S.; Tresso, E. New insights on laser-induced graphene electrodes for flexible supercapacitors: Tunable morphology and physical properties. Nanotechnology 2017, 28, 174002. [Google Scholar] [CrossRef]
- Rahimi, R.; Ochoa, M.; Yu, W.; Ziaie, B. Highly stretchable and sensitive unidirectional strain sensor via laser carbonization. ACS Appl. Mater. Interfaces 2015, 7, 4463–4470. [Google Scholar] [CrossRef]
- Wan, Z.; Umer, M.; Lobino, M.; Thiel, D.; Nguyen, N.-T.; Trinchi, A.; Shiddiky, M.J.; Gao, Y.; Li, Q. Laser induced self-N-doped porous graphene as an electrochemical biosensor for femtomolar miRNA detection. Carbon 2020, 163, 385–394. [Google Scholar] [CrossRef]
- Wang, L.; Wang, Z.; Bakhtiyari, A.N.; Zheng, H. A comparative study of laser-induced graphene by CO2 infrared laser and 355 nm ultraviolet (UV) laser. Micromachines 2020, 11, 1094. [Google Scholar] [CrossRef]
- Garland, N.T.; McLamore, E.S.; Cavallaro, N.D.; Mendivelso-Perez, D.; Smith, E.A.; Jing, D.; Claussen, J.C. Flexible laser-induced graphene for nitrogen sensing in soil. ACS Appl. Mater. 2018, 10, 39124–39133. [Google Scholar] [CrossRef] [Green Version]
- Biswas, R.K.; Farid, N.; O’Connor, G.; Scully, P. Improved conductivity of carbonized polyimide by CO2 laser graphitization. J. Mater. Chem. C 2020, 8, 4493–4501. [Google Scholar] [CrossRef]
- Singh, E.; Chen, Z.; Houshmand, F.; Ren, W.; Peles, Y.; Cheng, H.M.; Koratkar, N. Superhydrophobic graphene foams. Small 2013, 9, 75–80. [Google Scholar] [CrossRef]
- Beckham, J.L.; Li, J.T.; Stanford, M.G.; Chen, W.; McHugh, E.A.; Advincula, P.A.; Wyss, K.M.; Chyan, Y.; Boldman, W.L.; Rack, P.D. High-resolution laser-induced graphene from photoresist. ACS Nano 2021, 15, 8976–8983. [Google Scholar] [CrossRef] [PubMed]
- Le, T.S.D.; Park, S.; An, J.; Lee, P.S.; Kim, Y.J. Ultrafast Laser Pulses Enable One-Step Graphene Patterning on Woods and Leaves for Green Electronics. Adv. Funct. Mater. 2019, 29, 1902771. [Google Scholar] [CrossRef]
- Stanford, M.G.; Zhang, C.; Fowlkes, J.D.; Hoffman, A.; Ivanov, I.N.; Rack, P.D.; Tour, J.M. High-resolution laser-induced graphene. Flexible electronics beyond the visible limit. ACS Appl. Mater. Interfaces 2020, 12, 10902–10907. [Google Scholar] [CrossRef] [PubMed]
- Li, G. Direct laser writing of graphene electrodes. J. Appl. Phys. 2020, 127, 010901. [Google Scholar] [CrossRef] [Green Version]
- Chyan, Y.; Ye, R.; Li, Y.; Singh, S.P.; Arnusch, C.J.; Tour, J.M. Laser-induced graphene by multiple lasing: Toward electronics on cloth, paper, and food. ACS Nano 2018, 12, 2176–2183. [Google Scholar] [CrossRef]
- Gerringer, J.C.; Moran, A.G.; Habib, T.; Pospisil, M.J.; Oh, J.H.; Teipel, B.R.; Green, M.J. Radio frequency heating of laser-induced graphene on polymer surfaces for rapid welding. ACS Appl. Nano Mater. 2019, 2, 7032–7042. [Google Scholar] [CrossRef]
- Kaidarova, A.; Khan, M.A.; Marengo, M.; Swanepoel, L.; Przybysz, A.; Muller, C.; Fahlman, A.; Buttner, U.; Geraldi, N.R.; Wilson, R.P. Wearable multifunctional printed graphene sensors. NPJ Flex. Electron. 2019, 3, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Kaidarova, A.; Marengo, M.; Geraldi, N.R.; Duarte, C.M.; Kosel, J. Flexible conductivity, temperature, and depth sensor for marine environment monitoring. In Proceedings of the 2019 IEEE Sensors, Montreal, QC, Canada, 27–30 October 2019; pp. 1–4. [Google Scholar]
