Curing and Molecular Dynamics Simulation of MXene/Phenolic Epoxy Composites with Different Amine Curing Agent Systems
Abstract
:1. Introduction
2. Experimental
2.1. Materials
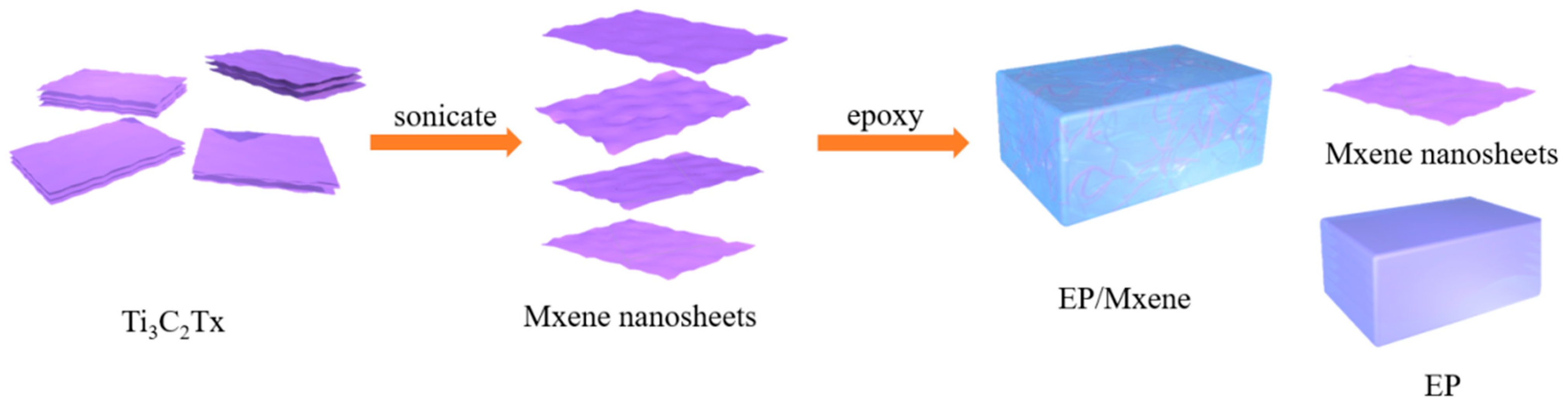
2.2. Preparation of MXene/Epoxy Resin Nanocomposites
2.3. Material Characterization
2.4. Curing Kinetics
2.5. Determination of Curing Degree
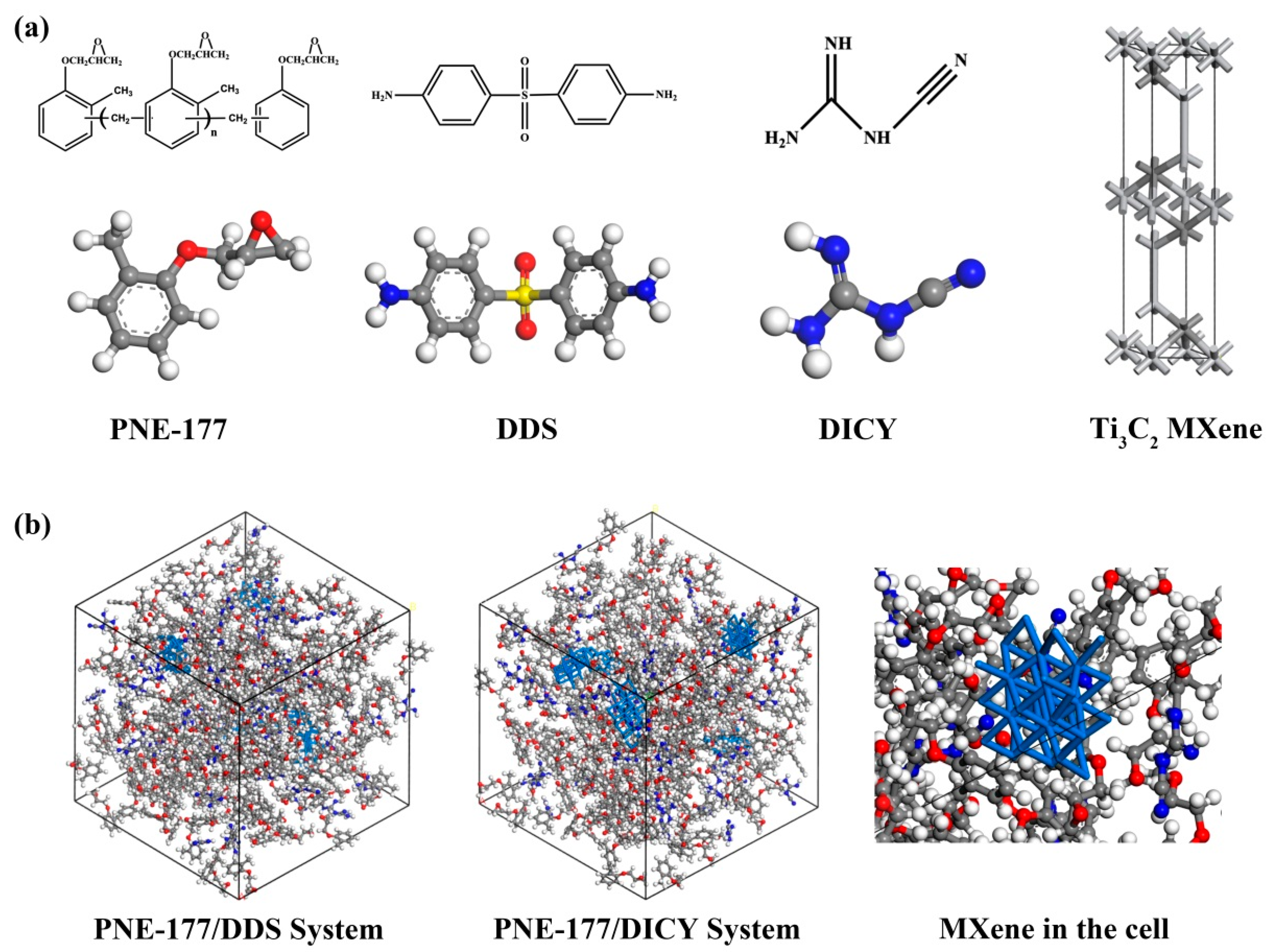
3. MD Simulations
4. Results and Discussion
4.1. Characterization of MXene Nanosheets
4.2. Curing Kinetics
Non-Isothermal DSC Analysis
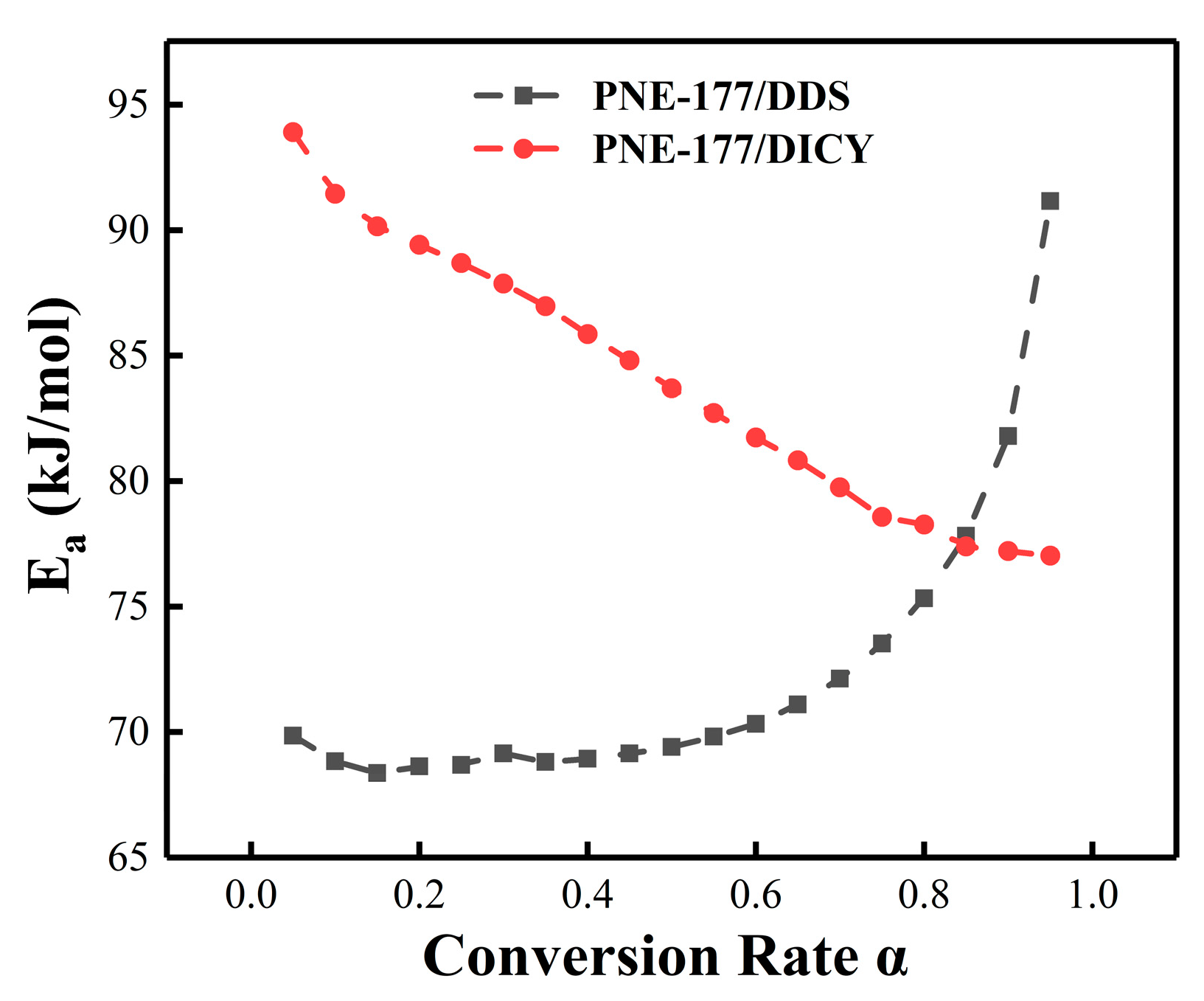
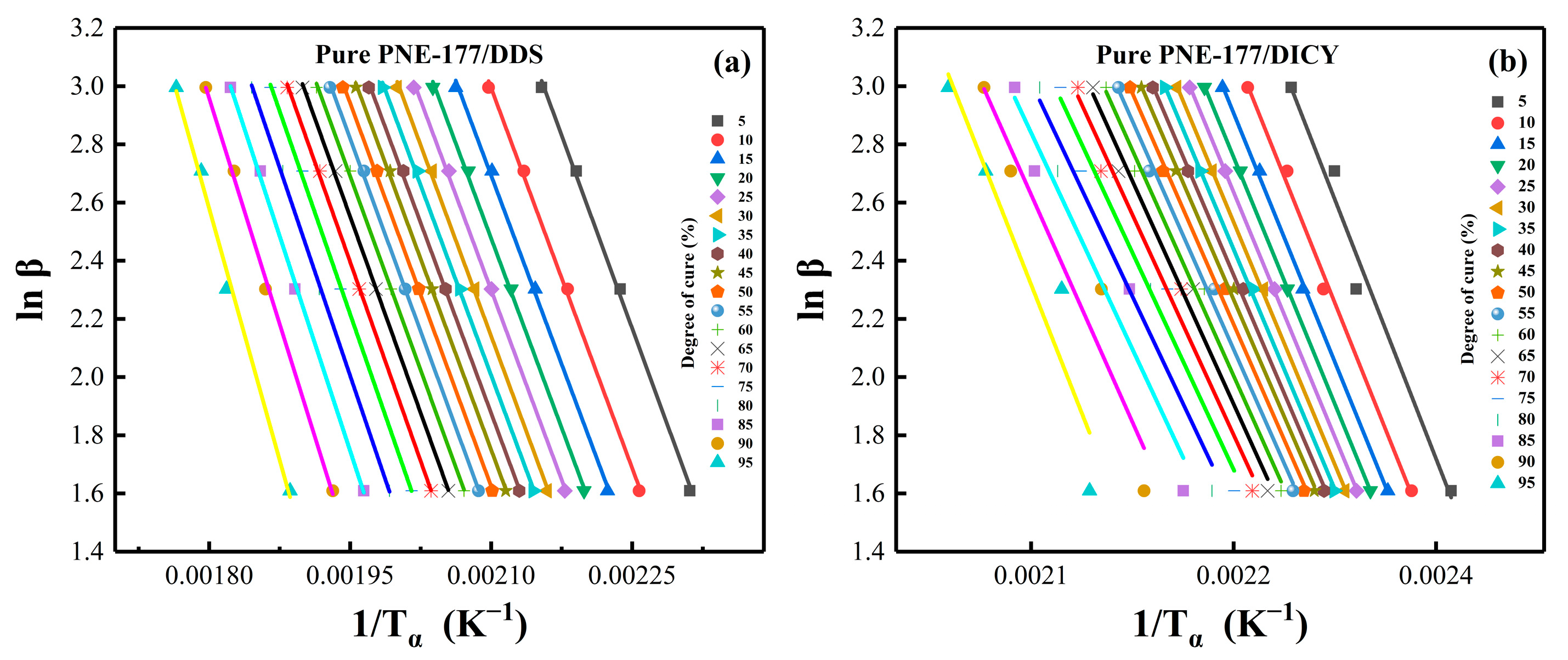
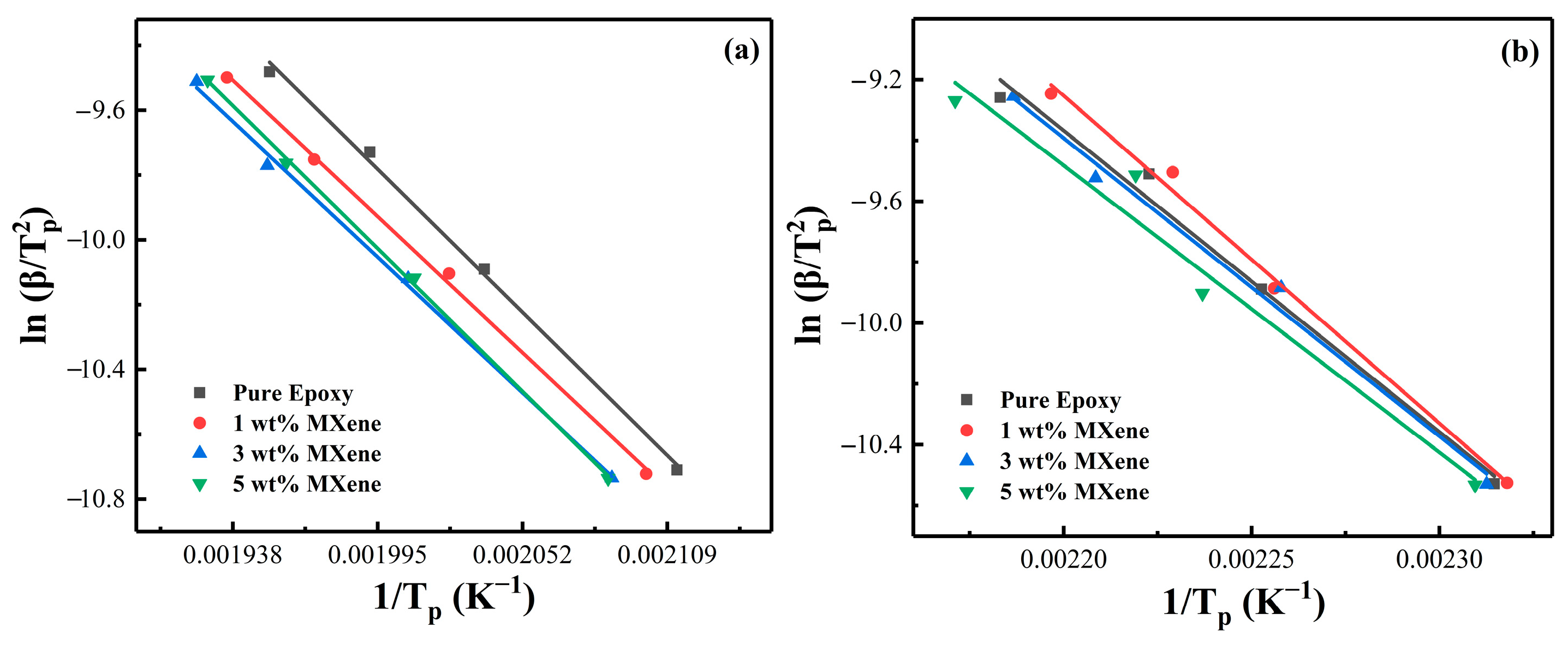
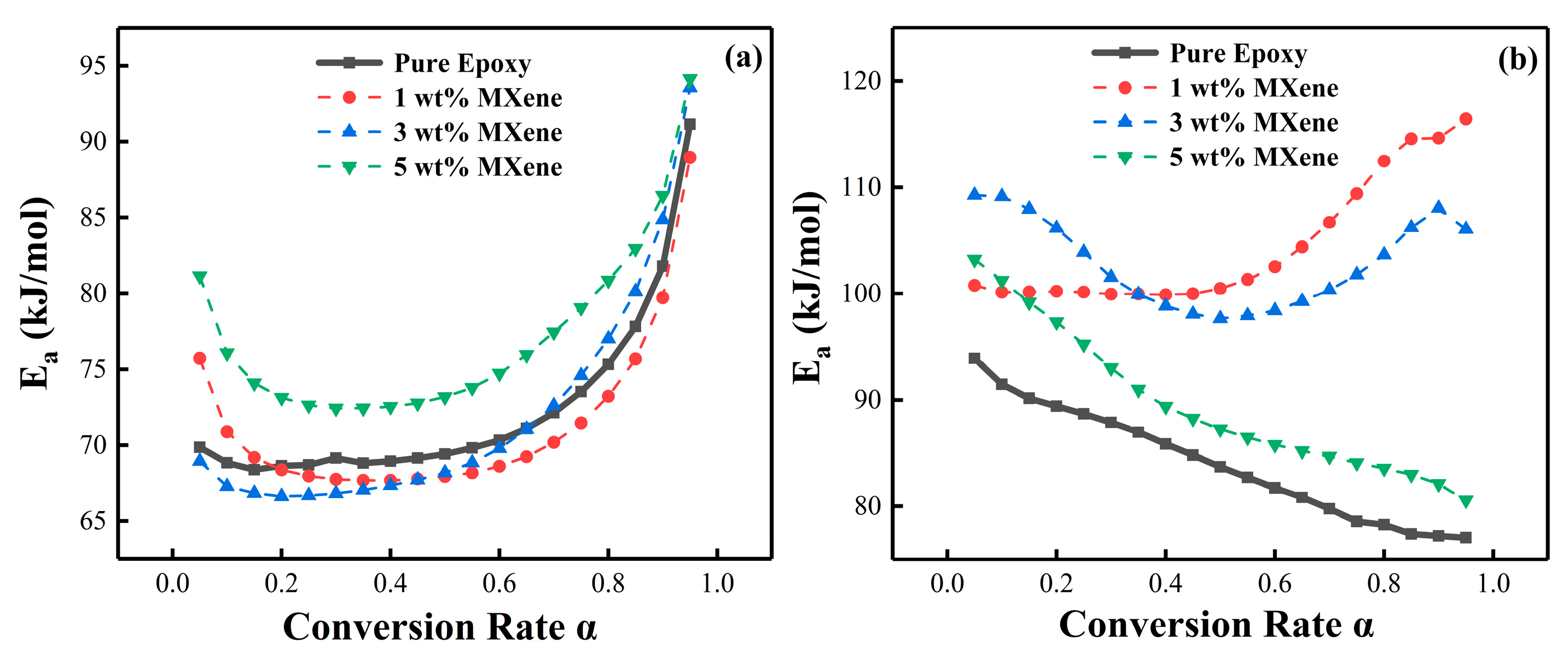
4.3. Reaction Activation Energy and Conversion Rate
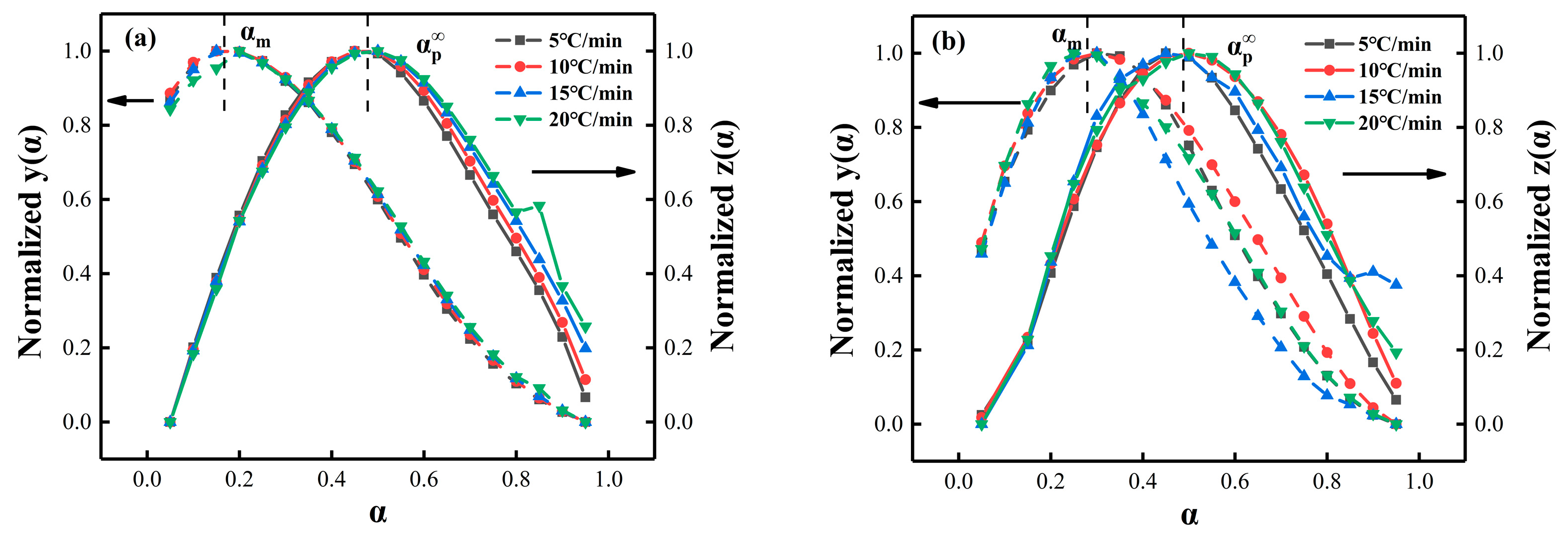
4.4. Málek Method Analysis
4.5. Curing Degree
4.6. Glass Transition Temperature
4.7. Free Volume
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fiorina, M.; Seman, A.; Castanié, B.; Ali, K.; Schwob, C.; Mezeix, L. Spring-in prediction for carbon/epoxy aerospace composite structure. Compos. Struct. 2017, 168, 739–745. [Google Scholar] [CrossRef]
- Hashjin, R.R.; Ranjbar, Z.; Yari, H. Modeling of electrical conductive graphene filled epoxy coatings. Prog. Org. Coat. 2018, 125, 411–419. [Google Scholar] [CrossRef]
- Merzah, Z.F.; Fakhry, S.; Allami, T.G.; Yuhana, N.Y.; Alamiery, A. Enhancement of the Properties of Hybridizing Epoxy and Nanoclay for Mechanical, Industrial, and Biomedical Applications. Polymers 2022, 14, 526. [Google Scholar] [CrossRef] [PubMed]
- Kitichatpayak, D.; Makcharoen, W.; Vittayakorn, N. Influence of various nanofillers on mechanical and electrical properties of epoxy resin composites. Polym. Plast. Technol. Mater. 2022, 03, 1–3. [Google Scholar] [CrossRef]
- Voto, G.; Sequeira, L.; Skordos, A.A. Formulation based predictive cure kinetics modelling of epoxy resins. Polymer 2021, 236, 124304. [Google Scholar] [CrossRef]
- Prime, R.B.; Turi, E.A. (Eds.) Thermal Characterization of Polymeric Materials; Academic Press: Cambridge, MA, USA, 1981; p. 435. [Google Scholar]
- Um, M.-K.; Daniel, I.M.; Hwang, B.-S. A study of cure kinetics by the use of dynamic differential scanning calorimetry. Compos. Sci. Technol. 2002, 62, 29–40. [Google Scholar] [CrossRef]
- Roşu, D.; Caşcaval, C.; Mustątǎ, F.; Ciobanu, C. Cure kinetics of epoxy resins studied by non-isothermal DSC data. Thermochim. Acta 2002, 383, 119–127. [Google Scholar] [CrossRef]
