The Efficient and Convenient Extracting Uranium from Water by a Uranyl-Ion Affine Microgel Container
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Instrumentation
2.3. Preparation of Phosphate Functionalized VPA-Microgel
2.4. Preparation of Micro-Container
2.5. Static Adsorption of Micro-Container
2.5.1. Hydrogen Ion Concentration Optimization
2.5.2. Adsorption Kinetic
2.5.3. Equilibrium Adsorption
2.5.4. Reflectance Spectral Change Test before and after Adsorption
3. Results and Discussion
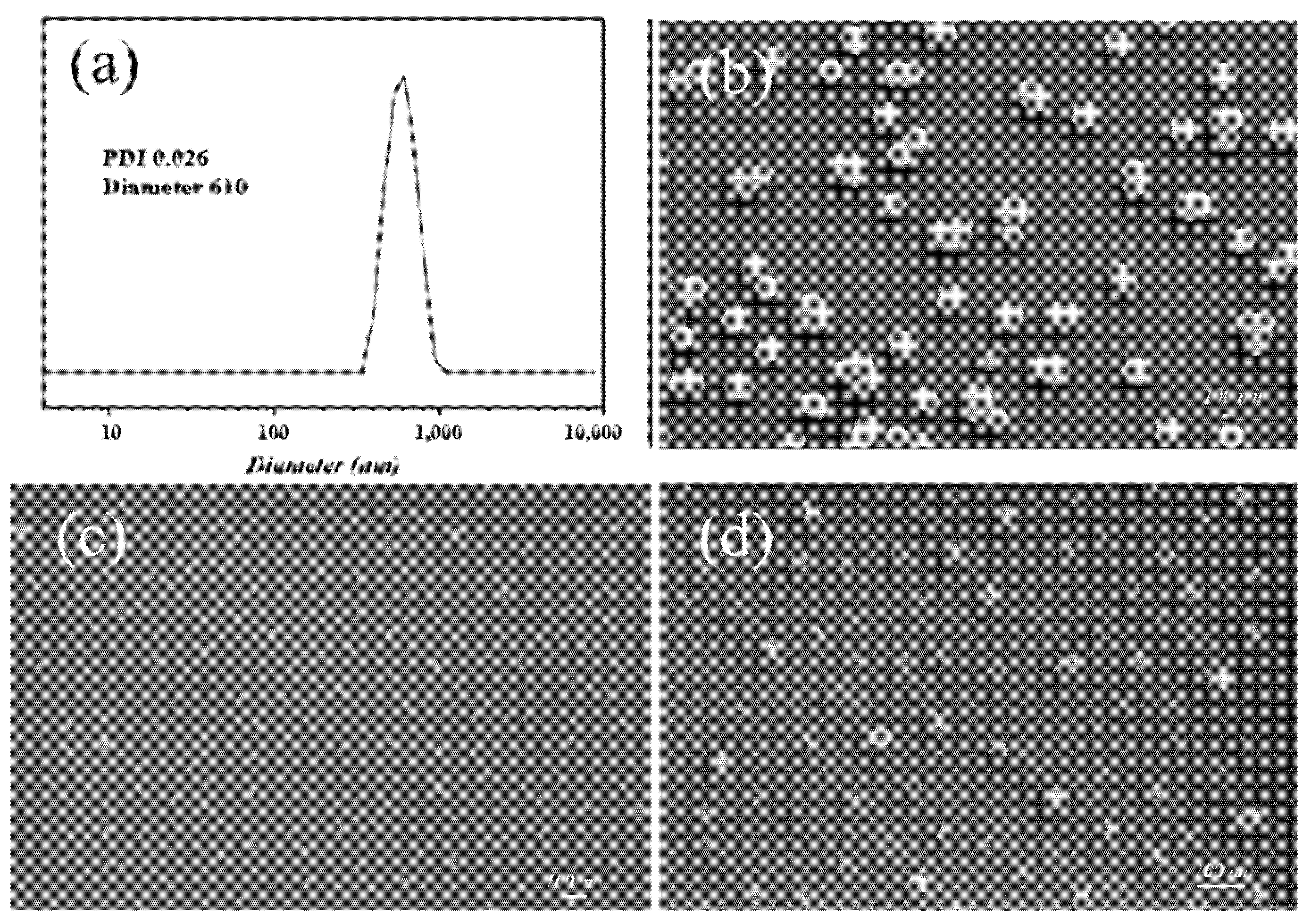
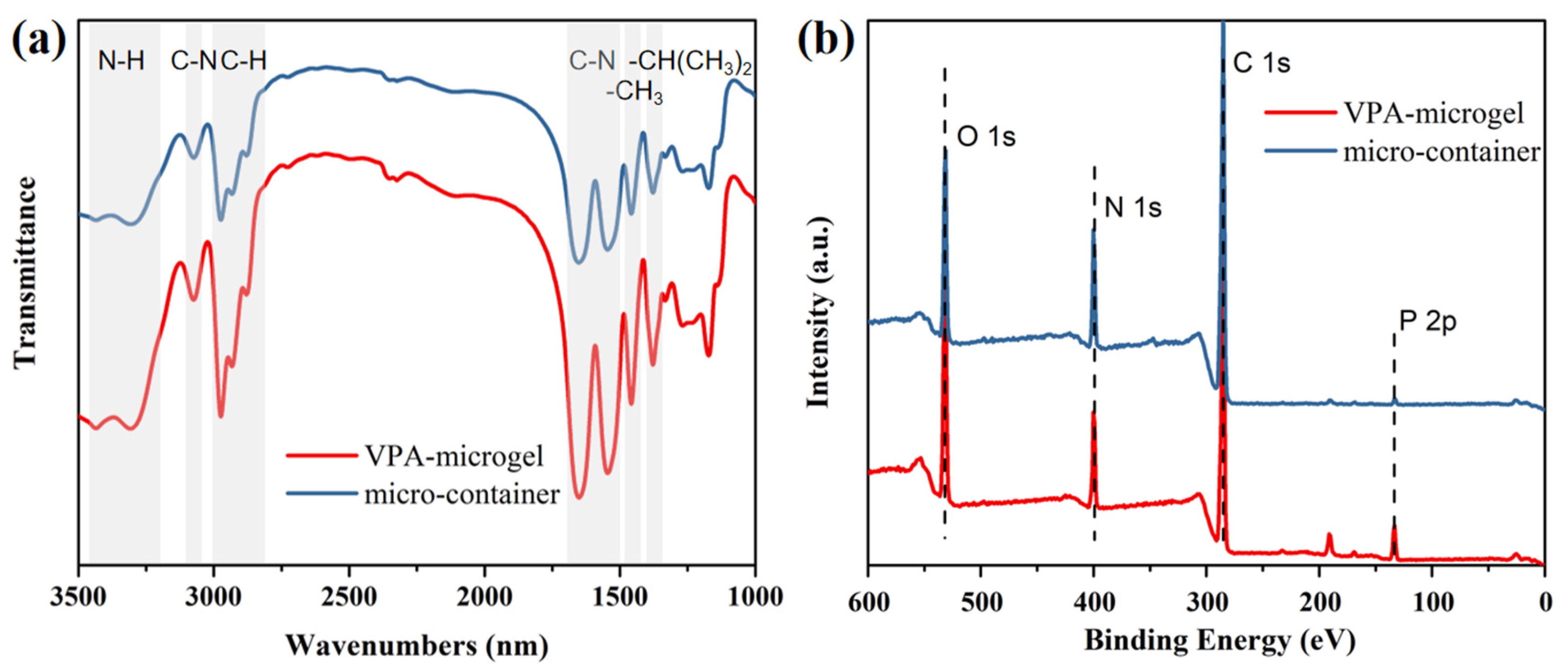
3.1. Morphology and Composition
3.2. Adsorption Experiment
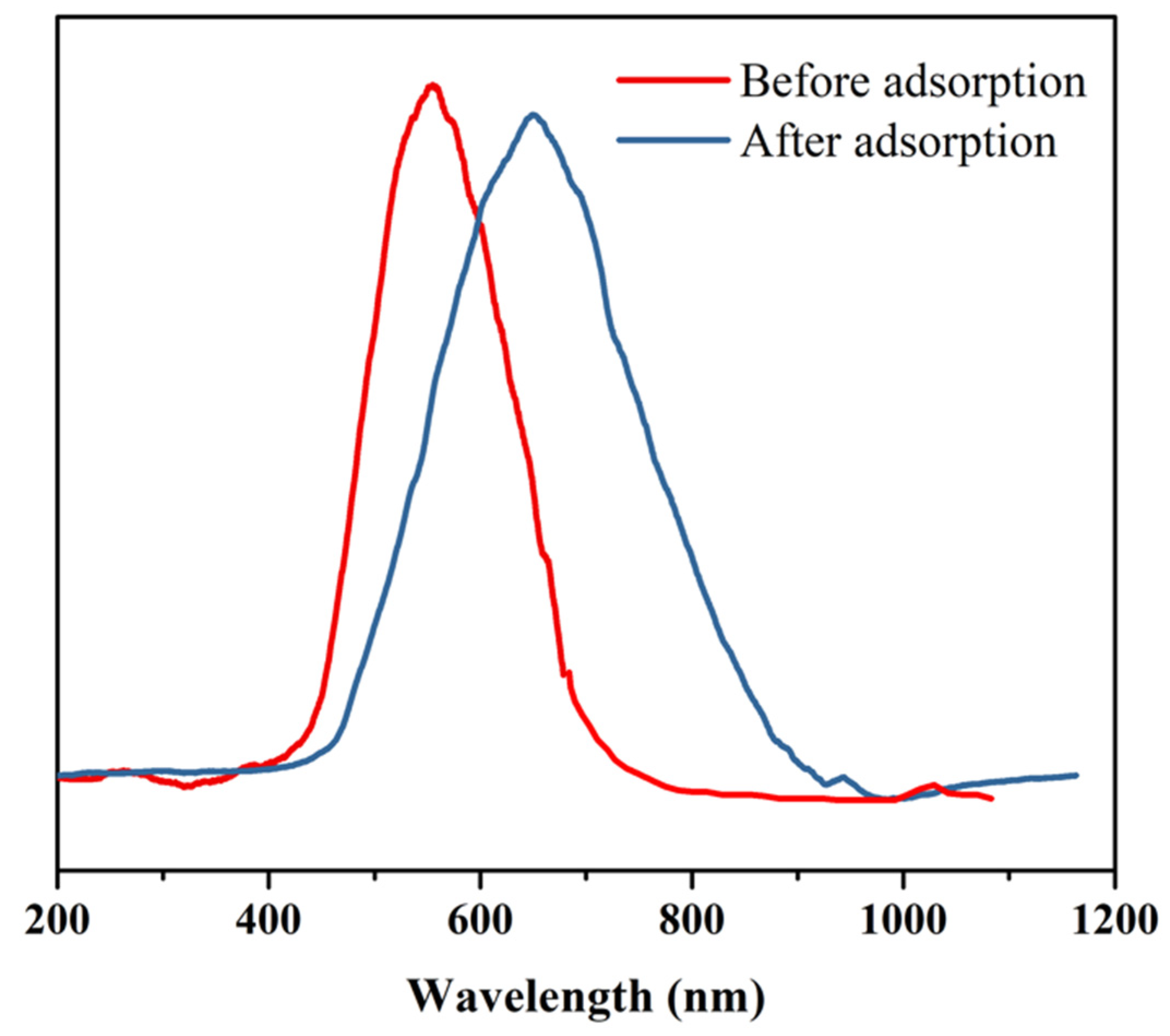
3.3. Reflection Spectra of Micro-Container
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sun, W.; Feng, L.; Zhang, J.; Lin, K.; Wang, H.; Yan, B.; Feng, T.; Cao, M.; Liu, T.; Yuan, Y.; et al. Amidoxime Group-Anchored Single Cobalt Atoms for Anti-Biofouling during Uranium Extraction from Seawater. Adv. Sci. 2022, 9, 2105008. [Google Scholar] [CrossRef] [PubMed]
- Bai, Z.; Liu, Q.; Zhang, H.; Yu, J.; Wang, J. Anti-Biofouling and Water—Stable Balanced Charged Metal Organic Framework-Based Polyelectrolyte Hydrogels for Extracting Uranium from Seawater. ACS Appl. Mater. Interfaces 2022, 12, 18012–18022. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.; Liu, J.; Xu, Y.; Ma, Y.; Liu, Z.; Pan, J. CO2 Pickering emulsion in water templated hollow porous sorbents for fast and highly selective uranium extraction. Chem. Eng. J. 2020, 387, 124096. [Google Scholar] [CrossRef]
- Bai, J.; Ma, X.; Gong, C.; Chen, Y.; Yan, H.; Wang, K.; Wang, J. A novel amidoxime functionalized porous resins for rapidly selective uranium uptake from solution. J. Mol. Liq. 2020, 320, 114443. [Google Scholar] [CrossRef]