- Kaidarova, A.; Marengo, M.; Marinaro, G.; Geraldi, N.; Duarte, C.M.; Kosel, J. Flexible and biofouling independent salinity sensor. Adv. Mater. Interfaces 2018, 5, 1801110. [Google Scholar] [CrossRef]
- Li, Y.; Luong, D.X.; Zhang, J.; Tarkunde, Y.R.; Kittrell, C.; Sargunaraj, F.; Ji, Y.; Arnusch, C.J.; Tour, J.M. Laser-induced graphene in controlled atmospheres: From superhydrophilic to superhydrophobic surfaces. Adv. Mater. 2017, 29, 1700496. [Google Scholar] [CrossRef] [PubMed]
- Luong, D.X.; Yang, K.; Yoon, J.; Singh, S.P.; Wang, T.; Arnusch, C.J.; Tour, J.M. Laser-induced graphene composites as multifunctional surfaces. ACS Nano 2019, 13, 2579–2586. [Google Scholar] [CrossRef] [PubMed]
- Peng, Z.; Ye, R.; Mann, J.A.; Zakhidov, D.; Li, Y.; Smalley, P.R.; Lin, J.; Tour, J.M. Flexible boron-doped laser-induced graphene microsupercapacitors. ACS Nano 2015, 9, 5868–5875. [Google Scholar] [CrossRef] [PubMed]
- Ye, R.; Chyan, Y.; Zhang, J.; Li, Y.; Han, X.; Kittrell, C.; Tour, J.M. Laser-induced graphene formation on wood. Adv. Mater. 2017, 29, 1702211. [Google Scholar] [CrossRef]
- Ye, R.; Peng, Z.; Wang, T.; Xu, Y.; Zhang, J.; Li, Y.; Nilewski, L.G.; Lin, J.; Tour, J.M. In situ formation of metal oxide nanocrystals embedded in laser-induced graphene. Acs Nano 2015, 9, 9244–9251. [Google Scholar] [CrossRef]
- Zhang, P.; Tang, X.; Pang, Y.; Bi, M.; Li, X.; Yu, J.; Zhang, J.; Yuan, M.; Luo, F. Flexible laser-induced-graphene omnidirectional sound device. Chem. Phys. Lett. 2020, 745, 137275. [Google Scholar] [CrossRef]
- Carvalho, A.F.; Fernandes, A.J.; Leitão, C.; Deuermeier, J.; Marques, A.C.; Martins, R.; Fortunato, E.; Costa, F.M. Laser-induced graphene strain sensors produced by ultraviolet irradiation of polyimide. Adv. Funct. Mater. 2018, 28, 1805271. [Google Scholar] [CrossRef]
- Zhang, Z.; Song, M.; Hao, J.; Wu, K.; Li, C.; Hu, C. Visible light laser-induced graphene from phenolic resin: A new approach for directly writing graphene-based electrochemical devices on various substrates. Carbon 2018, 127, 287–296. [Google Scholar] [CrossRef]
- Romero, F.J.; Salinas-Castillo, A.; Rivadeneyra, A.; Albrecht, A.; Godoy, A.; Morales, D.P.; Rodriguez, N. In-depth study of laser diode ablation of kapton polyimide for flexible conductive substrates. Nanomaterials 2018, 8, 517. [Google Scholar] [CrossRef] [Green Version]
- Cai, J.; Lv, C.; Watanabe, A. Cost-effective fabrication of high-performance flexible all-solid-state carbon micro-supercapacitors by blue-violet laser direct writing and further surface treatment. J. Mater. Chem. A 2016, 4, 1671–1679. [Google Scholar] [CrossRef]
- Carvalho, A.F.; Fernandes, A.J.; Martins, R.; Fortunato, E.; Costa, F.M. Laser-induced graphene piezoresistive sensors synthesized directly on cork insoles for gait analysis. Adv. Mater. Technol. 2020, 5, 2000630. [Google Scholar] [CrossRef]
- Stanford, M.G.; Li, J.T.; Chen, Y.; McHugh, E.A.; Liopo, A.; Xiao, H.; Tour, J.M. Self-sterilizing laser-induced graphene bacterial air filter. ACS Nano 2019, 13, 11912–11920. [Google Scholar] [CrossRef] [PubMed]
- Zhong, H.; Zhu, Z.; Lin, J.; Cheung, C.F.; Lu, V.L.; Yan, F.; Chan, C.-Y.; Li, G. Reusable and recyclable graphene masks with outstanding superhydrophobic and photothermal performances. ACS Nano 2020, 14, 6213–6221. [Google Scholar] [CrossRef] [PubMed]
- Kulyk, B.; Matos, M.; Silva, B.F.; Carvalho, A.F.; Fernandes, A.J.; Evtuguin, D.V.; Fortunato, E.; Costa, F.M. Conversion of paper and xylan into laser-induced graphene for environmentally friendly sensors. Diam. Relat. Mater. 2022, 108855. [Google Scholar] [CrossRef]
