MOCVD Growth and Structural Properties of ZnS Nanowires: A Case Study of Polytypism
Abstract
:1. Introduction
2. Materials and Methods
3. ZnS Thin Films: MOCVD Basic Studies
C2H5*Zn => Zn + C2H5*
2C2H5* => C4H10 or 2C2H5* => C2H6 + C2H4
3.1. MOCVD Growth Regimes
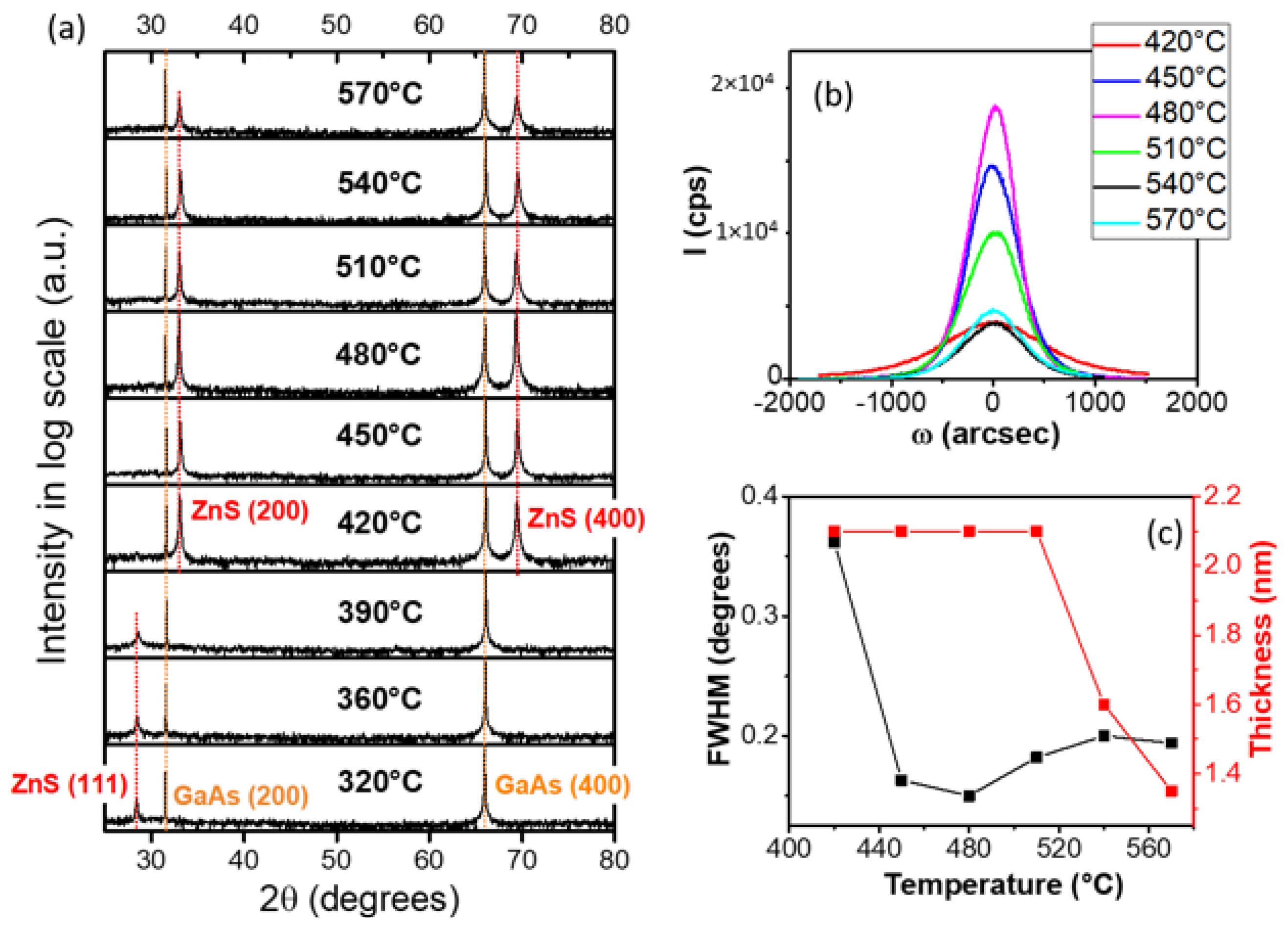
3.2. X-ray Diffraction Analysis of ZnS Buffer Layers
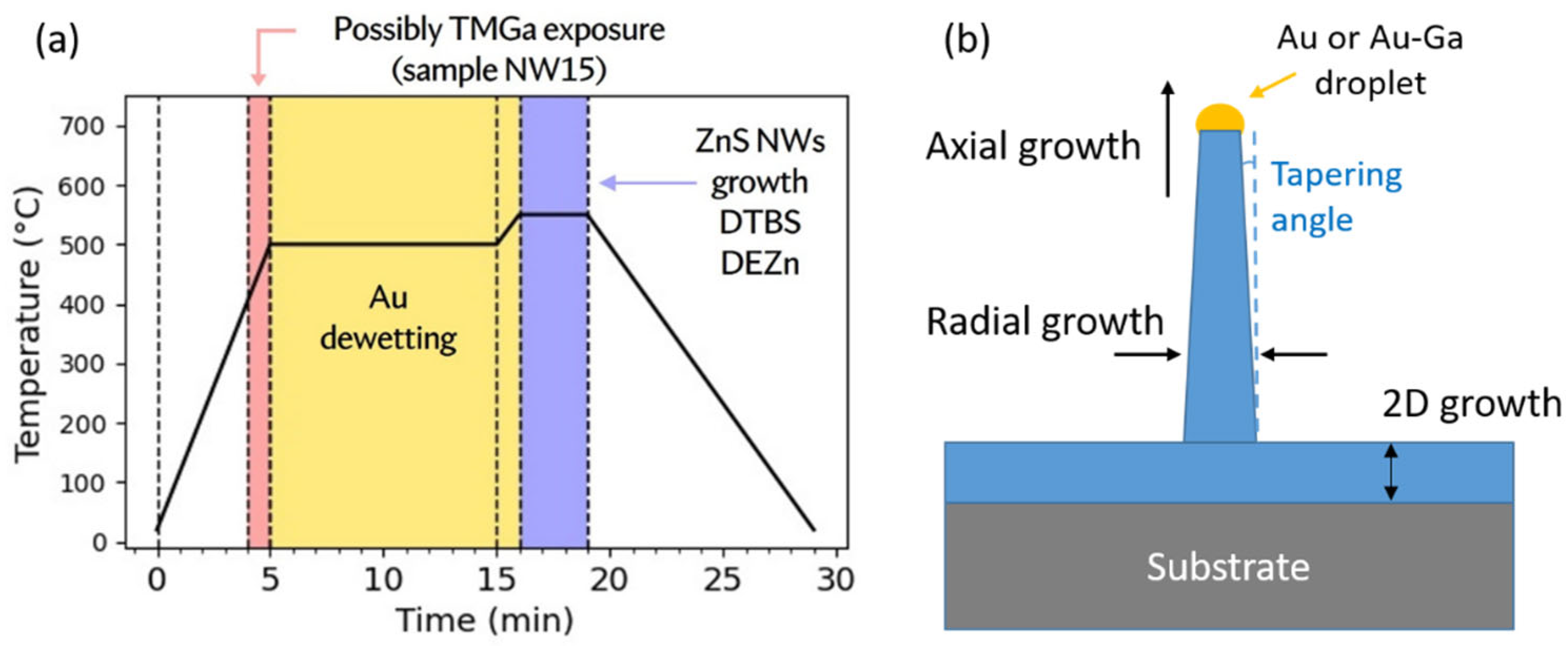
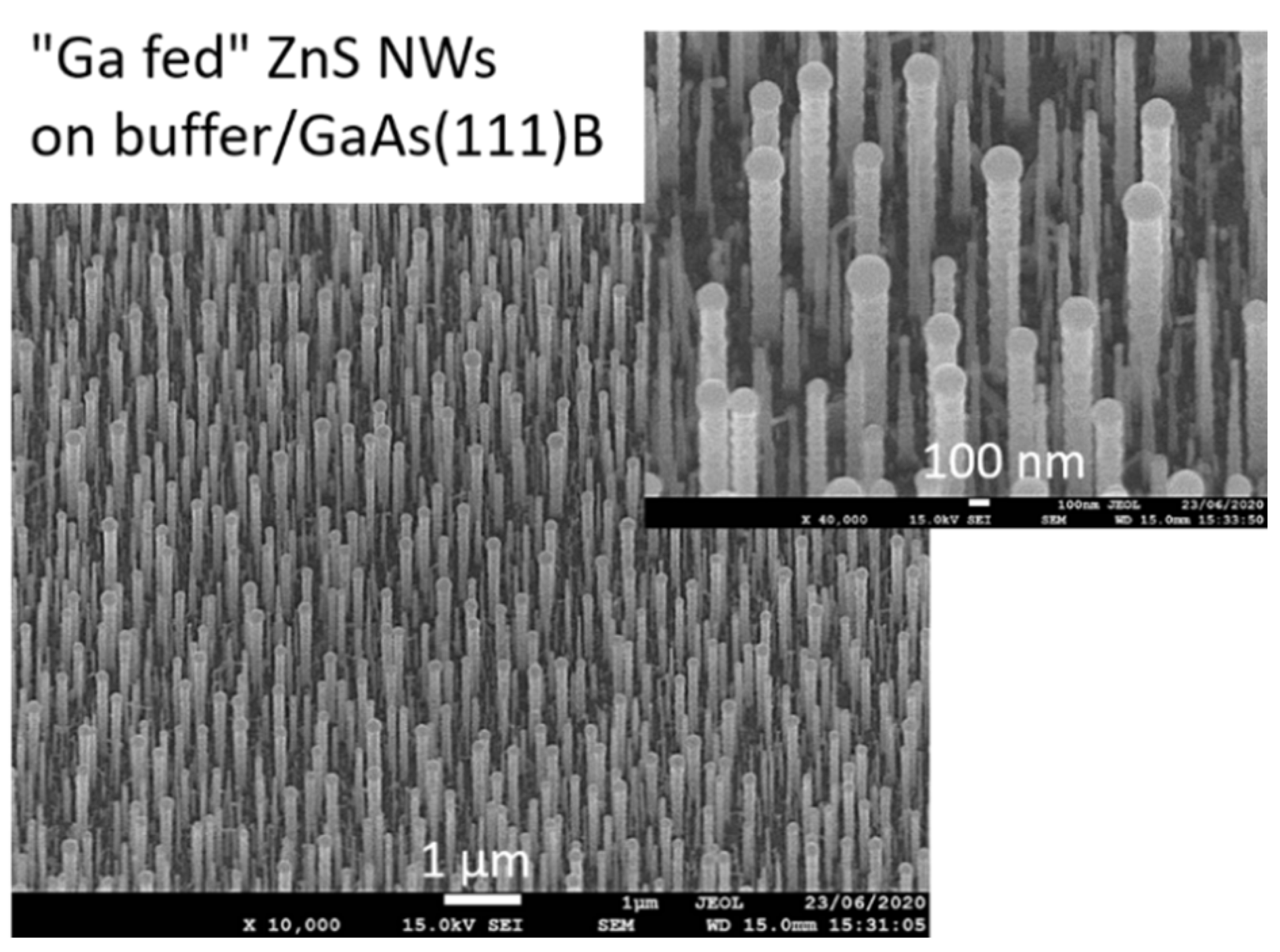
4. ZnS Nanowires Catalyzed Growth
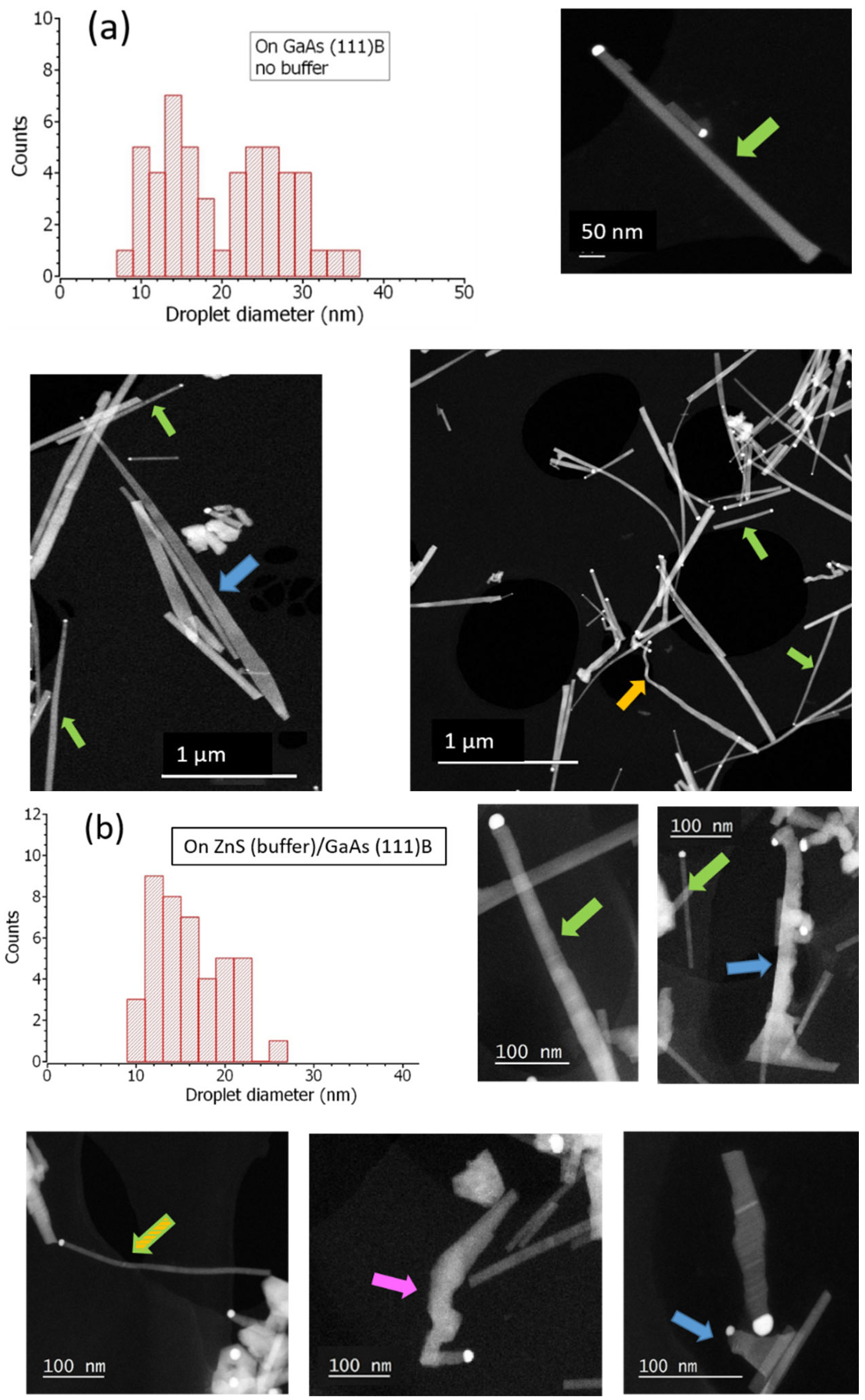
4.1. Au-Droplet Formation
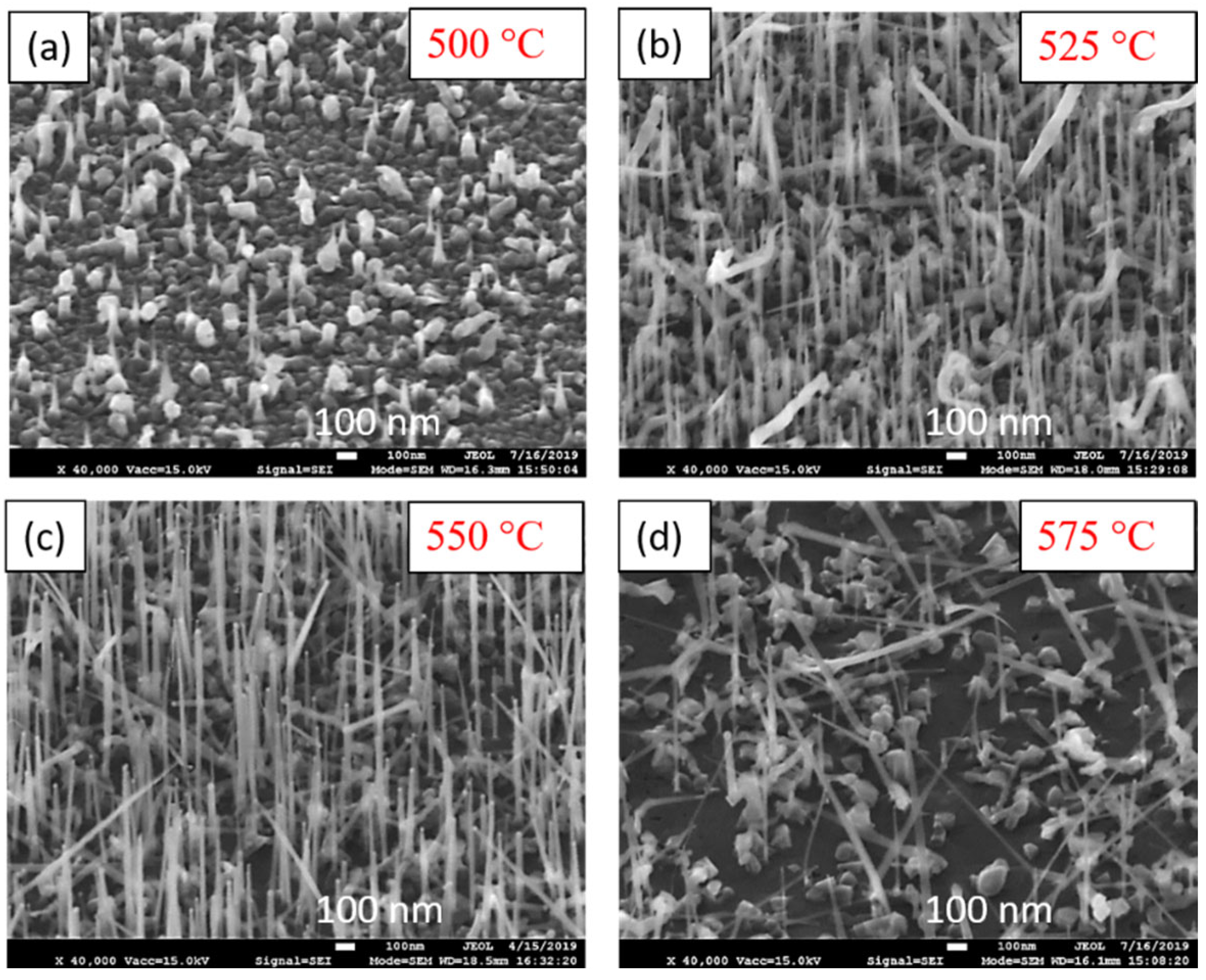
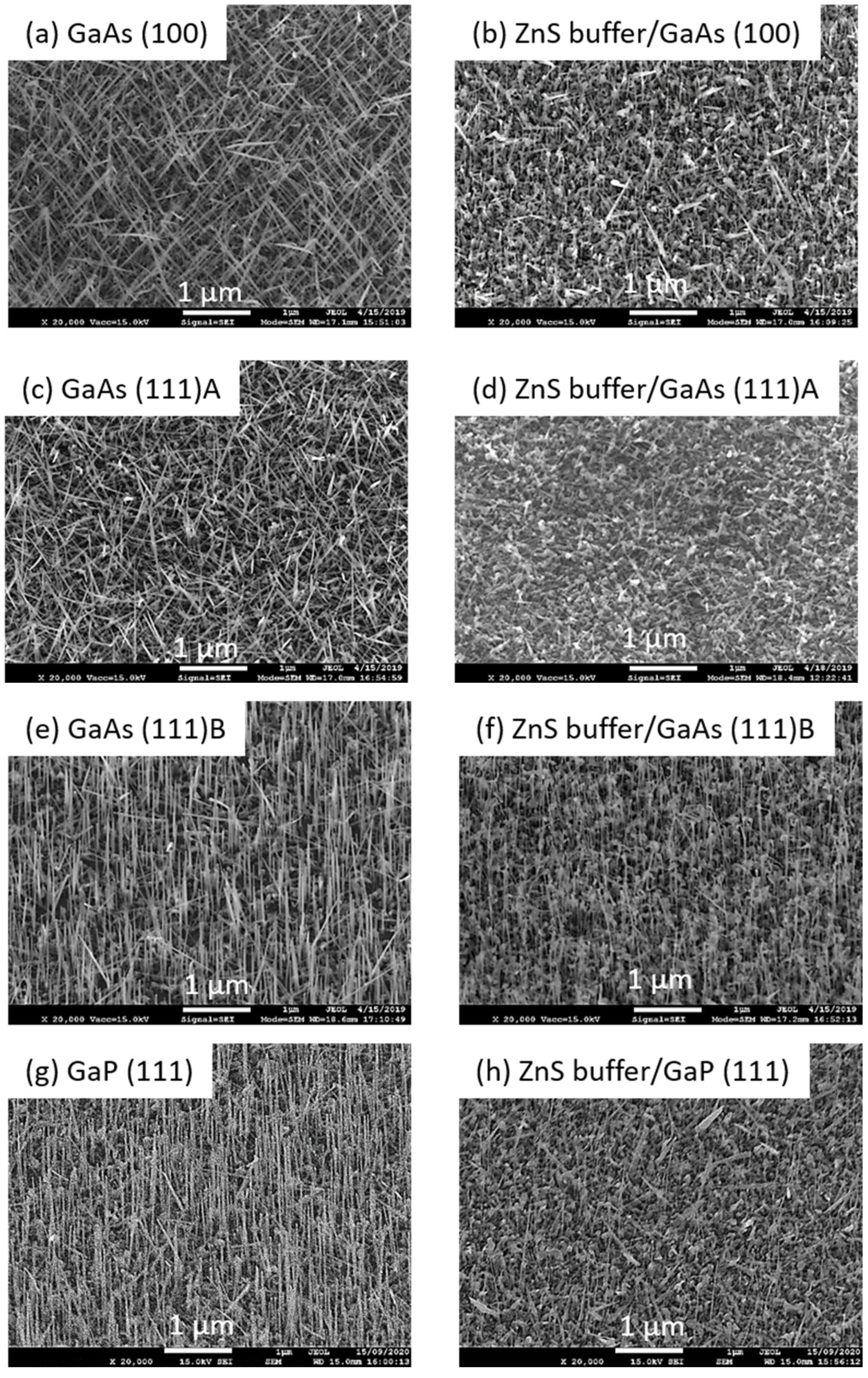
4.2. Growth Parameter Studies
4.3. Au-Droplet Size and Nanowire Diameter
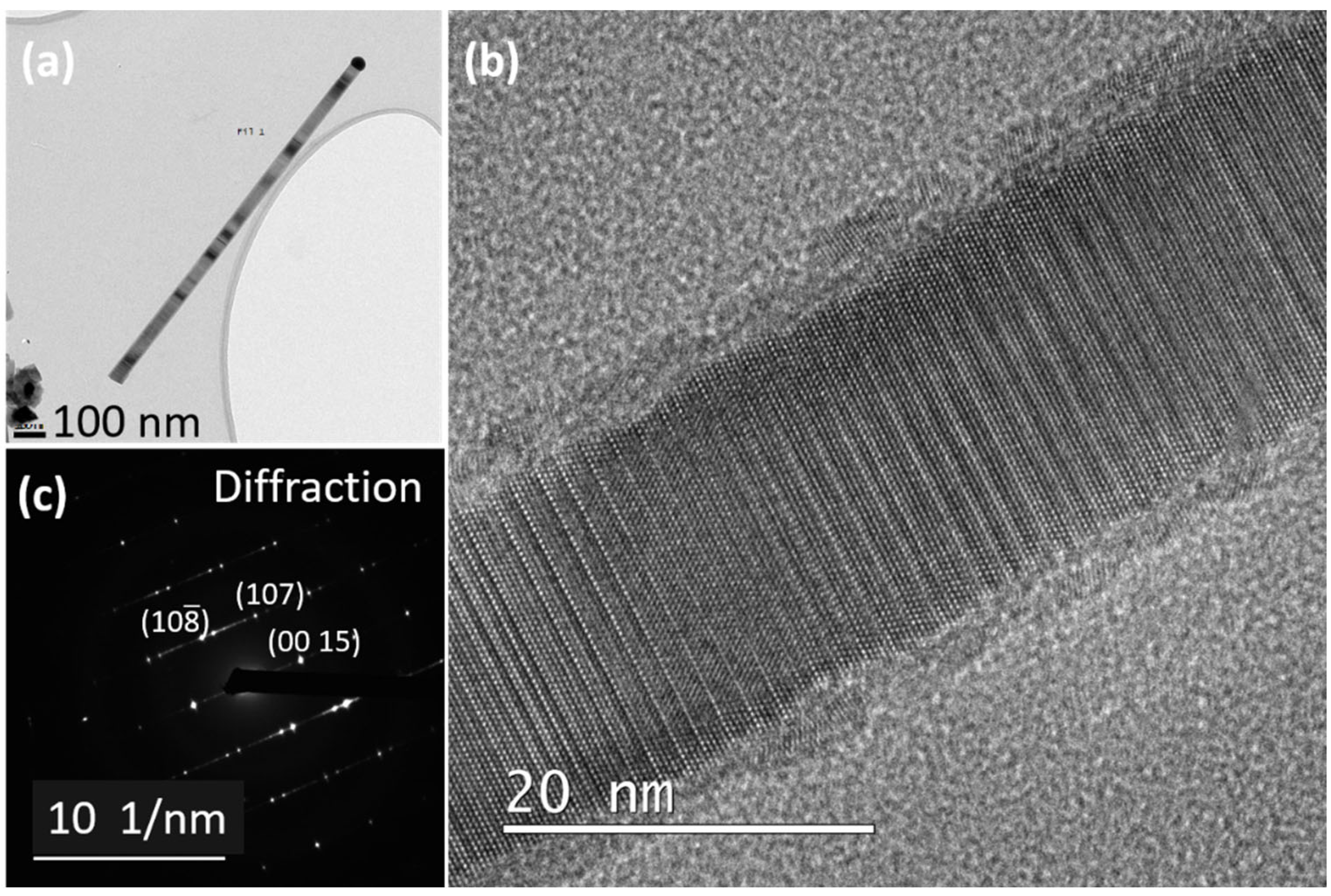
5. Structural Characterization by TEM and Discussion of the Growth Mechanisms
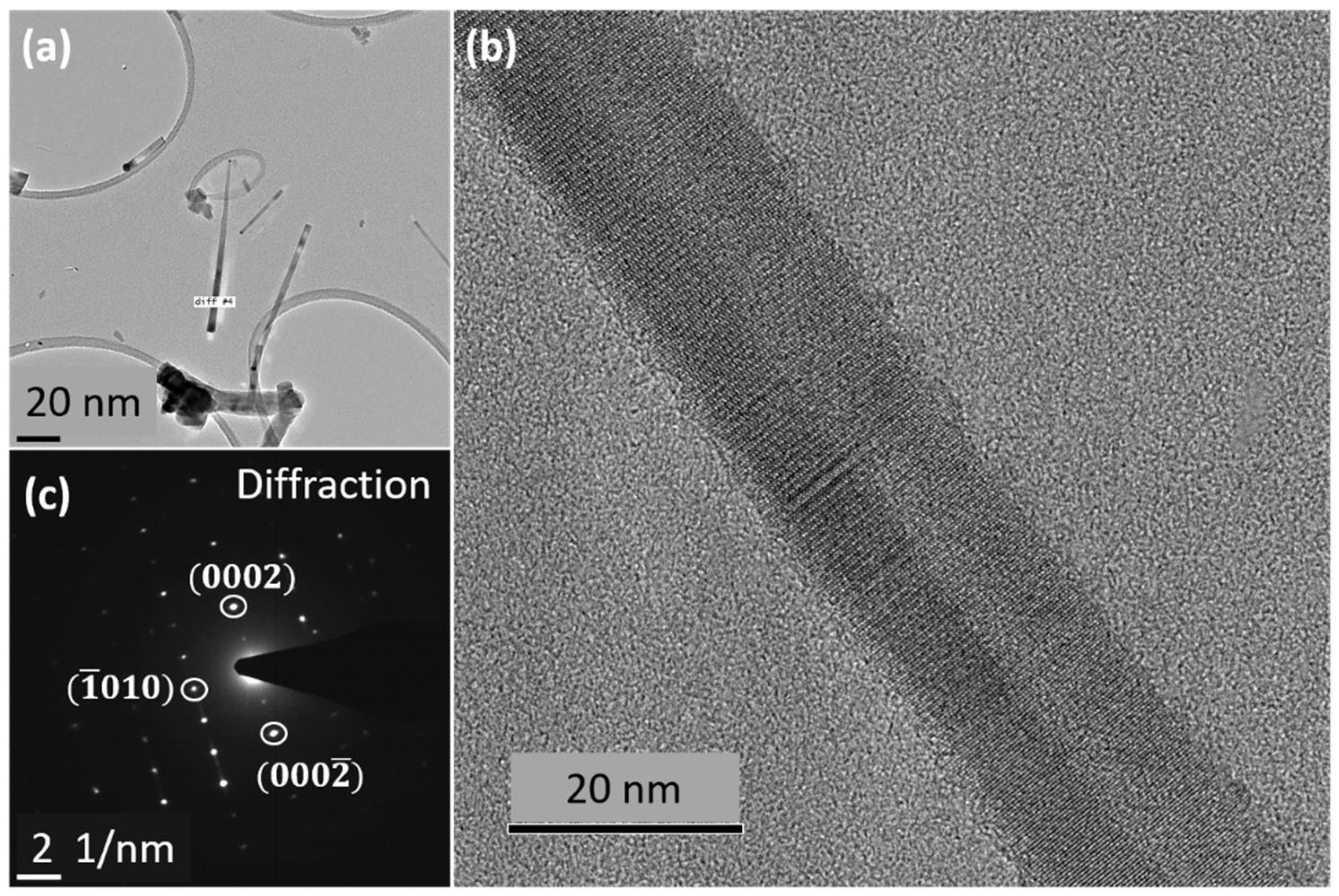
5.1. VLS versus VSS
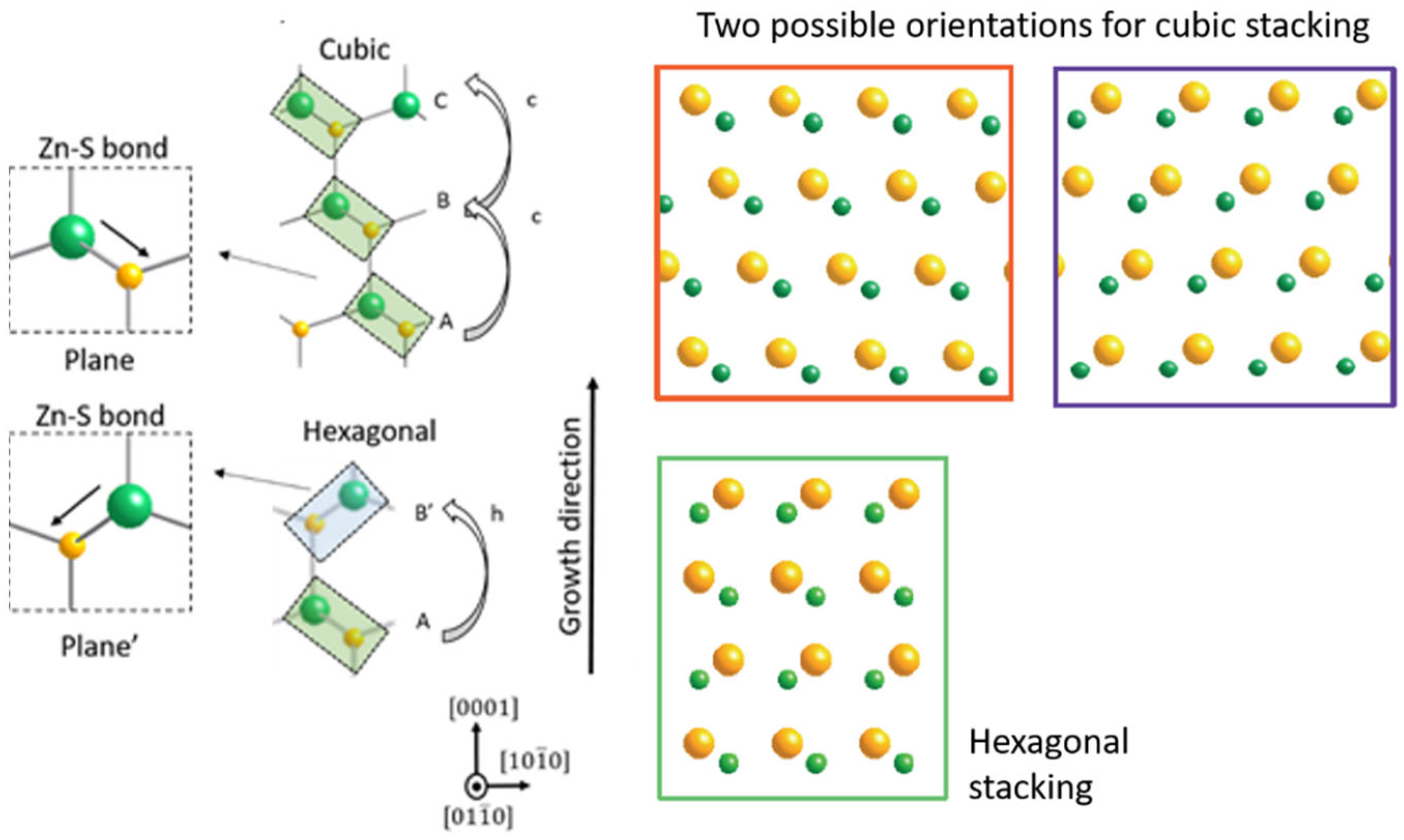
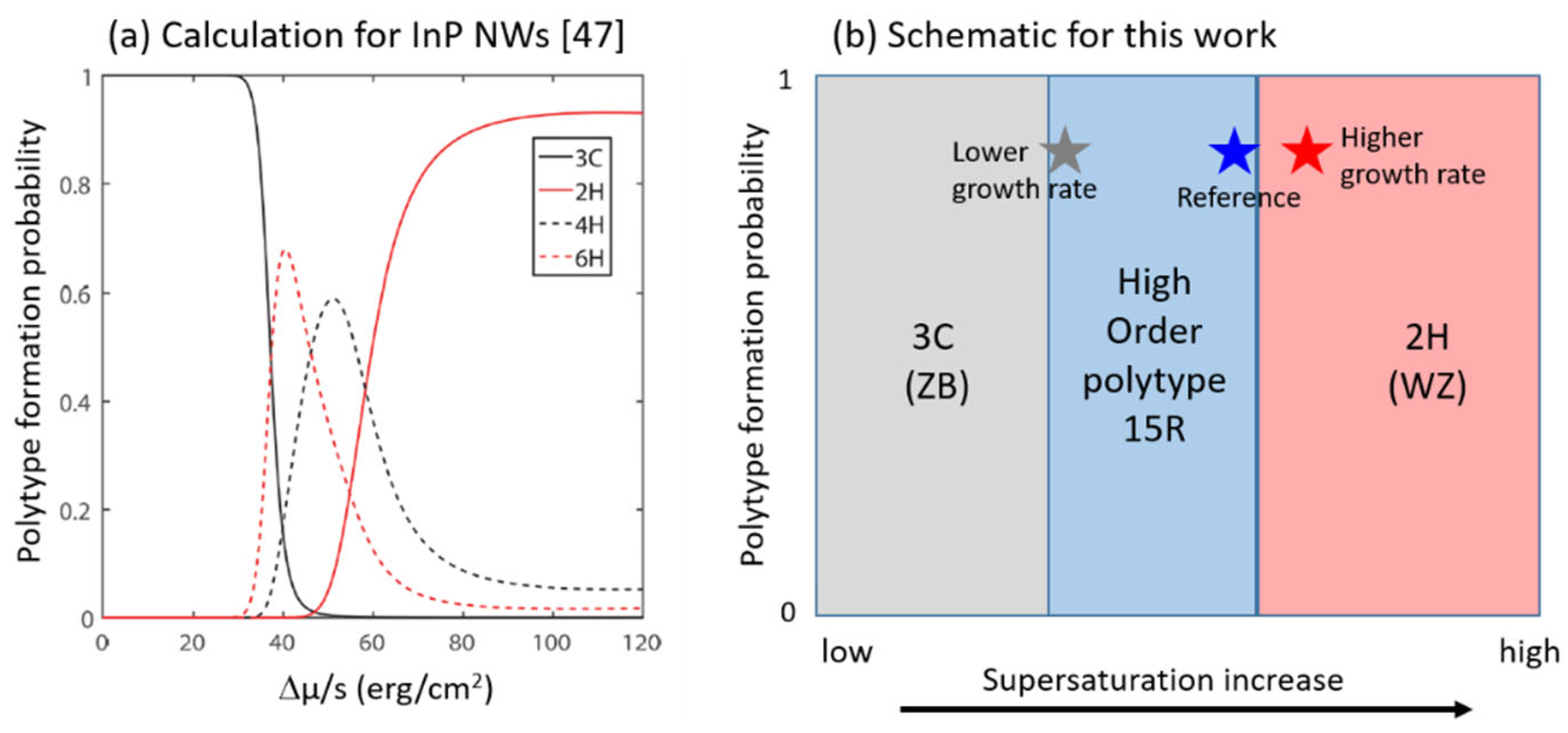
5.2. Controlling Crystal Phases: The Key Role of Supersaturation
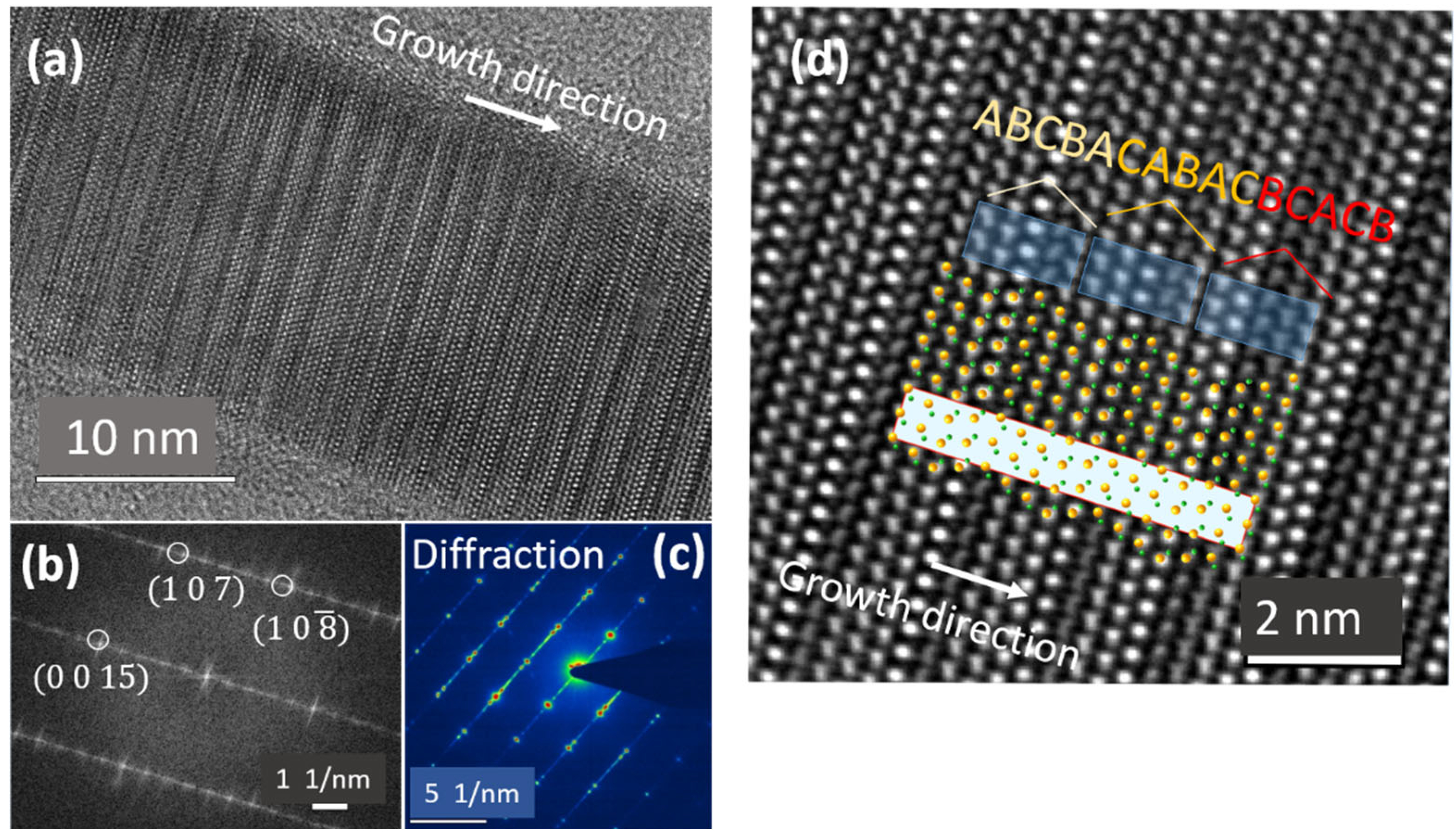
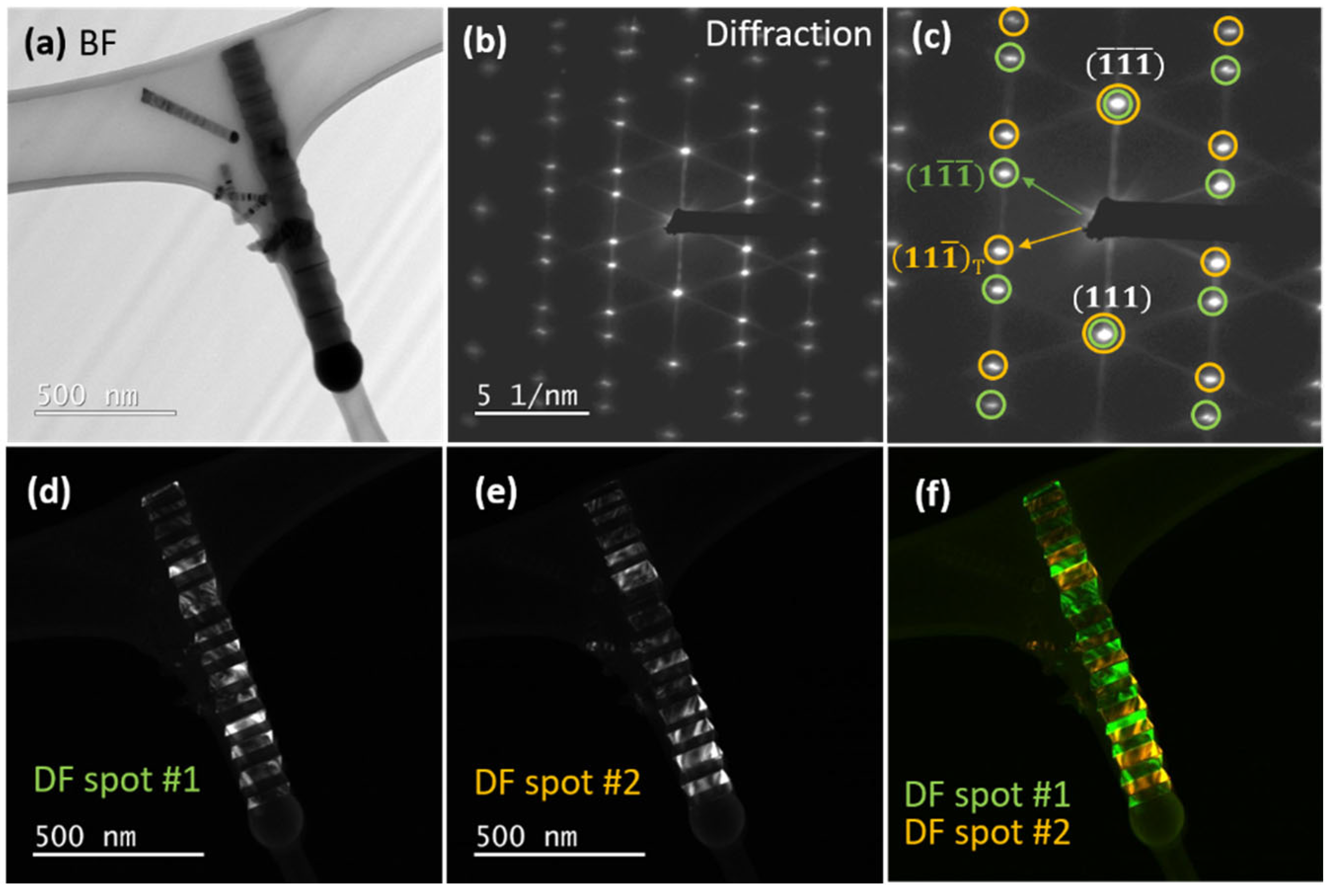
5.3. Twinning Superlattice Highlight in ZnS NWs
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Agarwal, R.; Lieber, C.M. Semiconductor Nanowires: Optics and Optoelectronics. Appl. Phys. A 2006, 85, 209. [Google Scholar] [CrossRef] [Green Version]
- Goktas, N.I.; Wilson, P.; Ghukasyan, A.; Wagner, D.; McNamee, S.; LaPierre, R.R. Nanowires for Energy: A Review. Appl. Phys. Rev. 2018, 5, 041305. [Google Scholar] [CrossRef]
- Li, Z.; Allen, J.; Allen, M.; Tan, H.H.; Jagadish, C.; Fu, L. Review on III-V Semiconductor Single Nanowire-Based Room Temperature Infrared Photodetectors. Materials 2020, 13, 1400. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wong-Leung, J.; Yang, I.; Li, Z.; Karuturi, S.K.; Fu, L.; Tan, H.H.; Jagadish, C. Engineering III–V Semiconductor Nanowires for Device Applications. Adv. Mater. 2020, 32, 1904359. [Google Scholar] [CrossRef]
