Cu Nanoparticles Modified Step-Scheme Cu2O/WO3 Heterojunction Nanoflakes for Visible-Light-Driven Conversion of CO2 to CH4
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials Synthesis
2.2. Characterization
2.3. Photocatalytic Performance Tests
3. Results and Discussion
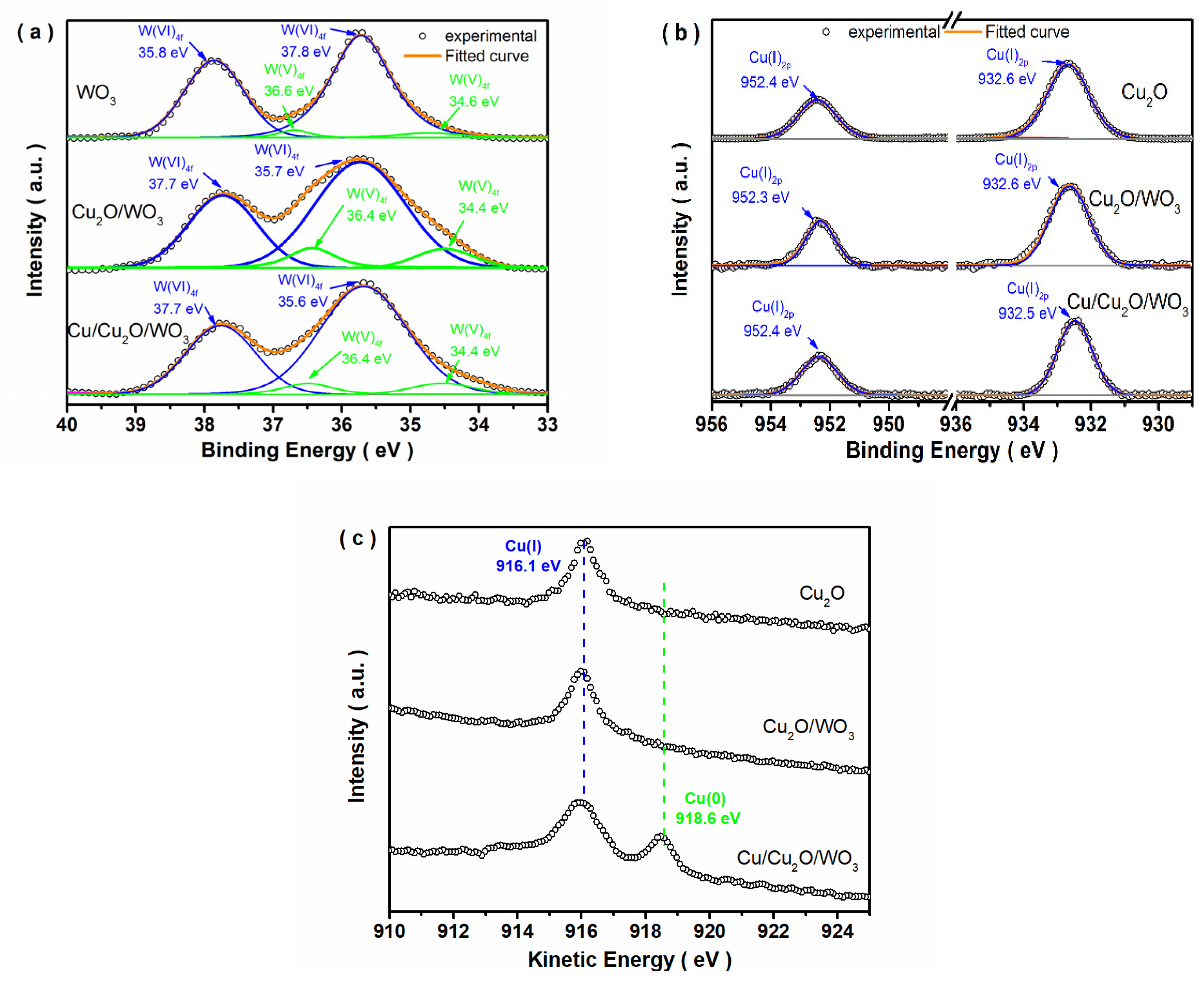
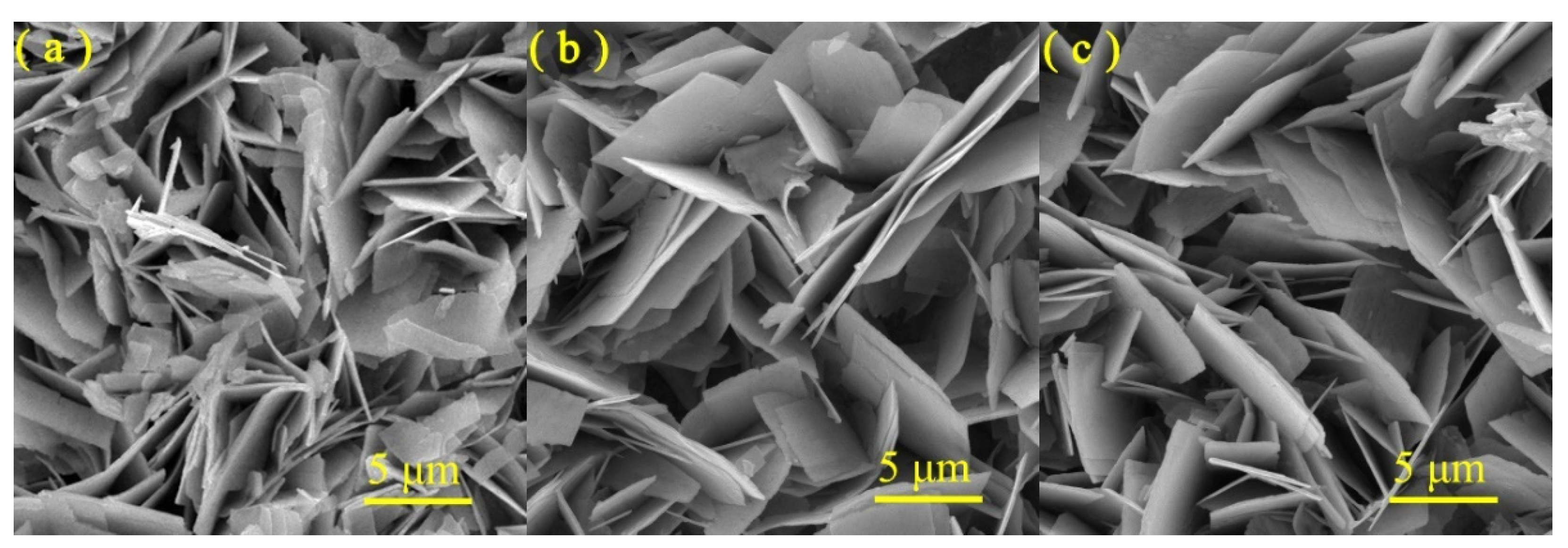
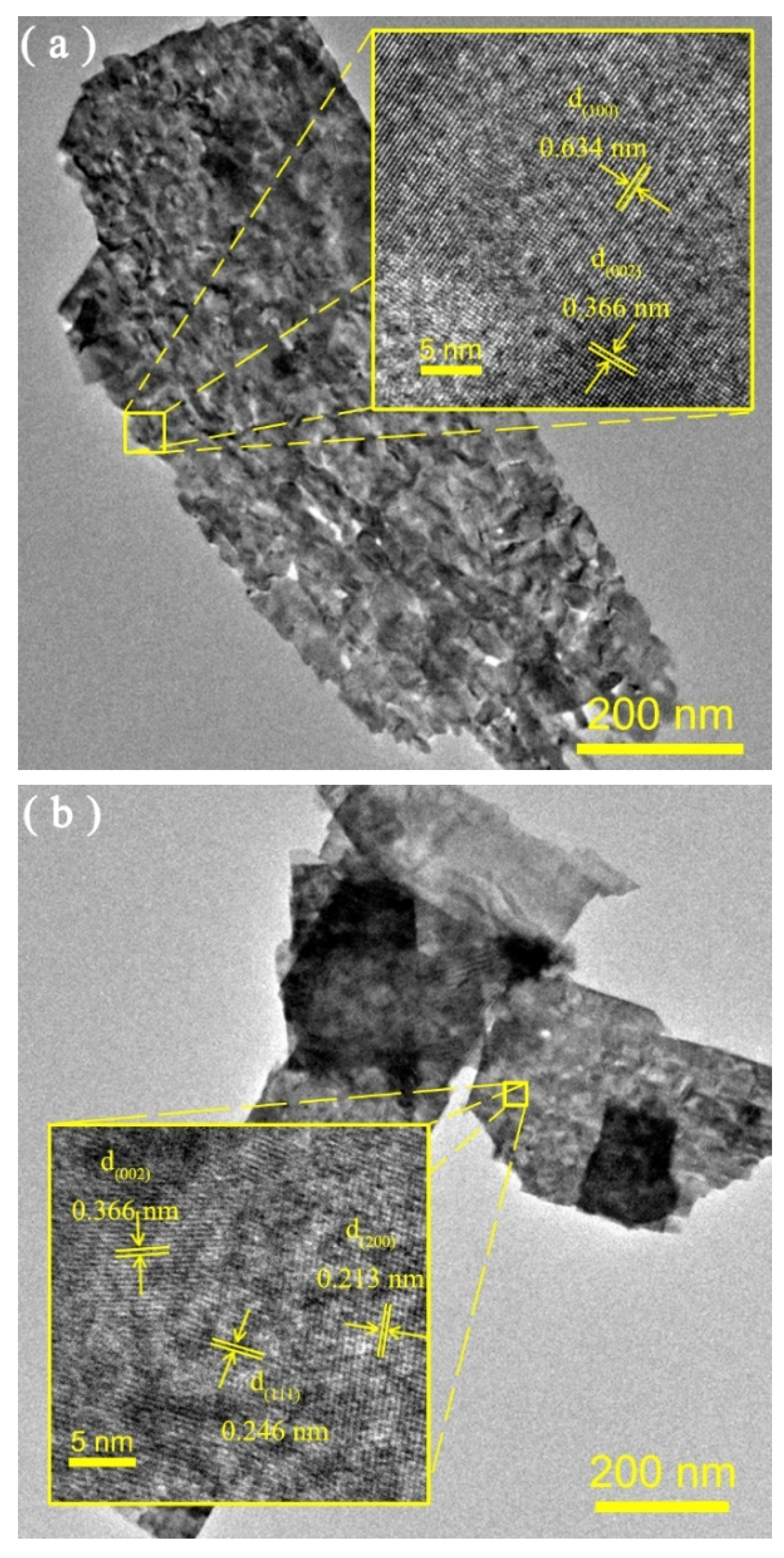
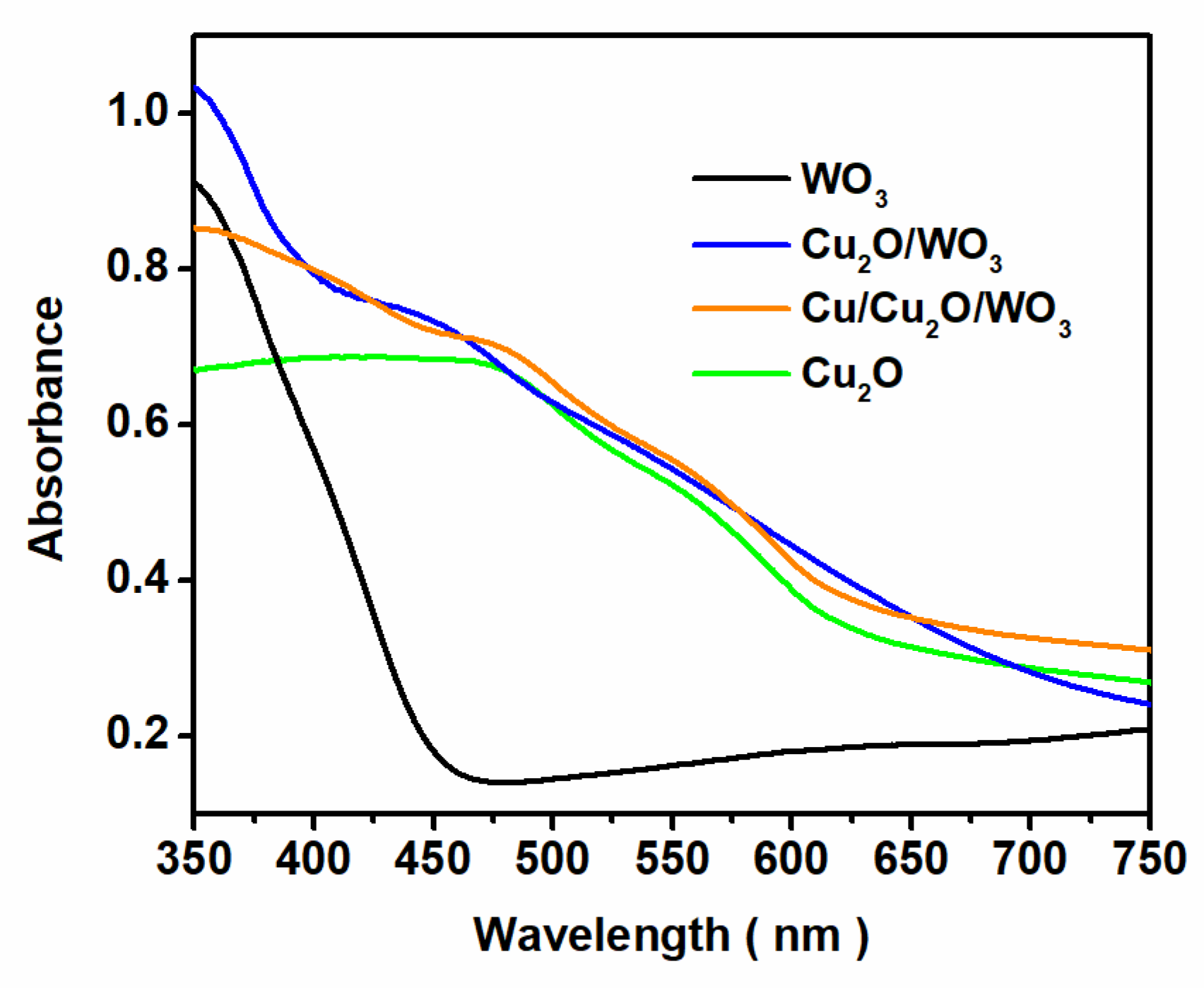
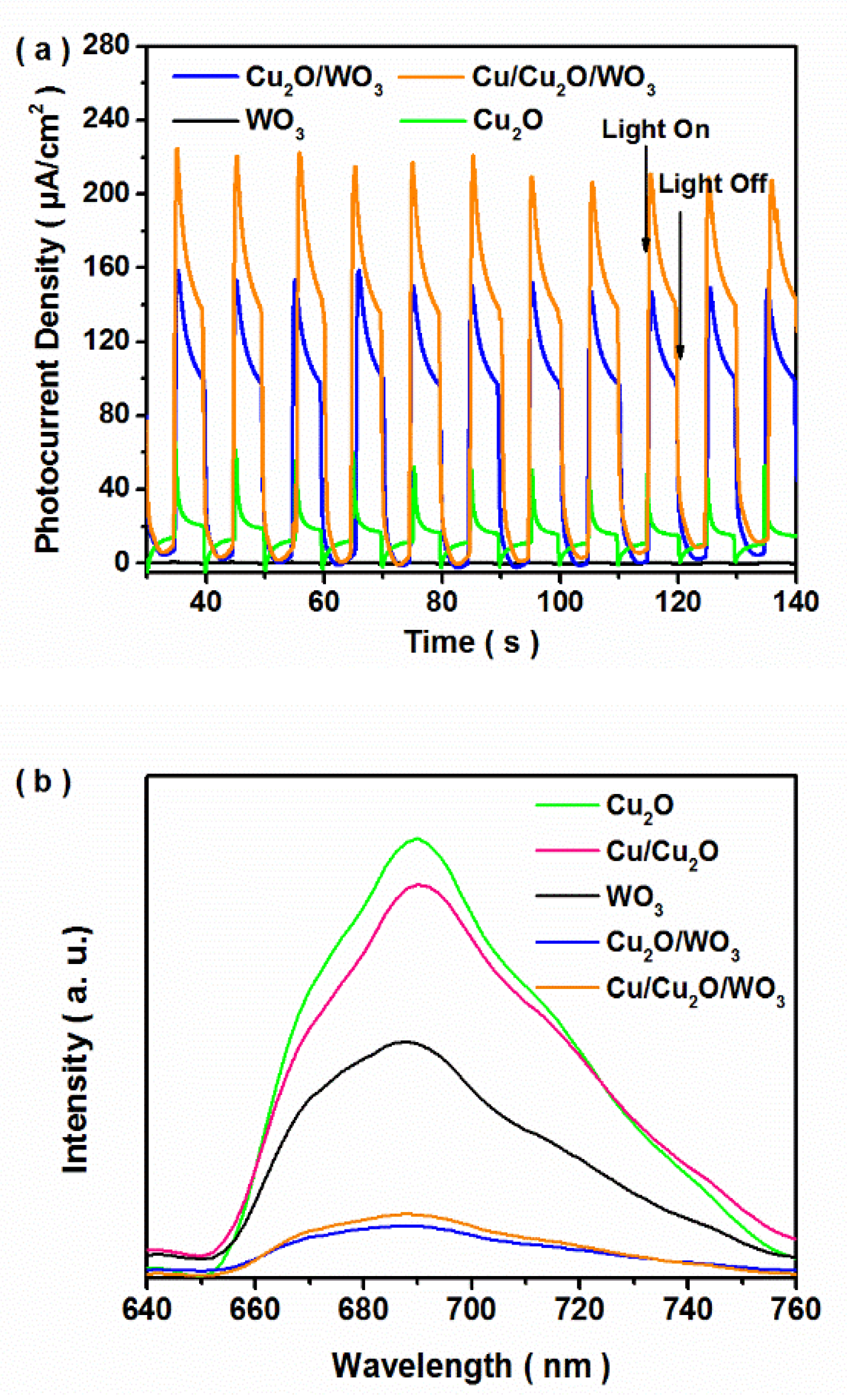
3.1. Structure, Composition and Morphology
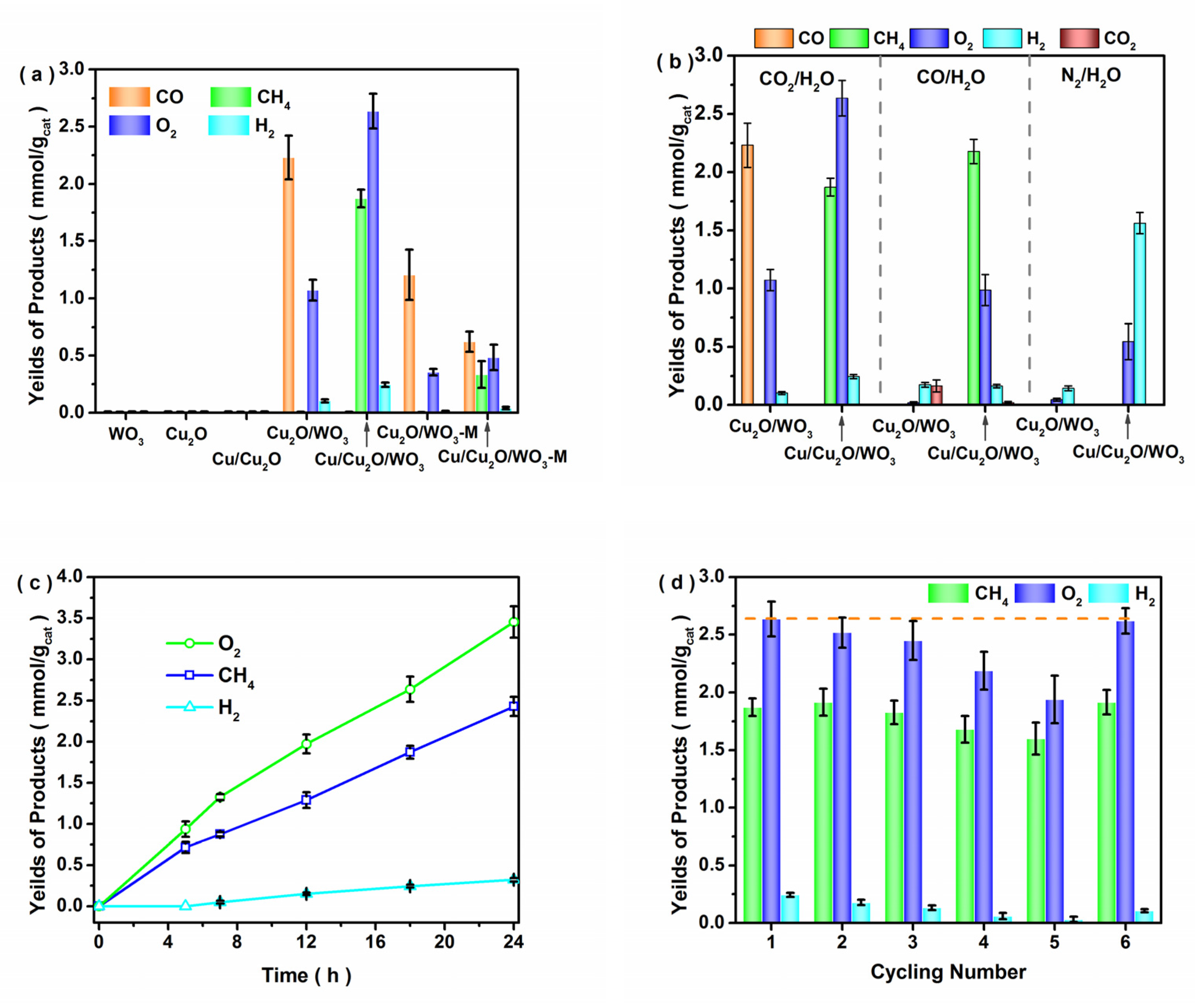
3.2. Photocatalytic Performance of CO2 Reduction
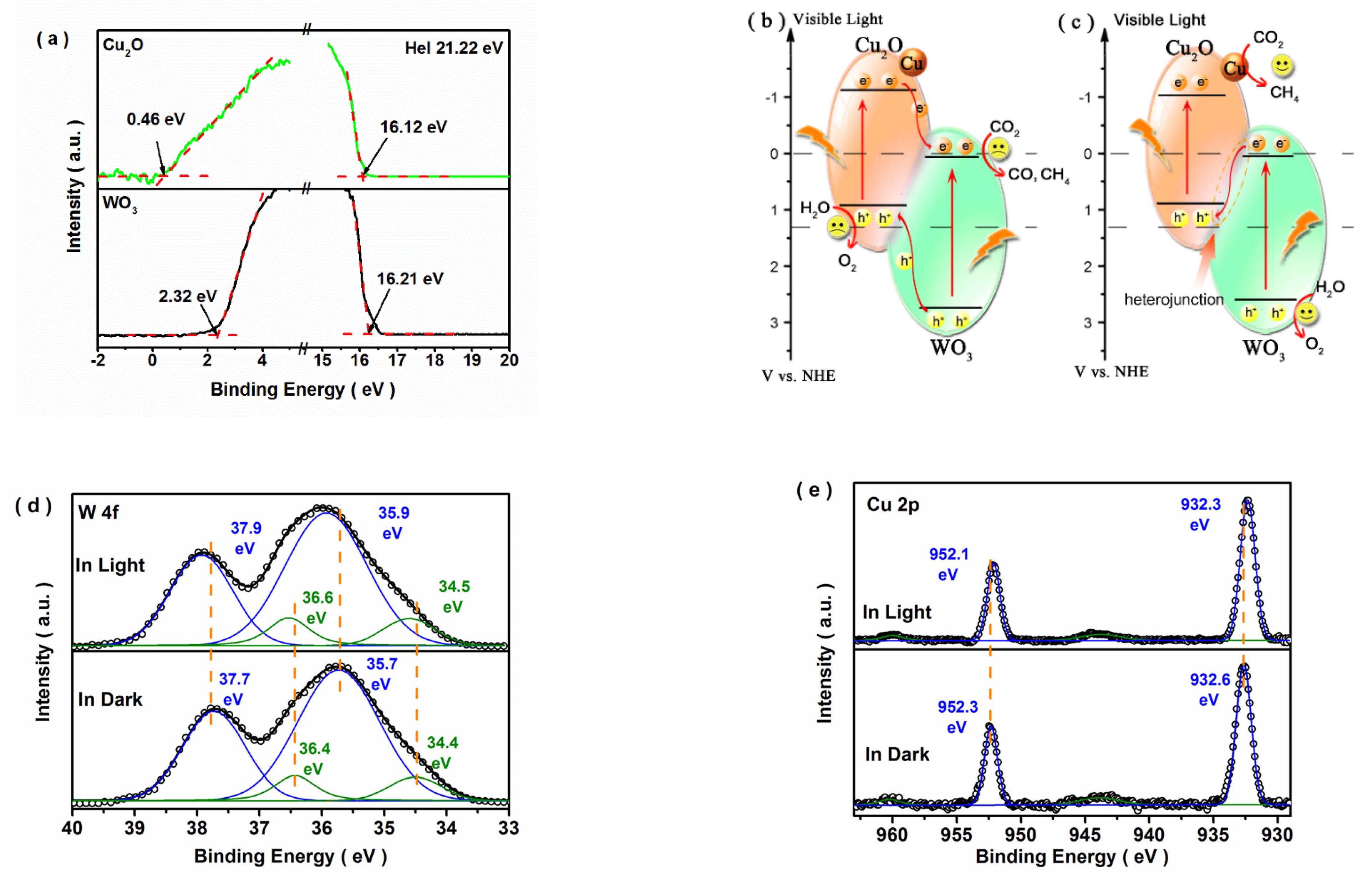
3.3. Possible Photocatalytic Mechanism
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wang, K.; Wang, T.; Islam, Q.A.; Wu, Y. Layered double hydroxide photocatalysts for solar fuel production. Chin. J. Catal. 2021, 42, 1944–1975. [Google Scholar] [CrossRef]
- Wang, H.N.; Zou, Y.H.; Sun, H.X.; Chen, Y.; Li, S.L.; Lan, Y.Q. Recent progress and perspectives in heterogeneous photocatalytic CO2 reduction through a solid-gas mode. Coord. Chem. Rev. 2021, 438, 213906. [Google Scholar] [CrossRef]
- Wang, B.; Liu, J.W.; Yao, S.; Liu, F.Y.; Li, Y.K.; He, J.Q.; Lin, Z.; Huang, F.; Liu, C.; Wang, M.Y. Vacancy engineering in nanostructured semiconductors for enhancing photocatalysis. J. Mater. Chem. A 2021, 9, 17143–17172. [Google Scholar] [CrossRef]
- Navarro-Jaen, S.; Virginie, M.; Bonin, J.; Robert, M.; Wojcieszak, R.; Khodakov, A.Y. Highlights and challenges in the selective reduction of carbon dioxide to methanol. Nat. Rev. Chem. 2021, 5, 564–579. [Google Scholar] [CrossRef]