- Trusovas, R.; Ratautas, K.; Račiukaitis, G.; Niaura, G. Graphene layer formation in pinewood by nanosecond and picosecond laser irradiation. Appl. Surf. Sci. 2019, 471, 154–161. [Google Scholar] [CrossRef]
- Singh, S.P.; Li, Y.; Zhang, J.; Tour, J.M.; Arnusch, C.J. Sulfur-doped laser-induced porous graphene derived from polysulfone-class polymers and membranes. ACS Nano 2018, 12, 289–297. [Google Scholar] [CrossRef]
- Yang, W.; Zhao, W.; Li, Q.; Li, H.; Wang, Y.; Li, Y.; Wang, G. Fabrication of smart components by 3D printing and laser-scribing technologies. ACS Appl. Mater. Interfaces 2019, 12, 3928–3935. [Google Scholar] [CrossRef]
- Lamberti, A.; Serrapede, M.; Ferraro, G.; Fontana, M.; Perrucci, F.; Bianco, S.; Chiolerio, A.; Bocchini, S. All-SPEEK flexible supercapacitor exploiting laser-induced graphenization. 2d Mater. 2017, 4, 035012. [Google Scholar] [CrossRef]
- Li, Z.; Lu, L.; Xie, Y.; Wang, W.; Lin, Z.; Tang, B.; Lin, N. Preparation of Laser-Induced Graphene Fabric from Silk and Its Application Examples for Flexible Sensor. Adv. Eng. Mater. 2021, 23, 2100195. [Google Scholar] [CrossRef]
- Li, Z.; Lin, J.; Li, B.; Yu, C.; Wang, H.; Li, Q. Construction of heteroatom-doped and three-dimensional graphene materials for the applications in supercapacitors: A review. J. Energy Storage 2021, 44, 103437. [Google Scholar] [CrossRef]
- Rixin, L.; JIANG, C.; Lin, L.; ZHANG, W.; XIANG, Y.; Hai, M.; ZHANG, H.; Gaoping, C.; Yun, D. Research progress of the regulation of nitrogen doping of graphene and the influence mechanism of supercapacitor capacitive performance. Energy Storage Sci. Technol. 2020, 9, 1657. [Google Scholar]
- Clerici, F.; Fontana, M.; Bianco, S.; Serrapede, M.; Perrucci, F.; Ferrero, S.; Tresso, E.; Lamberti, A. In situ MoS2 decoration of laser-induced graphene as flexible supercapacitor electrodes. ACS Appl. Mater. Interfaces 2016, 8, 10459–10465. [Google Scholar] [CrossRef] [PubMed]
- Chhetry, A.; Sharifuzzaman, M.; Yoon, H.; Sharma, S.; Xuan, X.; Park, J.Y. MoS2-decorated laser-induced graphene for a highly sensitive, hysteresis-free, and reliable piezoresistive strain sensor. ACS Appl. Mater. Interfaces 2019, 11, 22531–22542. [Google Scholar] [CrossRef]
- Zhang, F.; Alhajji, E.; Lei, Y.; Kurra, N.; Alshareef, H.N. Highly doped 3D graphene Na-ion battery anode by laser scribing polyimide films in nitrogen ambient. Adv. Energy Mater. 2018, 8, 1800353. [Google Scholar] [CrossRef]
- Yang, W.; Liu, Y.; Li, Q.; Wei, J.; Li, X.; Zhang, Y.; Liu, J. In situ formation of phosphorus-doped porous graphene via laser induction. RSC Adv. 2020, 10, 23953–23958. [Google Scholar] [CrossRef]
- Chen, L.; Hou, T.; Tan, Y.; Guo, C.; Wang, B.; Ge, L.; Li, F. Laser-Induced N-and B-Codoped Graphene Nanozymes with Intrinsic Peroxidase-Like Activities for Bactericidal Application. ACS Sustain. Chem. Eng. 2022, 10, 2750–2760. [Google Scholar] [CrossRef]
- Li, J.T.; Stanford, M.G.; Chen, W.; Presutti, S.E.; Tour, J.M. Laminated laser-induced graphene composites. ACS Nano 2020, 14, 7911–7919. [Google Scholar] [CrossRef]
- Ye, R.; James, D.K.; Tour, J.M. Laser-induced graphene. Acc. Chem. Res. 2018, 51, 1609–1620. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Y.; Zhang, P.; Liu, F.; Luo, S. Laser-Induced Freestanding Graphene Papers: A New Route of Scalable Fabrication with Tunable Morphologies and Properties for Multifunctional Devices and Structures. Small 2018, 14, 1802350. [Google Scholar] [CrossRef]
