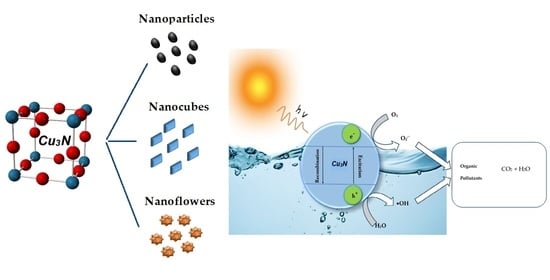
Surveying the Synthesis, Optical Properties and Photocatalytic Activity of Cu3N Nanomaterials
Abstract
:1. Introduction
2. Synthesis of Cu3N Nanomaterials
2.1. Gas-state Synthesis and the Ammonia Source
2.2. Solution-Based Synthesis
2.2.1. Effect of Solvent
2.2.2. Effect of Capping Agent
2.2.3. Effect of Reaction Time
2.2.4. Formation of Secondary Phases
2.3. Thin Film Deposition Techniques
3. Electronic Structure and Band Gap
4. Photocatalytic Activity of Cu3N Nanoparticles
5. Other Applications of Cu3N
6. Summary and Outlook
Author Contributions
Funding
Informed Consent Statement
Conflicts of Interest
References
- Biswas, P.; Wu, C.Y. Nanoparticles and the environment. J. Air Waste Manag. Assoc. 2005, 55, 708–746. [Google Scholar] [CrossRef] [PubMed]
- Jamkhande, P.G.; Ghule, N.W.; Bamer, A.H.; Kalaskar, M.G. Metal nanoparticles synthesis: An overview on methods of preparation, advantages and disadvantages, and applications. J. Drug Deliv. Sci. Technol. 2019, 53, 101174. [Google Scholar] [CrossRef]
- Panigrahi, S.; Kundu, S.; Ghosh, S.; Nath, S.; Pal, T. General method of synthesis for metal nanoparticles. J. Nanopart. Res. 2004, 6, 411–414. [Google Scholar] [CrossRef]
- Chen, P.; Xu, K.; Tong, Y.; Li, X.; Tao, S.; Fang, Z.; Chu, W.; Wu, X.; Wu, C. Cobalt nitrides as a class of metallic electrocatalysts for the oxygen evolution reaction. Inorg. Chem. Front. 2016, 3, 236–242. [Google Scholar] [CrossRef]
- Braic, M.; Balaceanu, M.; Braic, V.; Vladescu, A.; Pavelescu, G.; Albulescu, M. Synthesis and characterization of TiN, TiAIN and TiN/TiAIN biocompatible coatings. Surf. Coat. Technol. 2005, 200, 1014–1017. [Google Scholar] [CrossRef]
- Yu, R.; Zhan, Q.; De Jonghe, L.C. Crystal Structures of and Displacive Transitions in OsN2, IrN2, RuN2, and RhN2. Angew. Chem. Int. Ed. 2007, 46, 1136–1140. [Google Scholar] [CrossRef]
- Prabha, S.; Durgalakshmi, D.; Subramani, K.; Aruna, P.; Ganesan, S. Enhanced Emission of Zinc Nitride Colloidal Nanoparticles with Organic Dyes for Optical Sensors and Imaging Application. ACS Appl. Mater. Interfaces 2020, 12, 19245–19257. [Google Scholar] [CrossRef]
- Young, A.F.; Sanloup, C.; Gregoryanz, E.; Scandolo, S.; Hemley, R.J.; Mao, H.K. Synthesis of novel transition metal nitrides IrN2 and OsN2. Phys. Rev. Lett. 2006, 96, 155501. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Li, J.; Li, K.; Lin, Y.; Chen, J.; Gao, L.; Nicolosi, V.; Xiao, X.; Lee, J.-M. Transition metal nitrides for electrochemical energy applications. Chem. Soc. Rev. 2021, 50, 1354–1390. [Google Scholar] [CrossRef]
- Zhu, C.; Yang, P.; Chao, D.; Wang, X.; Zhang, X.; Chen, S.; Tay, B.K.; Huang, H.; Zhang, H.; Mai, W.; et al. All Metal Nitrides Solid-State Asymmetric Supercapacitors. Adv. Mater. 2015, 27, 4566–4571. [Google Scholar] [CrossRef]
- Xie, J.; Xie, Y. Transition Metal Nitrides for Electrocatalytic Energy Conversion: Opportunities and Challenges. Chem. A Eur. J. 2016, 22, 3588–3598. [Google Scholar] [CrossRef] [PubMed]
- Mosavati, N.; Salley, S.O.; Ng, K.Y.S. Characterization and electrochemical activities of nanostructured transition metal nitrides as cathode materials for lithium sulfur batteries. J. Power Sources 2017, 340, 210–216. [Google Scholar] [CrossRef] [Green Version]
- Hargreaves, J.S.J. Heterogeneous catalysis with metal nitrides. Coord. Chem. Rev. 2013, 257, 2015–2031. [Google Scholar] [CrossRef]
- Dong, S.; Chen, X.; Zhang, X.; Cui, G. Nanostructured transition metal nitrides for energy storage and fuel cells. Coord. Chem. Rev. 2013, 257, 1946–1956. [Google Scholar] [CrossRef]
- Zakutayev, A. Design of nitride semiconductors for solar energy conversion. J. Mater. Chem. A 2016, 4, 6742–6754. [Google Scholar] [CrossRef]