- Meng, J.; Li, Z. Schottky-Contacted Nanowire Sensors. Adv. Mater. 2020, 32, 2000130. [Google Scholar] [CrossRef]
- Yang, Y.; Yuan, W.; Kang, W.; Ye, Y.; Pan, Q.; Zhang, X.; Ke, Y.; Wang, C.; Qiu, Z.; Tang, Y. A Review on Silicon Nanowire-Based Anodes for next-Generation High-Performance Lithium-Ion Batteries from a Material-Based Perspective. Sustain. Energy Fuels 2020, 4, 1577–1594. [Google Scholar] [CrossRef]
- Liu, Y.; Ji, G.; Wang, J.; Liang, X.; Zuo, Z.; Shi, Y. Fabrication and Photocatalytic Properties of Silicon Nanowires by Metal-Assisted Chemical Etching: Effect of H2O2 Concentration. Nanoscale Res. Lett. 2012, 7, 663. [Google Scholar] [CrossRef] [Green Version]
- Güniat, L.; Caroff, P.; Fontcuberta i Morral, A. Vapor Phase Growth of Semiconductor Nanowires: Key Developments and Open Questions. Chem. Rev. 2019, 119, 8958–8971. [Google Scholar] [CrossRef] [Green Version]
- McIntyre, P.C.; Morral, A.F. i Semiconductor Nanowires: To Grow or Not to Grow? Mater. Today Nano 2020, 9, 100058. [Google Scholar] [CrossRef]
- Bagga, S.; Akhtar, J.; Mishra, S. Synthesis and Applications of ZnO Nanowire: A Review. AIP Conf. Proc. 2018, 1989, 020004. [Google Scholar] [CrossRef]
- Jeannin, M.; Artioli, A.; Rueda-Fonseca, P.; Bellet-Amalric, E.; Kheng, K.; André, R.; Tatarenko, S.; Cibert, J.; Ferrand, D.; Nogues, G. Light-Hole Exciton in a Nanowire Quantum Dot. Phys. Rev. B 2017, 95, 035305. [Google Scholar] [CrossRef] [Green Version]
- Huang, Y.; Duan, X.; Lieber, C.M. Nanowires for Integrated Multicolor Nanophotonics. Small 2005, 1, 142–147. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.; Needs, R.J.; Heine, V. Inter-Layer Interactions and the Origin of SiC Polytypes. J. Phys. C Solid State Phys. 1988, 21, 1049–1063. [Google Scholar] [CrossRef]