- Inoue, T.; Fujishima, A.; Konishi, S.; Honda, K. Photoelectrocatalytic reduction of carbon dioxide in aqueous suspensions of semiconductor powers. Nature 1979, 277, 637–638. [Google Scholar] [CrossRef]
- Lu, Q.Q.; Eid, K.; Li, W.P.; Li, W.; Abdullah, A.M.; Xu, G.B.; Varma, R.S. Engineering graphitic carbon nitride (g-C3N4) for catalytic reduction of CO2 to fuels and chemicals: Strategy and mechanism. Green Chem. 2021, 23, 5394–5428. [Google Scholar] [CrossRef]
- Hezam, A.; Namratha, K.; Drmosh, Q.A.; Ponnamma, D.; Wang, J.; Prasad, S.; Ahamed, M.; Cheng, C.; Byrappa, K. CeO2 nanostructures enriched with oxygen vacancies for photocatalytic CO2 reduction. ACS Appl. Energy Mater. 2020, 3, 138–148. [Google Scholar] [CrossRef] [Green Version]
- Zhang, M.; Cheng, G.; Wei, Y.; Wen, Z.; Chen, R.; Xiong, J.; Li, W.; Han, C.; Li, Z. Cuprous ion (Cu+) doping induced surface/interface engineering for enhancing the CO2 photoreduction capability of W18O49 nanowires. J. Colloid Interface Sci. 2020, 572, 306–317. [Google Scholar] [CrossRef]
- Liu, Y.; Shen, D.; Zhang, Q.; Lin, Y.; Peng, F. Enhanced photocatalytic CO2 reduction in H2O vapor by atomically thin Bi2WO6 nanosheets with hydrophobic and nonpolar surface. Appl. Catal. B 2021, 283, 119630. [Google Scholar] [CrossRef]
- Chen, D.; Zhang, X.; Lee, A.F. Synthetic strategies to nanostructured photocatalysts for CO2 reduction to solar fuels and chemicals. J. Mater. Chem. A 2015, 3, 14487–14516. [Google Scholar] [CrossRef] [Green Version]
- Zhao, G.; Huang, X.; Wang, X.; Wang, X. Progress in catalyst exploration for heterogeneous CO2 reduction and utilization: A critical review. J. Mater. Chem. A 2017, 5, 21625–21649. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, L.; Wang, K.; Sun, X.; Wang, W. Enhanced photocatalytic CO2 reduction to methane over WO3·0.33H2O via Mo doping. Appl. Catal. B 2019, 243, 771–779. [Google Scholar] [CrossRef]