- Dylewicz, R.; Patela, S.; Paszkiewicz, R. Applications of GaN-Based Materials in Modern Optoelectronics; SPIE: Bellingham, DC, USA, 2004; Volume 5484. [Google Scholar] [CrossRef]
- Bochkareva, N.I.; Gorbunov, R.I.; Klochkov, A.V.; Lelikov, Y.S.; Martynov, I.A.; Rebane, Y.T.; Belov, A.S.; Shreter, Y.G. Optical properties of blue light-emitting diodes in the InGaN/GaN system at high current densities. Semiconductors 2008, 42, 1355–1361. [Google Scholar] [CrossRef]
- Pankove, J.I. GaN: From fundamentals to applications. Mater. Sci. Eng. B 1999, 61–62, 305–309. [Google Scholar] [CrossRef]
- Lian, J.; Li, D.; Qi, Y.; Yang, N.; Zhang, R.; Xie, T.; Guan, N.; Li, L.; Zhang, F. Metal-seed assistant photodeposition of platinum over Ta3N5 photocatalyst for promoted solar hydrogen production under visible light. J. Energy Chem. 2021, 55, 444–448. [Google Scholar] [CrossRef]
- Menon, S.S.; Hafeez, H.Y.; Gupta, B.; Baskar, K.; Bhalerao, G.; Hussain, S.; Neppolian, B.; Singh, S. ZnO:InN oxynitride: A novel and unconventional photocatalyst for efficient UV–visible light driven hydrogen evolution from water. Renew. Energy 2019, 141, 760–769. [Google Scholar] [CrossRef]
- Singh, P.; Ruterana, P.; Morales, M.; Goubilleau, F.; Wojdak, M.; Carlin, J.F.; Ilegems, M.; Chateigner, D. Structural and optical characterisation of InN layers grown by MOCVD. Superlattices Microstruct. 2004, 36, 537–545. [Google Scholar] [CrossRef]
- Wang, L.; Zhao, W.; Hao, Z.-B.; Luo, Y. Photocatalysis of InGaN Nanodots Responsive to Visible Light. Chin. Phys. Lett. 2011, 28, 057301. [Google Scholar] [CrossRef]
- Sánchez, A.M.; Gass, M.; Papworth, A.J.; Goodhew, P.J.; Singh, P.; Ruterana, P.; Cho, H.K.; Choi, R.J.; Lee, H.J. V-defects and dislocations in InGaN/GaN heterostructures. Thin Solid Film. 2005, 479, 316–320. [Google Scholar] [CrossRef]
- Sithole, R.K.; Machogo, L.F.E.; Airo, M.A.; Gqoba, S.S.; Moloto, M.J.; Shumbula, P.; Van Wyk, J.; Moloto, N. Synthesis and characterization of Cu3N nanoparticles using pyrrole-2-carbaldpropyliminato Cu(ii) complex and Cu(NO3)2 as single-source precursors: The search for an ideal precursor. New J. Chem. 2018, 42, 3042–3049. [Google Scholar] [CrossRef]
- Xiao, J.; Qi, M.; Cheng, Y.; Jiang, A.; Zeng, Y.; Ma, J. Influences of nitrogen partial pressure on the optical properties of copper nitride films. RSC Adv. 2016, 6, 40895–40899. [Google Scholar] [CrossRef]
- Mondal, S.; Raj, C.R. Copper Nitride Nanostructure for the Electrocatalytic Reduction of Oxygen: Kinetics and Reaction Pathway. J. Phys. Chem. C 2018, 122, 18468–18475. [Google Scholar] [CrossRef]
- Nakamura, T.; Cheong, H.J.; Takamura, M.; Yoshida, M.; Uemura, S. Suitability of Copper Nitride as a Wiring Ink Sintered by Low-Energy Intense Pulsed Light Irradiation. Nanomaterials 2018, 8, 617. [Google Scholar] [CrossRef] [Green Version]
- Scigala, A.; Szłyk, E.; Rerek, T.; Wiśniewski, M.; Skowronski, L.; Trzcinski, M.; Szczesny, R. Copper Nitride Nanowire Arrays—Comparison of Synthetic Approaches. Materials 2021, 14, 603. [Google Scholar] [CrossRef]
- Lindahl, E.; Ottosson, M.; Carlsson, J.-O. Doping of metastable Cu3N at different Ni concentrations: Growth, crystallographic sites and resistivity. Thin Solid Film. 2018, 647, 1–8. [Google Scholar] [CrossRef]
- Jiang, A.; Xiao, J.; Gong, C.; Wang, Z.; Ma, S. Structure and electrical transport properties of Pb-doped copper nitride (Cu3N:Pb) films. Vacuum 2019, 164, 53–57. [Google Scholar] [CrossRef]
- Joo Kim, K.; Hyuk Kim, J.; Hoon Kang, J. Structural and optical characterization of Cu3N films prepared by reactive RF magnetron sputtering. J. Cryst. Growth 2001, 222, 767–772. [Google Scholar] [CrossRef]