- Sha, J.; Li, Y.; Villegas Salvatierra, R.; Wang, T.; Dong, P.; Ji, Y.; Lee, S.-K.; Zhang, C.; Zhang, J.; Smith, R.H. Three-dimensional printed graphene foams. Acs Nano 2017, 11, 6860–6867. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luong, D.X.; Subramanian, A.K.; Silva, G.A.L.; Yoon, J.; Cofer, S.; Yang, K.; Owuor, P.S.; Wang, T.; Wang, Z.; Lou, J. Laminated object manufacturing of 3D-printed laser-induced graphene foams. Adv. Mater. 2018, 30, 1707416. [Google Scholar] [CrossRef]
- Huang, L.; Su, J.; Song, Y.; Ye, R. Laser-induced graphene: En route to smart sensing. Nano Micro Lett. 2020, 12, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-Q.; Chen, Z.-D.; Mao, J.-W.; Han, D.-D.; Sun, X. Laser fabrication of graphene-based electronic skin. Front. Chem. 2019, 7, 461. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, W.; Lu, L.; Li, Z.; Lin, L.; Liang, Z.; Lu, X.; Xie, Y. Fingerprint-Inspired Strain Sensor with Balanced Sensitivity and Strain Range Using Laser-Induced Graphene. ACS Appl. Mater. Interfaces 2021. [Google Scholar] [CrossRef]
- Xu, Y.; Fei, Q.; Page, M.; Zhao, G.; Ling, Y.; Chen, D.; Yan, Z. Laser-induced graphene for bioelectronics and soft actuators. Nano Res. 2021, 14, 3033–3050. [Google Scholar] [CrossRef]
- Ye, R.; James, D.K.; Tour, J.M. Laser-induced graphene: From discovery to translation. Adv. Mater. 2019, 31, 1803621. [Google Scholar] [CrossRef]
- You, R.; Liu, Y.Q.; Hao, Y.L.; Han, D.D.; Zhang, Y.L.; You, Z. Laser fabrication of graphene-based flexible electronics. Adv. Mater. 2020, 32, 1901981. [Google Scholar] [CrossRef]
- Chae, H.; Kwon, H.-J.; Kim, Y.-K.; Won, Y.; Kim, D.; Park, H.-J.; Kim, S.; Gandla, S. Laser-processed nature-inspired deformable structures for breathable and reusable electrophysiological sensors toward controllable home electronic appliances and psychophysiological stress monitoring. ACS Appl. Mater. Interfaces 2019, 11, 28387–28396. [Google Scholar] [CrossRef]
- Dinh Le, T.-S.; An, J.; Huang, Y.; Vo, Q.; Boonruangkan, J.; Tran, T.; Kim, S.-W.; Sun, G.; Kim, Y.-J. Ultrasensitive anti-interference voice recognition by bio-inspired skin-attachable self-cleaning acoustic sensors. ACS Nano 2019, 13, 13293–13303. [Google Scholar] [CrossRef]
- Song, H.; Zhang, J.; Chen, D.; Wang, K.; Niu, S.; Han, Z.; Ren, L. Superfast and high-sensitivity printable strain sensors with bioinspired micron-scale cracks. Nanoscale 2017, 9, 1166–1173. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.; Hoang, P.T.; Liu, T. Direct laser writing for creating porous graphitic structures and their use for flexible and highly sensitive sensor and sensor arrays. Carbon 2016, 96, 522–531. [Google Scholar] [CrossRef]
- Dallinger, A.; Keller, K.; Fitzek, H.; Greco, F. Stretchable and skin-conformable conductors based on polyurethane/laser-induced graphene. ACS Appl. Mater. Interfaces 2020, 12, 19855–19865. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Li, Q.; Wu, R.; Sha, J.; Lu, Y.; Xuan, F. Laser direct writing of ultrahigh sensitive SiC-based strain sensor arrays on elastomer toward electronic skins. Adv. Funct. Mater. 2019, 29, 1806786. [Google Scholar] [CrossRef]
- Song, W.; Zhu, J.; Gan, B.; Zhao, S.; Wang, H.; Li, C.; Wang, J. Flexible, stretchable, and transparent planar microsupercapacitors based on 3D porous laser-induced graphene. Small 2018, 14, 1702249. [Google Scholar] [CrossRef]