- Rehman, S.; Gomez, J.; Villaro, E.; Cossey, D.; Karagiannidis, P.G. Bio-Based Epoxy/Amine Reinforced with Reduced Graphene Oxide (rGO) or GLYMO-rGO: Study of Curing Kinetics, Mechanical Properties, Lamination and Bonding Performance. Nanomaterials 2022, 12, 222. [Google Scholar] [CrossRef]
- Sun, Y.; Peng, Y.; Zhang, Y. A Study on the Synthesis, Curing Behavior and Flame Retardance of a Novel Flame Retardant Curing Agent for Epoxy Resin. Polymers 2022, 14, 245. [Google Scholar] [CrossRef]
- Startsev, O.V.; Vapirov, Y.M.; Lebedev, M.P.; Kychkin, A.K. Comparison of Glass-Transition Temperatures for Epoxy Polymers Obtained by Methods of Thermal Analysis. Polym. Mech. 2020, 56, 227–240. [Google Scholar] [CrossRef]
- Li, C.; Strachan, A. Molecular scale simulations on thermoset polymers: A review. J. Polym. Sci. Part B Polym. Phys. 2015, 53, 103–122. [Google Scholar] [CrossRef]
- Fan, H.B.; Yuen, M.M. Material properties of the cross-linked epoxy resin compound predicted by molecular dynamics simulation. Polymer 2007, 48, 2174–2178. [Google Scholar] [CrossRef]
- Estridge, C.E. The effects of competitive primary and secondary amine reactivity on the structural evolution and properties of an epoxy thermoset resin during cure: A molecular dynamics study. Polymer 2018, 141, 12–20. [Google Scholar] [CrossRef]
- Mohammad, R.S.; Hadi, R.; Meisam, S. Cure kinetics of epoxy/β-cyclodextrin-functionalized Fe3O4 nanocomposites: Experimental analysis, mathematical modeling, and molecular dynamics simulation. Prog. Org. Coat. 2017, 110, 172–181. [Google Scholar]
- Li, T.T.; Liu, X.Q.; Jiang, Y.H. Synthesis of epoxy curing agents containing different ring structures and properties investigation of the cured resins. J. Appl. Polym. Sci. 2016, 133, 1–9. [Google Scholar] [CrossRef]
- Rad, H.M.; Roosta, S.T.; Shariati, S.H.M.; Hosseini, S.G. Numerical Simulation of HTPB Resin Curing Process Using OpenFOAM and Study the Effect of Different Conditions on its Curing Time. Propellants Explos. Pyrotech. 2021, 46, 1447–1457. [Google Scholar] [CrossRef]
- Khan, A.; Savi, P.; Quaranta, S.; Rovere, M.; Giorcelli, M.; Tagliaferro, A.; Rosso, C.; Jia, C.Q. Low-Cost Carbon Fillers to Improve Mechanical Properties and Conductivity of Epoxy Composites. Polymers 2017, 9, 642. [Google Scholar] [CrossRef] [Green Version]
- Dong, M.; Zhang, H.; Tzounis, L. Multifunctional epoxy nanocomposites rein forced by two dimensional materials: A review. Carbon 2021, 185, 57–81. [Google Scholar] [CrossRef]
