Paper Biosensor for the Detection of NT-proBNP Using Silver Nanodisks as Electrochemical Labels
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
2.2. Instrumentation
2.3. Synthesis of Silver Nanodisks (AgNDs)
2.4. Preparation of Assay Components
2.5. Formation of the Metalloimmunoassay
2.6. Electrochemistry
3. Results
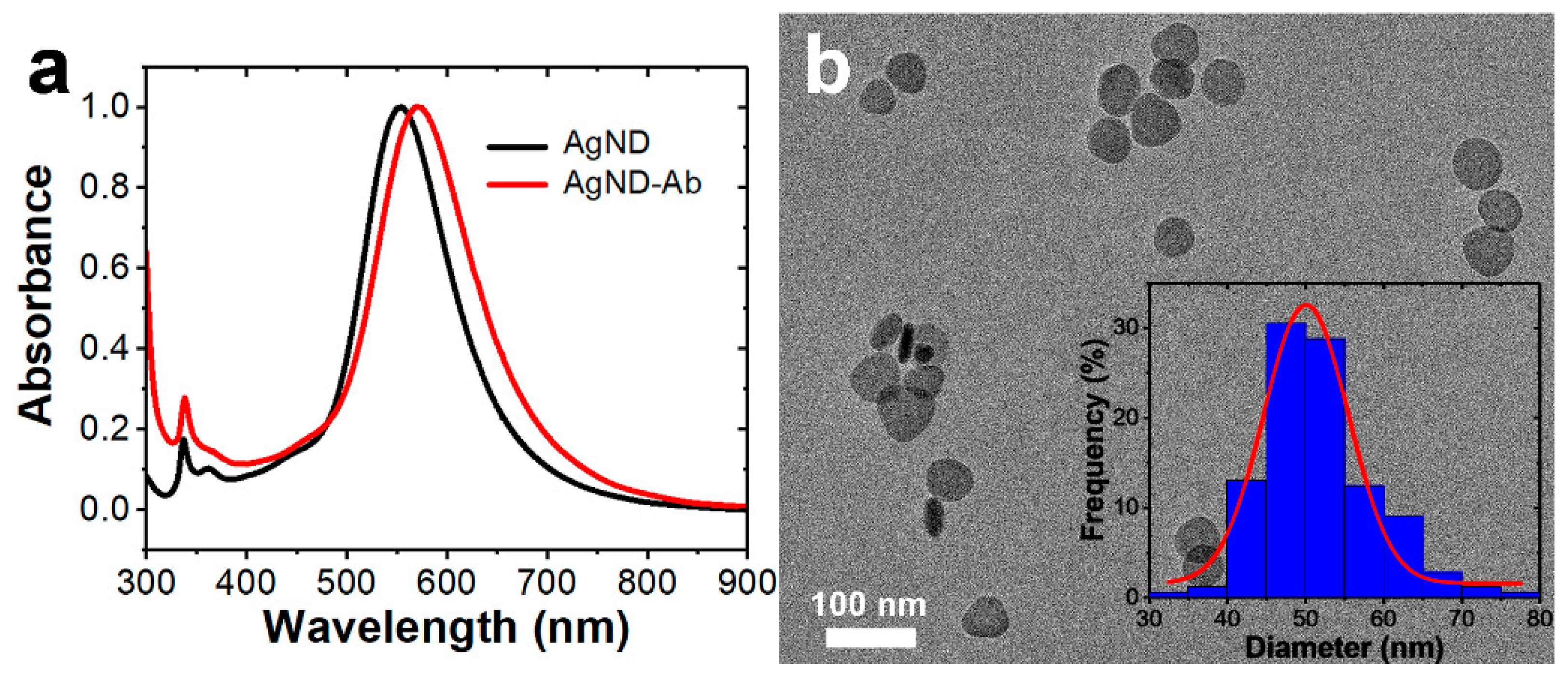
3.1. Characterization of AgNDs
3.2. Bioconjugation of Antibodies to AgNDs
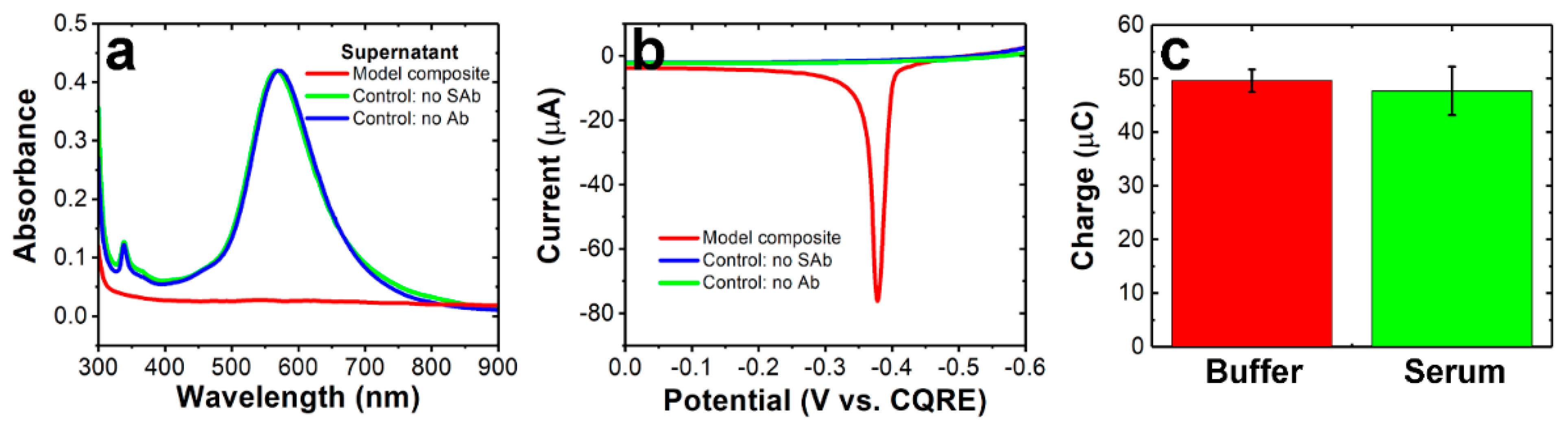
3.3. Formation of Model Composites
3.4. Detection of Model Composites
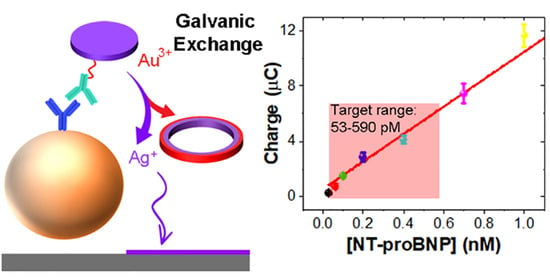
3.5. Detection of NT-proBNP
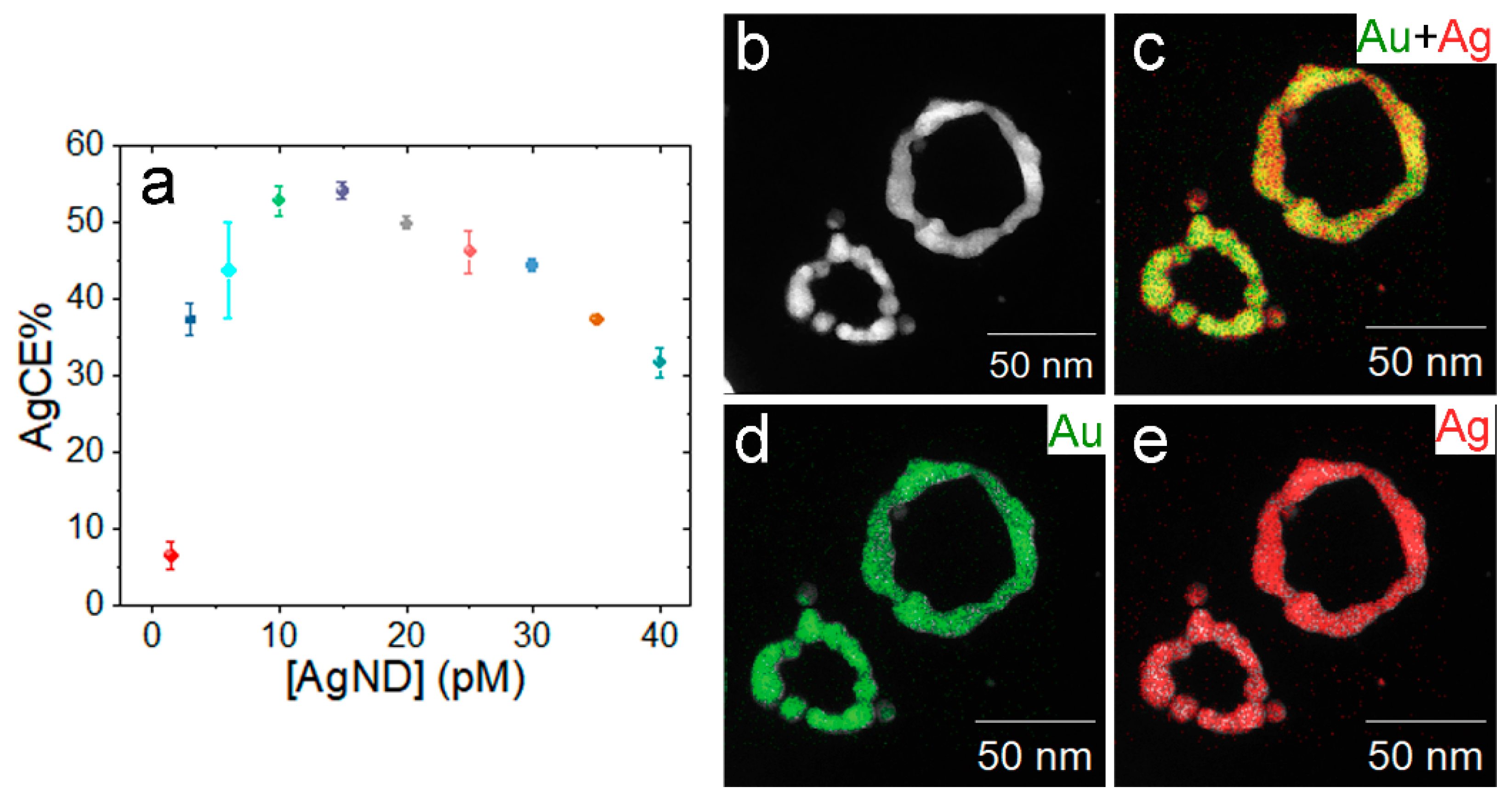
3.6. GE Mechanism for AgNDs
4. Summary and Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Roger, V.L. Epidemiology of Heart Failure. Circ. Res. 2013, 113, 646–659. [Google Scholar] [CrossRef] [PubMed]
- Semenov, A.G.; Tamm, N.N.; Seferian, K.R.; Postnikov, A.B.; Karpova, N.S.; Serebryanaya, D.V.; Koshkina, E.V.; Krasnoselsky, M.I.; Katrukha, A.G. Processing of Pro–B-Type Natriuretic Peptide: Furin and Corin as Candidate Convertases2. Clin. Chem. 2010, 56, 1166–1176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Januzzi, J.L.; Troughton, R. Are serial BNP measurements useful in heart failure management? Serial natriuretic peptide measurements are useful in heart failure management. Circulation 2013, 127, 500–507; discussion 508. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Masson, S.; Latini, R.; Anand, I.S.; Vago, T.; Angelici, L.; Barlera, S.; Missov, E.D.; Clerico, A.; Tognoni, G.; Cohn, J.N.; et al. Direct comparison of B-type natriuretic peptide (BNP) and amino-terminal proBNP in a large population of patients with chronic and symptomatic heart failure: The Valsartan Heart Failure (Val-HeFT) data. Clin. Chem. 2006, 52, 1528–1538. [Google Scholar] [CrossRef] [Green Version]
- Felker, G.M.; Ahmad, T.; Anstrom, K.J.; Adams, K.F.; Cooper, L.S.; Ezekowitz, J.A.; Fiuzat, M.; Houston-Miller, N.; Januzzi, J.L.; Leifer, E.S.; et al. Rationale and design of the GUIDE-IT study: Guiding evidence based therapy using biomarker intensified treatment in heart failure. JACC Heart Failure 2014, 2, 457–465. [Google Scholar] [CrossRef] [Green Version]
- DeGregory, P.R.; Tapia, J.; Wong, T.; Villa, J.; Richards, I.; Crooks, R.M. Managing Heart Failure at Home with Point-of-Care Diagnostics. IEEE J. Transl. Eng. Health Med. 2017, 5, 1–6. [Google Scholar] [CrossRef]
- Karabulut, A.; Kaplan, A.; Aslan, C.; Iltumur, K.; Toprak, G.; Toprak, N. The association between NT-proBNP levels, functional capacity and stage in patients with heart failure. Acta Cardiol. 2005, 60, 631–638. [Google Scholar] [CrossRef]
- Labib, M.; Sargent, E.H.; Kelley, S.O. Electrochemical Methods for the Analysis of Clinically Relevant Biomolecules. Chem. Rev. 2016, 116, 9001–9090. [Google Scholar] [CrossRef]
- Wongkaew, N.; Simsek, M.; Griesche, C.; Baeumner, A.J. Functional Nanomaterials and Nanostructures Enhancing Electrochemical Biosensors and Lab-on-a-Chip Performances: Recent Progress, Applications, and Future Perspective. Chem. Rev. 2019, 119, 120–194. [Google Scholar] [CrossRef]