- Xie, Y.P.; Liu, G.; Yin, L.; Cheng, H.M. Crystal facet-dependent photocatalytic oxidation and reduction reactivity of monoclinic WO3 for solar energy conversion. J. Mater. Chem. 2012, 22, 6746–6751. [Google Scholar] [CrossRef]
- Tian, F.H.; Zhao, L.; Xue, X.-Y.; Shen, Y.; Jia, X.; Chen, S.; Wang, Z. DFT study of CO sensing mechanism on hexagonal WO3 (0 0 1) surface: The role of oxygen vacancy. Appl. Surf. Sci. 2014, 311, 362–368. [Google Scholar] [CrossRef]
- Li, N.; Zhao, Y.; Wang, Y.; Lu, Y.; Song, Y.; Huang, Z.; Li, Y.; Zhao, J. Aqueous synthesis and visible-light photochromism of metastableh-WO3 hierarchical nanostructures. Eur. J. Inorg. Chem. 2015, 17, 2804–2812. [Google Scholar] [CrossRef]
- Sun, W.; Yeung, M.T.; Lech, A.T.; Lin, C.-W.; Lee, C.; Li, T.; Duan, X.; Zhou, J.; Kanar, R.B. High surface area tunnels in hexagonal WO3. Nano Lett. 2015, 15, 4834–4838. [Google Scholar] [CrossRef]
- Dong, P.; Hou, G.; Xi, X.; Shao, R.; Dong, F. WO3-based photocatalysts: Morphology control, activity enhancement and multifunctional applications. Environ. Sci. Nano 2017, 4, 539–557. [Google Scholar] [CrossRef]
- Yang, G.; Zhu, X.; Cheng, G.; Chen, R.; Xiong, J.; Li, W.; Li, Y. Engineered tungsten oxide-based photocatalysts for CO2 reduction: Categories and roles. J. Mater. Chem. A 2021, 9, 22781–22809. [Google Scholar] [CrossRef]
- Babu, V.J.; Vempati, S.; Uyar, T.; Ramakrishna, S. Review of one-dimensional and two-dimensional nanostructured materials for hydrogen generation. Phys. Chem. Chem. Phys. 2015, 17, 2960–2986. [Google Scholar] [CrossRef]