- Zhu, Y.; Cai, H.; Ding, H.; Pan, N.; Wang, X. Fabrication of low-cost and highly sensitive graphene-based pressure sensors by direct laser scribing polydimethylsiloxane. ACS Appl. Mater. Interfaces 2019, 11, 6195–6200. [Google Scholar] [CrossRef]
- La Torraca, P.; Bobinger, M.; Romero, F.J.; Rivadeneyra, A.; Ricci, Y.; Cattani, L.; Morales, D.P.; Rodríguez, N.; Salinas-Castillo, A.; Larcher, L. Acoustic characterization of laser-induced graphene film thermoacoustic loudspeakers. In Proceedings of the 2019 IEEE 19th International Conference on Nanotechnology (IEEE-NANO), Macao, China, 22–26 July 2019; pp. 5–8. [Google Scholar]
- Tao, L.-Q.; Liu, Y.; Ju, Z.-Y.; Tian, H.; Xie, Q.-Y.; Yang, Y.; Ren, T.-L. A flexible 360-degree thermal sound source based on laser induced graphene. Nanomaterials 2016, 6, 112. [Google Scholar] [CrossRef] [Green Version]
- Long, S.; Feng, Y.; He, F.; Zhao, J.; Bai, T.; Lin, H.; Cai, W.; Mao, C.; Chen, Y.; Gan, L. Biomass-derived, multifunctional and wave-layered carbon aerogels toward wearable pressure sensors, supercapacitors and triboelectric nanogenerators. Nano Energy 2021, 85, 105973. [Google Scholar] [CrossRef]
- Tian, Q.; Yan, W.; Li, Y.; Ho, D. Bean pod-inspired ultrasensitive and self-healing pressure sensor based on laser-induced graphene and polystyrene microsphere sandwiched structure. ACS Appl. Mater. Interfaces 2020, 12, 9710–9717. [Google Scholar] [CrossRef]
- Xia, S.-Y.; Long, Y.; Huang, Z.; Zi, Y.; Tao, L.-Q.; Li, C.-H.; Sun, H.; Li, J. Laser-Induced Graphene (LIG)-based Pressure Sensor and Triboelectric Nanogenerator towards High-Performance Self-Powered Measurement-Control Combined System. Nano Energy 2022, 107099. [Google Scholar] [CrossRef]
- Xiong, Y.; Shen, Y.; Tian, L.; Hu, Y.; Zhu, P.; Sun, R.; Wong, C.-P. A flexible, ultra-highly sensitive and stable capacitive pressure sensor with convex microarrays for motion and health monitoring. Nano Energy 2020, 70, 104436. [Google Scholar] [CrossRef]
- Li, Q.; Wu, T.; Zhao, W.; Ji, J.; Wang, G. Laser-Induced Corrugated Graphene Films for Integrated Multimodal Sensors. ACS Appl. Mater. Interfaces 2021, 13, 37433–37444. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Tai, H.; Yuan, Z.; Zhou, Y.; Su, Y.; Jiang, Y. A high-performances flexible temperature sensor composed of polyethyleneimine/reduced graphene oxide bilayer for real-time monitoring. Adv. Mater. Technol. 2019, 4, 1800594. [Google Scholar] [CrossRef]
- Xu, X.; Karis, A.J.; Buller, M.J.; Santee, W.R. Relationship between core temperature, skin temperature, and heat flux during exercise in heat. Eur. J. Appl. Physiol. 2013, 113, 2381–2389. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Zhao, D.; Ma, R.; Zhang, X.; Rao, J.; Yin, Y.; Wang, X.; Yi, F. Flexible temperature sensors based on carbon nanomaterials. J. Mater. Chem. B 2021, 9, 1941–1964. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Tao, L.-Q.; Wang, Y.; Zheng, K.; Yu, J.; Xiandong, L.; Chen, X.; Huang, Y. Graphene oxide humidity sensor with laser-induced graphene porous electrodes. Sens. Actuators B Chem. 2020, 325, 128790. [Google Scholar] [CrossRef]
- Kun, H.; Bin, L.; Orban, M.; Donghai, Q.; Hongbo, Y. Accurate Flexible Temperature Sensor Based on Laser-Induced Graphene Material. Shock Vib. 2021, 2021, 9938010. [Google Scholar] [CrossRef]
- Han, R.; Wang, L.; Tang, X.; Qian, J.; Yu, J.; Chen, X.; Huang, Y. Facile fabrication of rGO/LIG-based temperature sensor with high sensitivity. Mater. Lett. 2021, 304, 130637. [Google Scholar] [CrossRef]