- Zile, M.R.; Claggett, B.L.; Prescott, M.F.; McMurray, J.J.; Packer, M.; Rouleau, J.L.; Swedberg, K.; Desai, A.S.; Gong, J.; Shi, V.C.; et al. Prognostic Implications of Changes in N-Terminal Pro-B-Type Natriuretic Peptide in Patients with Heart Failure. J. Am. Coll. Cardiol. 2016, 68, 2425–2436. [Google Scholar] [CrossRef] [Green Version]
- Mainville, C.A.; Clark, G.H.; Esty, K.J.; Foster, W.M.; Hanscom, J.L.; Hebert, K.J.; Lyons, H.R. Analytical validation of an immunoassay for the quantification of N-terminal pro–B-type natriuretic peptide in feline blood. J. Vet. Diagn. Investig. 2015, 27, 414–421. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Yin, X.; Sun, D.; Xia, K.; Kang, C.; Chu, S.; Zhang, P.; Wang, H.; Qiu, Y. Detection of NT-pro BNP using fluorescent protein modified by streptavidin as a label in immunochromatographic assay. Sens. Bio-Sens. Res. 2016, 11, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Shi, L.; Li, X.; Zhu, W.; Wang, Y.; Du, B.; Cao, W.; Wei, Q.; Pang, X. Sandwich-Type Electrochemiluminescence Sensor for Detection of NT-proBNP by Using High Efficiency Quench Strategy of Fe3O4@PDA toward Ru(bpy)32+ Coordinated with Silver Oxalate. ACS Sens. 2017, 2, 1774–1778. [Google Scholar] [CrossRef]
- Chen, A.; Chatterjee, S. Nanomaterials based electrochemical sensors for biomedical applications. Chem. Soc. Rev. 2013, 42, 5425–5438. [Google Scholar] [CrossRef] [PubMed]