- Firouzjaei, M.D.; Karimiziarani, M.; Moradkhani, H.; Elliott, M.; Anasori, B. MXenes: The two-dimensional influencers. Mater. Today Adv. 2022, 13, 100202. [Google Scholar] [CrossRef]
- Xu, Z.; Sun, Y.; Zhuang, Y.; Jing, W.; Ye, H.; Cui, Z. Assembly of 2D MXene nanosheets and TiO2 nanoparticles for fabricating mesoporous TiO2-MXene membranes. J. Membr. Sci. 2018, 564, 35–43. [Google Scholar] [CrossRef]
- Gantayat, S.; Rout, D.; Swain, S.K. Carbon Nanomaterial–Reinforced Epoxy Composites: A Review. Polym. Technol. Eng. 2018, 57, 1–16. [Google Scholar] [CrossRef]
- Feng, A.L.; Hou, T.Q.; Jia, Z.R. Preparation and Characterization of Epoxy Resin Filled with Ti3C2Tx MXene Nanosheets with Excellent Electric Conductivity. Nanomaterials 2020, 10, 162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giménez, R.; Serrano, B.; San-Miguel, V.; Cabanelas, J.C. Recent Advances in MXene/Epoxy Composites: Trends and Prospects. Polymers 2022, 14, 1170. [Google Scholar] [CrossRef]
- Liu, L.; Ying, G.; Sun, C.; Min, H.; Zhang, J.; Zhao, Y.; Wang, C.; Wen, D.; Ji, Z.; Liu, X.; et al. MXene (Ti3C2Tx) Functionalized Short Carbon Fibers as a Cross-Scale Mechanical Reinforcement for Epoxy Composites. Polymers 2021, 13, 1825. [Google Scholar] [CrossRef]
- Sliozberg, Y.; Andzelm, J.; Hatter, C.B.; Anasori, B.; Gogotsi, Y.; Hall, A. Interface binding and mechanical properties of MXene-epoxy nanocomposites. Compos. Sci. Technol. 2020, 192, 108124. [Google Scholar] [CrossRef]
- Wang, D.; Wei, H.; Lin, Y.; Jiang, P.; Bao, H.; Huang, X. Achieving ultrahigh thermal conductivity in Ag/MXene/epoxy nanocomposites via filler-filler interface engineering. Compos. Sci. Technol. 2021, 213, 108953. [Google Scholar] [CrossRef]
- George, J.S.; Thomas, S. The Effect of Polymeric Inclusions and Nanofillers on Cure Kinetics of Epoxy Resin: A Review. Polym. Sci. Ser. A 2021, 63, 637–651. [Google Scholar] [CrossRef]
- Sbirrazzuoli, N. Model-free isothermal and nonisothermal predictions using advanced isoconversional methods. Thermochim. Acta 2020, 697, 178855. [Google Scholar] [CrossRef]
- Sbirrazzuoli, N. Interpretation and Physical Meaning of Kinetic Parameters Obtained from Isoconversional Kinetic Analysis of Polymers. Polymers 2020, 12, 1280. [Google Scholar] [CrossRef]
- Stanko, M.; Stommel, M. Kinetic Prediction of Fast Curing Polyurethane Resins by Model-Free Isoconversional Methods. Polymers 2018, 10, 698. [Google Scholar] [CrossRef] [Green Version]