- Guo, X.; Yang, H.; Liu, Q.; Liu, J.; Chen, R.; Zhang, H.; Yu, J.; Zhang, M.; Li, R.; Wang, J. A chitosan-graphene oxide/ZIF foam with anti-biofouling ability for uranium recovery from seawater. Chem. Eng. J. 2020, 382, 122850. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, R.; Chen, M.; Liu, Y.; Xie, Z.; Tang, S.; Wang, N. Vertically Aligned Polyamidoxime/Graphene Oxide Hybrid Sheets’ Membrane for Ultrafast and Selective Extraction of Uranium from Seawater. Adv. Funct. Mater. 2021, 19, 2111049. [Google Scholar] [CrossRef]
- Chaudhary, M.; Singh, L.; Rekha, P.; Srivastava, V.C.; Mohanty, P. Adsorption of uranium from aqueous solution as well as seawater conditions by nitrogen-enriched nanoporous polytriazine. Chem. Eng. J. 2019, 378, 122236. [Google Scholar] [CrossRef]
- Fu, M.; Ao, J.; Ma, L.; Kong, D.; Qi, S.; Zhang, P.; Xu, G.; Wu, M.; Ma, H. Uranium removal from waste water of the tailings with functional recycled plastic membrane. Sep. Purif. Technol. 2022, 424, 120572. [Google Scholar] [CrossRef]
- Broda, E.; Gładysz-Płaska, A.; Skwarek, E.; Payentko, V.V. Structural properties and adsorption of uranyl ions on the nanocomposite hydroxyapatite/white clay. Appl. Nanosci. 2022, 12, 1101–1110. [Google Scholar] [CrossRef]
- Feng, S.; Feng, L.; Wang, M.; Yuan, Y.; Yu, Q.; Feng, T.; Cao, M.; Wang, N.; Peng, Q. Highly efficient extraction of uranium from seawater by natural marine crab carapace. Chem. Eng. J. 2021, 430, 133038. [Google Scholar] [CrossRef]
- Skwarek, E.; Gładysz-Płaska, A.; Choromańska, J.B.; Broda, E. Adsorption of uranium ions on nano-hydroxyapatite and modified by Ca and Ag ions. Adsorption 2019, 25, 639–647. [Google Scholar] [CrossRef] [Green Version]