- Noviana, E.; McCord, C.P.; Clark, K.M.; Jang, I.; Henry, C.S. Electrochemical paper-based devices: Sensing approaches and progress toward practical applications. Lab Chip 2019, 20, 9–34, Correction in Lab Chip 2020, 20, 185. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Qi, X.; Zhang, G.; Wang, S.; Li, K.; Wu, J.; Wan, X.; Liu, Y.; Li, Q. Low-cost voltammetric sensors for robust determination of toxic Cd(II) and Pb(II) in environment and food based on shuttle-like α-Fe2O3 nanoparticles decorated β-Bi2O3 microspheres. Microchem. J. 2022, 179, 107515. [Google Scholar] [CrossRef]
- Li, G.; Qi, X.; Wu, J.; Xu, L.; Wan, X.; Liu, Y.; Chen, Y.; Li, Q. Ultrasensitive, label-free voltammetric determination of norfloxacin based on molecularly imprinted polymers and Au nanoparticle-functionalized black phosphorus nanosheet nanocomposite. J. Hazard. Mater. 2022, 436, 129107. [Google Scholar] [CrossRef]
- Cunningham, J.C.; Kogan, M.R.; Tsai, Y.-J.; Luo, L.; Richards, I.; Crooks, R.M. Paper-Based Sensor for Electrochemical Detection of Silver Nanoparticle Labels by Galvanic Exchange. ACS Sens. 2016, 1, 40–47. [Google Scholar] [CrossRef]
- Pollok, N.E.; Rabin, C.; Smith, L.; Crooks, R.M. Orientation-Controlled Bioconjugation of Antibodies to Silver Nanoparticles. Bioconjugate Chem. 2019, 30, 3078–3086. [Google Scholar] [CrossRef]
- Pollok, N.E.; Rabin, C.; Walgama, C.T.; Smith, L.; Richards, I.; Crooks, R.M. Electrochemical Detection of NT-proBNP Using a Metalloimmunoassay on a Paper Electrode Platform. ACS Sens. 2020, 5, 853–860. [Google Scholar] [CrossRef]