- Mardix, S. Polytypism: A Controlled Thermodynamic Phenomenon. Phys. Rev. B 1986, 33, 8677–8684. [Google Scholar] [CrossRef] [Green Version]
- Engel, G.E.; Needs, R.J. Total Energy Calculations on Zinc Sulphide Polytypes. J. Phys. Condens. Matter 1990, 2, 367–376. [Google Scholar] [CrossRef]
- Glas, F. A Simple Calculation of Energy Changes upon Stacking Fault Formation or Local Crystalline Phase Transition in Semiconductors. J. Appl. Phys. 2008, 104, 093520. [Google Scholar] [CrossRef]
- Kumar, S.; Fossard, F.; Amiri, G.; Chauveau, J.-M.; Sallet, V. Induced Structural Modifications in ZnS Nanowires via Physical State of Catalyst: Highlights of 15R Crystal Phase. Nano Res. 2021, 15, 377–385. [Google Scholar] [CrossRef]
- Akizuki, M. Investigation of phase transition of natural ZnS minerals by high resolution electron microscopy. Amer. Mineral. 1981, 66, 1006–1012. [Google Scholar]
- Borchers, C.; Stichtenoth, D.; Müller, S.; Schwen, D.; Ronning, C. Catalyst–Nanostructure Interaction and Growth of ZnS Nanobelts. Nanotechnology 2006, 17, 1067–1071. [Google Scholar] [CrossRef]
- Hao, Y.; Meng, G.; Wang, Z.L.; Ye, C.; Zhang, L. Periodically Twinned Nanowires and Polytypic Nanobelts of ZnS: The Role of Mass Diffusion in Vapor−Liquid−Solid Growth. Nano Lett. 2006, 6, 1650–1655. [Google Scholar] [CrossRef]
- Khanlary, M.R.; Alijarahi, S.; Reyhani, A. Growth Temperature Dependence of VLS-Grown Ultra-Long ZnS Nanowires Prepared by CVD Method. J. Theor. Appl. Phys. 2018, 12, 121–126. [Google Scholar] [CrossRef] [Green Version]
- Jiang, Y.; Meng, X.-M.; Liu, J.; Hong, Z.-R.; Lee, C.-S.; Lee, S.-T. ZnS Nanowires with Wurtzite Polytype Modulated Structure. Adv. Mater. 2003, 15, 1195–1198. [Google Scholar] [CrossRef]