- Chen, W.; Hou, X.; Shi, X.; Pan, H. Two-dimensional Janus transition metal oxides and chalcogenides: Multifunctional properties for photocatalysts, electronics. ACS Appl. Mater. Interfaces 2018, 10, 35289–35295. [Google Scholar] [CrossRef]
- Sumesh, C.K.; Peter, S.C. Two-dimensional semiconductor transition metal based chalcogenide based heterostructures for water splitting applications. Dalton Trans. 2019, 48, 12772–12802. [Google Scholar] [CrossRef] [PubMed]
- Hao, S.; Zhao, X.; Cheng, Q.; Xing, Y.; Ma, W.; Wang, X.; Zhao, G.; Xu, X. A mini review of the preparation and photocatalytic properties of two-dimensional materials. Front. Chem. 2020, 8, 582146. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, H.; Liu, L.; Li, W.; Cao, P. Controlled morphologies and growth direction of WO3 nanostructures hydrothermally synthesized with citric acid. Mater. Lett. 2014, 130, 248–251. [Google Scholar] [CrossRef]
- Peng, T.; Ke, D.; Xiao, J.; Wang, L.; Hu, J.; Zan, L. Hexagonal phase WO3 nanorods: Hydrothermal preparation, formation mechanism and its photocatalytic O2 production under visible-light irradiation. J. Solid State Chem. 2012, 194, 250–256. [Google Scholar] [CrossRef]
- Zheng, G.; Wang, J.; Liu, H.; Murugadoss, V.; Zu, G.; Che, H.; Lai, C.; Li, H.; Ding, T.; Gao, Q.; et al. Tungsten oxide nanostructures and nanocomposites for photoelectrochemical water splitting. Nanoscale 2019, 11, 18968–18994. [Google Scholar] [CrossRef]
- Lin, N.; Lin, Y.; Qian, X.; Wang, X.; Su, W. Construction of a 2D/2D WO3/LaTiO2N Direct Z-scheme photocatalyst for enhanced CO2 reduction performance under visible light. ACS Sustain. Chem. Eng. 2021, 9, 13686–13694. [Google Scholar] [CrossRef]