- Al-Mashat, L.; Shin, K.; Kalantar-Zadeh, K.; Plessis, J.D.; Han, S.H.; Kojima, R.W.; Kaner, R.B.; Li, D.; Gou, X.; Ippolito, S.J. Graphene/polyaniline nanocomposite for hydrogen sensing. J. Phys. Chem. C 2010, 114, 16168–16173. [Google Scholar] [CrossRef]
- Mackin, C.; Schroeder, V.; Zurutuza, A.; Su, C.; Kong, J.; Swager, T.M.; Palacios, T.s. Chemiresistive graphene sensors for ammonia detection. ACS Appl. Mater. Interfaces 2018, 10, 16169–16176. [Google Scholar] [CrossRef]
- Shokuhi Rad, A.; Esfahanian, M.; Maleki, S.; Gharati, G. Application of carbon nanostructures toward SO2 and SO3 adsorption: A comparison between pristine graphene and N-doped graphene by DFT calculations. J. Sulfur Chem. 2016, 37, 176–188. [Google Scholar] [CrossRef]
- Singh, E.; Meyyappan, M.; Nalwa, H.S. Flexible graphene-based wearable gas and chemical sensors. ACS Appl. Mater. Interfaces 2017, 9, 34544–34586. [Google Scholar] [CrossRef] [PubMed]
- Stanford, M.G.; Yang, K.; Chyan, Y.; Kittrell, C.; Tour, J.M. Laser-induced graphene for flexible and embeddable gas sensors. ACS Nano 2019, 13, 3474–3482. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Yi, N.; Zhu, J.; Cheng, Z.; Yin, X.; Zhang, X.; Zhu, H.; Cheng, H. Novel gas sensing platform based on a stretchable laser-induced graphene pattern with self-heating capabilities. J. Mater. Chem. A 2020, 8, 6487–6500. [Google Scholar] [CrossRef]
- Zhang, C.; Xie, Y.; Deng, H.; Tumlin, T.; Zhang, C.; Su, J.W.; Yu, P.; Lin, J. Monolithic and flexible ZnS/SnO2 ultraviolet photodetectors with lateral graphene electrodes. Small 2017, 13, 1604197. [Google Scholar] [CrossRef]
- Rackus, D.G.; Shamsi, M.H.; Wheeler, A.R. Electrochemistry, biosensors and microfluidics: A convergence of fields. Chem. Soc. Rev. 2015, 44, 5320–5340. [Google Scholar] [CrossRef]
- Marques, A.C.; Cardoso, A.R.; Martins, R.; Sales, M.G.F.; Fortunato, E. Laser-induced graphene-based platforms for dual biorecognition of molecules. ACS Appl. Nano Mater. 2020, 3, 2795–2803. [Google Scholar] [CrossRef]
- Cardoso, A.R.; Marques, A.C.; Santos, L.; Carvalho, A.F.; Costa, F.M.; Martins, R.; Sales, M.G.F.; Fortunato, E. Molecularly-imprinted chloramphenicol sensor with laser-induced graphene electrodes. Biosens. Bioelectron. 2019, 124, 167–175. [Google Scholar] [CrossRef]
- Kim, J.; Lee, M.; Shim, H.J.; Ghaffari, R.; Cho, H.R.; Son, D.; Jung, Y.H.; Soh, M.; Choi, C.; Jung, S. Stretchable silicon nanoribbon electronics for skin prosthesis. Nat. Commun. 2014, 5, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Trung, T.Q.; Ramasundaram, S.; Hwang, B.U.; Lee, N.E. An all-elastomeric transparent and stretchable temperature sensor for body-attachable wearable electronics. Adv. Mater. 2016, 28, 502–509. [Google Scholar] [CrossRef]
- Chhetry, A.; Sharma, S.; Barman, S.C.; Yoon, H.; Ko, S.; Park, C.; Yoon, S.; Kim, H.; Park, J.Y. Black Phosphorus@ Laser-Engraved Graphene Heterostructure-Based Temperature-Strain Hybridized Sensor for Electronic-Skin Applications. Adv. Funct. Mater. 2021, 31, 2007661. [Google Scholar] [CrossRef]
- Sinitskii, A.; Dimiev, A.; Corley, D.A.; Fursina, A.A.; Kosynkin, D.V.; Tour, J.M. Kinetics of diazonium functionalization of chemically converted graphene nanoribbons. ACS Nano 2010, 4, 1949–1954. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wei, D.; Gong, W.; Zheng, A.; Guan, Y. Hydrogen-bond assembly of poly (vinyl alcohol) and polyhexamethylene guanidine for nonleaching and transparent antimicrobial films. ACS Appl. Mater. Interfaces 2018, 10, 37535–37543. [Google Scholar] [CrossRef]