- Zhao, S.; Feng, T.; Feng, L.; Yan, B.; Sun, W.; Luo, G.; Wang, M.; Jian, Y.; Liu, T.; Yuan, Y.; et al. Rapid recovery of uranium with magnetic-single-molecular amidoxime adsorbent. Sep. Purif. Technol. 2022, 287, 120524. [Google Scholar] [CrossRef]
- Jiao, G.-J.; Ma, J.; Zhang, J.; Li, Y.; Liu, K.; Sun, R. Porous and biofouling-resistant amidoxime-based hybrid hydrogel with excellent interfacial compatibility for high-performance recovery of uranium from seawater. Sep. Purif. Technol. 2022, 287, 120571. [Google Scholar] [CrossRef]
- Zhao, W.-W.; Li, L.-H.; Liao, J.-Z.; Liang, R.-P.; Song, A.-M.; Zhang, F.-D.; Ke, H.; Qiu, J.-D. Regenerable and stable biomimetic hydroxyl-modified metal-organic frameworks for targeted uranium capture. Chem. Eng. J. 2021, 433, 133787. [Google Scholar] [CrossRef]
- Zhang, J.; Feng, L.; Jian, Y.; Luo, G.; Wang, M.; Hu, B.; Liu, T.; Li, J.; Yuan, Y.; Wang, N. Interlayer spacing adjusted zirconium phosphate with 2D ion channels for highly efficient removal of uranium contamination in radioactive effluent. Chem. Eng. J. 2022, 429, 132265. [Google Scholar] [CrossRef]
- Chen, F.; Lv, M.; Ye, Y.; Miao, S.; Tang, X.; Liu, Y.; Liang, B.; Qin, Z.; Chen, Y.; He, Z.; et al. Insights on uranium removal by ion exchange columns: The deactivation mechanisms, and an overlooked biological pathway. Chem. Eng. J. 2022, 434, 134708. [Google Scholar] [CrossRef]
- Pan, M.; Cui, C.; Tang, W.; Guo, Z.; Zhang, D.; Xu, X.; Li, J. Carbon cloth as an important electrode support for the high selective electrosorption of uranium from acidic uranium mine wastewater. Sep. Purif. Technol. 2022, 281, 119843. [Google Scholar] [CrossRef]