- Liang, Y.; Liang, H.; Xiao, X.; Hark, S. The Epitaxial Growth of ZnS Nanowire Arrays and Their Applications in UV-Light Detection. J. Mater. Chem. 2012, 22, 1199–1205. [Google Scholar] [CrossRef]
- Liu, Y.-H.; Chen, H.-Y.; Fan, H.-F.; Chen, Y.-H.; Wang, F. Unique Growth Pathway in Solution–Solid–Solid Nanowires: Cubic to Hexagonal Phase Transformation. ACS Omega 2020, 5, 18441–18448. [Google Scholar] [CrossRef] [PubMed]
- Rothman, A.; Forsht, T.; Danieli, Y.; Popovitz-Biro, R.; Rechav, K.; Houben, L.; Joselevich, E. Guided Growth of Horizontal ZnS Nanowires on Flat and Faceted Sapphire Surfaces. J. Phys. Chem. C 2018, 122, 12413–12420. [Google Scholar] [CrossRef]
- Maliakkal, C.B.; Tornberg, M.; Jacobsson, D.; Lehmann, S.; Dick, K.A. Vapor–Solid–Solid Growth Dynamics in GaAs Nanowires. Nanoscale Adv. 2021, 3, 5928–5940. [Google Scholar] [CrossRef]
- Jaffal, A.; Regreny, P.; Patriarche, G.; Chauvin, N.; Gendry, M. Density-Controlled Growth of Vertical InP Nanowires on Si(111) Substrates. Nanotechnology 2020, 31, 354003. [Google Scholar] [CrossRef]
- Orrù, M.; Robin, E.; Den Hertog, M.; Moratis, K.; Genuist, Y.; André, R.; Ferrand, D.; Cibert, J.; Bellet-Amalric, E. Nanowire Growth and Sublimation: CdTe Quantum Dots in ZnTe Nanowires. Phys. Rev. Mater. 2018, 2, 043404. [Google Scholar] [CrossRef] [Green Version]
- Liang, Y.; Xu, H.; Hark, S.K. Orientation and Structure Controllable Epitaxial Growth of ZnS Nanowire Arrays on GaAs Substrates. J. Phys. Chem. C 2010, 114, 8343–8347. [Google Scholar] [CrossRef]
- Wang, X.; Xie, Z.; Huang, H.; Liu, Z.; Chen, D.; Shen, G. Gas Sensors, Thermistor and Photodetector Based on ZnS Nanowires. J. Mater. Chem. 2012, 22, 6845–6850. [Google Scholar] [CrossRef]
- Fang, X.; Bando, Y.; Liao, M.; Gautam, U.K.; Zhi, C.; Dierre, B.; Liu, B.; Zhai, T.; Sekiguchi, T.; Koide, Y.; et al. Single-Crystalline ZnS Nanobelts as Ultraviolet-Light Sensors. Adv. Mater. 2009, 21, 2034–2039. [Google Scholar] [CrossRef]