- Zhu, W.; Zhang, X.; Fu, X.; Zhou, Y.; Luo, S.; Wu, X. Resistive-switching behavior and mechanism in copper-nitride thin films prepared by DC magnetron sputtering. Phys. Status Solidi 2012, 209, 1996–2001. [Google Scholar] [CrossRef]
- Kadzutu-Sithole, R.; Machogo-Phao, L.F.E.; Kolokoto, T.; Zimuwandeyi, M.; Gqoba, S.S.; Mubiayi, K.P.; Moloto, M.J.; Van Wyk, J.; Moloto, N. Elucidating the effect of precursor decomposition time on the structural and optical properties of copper(i) nitride nanocubes. RSC Adv. 2020, 10, 34231–34246. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.-C.; Liu, B.-H.; Su, C.-Y.; Liu, W.-S.; Kei, C.-C.; Wang, K.-W.; Perng, T.-P. Electronic Band Structure and Electrocatalytic Performance of Cu3N Nanocrystals. ACS Appl. Nano Mater. 2018, 1, 3673–3681. [Google Scholar] [CrossRef]
- Zhu, L.; Gong, C.; Xiao, J.; Wang, Z. Photocatalytic Properties of Copper Nitride/Molybdenum Disulfide Composite Films Prepared by Magnetron Sputtering. Coatings 2020, 10, 79. [Google Scholar] [CrossRef] [Green Version]
- Rahmati, A. Ti-containing Cu3N nanostructure thin films: Experiment and simulation on reactive magnetron sputter-assisted nitridation. IEEE Trans. Plasma Sci. 2015, 43, 1969–1973. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, Q.; Huang, S.; Zhang, J.; Ren, S.; Wang, H.; Wang, L.; Yang, T.; Yang, J.; Li, X. Effect of Magnetic Transition Metal (TM = V, Cr, and Mn) Dopant on Characteristics of Copper Nitride. J. Supercond. Nov. Magn. 2016, 29, 2351–2357. [Google Scholar] [CrossRef]
- Barman, D.; Paul, S.; Ghosh, S.; De, S.K. Cu3N Nanocrystals Decorated with Au Nanoparticles for Photocatalytic Degradation of Organic Dyes. ACS Appl. Nano Mater. 2019, 2, 5009–5019. [Google Scholar] [CrossRef]
- Cheng, Z.; Qi, W.; Pang, C.H.; Thomas, T.; Wu, T.; Liu, S.; Yang, M. Recent Advances in Transition Metal Nitride-Based Materials for Photocatalytic Applications. Adv. Funct. Mater. 2021, 31, 2100553. [Google Scholar] [CrossRef]
- Birkett, M.; Savory, C.N.; Fioretti, A.N.; Thompson, P.; Muryn, C.A.; Weerakkody, A.D.; Mitrovic, I.Z.; Hall, S.; Treharne, R.; Dhanak, V.R.; et al. Atypically small temperature-dependence of the direct band gap in the metastable semiconductor copper nitride Cu3N. Phys. Rev. B 2017, 95, 115201. [Google Scholar] [CrossRef] [Green Version]
- Zhu, S.; Wang, D. Photocatalysis: Basic Principles, Diverse Forms of Implementations and Emerging Scientific Opportunities. Adv. Energy Mater. 2017, 7, 1700841. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.; Li, X.A.; Zhao, J.; Wu, Z.; Yang, T.; Ma, Y.; Huang, W.; Yao, K. First principles study on the influence of electronic configuration of M on Cu3NM: M = Sc, Ti, V, Cr, Mn, Fe, Co, Ni. Comput. Theor. Chem. 2014, 1027, 33–38. [Google Scholar] [CrossRef]
- Ashraf, I.; Rizwan, S.; Iqbal, M. A Comprehensive Review on the Synthesis and Energy Applications of Nano-structured Metal Nitrides. Front. Mater. 2020, 7, 181. [Google Scholar] [CrossRef]
- Rao, T.; Cai, W.; Zhang, H.; Liao, W. Nanostructured metal nitrides for photocatalysts. J. Mater. Chem. C 2021, 9, 5323–5342. [Google Scholar] [CrossRef]
- Mazumder, B.; Hector, A.L. Synthesis and applications of nanocrystalline nitride materials. J. Mater. Chem. 2009, 19, 4673–4686. [Google Scholar] [CrossRef]
- Panda, C.; Menezes, P.W.; Zheng, M.; Orthmann, S.; Driess, M. In Situ Formation of Nanostructured Core–Shell Cu3N–CuO to Promote Alkaline Water Electrolysis. ACS Energy Lett. 2019, 4, 747–754. [Google Scholar] [CrossRef]
- Wu, H.; Chen, W. Copper Nitride Nanocubes: Size-Controlled Synthesis and Application as Cathode Catalyst in Alkaline Fuel Cells. J. Am. Chem. Soc. 2011, 133, 15236–15239. [Google Scholar] [CrossRef]