- Feng, M.L.; Sarma, D.; Qi, X.H.; Du, K.Z.; Huang, X.Y.; Kanatzidis, M.G. Efficient removal and recovery of uranium by a layered organic-inorganic hybrid thiostannate. J. Am. Chem. Sci. 2016, 138, 12578–12585. [Google Scholar] [CrossRef]
- Yuan, Y.; Niu, B.; Yu, Q.; Guo, X.; Guo, Z.; Wen, J.; Liu, T.; Zhang, H.; Wang, N. Photoinduced Multiple Effects to Enhance Uranium Extraction from Natural Seawater by Black Phosphorus Nanosheets. Angew. Chem. Int. Ed. 2020, 59, 1220. [Google Scholar] [CrossRef]
- Estes, S.L.; Powell, B.A. Response to Comment on “Enthalpy of Uranium Adsorption onto Hematite”. Environ. Sci. Technol. 2021, 55, 3444–3446. [Google Scholar] [CrossRef]
- Ruan, Y.; Zhang, H.; Yu, Z.; Diao, Z.; Song, G.; Su, M.; Hou, L.; Chen, D.; Wang, S.; Kong, L. Phosphate enhanced uranium stable immobilization on biochar supported nano zero valent iron. J. Hazard. Mater. 2022, 424, 127119. [Google Scholar] [CrossRef] [PubMed]
- Wen, S.; Sun, Y.; Liu, R.; Chen, L.; Wang, J.; Peng, S.; Ma, C.; Yuan, Y.; Gong, W.; Wang, N. Supramolecularly Poly(amidoxime)-Loaded Macroporous Resin for Fast Uranium Recovery from Seawater and Uranium-Containing Wastewater. ACS Appl. Mater. Interfaces 2021, 13, 3246–3258. [Google Scholar] [CrossRef]
- Feng, L.; Wang, H.; Feng, T.; Yan, B.; Yu, Q.; Zhang, J.; Guo, Z.; Yuan, Y.; Ma, C.; Liu, T.; et al. In Situ Synthesis of Uranyl-Imprinted Nanocage for Selective Uranium Recovery from Seawater. Angew. Chem. Int. Ed. 2022, 134, e202101015. [Google Scholar] [CrossRef]
- He, Y.; Lin, X.; Yan, T.; Zhang, X.; Zhou, J.; Chen, Y.; Luo, X. Selective adsorption of uranium from salt lake-simulated solution by phenolic-functionalized hollow sponge-like adsorbent. J. Chem. Technol. Biotechnol. 2019, 94, 455–467. [Google Scholar] [CrossRef]