- Gholinezhad, F.; Golhosseini, R.; Jazani, O.M. Non-isothermal DSC curing kinetics study of silicone-modified epoxy/ABS/GO nanocomposite. Polym. Compos. 2022, 43, 3794–3808. [Google Scholar] [CrossRef]
- Liu, F.; Yu, W.; Wang, Y.; Shang, R.; Zheng, Q. Curing kinetics and thixotropic properties of epoxy resin composites with different kinds of fillers. J. Mater. Res. Technol. 2022, 18, 2125–2139. [Google Scholar] [CrossRef]
- Filipe, H.A.L.; Loura, L.M.S. Molecular Dynamics Simulations: Advances and Applications. Molecules 2022, 27, 2105. [Google Scholar] [CrossRef] [PubMed]
- Sarafrazi, M.; Ghasemi, A.R.; Hamadanian, M. A Semi-analytical and Experimental Approach Using Molecular Dynamic Simulation for Thermo-mechanical Properties of Surface Functionalized Epoxy/Polyurethane/MWCNT/ZnMoO4 Nanocomposites. Fibers Polym. 2021, 22, 2306–2315. [Google Scholar] [CrossRef]
- Wang, M.; Liu, Y.; Zhang, H.; Wu, Y.; Pan, L. Thermal conductivities of Ti3C2Tx MXenes and their interfacial thermal performance in MXene/epoxy composites—A molecular dynamics simulation. Int. J. Heat Mass Transf. 2022, 194. [Google Scholar] [CrossRef]
- Feng, Y.; Luo, C.; Chen, X.; Gu, J.; Zhang, Y.; Chao, M.; Li, M.; Chen, T.; Chen, X.; Wang, X.; et al. Shell inspired heterogeneous membrane with smaller bandgap toward sunlight-activated sustainable water purification. Chem. Eng. J. 2022, 440, 135910. [Google Scholar] [CrossRef]
- Montserrat, S.; Málek, J. A kinetic analysis of the curing reaction of an epoxy resin. Thermochim. Acta 1993, 228, 47–60. [Google Scholar] [CrossRef]
- Montserrat, S.; Flaqué, C.; Pagès, P.; Málek, J. Effect of the crosslinking degree on curing kinetics of an epoxy–anhydride system. J. Appl. Polym. Sci. 1995, 56, 1413–1421. [Google Scholar] [CrossRef]
- Mustata, F.; Tudorachi, N. Curing kinetics and thermal characterization of epoxy resin cured with amidodicarboxylic acids. Appl. Therm. Eng. 2017, 125, 285–296. [Google Scholar] [CrossRef]
- Vyazovkin, S.A.; Wight, C. Model-free and model-fitting approaches to kinetic analysis of isothermal and nonisothermal data. Thermochim. Acta 1999, 340-341, 53–68. [Google Scholar] [CrossRef]
- Flynn, J.H.; Wall, L.A. General treatment of the thermogravimetry of polymers. J. Res. Natl. Bur. Stand. Sect. A Phys. Chem. 1966, 70A, 487–523. [Google Scholar] [CrossRef] [PubMed]