- Thakur, A.K.; Singh, S.P.; Thamaraiselvan, C.; Kleinberg, M.N.; Arnusch, C.J. Graphene oxide on laser-induced graphene filters for antifouling, electrically conductive ultrafiltration membranes. J. Membr. Sci. 2019, 591, 117322. [Google Scholar] [CrossRef]
- Sohrabi, C.; Alsafi, Z.; O'neill, N.; Khan, M.; Kerwan, A.; Al-Jabir, A.; Iosifidis, C.; Agha, R. World Health Organization declares global emergency: A review of the 2019 novel coronavirus (COVID-19). Int. J. Surg. 2020, 76, 71–76. [Google Scholar] [CrossRef]
- Huang, L.; Gu, M.; Wang, Z.; Tang, T.W.; Zhu, Z.; Yuan, Y.; Wang, D.; Shen, C.; Tang, B.Z.; Ye, R. Highly Efficient and Rapid Inactivation of Coronavirus on Non-Metal Hydrophobic Laser-Induced Graphene in Mild Conditions. Adv. Funct. Mater. 2021, 31, 2101195. [Google Scholar] [CrossRef]
- Li, G.; Law, W.-C.; Chan, K.C. Floating, highly efficient, and scalable graphene membranes for seawater desalination using solar energy. Green Chem. 2018, 20, 3689–3695. [Google Scholar] [CrossRef]
- Luo, Y.T.; Chen, Z.C.; Li, Q.; Chen, X.M. Laser-Induced Porous Graphene on a Polyimide Membrane with a Melamine Sponge Framework (PI@MS) for Long-Term Stable Steam Generation. Acs Appl. Energy Mater. 2021, 4, 9766–9774. [Google Scholar] [CrossRef]
- Bobinger, M.R.; Romero, F.J.; Salinas-Castillo, A.; Becherer, M.; Lugli, P.; Morales, D.P.; Rodríguez, N.; Rivadeneyra, A. Flexible and robust laser-induced graphene heaters photothermally scribed on bare polyimide substrates. Carbon 2019, 144, 116–126. [Google Scholar] [CrossRef]
- Chen, J.; Wang, Y.; Liu, F.; Luo, S. Laser-induced graphene paper heaters with multimodally patternable electrothermal performance for low-energy manufacturing of composites. ACS Appl. Mater. Interfaces 2020, 12, 23284–23297. [Google Scholar] [CrossRef]
- Noh, S.H.; Eom, W.; Lee, W.J.; Park, H.; Ambade, S.B.; Kim, S.O.; Han, T.H. Joule heating-induced sp2-restoration in graphene fibers. Carbon 2019, 142, 230–237. [Google Scholar] [CrossRef]
- He, M.; Wang, G.; Zhu, Y.; Wang, Y.; Liu, F.; Luo, S. In-situ joule heating-triggered nanopores generation in laser-induced graphene papers for capacitive enhancement. Carbon 2022, 186, 215–226. [Google Scholar]
- Yuan, Y.; Jiang, L.; Li, X.; Zuo, P.; Xu, C.; Tian, M.; Zhang, X.; Wang, S.; Lu, B.; Shao, C. Laser photonic-reduction stamping for graphene-based micro-supercapacitors ultrafast fabrication. Nat. Commun. 2020, 11, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Parmeggiani, M.; Zaccagnini, P.; Stassi, S.; Fontana, M.; Bianco, S.; Nicosia, C.; Pirri, C.F.; Lamberti, A. PDMS/polyimide composite as an elastomeric substrate for multifunctional laser-induced graphene electrodes. ACS Appl. Mater. Interfaces 2019, 11, 33221–33230. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.; Li, J.; Li, Y.; Wang, H.; Zhang, Q.; Kaner, R.B. Flexible quasi-solid-state planar micro-supercapacitor based on cellular graphene films. Mater. Horiz. 2017, 4, 1145–1150. [Google Scholar] [CrossRef]
- Yun, J.; Song, C.; Lee, H.; Park, H.; Jeong, Y.R.; Kim, J.W.; Jin, S.W.; Oh, S.Y.; Sun, L.; Zi, G. Stretchable array of high-performance micro-supercapacitors charged with solar cells for wireless powering of an integrated strain sensor. Nano Energy 2018, 49, 644–654. [Google Scholar] [CrossRef]
- Yang, Z.; Xu, C.; Yan, H.; Liu, Y.; Yue, C.; Zhang, L.; Shui, M.; Hu, F.; Shu, J. Laser-Induced Graphene Assisting Self-Conversion Reaction for Sulfur-Free Aqueous Cu-S Battery. Adv. Funct. Mater. 2021, 31, 2103893. [Google Scholar] [CrossRef]