- Pazos, M.C.; Cota, A.; Osuna, F.J.; Pavón, E.; Alba, M.D. Self-Assembling of Tetradecylammonium Chain on Swelling High Charge Micas (Na-Mica-3 and Na-Mica-2): Effect of Alkylammonium Concentration and Mica Layer Charge. Langmuir 2015, 31, 4394–4401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, W.; Wang, Q.; Wang, H.; Xin, Q.; Hou, W.; Hu, E.; Lei, Z. Adsorption of uranium by chitosan/Chlorella pyrenoidosa composite adsorbent bearing phosphate ligand. Chemosphere 2022, 287, 132193. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.-R.; Cui, W.-R.; Niu, C.-P.; Yi, S.-M.; Liang, R.-P.; Qi, J.-X.; Chen, X.-J.; Jiang, W.; Zhang, L.; Qiu, J.-D. rGO-based covalent organic framework hydrogel for synergistically enhance uranium capture capacity through photothermal desalination. Chem. Eng. J. 2022, 428, 131178. [Google Scholar] [CrossRef]
- Yan, B.; Ma, C.; Gao, J.; Yuan, Y.; Wang, N. An Ion-Crosslinked Supramolecular Hydrogel for Ultrahigh and Fast Uranium Recovery from Seawater. Adv. Mater. 2022, 32, e1906615. [Google Scholar] [CrossRef]
- Zhang, M.-K.; Zhang, X.-H.; Han, G.-Z. Magnetic alginate/PVA hydrogel microspheres with selective adsorption performance for aromatic compounds. Sep. Purif. Technol. 2021, 278, 119547. [Google Scholar] [CrossRef]
- Ma, C.; Gao, J.; Wang, D.; Yuan, Y.; Wen, J.; Yan, B.; Zhao, S.; Zhao, X.; Sun, Y.; Wang, X.; et al. Sunlight Polymerization of Poly(amidoxime) Hydrogel Membrane for Enhanced Uranium Extraction from Seawater. Adv. Sci. 2019, 6, 1900085. [Google Scholar] [CrossRef] [Green Version]
- Yue, H.Y.; Shang, Z.J.; Xu, P.; Feng, D.Y.; Li, X.X. Preparation of EDTA Modified Chitooligosaccharide/Sodium Alginate/Ca2+ Physical Double Network Hydrogel by Using of High-Salinity Oilfield Produced Water for Adsorption of Zn2+, Ni2+ and Mn2+. Sep. Purif. Technol. 2020, 280, 119767. [Google Scholar] [CrossRef]