- Ozawa, T. A New Method of Analyzing Thermogravimetric Data. Bull. Chem. Soc. Jpn. 1965, 38, 1881–1886. [Google Scholar] [CrossRef] [Green Version]
- Kissinger, H.E. Reaction Kinetics in Differential Thermal Analysis. Anal. Chem. 1957, 29, 1702–1706. [Google Scholar] [CrossRef]
- Akahira, T.; Sunose, T. Method of determining activation deterioration constant ofelectrical insulating materials. Res. Rep. Chiba Inst. Technol. (Sci. Technol.) 1971, 16, 22–31. [Google Scholar]
- Senum, G.I.; Yang, R.T. Rational approximations of the integral of the Arrhenius function. J. Therm. Anal. 1977, 11, 445–447. [Google Scholar] [CrossRef]
- Shenogina, N.B.; Tsige, M.; Patnaik, S.S.; Mukhopadhyay, S.M. Molecular Modeling Approach to Prediction of Thermo-Mechanical Behavior of Thermoset Polymer Networks. Macromolecules 2012, 45, 5307–5315. [Google Scholar] [CrossRef]
- Sun, H.; Jin, Z.; Yang, C.; Akkermans, R.L.; Robertson, S.H.; Spenley, N. A COMPASS II: Extended coverage for polymer and drug-like molecule databases. J. Mol. Modeling 2016, 47, 1–10. [Google Scholar] [CrossRef]
- Lei, D.; Liu, N.; Su, T.; Zhang, Q.; Wang, L.; Ren, Z.; Gao, Y. Roles of MXene in Pressure Sensing: Preparation, Composite Structure Design, and Mechanism. Adv. Mater. 2022. [Google Scholar] [CrossRef]
- Sbirrazzuoli, N.; Mititelu-Mija, A.; Vincent, L.; Alzina, C. Isoconversional kinetic analysis of stoichiometric and off-stoichiometric epoxy-amine cures. Thermochim. Acta 2006, 447, 167–177. [Google Scholar] [CrossRef]
- Lucci, A.; Tamanini, M. Methods of non-isothermal kinetic analysis by DSC curves: An investigation on lattice reorganization in copper. Thermochim. Acta 1975, 13, 147–163. [Google Scholar] [CrossRef]
- Feng, L.; Wang, Y.; Wang, Y.; Liu, H.; Zhao, J. Study on reaction kinetics of epoxy resin cured by a modified dicyandiamide. J. Appl. Polym. Sci. 2013, 127, 1895–1900. [Google Scholar] [CrossRef]
- Chen, X.; Jiang, W.; Hu, B.; Liang, Z.; Zhang, Y.; Kang, J.; Cao, Y.; Xiang, M. Effects of graphene oxide size on curing kinetics of epoxy resin. RSC Adv. 2021, 11, 29215–29226. [Google Scholar] [CrossRef] [PubMed]
- Fang, M.; Wang, K.; Lu, H.; Yang, Y.; Nutt, S. Covalent polymer functionalization of graphene nanosheets and mechanical properties of composites. J. Mater. Chem. 2009, 19, 7098–7105. [Google Scholar] [CrossRef]
- Miller, R.; Oebser, M. Determination of cure times and activation energies of one-container epoxy resin systems by isothermal DSC. Thermochim. Acta 1980, 36, 121–131. [Google Scholar] [CrossRef]