- Thiandoume, C.; Sallet, V.; Triboulet, R.; Gorochov, O. Decomposition Kinetics of Tertiarybutanol and Diethylzinc Used as Precursor Sources for the Growth of ZnO. J. Cryst. Growth 2009, 311, 1411–1415. [Google Scholar] [CrossRef]
- Dumont, H.; Marbeuf, A.; Bourée, J.E.; Gorochov, O. Mass-Spectrometric Study of Thermal Decomposition of Diethylzinc and Diethyltellurium. J. Mater. Chem. 1992, 2, 923–930. [Google Scholar] [CrossRef]
- Stringfellow, G.B. Organometallic Vapor-Phase Epitaxy: Theory and Practice, 2nd ed.; Academic Press: London, UK, 1999. [Google Scholar]
- Granqvist, C.G.; Buhrman, R.A. Size distributions for supported metal catalysts: Coalescence growth versus ostwald ripening. J. Catal. 1976, 42, 477–479. [Google Scholar] [CrossRef]
- Xu, H.; Guo, Y.; Sun, W.; Liao, Z.; Burgess, T.; Lu, H.; Gao, Q.; Tan, H.H.; Jagadish, C.; Zou, J. Quantitative Study of GaAs Nanowires Catalyzed by Au Film of Different Thicknesses. Nanoscale Res. Lett. 2012, 7, 589. [Google Scholar] [CrossRef] [Green Version]
- Gomes, U.P.; Ercolani, D.; Zannier, V.; Beltram, F.; Sorba, L. Controlling the Diameter Distribution and Density of InAs Nanowires Grown by Au-Assisted Methods. Semicond. Sci. Technol. 2015, 30, 115012. [Google Scholar] [CrossRef]
- Harmand, J.C.; Patriarche, G.; Péré-Laperne, N.; Mérat-Combes, M.-N.; Travers, L.; Glas, F. Analysis of Vapor-Liquid-Solid Mechanism in Au-Assisted GaAs Nanowire Growth. Appl. Phys. Lett. 2005, 87, 203101. [Google Scholar] [CrossRef]
- Han, N.; Wang, F.; Hou, J.J.; Yip, S.; Lin, H.; Fang, M.; Xiu, F.; Shi, X.; Hung, T.; Ho, J.C. Manipulated Growth of GaAs Nanowires: Controllable Crystal Quality and Growth Orientations via a Supersaturation-Controlled Engineering Process. Cryst. Growth Des. 2012, 12, 6243–6249. [Google Scholar] [CrossRef]