- Sithole, R.K.; Machogo, L.F.E.; Moloto, M.J.; Gqoba, S.S.; Mubiayi, K.P.; Van Wyk, J.; Moloto, N. One-step synthesis of Cu3N, Cu2S and Cu9S5 and photocatalytic degradation of methyl orange and methylene blue. J. Photochem. Photobiol. A Chem. 2020, 397, 112577. [Google Scholar] [CrossRef]
- Wang, D.; Li, Y. Controllable synthesis of Cu-based nanocrystals in ODA solvent. Chem. Commun. 2011, 47, 3604–3606. [Google Scholar] [CrossRef]
- Xi, P.; Xu, Z.; Gao, D.; Chen, F.; Xue, D.; Tao, C.-L.; Chen, Z.-N. Solvothermal synthesis of magnetic copper nitride nanocubes with highly electrocatalytic reduction properties. RSC Adv. 2014, 4, 14206–14209. [Google Scholar] [CrossRef]
- Egeberg, A.; Warmuth, L.; Riegsinger, S.; Gerthsen, D.; Feldmann, C. Pyridine-based low-temperature synthesis of CoN, Ni3N and Cu3N nanoparticles. Chem. Commun. 2018, 54, 9957–9960. [Google Scholar] [CrossRef]
- Sithole, R.K.; Kolokoto, T.; Machogo, L.F.E.; Ngubeni, G.N.; Moloto, M.J.; Van Wyk, J.; Moloto, N. Simultaneous capping and substitution of nitrogen ions of Cu3N nanocrystals with sulfur ions using DDT as a co-surfactant to form chalcocite and digenite nanocrystals. Mater. Chem. Phys. 2020, 251, 123074. [Google Scholar] [CrossRef]
- Nakamura, T.; Hayashi, H.; Hanaoka, T.-A.; Ebina, T. Preparation of Copper Nitride (Cu3N) Nanoparticles in Long-Chain Alcohols at 130–200 °C and Nitridation Mechanism. Inorg. Chem. 2014, 53, 710–715. [Google Scholar] [CrossRef] [PubMed]
- Deshmukh, R.; Zeng, G.; Tervoort, E.; Staniuk, M.; Wood, D.W.; Niederberger, M. Ultrasmall Cu3N Nanoparticles: Surfactant-Free Solution-Phase Synthesis, Nitridation Mechanism, and Application for Lithium Storage. Chem. Mater. 2015, 27, 8282–8288. [Google Scholar] [CrossRef]
- Li, X.; Hector, A.L.; Owen, J.R. Evaluation of Cu3N and CuO as Negative Electrode Materials for Sodium Batteries. J. Phys. Chem. C 2014, 118, 29568–29573. [Google Scholar] [CrossRef]
- Nakamura, T.; Hiyoshi, N.; Hayashi, H.; Ebina, T. Preparation of plate-like copper nitride nanoparticles from a fatty acid copper(II) salt and detailed observations by high resolution transmission electron microscopy and high-angle annular dark-field scanning transmission electron microscopy. Mater. Lett. 2015, 139, 271–274. [Google Scholar] [CrossRef] [Green Version]
- Miura, A.; Takei, T.; Kumada, N. Synthesis of Cu3N from CuO and NaNH2. J. Asian Ceram. Soc. 2014, 2, 326–328. [Google Scholar] [CrossRef] [Green Version]
- Szczęsny, R.; Szłyk, E.; Wiśniewski, M.A.; Hoang, T.K.A.; Gregory, D.H. Facile preparation of copper nitride powders and nanostructured films. J. Mater. Chem. C 2016, 4, 5031–5037. [Google Scholar] [CrossRef] [Green Version]
- Choi, J.; Gillan, E.G. Solvothermal Synthesis of Nanocrystalline Copper Nitride from an Energetically Unstable Copper Azide Precursor. Inorg. Chem. 2005, 44, 7385–7393. [Google Scholar] [CrossRef]
- Paniconi, G.; Stoeva, Z.; Doberstein, H.; Smith, R.I.; Gallagher, B.L.; Gregory, D.H. Structural chemistry of Cu3N powders obtained by ammonolysis reactions. Solid State Sci. 2007, 9, 907–913. [Google Scholar] [CrossRef]
- Vaughn Ii, D.D.; Araujo, J.; Meduri, P.; Callejas, J.F.; Hickner, M.A.; Schaak, R.E. Solution Synthesis of Cu3PdN Nanocrystals as Ternary Metal Nitride Electrocatalysts for the Oxygen Reduction Reaction. Chem. Mater. 2014, 26, 6226–6232. [Google Scholar] [CrossRef]
- Reichert, M.D.; White, M.A.; Thompson, M.J.; Miller, G.J.; Vela, J. Preparation and Instability of Nanocrystalline Cuprous Nitride. Inorg. Chem. 2015, 54, 6356–6362. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Primc, D.; Indrizzi, L.; Tervoort, E.; Xie, F.; Niederberger, M. Synthesis of Cu3N and Cu3N–Cu2O multicomponent mesocrystals: Non-classical crystallization and nanoscale Kirkendall effect. Nanoscale 2021, 13, 17521–17529. [Google Scholar] [CrossRef] [PubMed]