- Moniz, S.J.A.; Shevlin, S.; Martin, D.; Guo, Z.X.; Tang, J.J. Visible-light driven heterojunction photocatalystsfor water splitting-a critical review. Energy Environ. Sci. 2015, 8, 731–759. [Google Scholar] [CrossRef]
- Xu, Q.; Zhang, L.; Cheng, B.; Fan, J.; Yu, J. S-scheme heterojunction photocatalyst. Chem 2020, 6, 1543–1559. [Google Scholar] [CrossRef]
- Wang, J.-C.; Zhang, L.; Fang, W.-X.; Ren, J.; Li, Y.-Y.; Yao, H.-C.; Wang, J.-S.; Li, Z.-J. Enhanced photoreduction CO2 activity over direct Z-scheme alpha-Fe2O3/Cu2O heterostructures under visible light irradiation. ACS Appl. Mater. Interfaces 2015, 16, 8631–8639. [Google Scholar] [CrossRef]
- Zhai, Q.; Xie, S.; Fan, W.; Zhang, Q.; Wang, Y.; Deng, W.; Wang, Y. Photocatalytic conversion of carbon dioxide with water into methane: Platinum and copper(I) oxide co-catalysts with a core-shell structure. Angew. Chem. Int. Ed. 2013, 52, 5776–5779. [Google Scholar] [CrossRef]
- Ali, H.; Guler, A.C.; Masar, M.; Urbanek, P.; Urbanek, M.; Skoda, D.; Suly, P.; Machovsky, M.; Galusek, D.; Kuritka, I. Solid-state synthesis of direct Z-scheme Cu2O/WO3 nanocomposites with enhanced visible-light photocatalytic performance. Catalysts 2021, 11, 293. [Google Scholar] [CrossRef]
- Hao, J.; Qi, B.; Wei, J.; Li, D.; Zeng, F. A Z-scheme Cu2O/WO3 heterojunction for production of renewable hydrocarbon fuel from carbon dioxide. Fuel 2021, 287, 119439. [Google Scholar] [CrossRef]
- Zhang, J.; Ma, H.P.; Liu, Z.F. Highly efficient photocatalyst based on all oxides WO3/Cu2O heterojunction for photoelectrochemical water splitting. Appl. Catal. B 2017, 201, 84–91. [Google Scholar] [CrossRef]
- Guo, J.; Wan, Y.; Zhu, Y.; Zhao, M.; Tang, Z. Advanced photocatalysts based on metal nanoparticle/metalorganic framework composites. Nano Res. 2021, 14, 2037–2052. [Google Scholar] [CrossRef]