- Ma, H.; Zhang, X.; Ju, F.; Tsai, S.-B. A Study on Curing Kinetics of Nano-Phase Modified Epoxy Resin. Sci. Rep. 2018, 8, 3045. [Google Scholar] [CrossRef]
- Wazalwar, R.; Raichur, A.M. Model-free cure kinetics of tetra-functional epoxy reinforced with GO and p-Phenylenediamine modified GO. Thermochim. Acta 2020, 697, 178857. [Google Scholar] [CrossRef]
- Wu, F.; Zhou, X.P.; Yu, X.H. Reaction mechanism, cure behavior and properties of a multifunctional epoxy resin, TGDDM, with latent curing agent dicyandiamide. RSC Adv. 2018, 8, 8248–8258. [Google Scholar] [CrossRef] [Green Version]
- Gao, L.; Zhang, Q.J.; Li, H. Effect of epoxy monomer structure on the curing process and thermo-mechanical characteristics of tri-functional epoxy/amine systems: A methodology combined atomistic molecular simulation with experimental analyses. Polym. Chem. 2017, 8, 2016–2027. [Google Scholar] [CrossRef]
- Nayak, L.; Rahaman, M.; Khastgir, D.; Chaki, T.K. Thermal degradation kinetics of polyimide nanocomposites from different carbon nanofillers: Applicability of different theoretical models. J. Appl. Polym. Sci. 2017, 135. [Google Scholar] [CrossRef]
- Vertuccio, L.; Russo, S.; Raimondo, M.; Lafdi, K.; Guadagno, L. Influence of carbon nanofillers on the curing kinetics of epoxy-amine resin. RSC Adv. 2015, 110, 90437–90450. [Google Scholar] [CrossRef]
- Bednarczyk, P.; Irska, I.; Gziut, K.; Ossowicz-Rupniewska, P. Synthesis of Epoxy Methacrylate Resin and Coatings Preparation by Cationic and Radical Photocrosslinking. Molecules 2021, 26, 7663. [Google Scholar] [CrossRef] [PubMed]
- Šesták, J.; Berggren, G. Study of the kinetics of the mechanism of solid-state reactions at increasing temperatures. Thermochim. Acta 1971, 3, 1–12. [Google Scholar] [CrossRef]
- Gibson, R.L.; Simmons, M.J.; Stitt, E.H.; West, J.; Wilkinson, S.K.; Gallen, R.W. Kinetic modelling of thermal processes using a modified Sestak-Berggren equation. Chem. Eng. J. 2020, 408, 127318. [Google Scholar] [CrossRef]
- Tao, Q.; Pinter, G.; Krivec, T. Influence of cooling rate and annealing on the DSC Tg of an epoxy resin. Microelectron. Reliab. 2017, 78, 396–400. [Google Scholar] [CrossRef]
- Kang, R.; Zhang, Z.; Guo, L.; Cui, J.; Chen, Y.; Hou, X.; Wang, B.; Lin, C.-T.; Jiang, N.; Yu, J. Enhanced Thermal Conductivity of Epoxy Composites Filled with 2D Transition Metal Carbides (MXenes) with Ultralow Loading. Sci. Rep. 2019, 9, 9135. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Wang, L.; Chen, Q.; Li, P.; Zhou, A.; Cao, X.; Hu, Q. Preparation, mechanical and anti-friction performance of MXene/polymer composites. Mater. Des. 2016, 92, 682–689. [Google Scholar] [CrossRef]