- Mukhopadhyay, A.K.; Momin, M.A.; Roy, A.; Das, S.C.; Majumdar, A. Optical and Electronic Structural Properties of Cu3N Thin Films: A First-Principles Study (LDA + U). ACS Omega 2020, 5, 31918–31924. [Google Scholar] [CrossRef]
- Jiang, A.; Shao, H.; Zhu, L.; Ma, S.; Xiao, J. Preparation of Copper Nitride Films with Superior Photocatalytic Activity through Magnetron Sputtering. Materials 2020, 13, 4325. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, G.; Meher, S.R.; Jain, M.K. Room temperature growth of high crystalline quality Cu3N thin films by modified activated reactive evaporation. Mater. Sci. Eng. B 2015, 191, 7–14. [Google Scholar] [CrossRef]
- Ali, H.T.; Tanveer, Z.; Javed, M.R.; Mahmood, K.; Amin, N.; Ikram, S.; Ali, A.; Shah Gilani, M.R.H.; Sajjad, M.A.; Yusuf, M. A new approach for the growth of copper nitrides thin films by thermal evaporation using nitrogen as source gas. Optik 2021, 245, 167666. [Google Scholar] [CrossRef]
- Caskey, C.M.; Richards, R.M.; Ginley, D.S.; Zakutayev, A. Thin film synthesis and properties of copper nitride, a metastable semiconductor. Mater. Horiz. 2014, 1, 424–430. [Google Scholar] [CrossRef]
- Xiao, J.; Qi, M.; Gong, C.; Wang, Z.; Jiang, A.; Ma, J.; Cheng, Y. Crystal structure and optical properties of silver-doped copper nitride films (Cu3N:Ag) prepared by magnetron sputtering. J. Phys. D Appl. Phys. 2018, 51, 055305. [Google Scholar] [CrossRef]
- Su, C.Y.; Liu, B.H.; Lin, T.J.; Chi, Y.M.; Kei, C.C.; Wang, K.W.; Perng, T.P. Carbon nanotube-supported Cu3N nanocrystals as a highly active catalyst for oxygen reduction reaction. J. Mater. Chem. A 2015, 3, 18983–18990. [Google Scholar] [CrossRef]
- Deshmukh, R.; Schubert, U. Synthesis of CuO and Cu3N Nanoparticles in and on Hollow Silica Spheres. Eur. J. Inorg. Chem. 2013, 2013, 2498–2504. [Google Scholar] [CrossRef] [Green Version]
- Dongil, A.B. Recent Progress on Transition Metal Nitrides Nanoparticles as Heterogeneous Catalysts. Nanomaterials 2019, 9, 1111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alexander, A.M.; Hargreaves, J.S.J.; Mitchell, C. The Reduction of Various Nitrides under Hydrogen: Ni3N, Cu3N, Zn3N2 and Ta3N5. Top. Catal. 2012, 55, 1046–1053. [Google Scholar] [CrossRef]
- Szczęsny, R.; Hoang, T.K.A.; Dobrzańska, L.; Gregory, D.H. Solution/Ammonolysis Syntheses of Unsupported and Silica-Supported Copper(I) Nitride Nanostructures from Oxidic Precursors. Molecules 2021, 26, 4926. [Google Scholar] [CrossRef] [PubMed]
- Rasaki, S.A.; Zhang, B.; Anbalgam, K.; Thomas, T.; Yang, M. Synthesis and application of nano-structured metal nitrides and carbides: A review. Prog. Solid State Chem. 2018, 50, 1–15. [Google Scholar] [CrossRef]
- Tareen, A.K.; Priyanga, G.S.; Behara, S.; Thomas, T.; Yang, M. Mixed ternary transition metal nitrides: A comprehensive review of synthesis, electronic structure, and properties of engineering relevance. Prog. Solid State Chem. 2019, 53, 1–26. [Google Scholar] [CrossRef]
- Lord, R.W.; Holder, C.F.; Fenton, J.L.; Schaak, R.E. Seeded Growth of Metal Nitrides on Noble-Metal Nanoparticles To Form Complex Nanoscale Heterostructures. Chem. Mater. 2019, 31, 4605–4613. [Google Scholar] [CrossRef]
- Polte, J. Fundamental growth principles of colloidal metal nanoparticles—A new perspective. CrystEngComm 2015, 17, 6809–6830. [Google Scholar] [CrossRef] [Green Version]
- Kieda, N.; Messing, G.L. Microfoamy particles of copper oxide and nitride by spray pyrolysis of copper–ammine complex solutions. J. Mater. Sci. Lett. 1998, 17, 299–301. [Google Scholar] [CrossRef]
- Cremer, R.; Witthaut, M.; Neuschütz, D.; Trappe, C.; Laurenzis, M.; Winkler, O.; Kurz, H. Deposition and Characterization of Metastable Cu3N Layers for Applications in Optical Data Storage. Microchim. Acta 2000, 133, 299–302. [Google Scholar] [CrossRef]