- Cho, H.; Kim, W.D.; Yu, J.; Lee, S.; Lee, D.C. Molecule-driven shape control of metal co-catalysts for selective CO2 conversion photocatalysis. ChemCatChem 2018, 10, 5679–5688. [Google Scholar] [CrossRef]
- Qiu, G.; Wang, R.; Han, F.; Tao, X.; Xiao, Y.; Li, B. One-step synthesized Au-Bi2WO6 hybrid nanostructures: Synergistic effects of Au nanoparticles and oxygen vacancies for promoting selective oxidation under visible light. Ind. Eng. Chem. Res. 2019, 58, 17389–17398. [Google Scholar] [CrossRef]
- Li, R.; Luan, Q.; Dong, C.; Dong, W.; Tang, W.; Wang, G.; Lu, Y. Light-facilitated structure reconstruction on self-optimized photocatalyst TiO2@BiOCl for selectively efficient conversion of CO2 to CH4. Appl. Catal. B 2021, 286, 119832. [Google Scholar] [CrossRef]
- Wang, S.; Teramura, K.; Hisatomi, T.; Domen, K.; Asakura, H.; Hosokawa, S.; Tanaka, T. Highly selective photocatalytic conversion of carbon dioxide by water over Al-SrTiO3 photocatalyst modified with silver-metal dual cocatalysts. ACS Sustain. Chem. Eng. 2021, 9, 9327–9335. [Google Scholar] [CrossRef]
- Albo, J.; Qadir, M.I.; Samperi, M.; Fernandes, J.A.; Pedro, I.D.; Dupont, J. Use of an optofluidic microreactor and Cu nanoparticles synthesized in ionic liquid and embedded in TiO2 for an efficient photoreduction of CO2 to methanol. Chem. Eng. J. 2021, 404, 126643. [Google Scholar] [CrossRef]
- Ojha, N.; Bajpai, A.; Kumar, S. Visible light-driven enhanced CO2 reduction by water over Cu modified S-doped g-C3N4. Catal. Sci. Technol. 2019, 9, 4598–4613. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, P.; Wang, T.; Gong, J. Monoclinic WO3 nanomultilayers with preferentially exposed (002) facets for photoelectrochemical water splitting. Nano Energy 2015, 11, 189–195. [Google Scholar] [CrossRef]