- Dayeh, S.A.; Yu, E.T.; Wang, D. III−V Nanowire Growth Mechanism: V/III Ratio and Temperature Effects. Nano Lett. 2007, 7, 2486–2490. [Google Scholar] [CrossRef]
- Rueda-Fonseca, P.; Bellet-Amalric, E.; Vigliaturo, R.; den Hertog, M.; Genuist, Y.; André, R.; Robin, E.; Artioli, A.; Stepanov, P.; Ferrand, D.; et al. Structure and Morphology in Diffusion-Driven Growth of Nanowires: The Case of ZnTe. Nano Lett. 2014, 14, 1877–1883. [Google Scholar] [CrossRef]
- Sallet, V.; Sartel, C.; Vilar, C.; Lusson, A.; Galtier, P. Opposite Crystal Polarities Observed in Spontaneous and Vapour-Liquid-Solid Grown ZnO Nanowires. Appl. Phys. Lett. 2013, 102, 182103. [Google Scholar] [CrossRef]
- Bellet-Amalric, E.; Elouneg-Jamroz, M.; Bougerol, C.; Den Hertog, M.; Genuist, Y.; Bounouar, S.; Poizat, J.P.; Kheng, K.; André, R.; Tatarenko, S. Epitaxial Growth of ZnSe and ZnSe/CdSe Nanowires on ZnSe. Phys. Status Solidi C 2010, 7, 1526–1529. [Google Scholar] [CrossRef]
- Zannier, V.; Grillo, V.; Martelli, F.; Plaisier, J.R.; Lausi, A.; Rubini, S. Tuning the Growth Mode of Nanowires via the Interaction among Seeds, Substrates and Beam Fluxes. Nanoscale 2014, 6, 8392–8399. [Google Scholar] [CrossRef]
- Takeuchi, S.; Suzuki, K.; Maeda, K.; Iwanaga, H. Stacking-fault energy of II–VI compounds. Philos. Mag. A 1985, 50, 171–178. [Google Scholar] [CrossRef]
- Glas, F.; Harmand, J.-C.; Patriarche, G. Why Does Wurtzite Form in Nanowires of III-V Zinc Blende Semiconductors? Phys. Rev. Lett. 2007, 99, 146101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johansson, J.; Zanolli, Z.; Dick, K.A. Polytype attainability in III–Vsemiconductor nanowires. Cryst. Growth Des. 2016, 16, 371–379. [Google Scholar] [CrossRef]
- Panciera, F.; Baraissov, Z.; Patriarche, G.; Dubrovskii, V.G.; Glas, F.; Travers, L.; Mirsaidov, U.; Harmand, J.-C. Phase Selection in Self-Catalyzed GaAs Nanowires. Nano Lett. 2020, 20, 1669–1675. [Google Scholar] [CrossRef]