- Kaur, N.; Choudhary, N.; Goyal, R.N.; Viladkar, S.; Matai, I.; Gopinath, P.; Chockalingam, S.; Kaur, D. Magnetron sputtered Cu3N/NiTiCu shape memory thin film heterostructures for MEMS applications. J. Nanopart. Res. 2013, 15, 1468. [Google Scholar] [CrossRef]
- Yuan, N.Y.; Wang, S.Y.; Ding, J.N.; Qiu, J.H.; Wang, X.Q.; Huang, W.H. The Componential and Morphological Characteristics of Cu3N Induced by Femtosecond Laser Pulses. Key Eng. Mater. 2014, 609–610, 135–140. [Google Scholar] [CrossRef]
- Shi, J.; Jiang, B.; Li, C.; Yan, F.; Wang, D.; Yang, C.; Wan, J. Review of Transition Metal Nitrides and Transition Metal Nitrides/Carbon nanocomposites for supercapacitor electrodes. Mater. Chem. Phys. 2020, 245, 122533. [Google Scholar] [CrossRef]
- Zhu, L.; Cao, X.; Xiao, J.; Ma, S.; Ta, S. Structure and photocatalytic properties of TiO2/Cu3N composite films prepared by magnetron sputtering. Mater. Today Commun. 2021, 26, 101739. [Google Scholar] [CrossRef]
- Kuzmin, A.; Anspoks, A.; Kalinko, A.; Timoshenko, J.; Nataf, L.; Baudelet, F.; Irifune, T. Origin of Pressure-Induced Metallization in Cu3N: An X-ray Absorption Spectroscopy Study. Phys. Status Solidi 2018, 255, 1800073. [Google Scholar] [CrossRef]
- Jiang, A.; Qi, M.; Xiao, J. Preparation, structure, properties, and application of copper nitride (Cu3N) thin films: A review. J. Mater. Sci. Technol. 2018, 34, 1467–1473. [Google Scholar] [CrossRef]
- Yee, Y.S.; Inoue, H.; Hultqvist, A.; Hanifi, D.; Salleo, A.; Magyari-Köpe, B.; Nishi, Y.; Bent, S.F.; Clemens, B.M. Copper interstitial recombination centers in Cu3N. Phys. Rev. B 2018, 97, 245201. [Google Scholar] [CrossRef] [Green Version]
- Ścigała, A.; Szłyk, E.; Dobrzańska, L.; Gregory, D.H.; Szczęsny, R. From binary to multinary copper based nitrides-Unlocking the potential of new applications. Coord. Chem. Rev. 2021, 436, 213791. [Google Scholar] [CrossRef]
- Hahn, U.; Weber, W. Electronic structure and chemical-bonding mechanism of Cu3N, Cu3NPd, and related Cu(I) compounds. Phys. Rev. B Condens. Matter 1996, 53, 12684–12693. [Google Scholar] [CrossRef]
- Moreno-Armenta, M.G.; Martínez-Ruiz, A.; Takeuchi, N. Ab initio total energy calculations of copper nitride: The effect of lattice parameters and Cu content in the electronic properties. Solid State Sci. 2004, 6, 9–14. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, H.; Zhu, J.; Lü, X.; Li, S.; Zou, R.; Zhao, Y. Antiperovskites with Exceptional Functionalities. Adv. Mater. 2020, 32, 1905007. [Google Scholar] [CrossRef]
- Lee, B.S.; Yi, M.; Chu, S.Y.; Lee, J.Y.; Kwon, H.R.; Lee, K.R.; Kang, D.; Kim, W.S.; Lim, H.B.; Lee, J.; et al. Copper nitride nanoparticles supported on a superparamagnetic mesoporous microsphere for toxic-free click chemistry. Chem. Commun. 2010, 46, 3935–3937. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.G.; You, S.J.; Yang, L.X.; Jin, C.Q. Structural phase transition of Cu3N under high pressure. Solid State Commun. 2010, 150, 1521–1524. [Google Scholar] [CrossRef]
- Tilemachou, A.; Zervos, M.; Othonos, A.; Pavloudis, T.; Kioseoglou, J. p-Type Iodine-Doping of Cu3N and Its Conversion to γCuI I for the Fabrication of γCuI/Cu3N p-n Heterojunctions. Electron. Mater. 2022, 3, 2. [Google Scholar] [CrossRef]
- Zakutayev, A.; Caskey, C.M.; Fioretti, A.N.; Ginley, D.S.; Vidal, J.; Stevanovic, V.; Tea, E.; Lany, S. Defect Tolerant Semiconductors for Solar Energy Conversion. J. Phys. Chem. Lett. 2014, 5, 1117–1125. [Google Scholar] [CrossRef]
- Kong, F.; Hu, Y.; Wang, Y.; Wang, B.; Tang, L. Structural, elastic and thermodynamic properties of anti-ReO3 type Cu3N under pressure from first principles. Comput. Mater. Sci. 2012, 65, 247–253. [Google Scholar] [CrossRef]