- Li, B.; Kappl, M.; Han, L.; Cui, J.; Zhou, F.; del Campo, A. Goosebumps-Inspired Microgel Patterns with Switchable Adhesion and Friction. Small 2019, 15, 1902376. [Google Scholar] [CrossRef] [PubMed]
- Rommel, D.; Mork, M.; Vedaraman, S.; Bastard, C.; Guerzoni, L.P.B.; Kittel, Y.; Vinokur, R.; Born, N.; Haraszti, T.; De Laporte, L. Functionalized Microgel Rods Interlinked into Soft Macroporous Structures for 3D Cell Culture. Adv. Sci. 2022, 9, 2103554. [Google Scholar] [CrossRef] [PubMed]
- Xin, S.; Wyman, O.M.; Alge, D.L. Assembly of PEG Microgels into Porous Cell-Instructive 3D Scaffolds via Thiol-Ene Click Chemistry. Adv. Heal. Mater. 2018, 7, 1800160. [Google Scholar] [CrossRef]
- Switacz, V.K.; Wypysek, S.K.; Degen, R.; Crassous, J.J.; Spehr, M.; Richtering, W. Influence of Size and Cross-Linking Density of Microgels on Cellular Uptake and Uptake Kinetics. Biomacromolecules 2021, 21, 4532–4544. [Google Scholar] [CrossRef]
- Yan, J.; Wang, Y.; Ran, M.; Mustafa, R.A.; Luo, H.; Wang, J.; Smått, J.; Rosenholm, J.M.; Cui, W.; Lu, Y.; et al. Peritumoral Microgel Reservoir for Long-Term Light-Controlled Triple-Synergistic Treatment of Osteosarcoma with Single Ultra-Low Dose. Small 2021, 17, 2100479. [Google Scholar] [CrossRef]
- Cutright, C.C.; Harris, J.L.; Ramesh, S.; Khan, S.A.; Genzer, J.; Menegatti, S. Surface-Bound Microgels for Separation, Sensing, and Biomedical Applications. Adv. Funct. Mater. 2021, 31, 2104164. [Google Scholar] [CrossRef]
- Ma, Y.; He, P.; Xie, W.; Zhang, Q.; Yin, W.; Pan, J.; Wang, M.; Zhao, X.; Pan, G. Dynamic Colloidal Photonic Crystal Hydrogels with Self-Recovery and Injectability. Research 2021, 2021, 9565402. [Google Scholar] [CrossRef] [PubMed]