- Walgama, C.; Nguyen, M.P.; Boatner, L.M.; Richards, I.; Crooks, R.M. Hybrid paper and 3D-printed microfluidic device for electrochemical detection of Ag nanoparticle labels. Lab Chip 2020, 20, 1648–1657. [Google Scholar] [CrossRef] [PubMed]
- Pollok, N.E.; Peng, Y.; Rabin, C.; Richards, I.; Crooks, R.M. Effect of Serum on Electrochemical Detection of Bioassays Having Ag Nanoparticle Labels. ACS Sens. 2021, 6, 1956–1962. [Google Scholar] [CrossRef] [PubMed]
- Bard, A.; Faulkner, L.R. Electrochemical Methods: Fundamentals and Applications, 2nd ed.; Wiley: New York, NY, USA, 2001. [Google Scholar]
- Kogan, M.R.; Pollok, N.E.; Crooks, R.M. Detection of Silver Nanoparticles by Electrochemically Activated Galvanic Exchange. Langmuir 2018, 34, 15719–15726. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Rabin, C.; Walgama, C.T.; Pollok, N.E.; Smith, L.; Richards, I.; Crooks, R.M. Silver Nanocubes as Electrochemical Labels for Bioassays. ACS Sens. 2021, 6, 1111–1119. [Google Scholar] [CrossRef]
- Pollok, N.E.; Peng, Y.; Raj, N.; Walgama, C.; Crooks, R.M. Dual-Shaped Silver Nanoparticle Labels for Electrochemical Detection of Bioassays. ACS Appl. Nano Mater. 2021, 4, 10764–10770. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, N.; Goebl, J.; Lu, Z.; Yin, Y. A Systematic Study of the Synthesis of Silver Nanoplates: Is Citrate a “Magic” Reagent? J. Am. Chem. Soc. 2011, 133, 18931–18939. [Google Scholar] [CrossRef]
- Thermofisher. Dynabeads MyOne Streptavidin T1 Protocol. Available online: https://tools.thermofisher.com/content/sfs/manuals/dynabeads_myone_savT1_man.pdf (accessed on 1 March 2006).