- Soldera, A.; Metatla, N. Glass transition of polymers: Atomistic simulation versus experiments. Phys. Rev. E 2006, 74, 061803. [Google Scholar] [CrossRef]
- Červinka, C.; Fulem, M. Structure and Glass Transition Temperature of Amorphous Dispersions of Model Pharmaceuticals with Nucleobases from Molecular Dynamics. Pharmaceutics 2021, 13, 1253. [Google Scholar] [CrossRef]
- Li, W.; de la Cruz, M.O. Glass transition of ion-containing polymer melts in bulk and thin films. Soft Matter 2021, 17, 8420–8433. [Google Scholar] [CrossRef]
- Zhang, W.Q.; Li, H.; Gao, L. Molecular Simulation and Experimental Analysis on Thermal and Mechanical Properties of Carbon Nanotube/Epoxy Resin Composites with Different Curing Agents at High-Low Temperature. Polym. Compos. 2018, 39, E945–E954. [Google Scholar] [CrossRef]
- Moynihan, C.T.; Easteal, A.J.; Wilder, J.; Tucker, J. Dependence of the glass transition temperature on heating and cooling rate. J. Phys. Chem. 1974, 78, 2673–2677. [Google Scholar] [CrossRef]
- Montserrat, S.; Calventus, Y.; Hutchinson, J. Effect of cooling rate and frequency on the calorimetric measurement of the glass transition. Polymer 2005, 46, 12181–12189. [Google Scholar] [CrossRef]
- White, R.P.; Lipson, J.E.G. Polymer Free Volume and Its Connection to the Glass Transition. Macromolecules 2016, 49, 3987–4007. [Google Scholar] [CrossRef]
- Song, M.; Yue, X.; Wang, X.; Cao, F.; Li, Y.; Su, C.; Qin, Q. Effect of Hindered Phenol AO-80 on the Damping Properties for Nitrile-Butadiene Rubber/Phenolic Resin: Molecular Simulation and Experimental Study. Macromol. Mater. Eng. 2020, 305. [Google Scholar] [CrossRef]

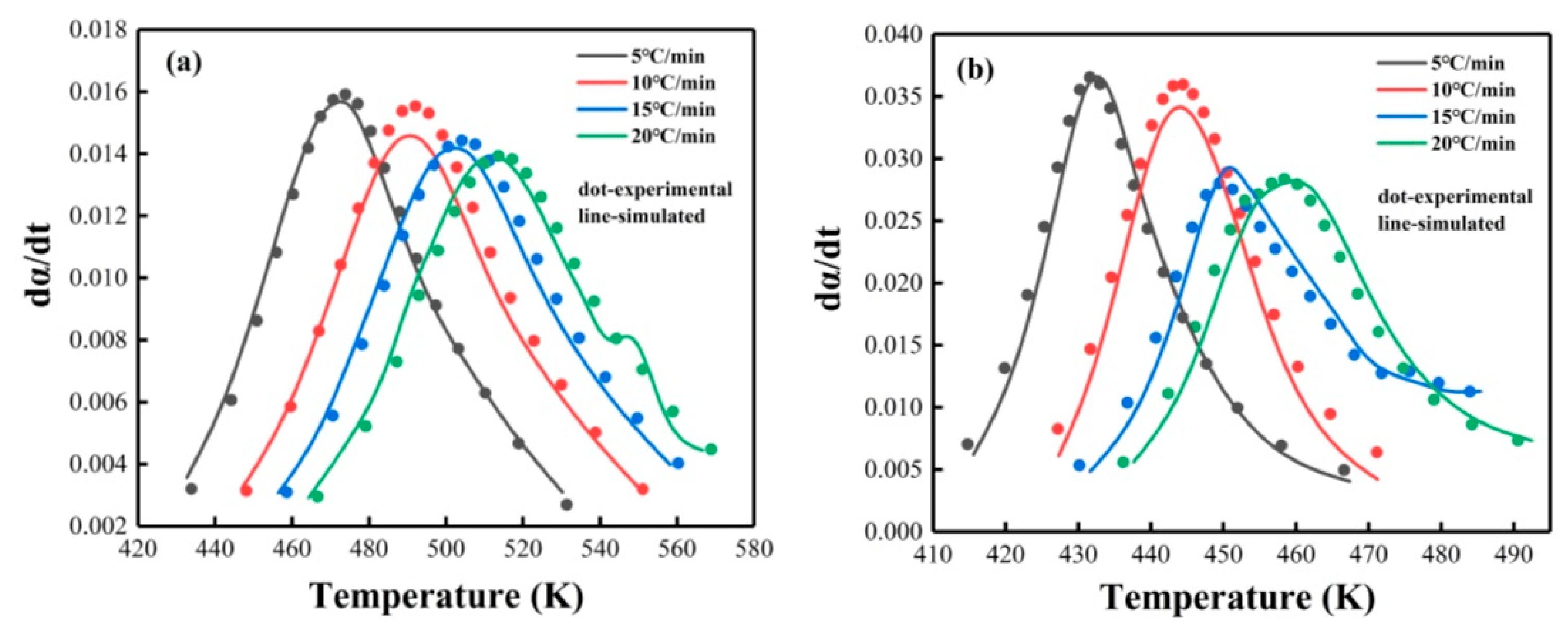
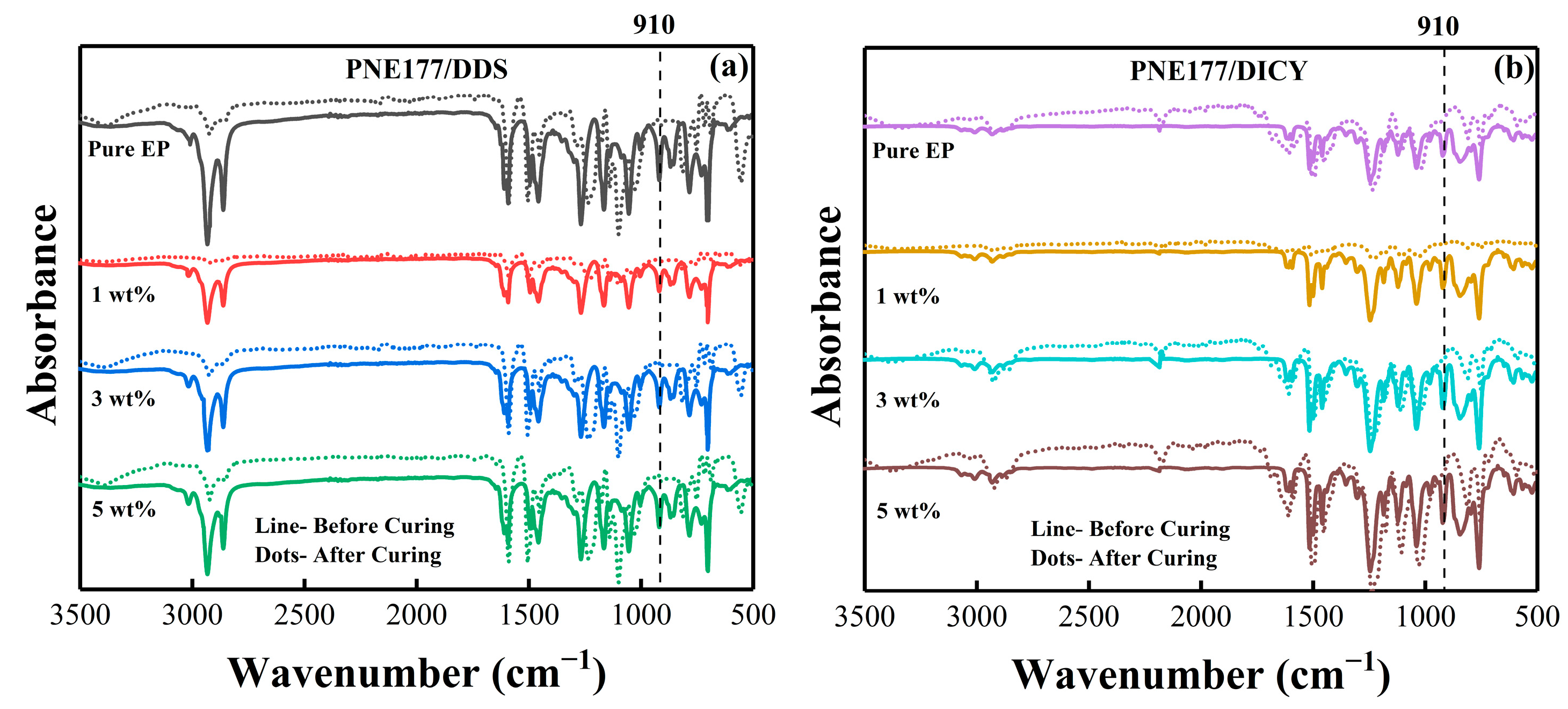

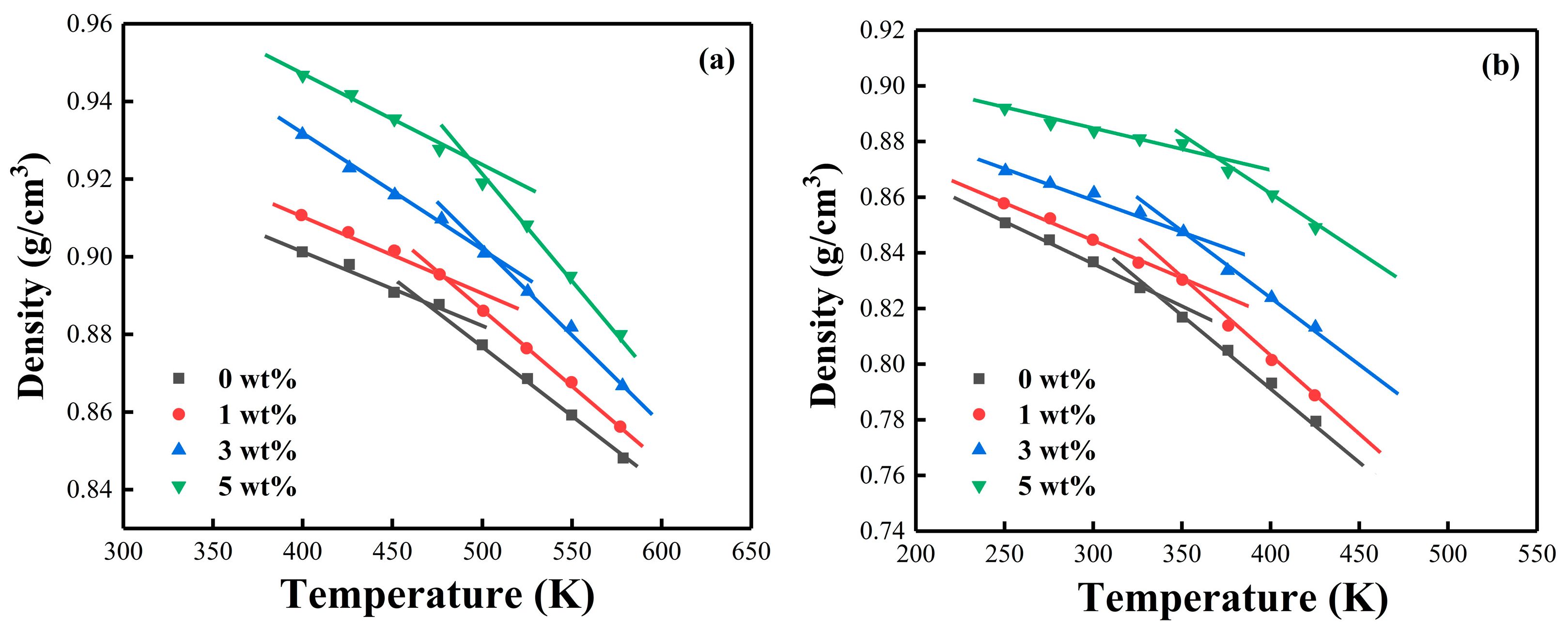
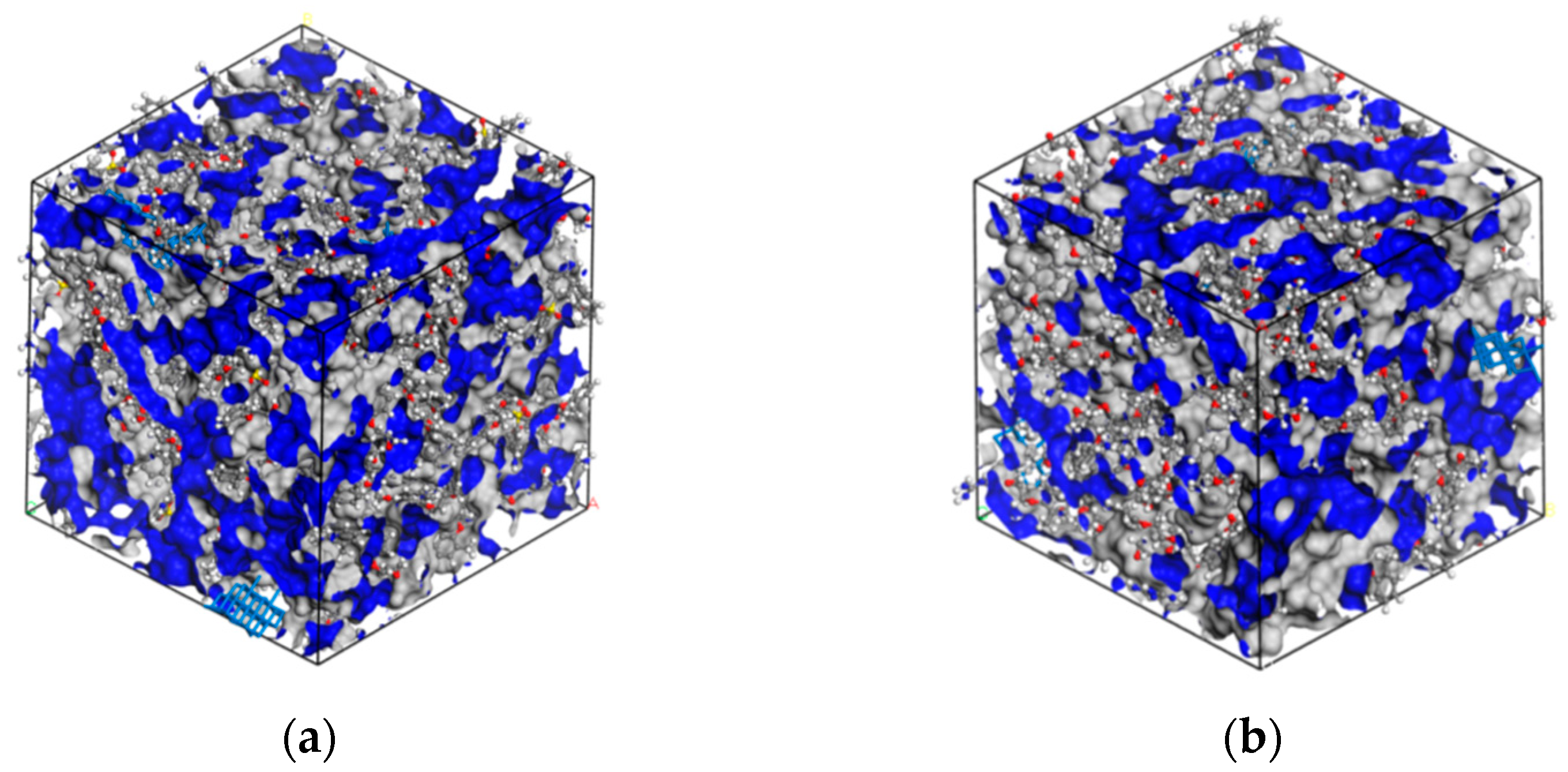
| Epoxy System | Β (°C/min) | Ti (°C) | Tp (°C) | Tf (°C) |
|---|---|---|---|---|
| PNE-177/DDS | 5 | 114.55 | 200.16 | 285.77 |
| 10 | 120.01 | 217.77 | 298.77 | |
| 15 | 124.47 | 228.87 | 300.25 | |
| 20 | 129.00 | 239.03 | 306.01 | |
| PNE-177/DICY | 5 | 113.8 | 158.89 | 204.77 |
| 10 | 121.88 | 170.77 | 207.51 | |
| 5 | 125.44 | 176.75 | 215.31 | |
| 20 | 131.83 | 184.91 | 224.61 |
| Epoxy System | MXene (wt%) | Ti (°C) | Tp (°C) | Tf (°C) |