- Sorrell, C.D.; Carter, M.C.D.; Serpe, M.J. Color Tunable Poly (N-Isopropylacrylamide)-co-Acrylic Acid Microgel-Au Hybrid Assemblies. Adv. Funct. Mater. 2021, 21, 425–433. [Google Scholar] [CrossRef]
- Sorrell, C.D.; Serpe, M.J. Reflection Order Selectivity of Color-Tunable Poly(N-isopropylacrylamide) Microgel Based Etalons. Adv. Mater. 2011, 23, 4088–4092. [Google Scholar] [CrossRef]
- Pan, G.; Shinde, S.; Yeung, S.Y.; Jakštaitė, M.; Li, Q.; Wingren, A.G.; Sellergren, B. An Epitope-Imprinted Biointerface with Dynamic Bioactivity for Modulating Cell-Biomaterial Interactions. Angew. Chem. Int. Ed. 2017, 56, 15959. [Google Scholar] [CrossRef] [PubMed]
- Yuan, D.; Wang, Y.; Qian, Y.; Liu, Y.; Feng, G.; Huang, B.; Zhao, X. Highly selective adsorption of uranium in strong HNO3 media achieved on a phosphonic acid functionalized nanoporous polymer. J. Mater. Chem. A 2017, 5, 22735–22742. [Google Scholar] [CrossRef]
- Xu, Q.; Chen, B.; He, M.; Hu, B. Ti (IV) modified vinyl phosphate magnetic nanoparticles for simultaneous preconcentration of multiple arsenic species from chicken samples followed by HPLC-ICP-MS analysis. Electrophoresis 2021, 42, 465–472. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wang, P.; Zhou, M.; Ma, Y.; Niu, X.; Pan, G.; Pan, J. Tailored Janus silica nanosheets integrating bispecific artificial receptors for simultaneous adsorption of 2,6-dichlorophenol and Pb(ii). J. Mater. Chem. A 2019, 7, 16161–16175. [Google Scholar] [CrossRef]
- Bai, X.; Wang, Y.; Li, H.; Tian, X.; Ma, Y.; Pan, J. Stalagmites in Karst Cave Inspired Construction: Lotus Root-type Adsorbent with Porous Surface Derived from CO2-in-Water Pickering Emulsion for Selective and Ultrafast Uranium Extraction. J. Hazard. Mater. 2021, 419, 126398. [Google Scholar] [CrossRef] [PubMed]