- Gordillo, N.; Gonzalez-Arrabal, R.; Diaz-Chao, P.; Ares, J.R.; Ferrer, I.J.; Yndurain, F.; Agulló-López, F. Electronic structure of copper nitrides as a function of nitrogen content. Thin Solid Film. 2013, 531, 588–591. [Google Scholar] [CrossRef] [Green Version]
- Han, N.; Liu, P.; Jiang, J.; Ai, L.; Shao, Z.; Liu, S. Recent advances in nanostructured metal nitrides for water splitting. J. Mater. Chem. A 2018, 6, 19912–19933. [Google Scholar] [CrossRef]
- Ningthoujam, R.S.; Gajbhiye, N.S. Synthesis, electron transport properties of transition metal nitrides and applications. Prog. Mater. Sci. 2015, 70, 50–154. [Google Scholar] [CrossRef]
- Jiang, H.; Li, X.; Zang, S.; Zhang, W. Mixed cobalt-nitrides CoxN and Ta2N bifunction-modified Ta3N5 nanosheets for enhanced photocatalytic water-splitting into hydrogen. J. Alloys Compd. 2021, 854, 155328. [Google Scholar] [CrossRef]
- Guo, Q.; Zhao, J.; Yang, Y.; Huang, J.; Tang, Y.; Zhang, X.; Li, Z.; Yu, X.; Shen, J.; Zhao, J. Mesocrystalline Ta3N5 superstructures with long-lived charges for improved visible light photocatalytic hydrogen production. J. Colloid Interface Sci. 2020, 560, 359–368. [Google Scholar] [CrossRef]
- Sun, Z.; Chen, H.; Zhang, L.; Lu, D.; Du, P. Enhanced photocatalytic H2 production on cadmium sulfide photocatalysts using nickel nitride as a novel cocatalyst. J. Mater. Chem. A 2016, 4, 13289–13295. [Google Scholar] [CrossRef]
- Chen, L.; Huang, H.; Zheng, Y.; Sun, W.; Zhao, Y.; Francis, P.S.; Wang, X. Noble-metal-free Ni3N/g-C3N4 photocatalysts with enhanced hydrogen production under visible light irradiation. Dalton Trans. 2018, 47, 12188–12196. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wu, Y.; Sun, K.; Mi, Z. A quadruple-band metal–nitride nanowire artificial photosynthesis system for high efficiency photocatalytic overall solar water splitting. Mater. Horiz. 2019, 6, 1454–1462. [Google Scholar] [CrossRef]
- Di Valentin, C.; Diebold, U.; Selloni, A. Doping and functionalization of photoactive semiconducting metal oxides. Chem. Phys. 2007, 339, vii–viii. [Google Scholar] [CrossRef]
- Xu, W.; Liu, H.; Zhou, D.; Chen, X.; Ding, N.; Song, H.; Ågren, H. Localized surface plasmon resonances in self-doped copper chalcogenide binary nanocrystals and their emerging applications. Nano Today 2020, 33, 100892. [Google Scholar] [CrossRef]
- Zhang, L.; Ding, N.; Lou, L.; Iwasaki, K.; Wu, H.; Luo, Y.; Li, D.; Nakata, K.; Fujishima, A.; Meng, Q. Localized Surface Plasmon Resonance Enhanced Photocatalytic Hydrogen Evolution via Pt@Au NRs/C3N4 Nanotubes under Visible-Light Irradiation. Adv. Funct. Mater. 2019, 29, 1806774. [Google Scholar] [CrossRef]
- Brongersma, M.L.; Halas, N.J.; Nordlander, P. Plasmon-induced hot carrier science and technology. Nat. Nanotechnol. 2015, 10, 25–34. [Google Scholar] [CrossRef]
- Lalisse, A.; Tessier, G.; Plain, J.; Baffou, G. Plasmonic efficiencies of nanoparticles made of metal nitrides (TiN, ZrN) compared with gold. Sci. Rep. 2016, 6, 38647. [Google Scholar] [CrossRef]
- Askes, S.H.C.; Schilder, N.J.; Zoethout, E.; Polman, A.; Garnett, E.C. Tunable plasmonic HfN nanoparticles and arrays. Nanoscale 2019, 11, 20252–20260. [Google Scholar] [CrossRef]
- Beierle, A.; Gieri, P.; Pan, H.; Heagy, M.D.; Manjavacas, A.; Chowdhury, S. Titanium nitride nanoparticles for the efficient photocatalysis of bicarbonate into formate. Sol. Energy Mater. Sol. Cells 2019, 200, 109967. [Google Scholar] [CrossRef]
- Sajeev, A.; Paul, A.M.; Nivetha, R.; Gothandapani, K.; Gopal, T.S.; Jacob, G.; Muthuramamoorty, M.; Pandiaraj, S.; Alodhayb, A.; Kim, S.Y.; et al. Development of Cu3N electrocatalyst for hydrogen evolution reaction in alkaline medium. Sci. Rep. 2022, 12, 2004. [Google Scholar] [CrossRef]