- Liu, J.; Yu, S.; Zhu, W.; Yan, X. Oxygen vacancy-enhanced visible light-driven photocatalytic activity of TiO2 sphere-W18O49 nanowire bundle heterojunction. Appl. Catal. A 2015, 500, 30–39. [Google Scholar] [CrossRef]
- Yue, C.; Zhu, X.; Rigutto, M.; Hensen, E. Acid catalytic properties of reduced tungsten and niobium-tungsten oxides. Appl. Catal. B 2015, 163, 370–381. [Google Scholar] [CrossRef]
- Lee, K.Y.; Hwang, H.; Shin, D.; Choi, W. Enhanced thermopower wave via nanowire bonding and grain boundary fusion in combustion of fuel/CuO-Cu2O-Cu hybrid composites. J. Mater. Chem. A 2015, 3, 5457–5466. [Google Scholar] [CrossRef]
- Mao, Y.; He, J.; Sun, X.; Li, W.; Lu, X.; Gan, J.; Liu, Z.; Gong, L.; Chen, J.; Liu, P.; et al. Electrochemical synthesis of hierarchical Cu2O stars with enhanced photoelectrochemical properties. Electrochim. Acta 2012, 62, 1–7. [Google Scholar] [CrossRef]
- Moretti, G.; Beck, H.P. On the Auger parameter of Cu(II) compounds. Surf. Interface Anal. 2022, 54, 803–812. [Google Scholar] [CrossRef]
- Liu, S.; Xia, J.; Yu, J. Amine-functionalized titanate nanosheet-assembled yolk@shell microspheres for efficient cocatalyst-free visible-light photocatalytic CO2 Reduction. ACS Appl. Mater. Interfaces 2015, 7, 8166–8175. [Google Scholar] [CrossRef]
- Liu, J.; Liu, Y.; Liu, N.; Han, Y.; Zhang, X.; Huang, H.; Lifshitz, Y.; Lee, S.-T.; Zhong, J.; Kang, Z. Metal-free efficient photocatalyst for stable visible water splitting via a two-electron pathway. Science 2015, 347, 970–974. [Google Scholar] [CrossRef]