- Bio-Rad Laboratories. LYNX Rapid Plus Biotin (Type 2) Antibody Conjugation Kit Protocol. Available online: https://www.bio-rad-antibodies.com/kit/biotin-type-2-conjugation-kit-lnk27.html?f=kit (accessed on 15 November 2011).
- Tang, S.; Tang, Y.; Zhu, S.; Lu, H.; Meng, X. Synthesis and characterization of silica–silver core–shell composite particles with uniform thin silver layers. J. Solid State Chem. 2007, 180, 2871–2876. [Google Scholar] [CrossRef]
- Ghosh, T.; Satpati, B.; Senapati, D. Characterization of bimetallic core–shell nanorings synthesized via ascorbic acid-controlled galvanic displacement followed by epitaxial growth. J. Mater. Chem. C 2014, 2, 2439–2447. [Google Scholar] [CrossRef]
- Liu, M.; Leng, M.; Yu, C.; Wang, X.; Wang, C. Selective synthesis of hexagonal Ag nanoplates in a solution-phase chemical reduction process. Nano Res. 2010, 3, 843–851. [Google Scholar] [CrossRef] [Green Version]
- Khlebtsov, N.G. Determination of Size and Concentration of Gold Nanoparticles from Extinction Spectra. Anal. Chem. 2008, 80, 6620–6625. [Google Scholar] [CrossRef]
- Wang, Z.L. Transmission Electron Microscopy of Shape-Controlled Nanocrystals and Their Assemblies. J. Phys. Chem. B 2000, 104, 1153–1175. [Google Scholar] [CrossRef]
- Qian, H.; Xu, M.; Li, X.; Ji, M.; Cheng, L.; Shoaib, A.; Liu, J.; Jiang, L.; Zhu, H.; Zhang, J. Surface micro/nanostructure evolution of Au–Ag alloy nanoplates: Synthesis, simulation, plasmonic photothermal and surface-enhanced Raman scattering applications. Nano Res. 2016, 9, 876–885. [Google Scholar] [CrossRef]
- Tsuji, M.; Kidera, T.; Yajima, A.; Hamasaki, M.; Hattori, M.; Tsuji, T.; Kawazumi, H. Synthesis of Ag–Au and Ag–Pd alloy triangular hollow nanoframes by galvanic replacement reactions without and with post-treatment using NaCl in an aqueous solution. CrystEngComm 2014, 16, 2684–2691. [Google Scholar] [CrossRef]
- Shahjamali, M.M.; Salvador, M.; Bosman, M.; Ginger, D.S.; Xue, C. Edge-Gold-Coated Silver Nanoprisms: Enhanced Stability and Applications in Organic Photovoltaics and Chemical Sensing. J. Phys. Chem. C 2014, 118, 12459–12468. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, Y.; Raj, N.; Strasser, J.W.; Crooks, R.M. Paper Biosensor for the Detection of NT-proBNP Using Silver Nanodisks as Electrochemical Labels. Nanomaterials 2022, 12, 2254. https://doi.org/10.3390/nano12132254
Peng Y, Raj N, Strasser JW, Crooks RM. Paper Biosensor for the Detection of NT-proBNP Using Silver Nanodisks as Electrochemical Labels. Nanomaterials. 2022; 12(13):2254. https://doi.org/10.3390/nano12132254
Chicago/Turabian StylePeng, Yi, Nikhil Raj, Juliette W. Strasser, and Richard M. Crooks. 2022. "Paper Biosensor for the Detection of NT-proBNP Using Silver Nanodisks as Electrochemical Labels" Nanomaterials 12, no. 13: 2254. https://doi.org/10.3390/nano12132254
APA StylePeng, Y., Raj, N., Strasser, J. W., & Crooks, R. M. (2022). Paper Biosensor for the Detection of NT-proBNP Using Silver Nanodisks as Electrochemical Labels. Nanomaterials, 12(13), 2254. https://doi.org/10.3390/nano12132254