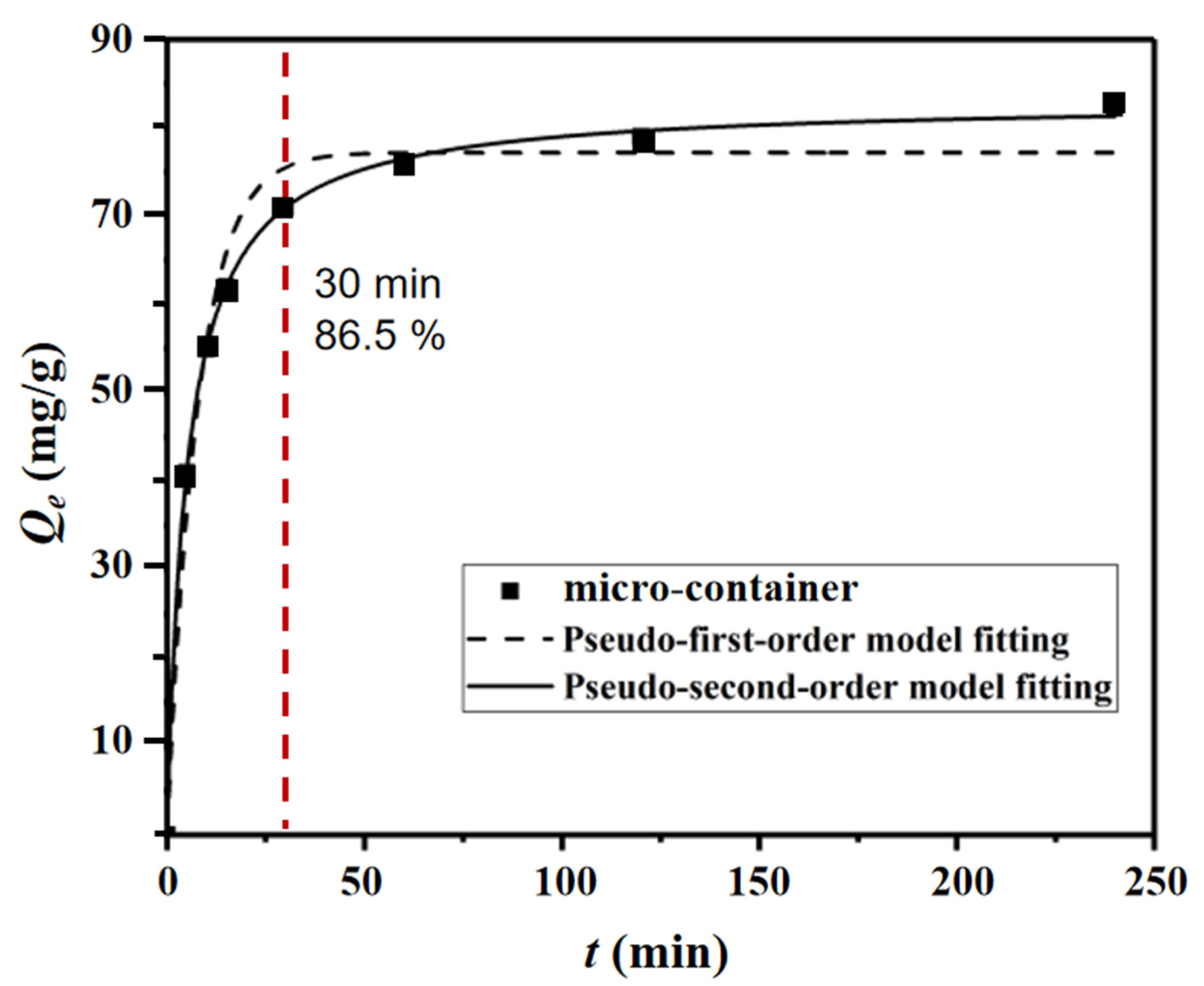
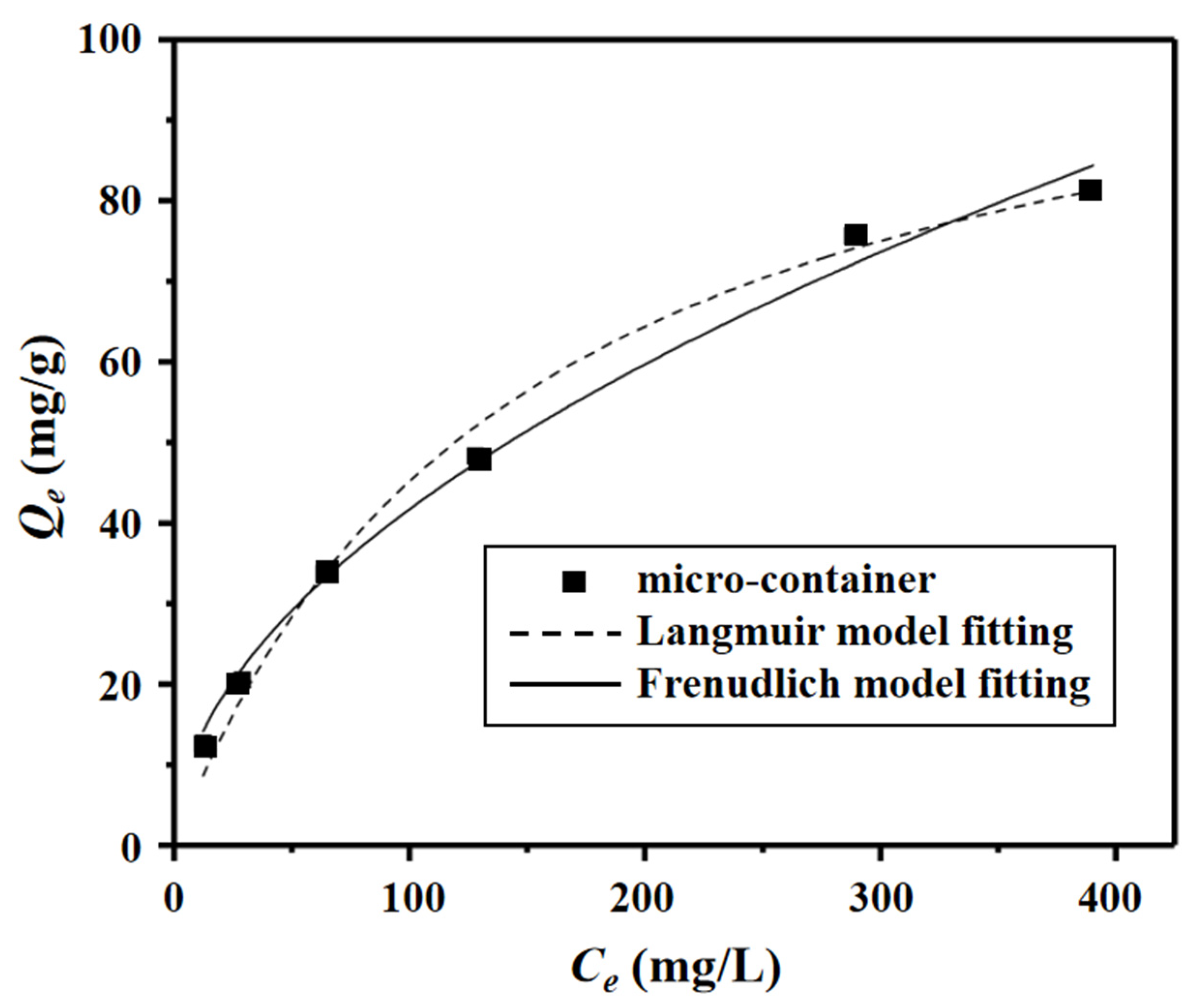
| Pseudo-First-Order Model Fitting a | Pseudo-Second-Order Model Fitting a | |||||||
|---|---|---|---|---|---|---|---|---|
| Qt (mg/g) | Qe (mg/g) | k1 (L/min) | R2 | Qe (mg/g) | k2 (g/(mg·min)) | R2 | h (mg/(g·min)) | t1/2 (min) |
| 82.1 | 77.2 | 0.1284 | 0.966 | 83.1 | 0.0024 | 9.997 | 16.57 | 5.014 |
| T (K) | Langmuir Model a | Freundlich Model b | |||||
|---|---|---|---|---|---|---|---|
| Qm (mg/g) | KL × 102 (L/min) | R2 | RL | KF (g/mg)·(L/mg)1/n | R2 | 1/n | |
| 273 | 111.9 | 0.676 | 0.991 | 0.228 | 3.85 | 0.980 | 0.5173 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, P.; Shen, M.; Xie, W.; Ma, Y.; Pan, J. The Efficient and Convenient Extracting Uranium from Water by a Uranyl-Ion Affine Microgel Container. Nanomaterials 2022, 12, 2259. https://doi.org/10.3390/nano12132259
He P, Shen M, Xie W, Ma Y, Pan J. The Efficient and Convenient Extracting Uranium from Water by a Uranyl-Ion Affine Microgel Container. Nanomaterials. 2022; 12(13):2259. https://doi.org/10.3390/nano12132259
Chicago/Turabian StyleHe, Peiyan, Minghao Shen, Wanli Xie, Yue Ma, and Jianming Pan. 2022. "The Efficient and Convenient Extracting Uranium from Water by a Uranyl-Ion Affine Microgel Container" Nanomaterials 12, no. 13: 2259. https://doi.org/10.3390/nano12132259
APA StyleHe, P., Shen, M., Xie, W., Ma, Y., & Pan, J. (2022). The Efficient and Convenient Extracting Uranium from Water by a Uranyl-Ion Affine Microgel Container. Nanomaterials, 12(13), 2259. https://doi.org/10.3390/nano12132259