- Khoo, K.S.; Chia, W.Y.; Wang, K.; Chang, C.-K.; Leong, H.Y.; Maaris, M.N.B.; Show, P.L. Development of proton-exchange membrane fuel cell with ionic liquid technology. Sci. Total Environ. 2021, 793, 148705. [Google Scholar] [CrossRef]
- Mehta, V.; Cooper, J.S. Review and analysis of PEM fuel cell design and manufacturing. J. Power Sources 2003, 114, 32–53. [Google Scholar] [CrossRef]
- Tiliakos, A.; Trefilov, A.M.; Tanasă, E.; Balan, A.; Stamatin, I. Laser-induced graphene as the microporous layer in proton exchange membrane fuel cells. Appl. Surf. Sci. 2020, 504, 144096. [Google Scholar] [CrossRef]
- Kong, X.; Gai, P.; Ge, L.; Li, F. Laser-scribed N-doped graphene for integrated flexible enzymatic biofuel cells. ACS Sustain. Chem. 2020, 8, 12437–12442. [Google Scholar] [CrossRef]



| Raw Materials | Doped Materials | Prepared Graphene | Process Method | Performance | Application | Refs. |
|---|---|---|---|---|---|---|
| PI | Metal oxide nanoparticle (Co3O4, MoO2, and Fe3O4) | MO-LIG | Single irradiation step | Efficient electrocatalytic activity and catalytic stability | Electrocatalyst | [67] |
| H3BO3 | B-LIG | Single irradiation step | High energy density, excellent recyclability, and flexibility | Metal-free oxygen reduction reaction catalyst, solar cells, field emission transistors, and lithium ion batteries. | [65] | |
| Urea | N-LIG | Single irradiation step | Excellent coulombic efficiency, cycling stability, and rate capabilities | Metal ion battery anodes | [86] | |
| Solid hydrocarbons, elastomers, epoxy, cement, and geopolymer | LIGC | Single irradiation step | Superhydrophobicity, high electrical conductivity | Wearable thermal therapy devices, deicing, anti-icing, antibiofouling and antimicrobial applications | [64] | |
| Ammonium polyphosphate | P-LIG | Single irradiation step | Good electrochemical performance, and high specific capacitance | Supercapacitor | [87] | |
| H3BO3 | NB-LIG | Multiple irradiation steps | High peroxidase-like catalytic activity, excellent bactericidal efficiency and capacitive performance | Sterilization, supercapacitor | [29,88] | |
| Fluorinated ethylene propylene | F-LIG | Single irradiation step | Excellent and stable electrical conductivity and hydrophobicity | Electrode material | [45] | |
| PSU, PES, PPSU | - | S-LIG | Single irradiation step | Electrochemical and antifouling properties | Wastewater purification, fouling-resistant cathodes in microbial fuel cells | [78] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, Y.; Zhang, C.; Chen, Y.; Nie, Z. Research Progress on the Preparation and Applications of Laser-Induced Graphene Technology. Nanomaterials 2022, 12, 2336. https://doi.org/10.3390/nano12142336
Guo Y, Zhang C, Chen Y, Nie Z. Research Progress on the Preparation and Applications of Laser-Induced Graphene Technology. Nanomaterials. 2022; 12(14):2336. https://doi.org/10.3390/nano12142336
Chicago/Turabian StyleGuo, Yani, Cheng Zhang, Ye Chen, and Zhengwei Nie. 2022. "Research Progress on the Preparation and Applications of Laser-Induced Graphene Technology" Nanomaterials 12, no. 14: 2336. https://doi.org/10.3390/nano12142336
APA StyleGuo, Y., Zhang, C., Chen, Y., & Nie, Z. (2022). Research Progress on the Preparation and Applications of Laser-Induced Graphene Technology. Nanomaterials, 12(14), 2336. https://doi.org/10.3390/nano12142336