- Algra, R.E.; Verheijen, M.A.; Borgström, M.T.; Feiner, L.-F.; Immink, G.; van Enckevort, W.J.P.; Vlieg, E.; Bakkers, E.P.A.M. Twinning Superlattices in Indium Phosphide Nanowires. Nature 2008, 456, 369–372. [Google Scholar] [CrossRef] [Green Version]
- Caroff, P.; Dick, K.A.; Johansson, J.; Messing, M.E.; Deppert, K.; Samuelson, L. Controlled Polytypic and Twin-Plane Superlattices in III–V Nanowires. Nat. Nanotechnol. 2009, 4, 50–55. [Google Scholar] [CrossRef]
- Burgess, T.; Breuer, S.; Caroff, P.; Wong-Leung, J.; Gao, Q.; Hoe Tan, H.; Jagadish, C. Twinning Superlattice Formation in GaAs Nanowires. ACS Nano 2013, 7, 8105–8114. [Google Scholar] [CrossRef]
- Wang, Y.Q.; Philipose, U.; Xu, T.; Ruda, H.E.; Kavanagh, K.L. Twinning Modulation in ZnSe Nanowires. Semicond. Sci. Technol. 2007, 22, 175–178. [Google Scholar] [CrossRef]
- Wang, D.-H.; Xu, D.; Wang, Q.; Hao, Y.-J.; Jin, G.-Q.; Guo, X.-Y.; Tu, K.N. Periodically Twinned SiC Nanowires. Nanotechnology 2008, 19, 215602. [Google Scholar] [CrossRef] [PubMed]
- Goktas, N.I.; Sokolovskii, A.; Dubrovskii, V.G.; LaPierre, R.R. Formation Mechanism of Twinning Superlattices in Doped GaAs Nanowires. Nano Lett. 2020, 20, 3344–3351. [Google Scholar] [CrossRef] [PubMed]



| Study | Ref. of Sample | Tg (°C) | PDEZn (Pa) | PDTBS (Pa) | VI/II Ratio | Substrate | |
|---|---|---|---|---|---|---|---|
| Thin films | TF1 | 320 | 15.6 | 2 | |||
| TF2 | 360 | 15.6 | 2 | ||||
| TF3 | 390 | 15.6 | 2 | ||||
| TF4 | 420 | 15.6 | 2 | ||||
| Temperature | TF5 | 450 | 15.6 | 2 | |||
| TF6 | 480 | 15.6 | 2 | ||||
| TF7 | 510 | 7.8 | 15.6 | 2 | GaAs (100) | ||
| TF8 | 540 | 15.6 | 2 | ||||
| _____ | TF9 | 570 | 15.6 | 2 | |||
| TF10 | 450 | 5.1 | 0.6 | ||||
| TF11 | 450 | 7.8 | 1 | ||||