|---|---|---|---|---|
| PNE-177/DDS | 0 | 110.05 | 189.53 | 282.15 |
| 1 | 121.62 | 191.73 | 286.91 | |
| 3 | 122.875 | 195.2 | 276.05 | |
| 5 | 129.72 | 195.8 | 271.25 | |
| PNE-177/DICY | 0 | 108.83 | 151.82 | 196.22 |
| 1 | 111.99 | 152.27 | 196.64 | |
| 3 | 118.04 | 152.07 | 192.87 | |
| 5 | 117.7 | 153.08 | 191.85 |
| Epoxy System | MXene (wt%) | FWO Ea (kJ/mol) | KAS Ea (kJ/mol) |
|---|---|---|---|
| PNE-177/DDS | 0 | 72.25 | 64.40 |
| 1 | 71.37 | 61.28 | |
| 3 | 71.88 | 61.02 | |
| 5 | 77.14 | 64.29 | |
| PNE-177/DICY | 0 | 85.14 | 82.33 |
| 1 | 104.42 | 89.51 | |
| 3 | 102.85 | 81.47 | |
| 5 | 89.49 | 78.42 |
| Epoxy System | MXene (wt%) | Experimental Tg (K) | Simulation Tg (K) |
|---|---|---|---|
| PNE-177/DDS | 0 | 348.33 | 366.67 |
| 1 | 354.27 | 375.31 | |
| 3 | 372.77 | 389.12 | |
| 5 | 375.54 | 396.08 | |
| PNE-177/DICY | 0 | 425.50 | 437.27 |
| 1 | 437.06 | 449.58 | |
| 3 | 439.28 | 452.09 | |
| 5 | 456.61 | 471.34 |
| Epoxy System | MXene (wt%) | Vf (Å3) | VT (Å3) | FFV (%) |
|---|---|---|---|---|
| PNE-177/DDS | 0 | 2578.3 | 12,710.9 | 20.28 |
| 1 | 2331.88 | 12,797.6 | 18.22 | |
| 3 | 2207.69 | 12,840.4 | 17.19 | |
| 5 | 2134.34 | 12,894.5 | 16.55 | |
| PNE-177/DICY | 0 | 2112.54 | 11,548.2 | 18.29 |
| 1 | 1899.41 | 11,625.8 | 16.34 | |
| 3 | 1802.55 | 11,709.9 | 15.39 | |
| 5 | 1743.09 | 11,774.3 | 14.80 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cai, R.; Zhao, J.; Lv, N.; Fu, A.; Yin, C.; Song, C.; Chao, M. Curing and Molecular Dynamics Simulation of MXene/Phenolic Epoxy Composites with Different Amine Curing Agent Systems. Nanomaterials 2022, 12, 2249. https://doi.org/10.3390/nano12132249
Cai R, Zhao J, Lv N, Fu A, Yin C, Song C, Chao M. Curing and Molecular Dynamics Simulation of MXene/Phenolic Epoxy Composites with Different Amine Curing Agent Systems. Nanomaterials. 2022; 12(13):2249. https://doi.org/10.3390/nano12132249
Chicago/Turabian StyleCai, Rui, Jinlong Zhao, Naixin Lv, Anqing Fu, Chengxian Yin, Chengjun Song, and Min Chao. 2022. "Curing and Molecular Dynamics Simulation of MXene/Phenolic Epoxy Composites with Different Amine Curing Agent Systems" Nanomaterials 12, no. 13: 2249. https://doi.org/10.3390/nano12132249
APA StyleCai, R., Zhao, J., Lv, N., Fu, A., Yin, C., Song, C., & Chao, M. (2022). Curing and Molecular Dynamics Simulation of MXene/Phenolic Epoxy Composites with Different Amine Curing Agent Systems. Nanomaterials, 12(13), 2249. https://doi.org/10.3390/nano12132249