- Pu, Y.C.; Lin, W.H.; Hsu, Y.-C. Modulation of charge carrier dynamics of NaxH2−xTi3O7-Au-Cu2O Z-scheme nanoheterostructures through size effect. Appl. Catal. B 2015, 163, 343–351. [Google Scholar] [CrossRef]
- Cui, D.; Hao, W.; Chen, J. The synergistic effect of heteroatom doping and vacancy on the reduction of CO2 by photocatalysts. ChemNanoMat 2021, 7, 894–901. [Google Scholar] [CrossRef]
- Oropeza, F.E.; Dzade, N.Y.; Pons-Marti, A.; Yang, Z.; Zhang, K.H.L.; de Leeuw, N.H.; Hensen, E.J.M.; Hofmann, J.P. Electronic structure and interface energetics of CuBi2O4 photoelectrodes. J. Phys. Chem. C 2020, 124, 22416–22425. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, W.; Wang, J.-C.; Chen, A.; Xu, X.; Wang, S.; Li, R.; Zhang, W.; Hou, Y. Cu Nanoparticles Modified Step-Scheme Cu2O/WO3 Heterojunction Nanoflakes for Visible-Light-Driven Conversion of CO2 to CH4. Nanomaterials 2022, 12, 2284. https://doi.org/10.3390/nano12132284
Shi W, Wang J-C, Chen A, Xu X, Wang S, Li R, Zhang W, Hou Y. Cu Nanoparticles Modified Step-Scheme Cu2O/WO3 Heterojunction Nanoflakes for Visible-Light-Driven Conversion of CO2 to CH4. Nanomaterials. 2022; 12(13):2284. https://doi.org/10.3390/nano12132284
Chicago/Turabian StyleShi, Weina, Ji-Chao Wang, Aimin Chen, Xin Xu, Shuai Wang, Renlong Li, Wanqing Zhang, and Yuxia Hou. 2022. "Cu Nanoparticles Modified Step-Scheme Cu2O/WO3 Heterojunction Nanoflakes for Visible-Light-Driven Conversion of CO2 to CH4" Nanomaterials 12, no. 13: 2284. https://doi.org/10.3390/nano12132284
APA StyleShi, W., Wang, J.-C., Chen, A., Xu, X., Wang, S., Li, R., Zhang, W., & Hou, Y. (2022). Cu Nanoparticles Modified Step-Scheme Cu2O/WO3 Heterojunction Nanoflakes for Visible-Light-Driven Conversion of CO2 to CH4. Nanomaterials, 12(13), 2284. https://doi.org/10.3390/nano12132284