| VI/II ratio | TF11 | 450 | 11.7 | 1.5 | |||
| TF13 | 450 | 15.6 | 2 | ||||
| TF14 | 450 | 25 | 3.2 | ||||
| Nanowires | NW1 | 450 | 15.6 | 2 | |||
| NW2 | 500 | 15.6 | 2 | ||||
| Temperature | NW3 | 525 | 15.6 | 2 | |||
| NW4 | 550 | 15.6 | 2 | ||||
| _____ | NW5 | 575 | 7.8 | 15.6 | 2 | GaAs (111)B | |
| NW6 | 550 | 5.1 | 0.6 | ||||
| VI/II ratio | NW7 | 550 | 7.8 | 1 | |||
| NW8 | 550 | 11.7 | 1.5 | ||||
| _____ | NW9 | 550 | 25 | 3.2 | |||
| Substrate | NW10 | 550 | 15.6 | 2 | GaAs (100) | ||
| and ZnS buffer | |||||||
| Substrate | NW11 | 550 | 15.6 | 2 | GaAs (111)A | ||
| and ZnS buffer | |||||||
| Substrate | NW12 | 550 | 15.6 | 2 | GaP (111) | ||
| and ZnS buffer | |||||||
| High Vg | NW13 | 550 | 12 | 24 | 2 | GaAs (111)B | |
| Low Vg | NW14 | 550 | 2 | 4 | 2 | GaAs (111)B | |
| Ga feeding nanowires | TMGa | NW15 | 550 | 2 | 4 | 2 | ZnS buffer |
| 4.5 Pa |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumar, S.; Fossard, F.; Amiri, G.; Chauveau, J.-M.; Sallet, V. MOCVD Growth and Structural Properties of ZnS Nanowires: A Case Study of Polytypism. Nanomaterials 2022, 12, 2323. https://doi.org/10.3390/nano12142323
Kumar S, Fossard F, Amiri G, Chauveau J-M, Sallet V. MOCVD Growth and Structural Properties of ZnS Nanowires: A Case Study of Polytypism. Nanomaterials. 2022; 12(14):2323. https://doi.org/10.3390/nano12142323
Chicago/Turabian StyleKumar, Sumit, Frédéric Fossard, Gaelle Amiri, Jean-Michel Chauveau, and Vincent Sallet. 2022. "MOCVD Growth and Structural Properties of ZnS Nanowires: A Case Study of Polytypism" Nanomaterials 12, no. 14: 2323. https://doi.org/10.3390/nano12142323
APA StyleKumar, S., Fossard, F., Amiri, G., Chauveau, J.-M., & Sallet, V. (2022). MOCVD Growth and Structural Properties of ZnS Nanowires: A Case Study of Polytypism. Nanomaterials, 12(14), 2323. https://doi.org/10.3390/nano12142323