- Yin, Z.; Yu, C.; Zhao, Z.; Guo, X.; Shen, M.; Li, N.; Muzzio, M.; Li, J.; Liu, H.; Lin, H.; et al. Cu3N Nanocubes for Selective Electrochemical Reduction of CO2 to Ethylene. Nano Lett. 2019, 19, 8658–8663. [Google Scholar] [CrossRef] [PubMed]
- Ni, D.; Shen, H.; Li, H.; Ma, Y.; Zhai, T. Synthesis of high efficient Cu/TiO2 photocatalysts by grinding and their size-dependent photocatalytic hydrogen production. Appl. Surf. Sci. 2017, 409, 241–249. [Google Scholar] [CrossRef]
- Cao, S.; Jiang, J.; Zhu, B.; Yu, J. Shape-dependent photocatalytic hydrogen evolution activity over a Pt nanoparticle coupled g-C3N4 photocatalyst. Phys. Chem. Chem. Phys. 2016, 18, 19457–19463. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Yao, C.; Kong, X.; Li, Z.; Jiang, M.; Zhang, F.; Lei, X. Boosting Hydrogen Production by Electrooxidation of Urea over 3D Hierarchical Ni4N/Cu3N Nanotube Arrays. ACS Sustain. Chem. Eng. 2019, 7, 13278–13285. [Google Scholar] [CrossRef]
- Pereira, N.; Dupont, L.; Tarascon, J.M.; Klein, L.C.; Amatucci, G.G. Electrochemistry of Cu3N with Lithium. J. Electrochem. Soc. 2003, 150, A1273. [Google Scholar] [CrossRef]
- Chen, W.; Zhang, H.; Yang, B.; Li, B.; Li, Z. Characterization of Cu3N/CuO thin films derived from annealed Cu3N for electrode application in Li-ion batteries. Thin Solid Film. 2019, 672, 157–164. [Google Scholar] [CrossRef]
- Matsuzaki, K.; Okazaki, T.; Lee, Y.-S.; Hosono, H.; Susaki, T. Controlled bipolar doping in Cu3N (100) thin films. Appl. Phys. Lett. 2014, 105, 222102. [Google Scholar] [CrossRef]
- Yue, G.H.; Yan, P.X.; Liu, J.Z.; Wang, M.X.; Li, M.; Yuan, X.M. Copper nitride thin film prepared by reactive radio-frequency magnetron sputtering. J. Appl. Phys. 2005, 98, 103506. [Google Scholar] [CrossRef]
- Li, X.; Liu, Z.; Zuo, A.; Yuan, Z.; Yang, J.; Yao, K. Properties of Al-doped copper nitride films prepared by reactive magnetron sputtering. J. Wuhan Univ. Technol. Mater. Sci. Ed. 2007, 22, 446–449. [Google Scholar] [CrossRef]
- Du, Y.; Yin, Y.; Wang, J.; Wang, Z.; Li, C.; Baunack, S.; Ma, L.; Schmidt, O.G. Nanoporous Copper Pattern Fabricated by Electron Beam Irradiation on Cu3N Film for SERS Application. Phys. Status Solidi 2019, 256, 1800378. [Google Scholar] [CrossRef]
- Song, R.; Zhang, L.; Zhu, F.; Li, W.; Fu, Z.; Chen, B.; Chen, M.; Zeng, H.; Pan, D. Hierarchical Nanoporous Copper Fabricated by One-Step Dealloying Toward Ultrasensitive Surface-Enhanced Raman Sensing. Adv. Mater. Interfaces 2018, 5, 1800332. [Google Scholar] [CrossRef]
- Jaque, D.; Richard, C.; Viana, B.; Soga, K.; Liu, X.; García Solé, J. Inorganic nanoparticles for optical bioimaging. Adv. Opt. Photon. 2016, 8, 1–103. [Google Scholar] [CrossRef]
- Wang, Y.; Song, W.; Ruan, W.; Yang, J.; Zhao, B.; Lombardi, J.R. SERS Spectroscopy Used To Study an Adsorbate on a Nanoscale Thin Film of CuO Coated with Ag. J. Phys. Chem. C 2009, 113, 8065–8069. [Google Scholar] [CrossRef]
- Wu, L.; Tan, J.; Chen, S.; Liu, X. Catalyst-enhanced micro-galvanic effect of Cu3N/Cu-bearing NiTi alloy surface for selective bacteria killing. Chem. Eng. J. 2022, 447, 137484. [Google Scholar] [CrossRef]
- Blaznik, U.; Krušič, S.; Hribar, M.; Kušar, A.; Žmitek, K.; Pravst, I. Use of Food Additive Titanium Dioxide (E171) before the Introduction of Regulatory Restrictions Due to Concern for Genotoxicity. Foods 2021, 10, 1910. [Google Scholar] [CrossRef]
- Shanker, U.; Rani, M.; Jassal, V. Degradation of hazardous organic dyes in water by nanomaterials. Environ. Chem. Lett. 2017, 15, 623–642. [Google Scholar] [CrossRef]
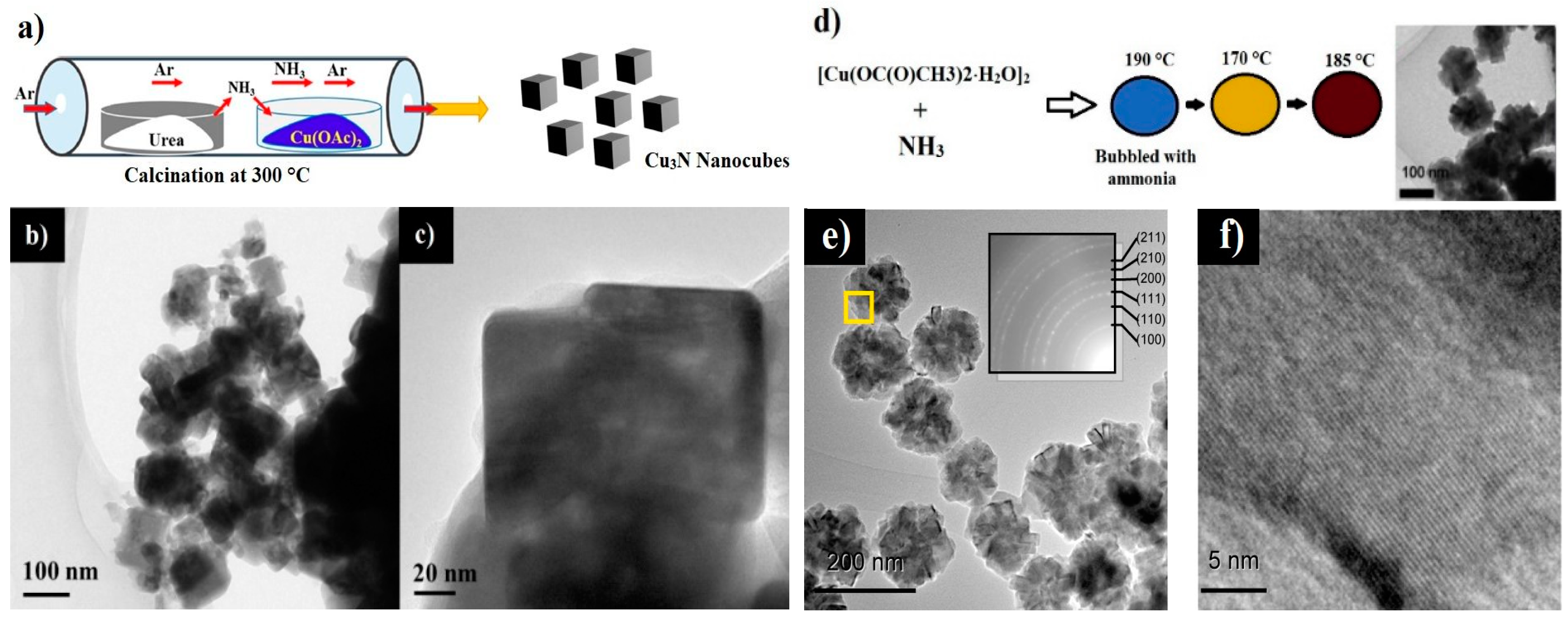
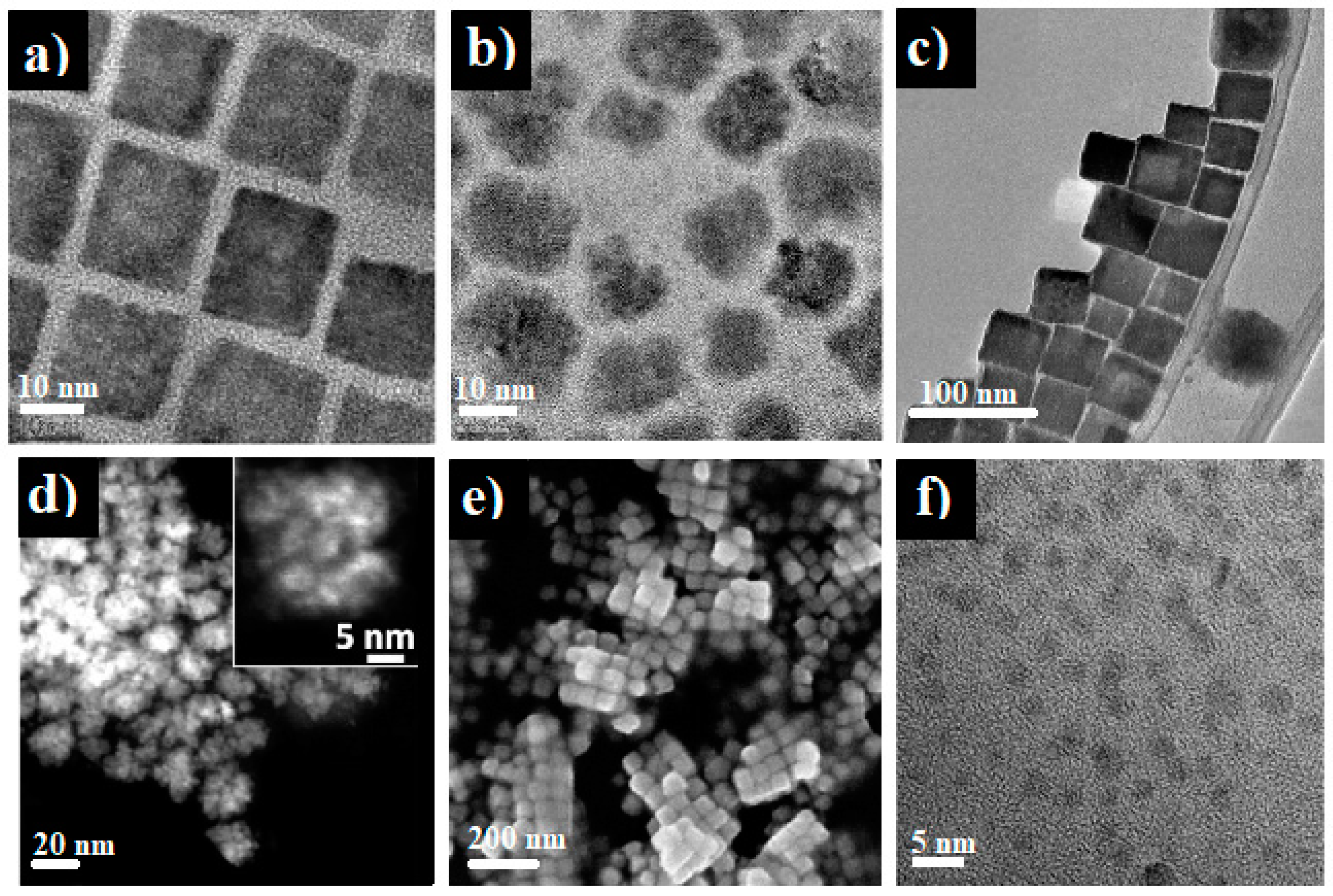
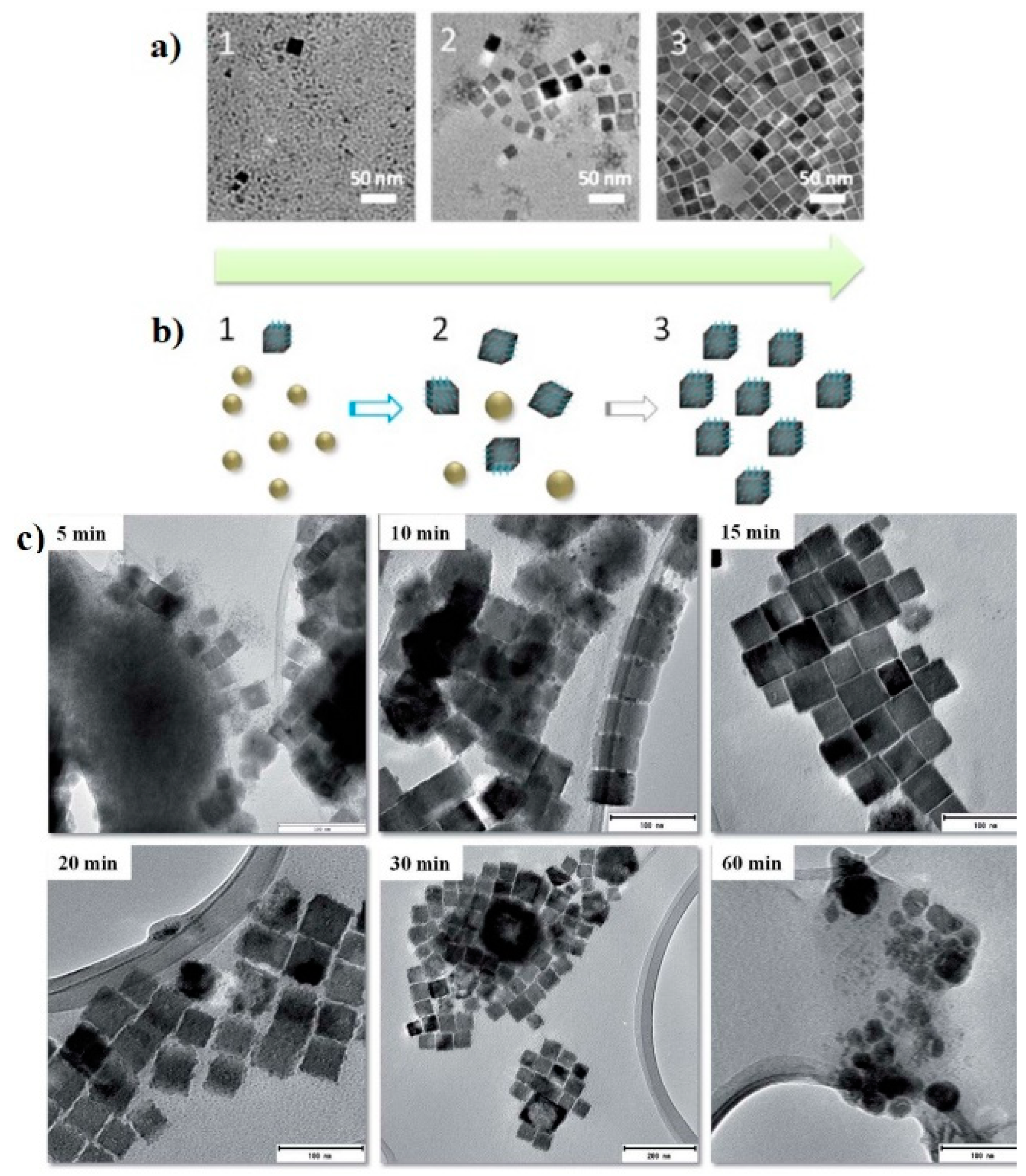
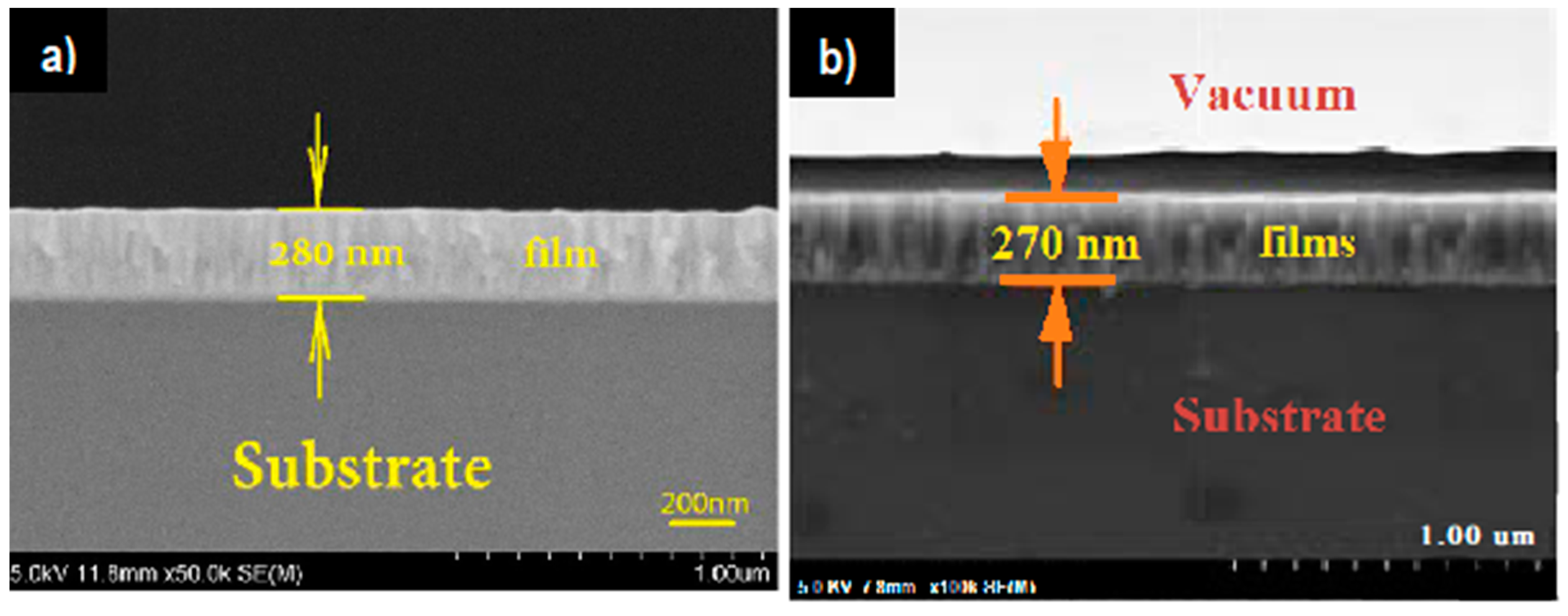
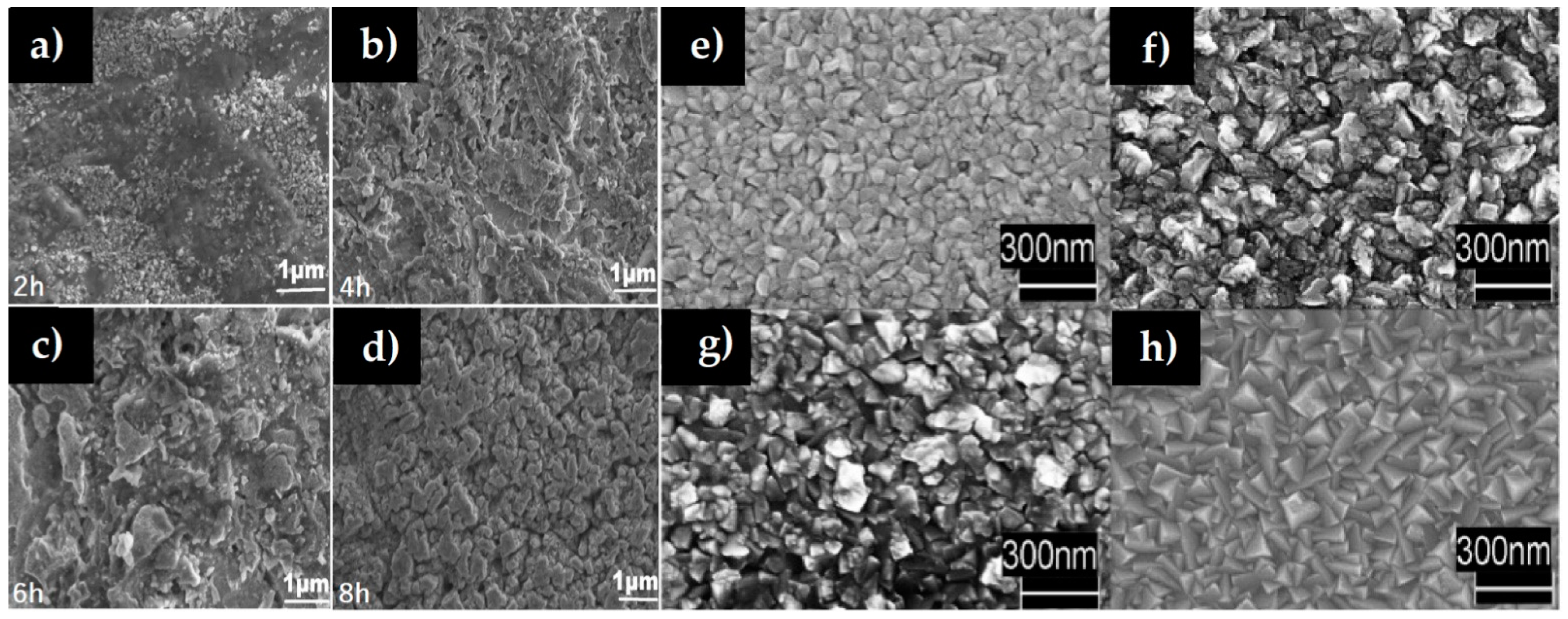
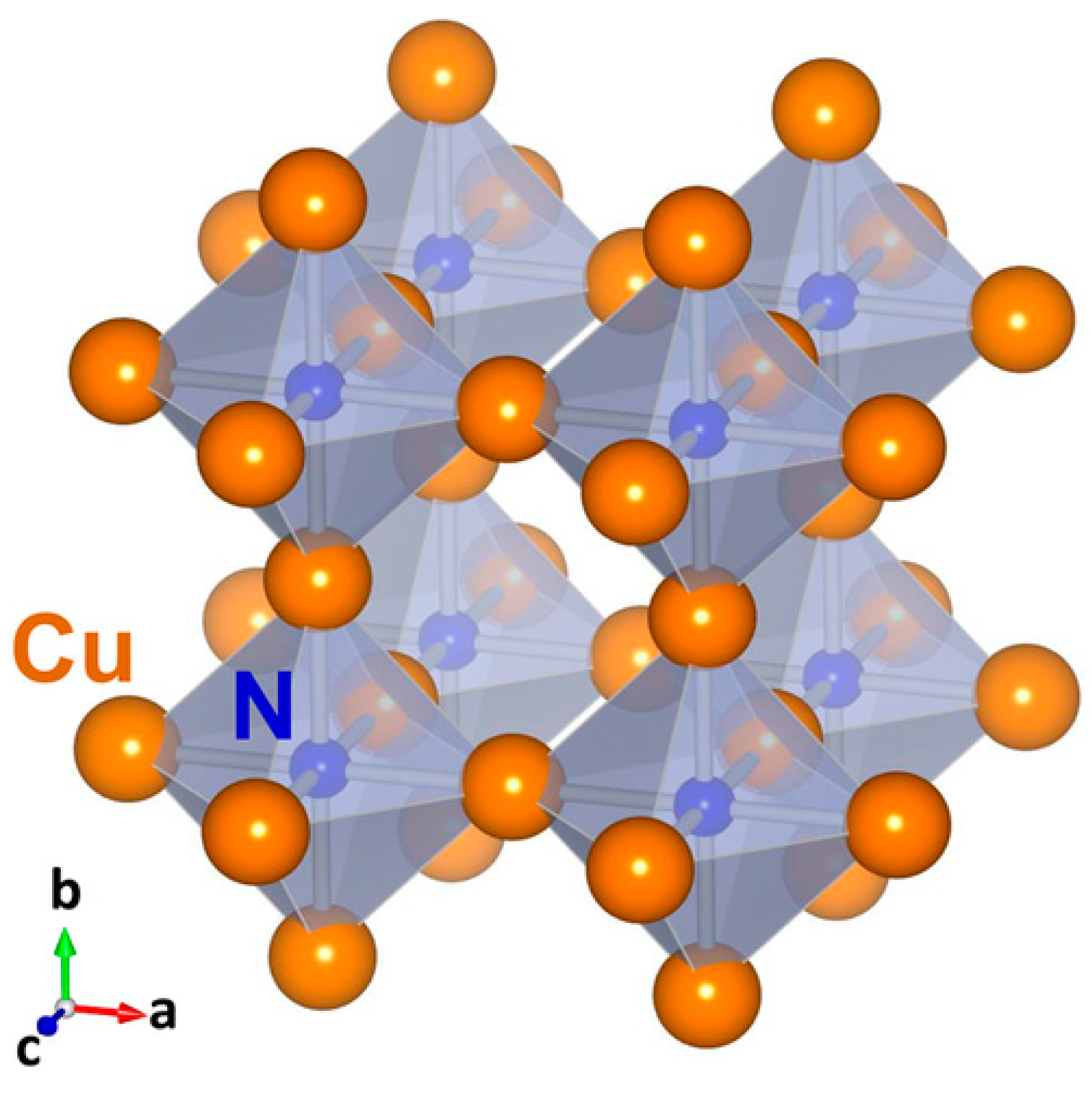
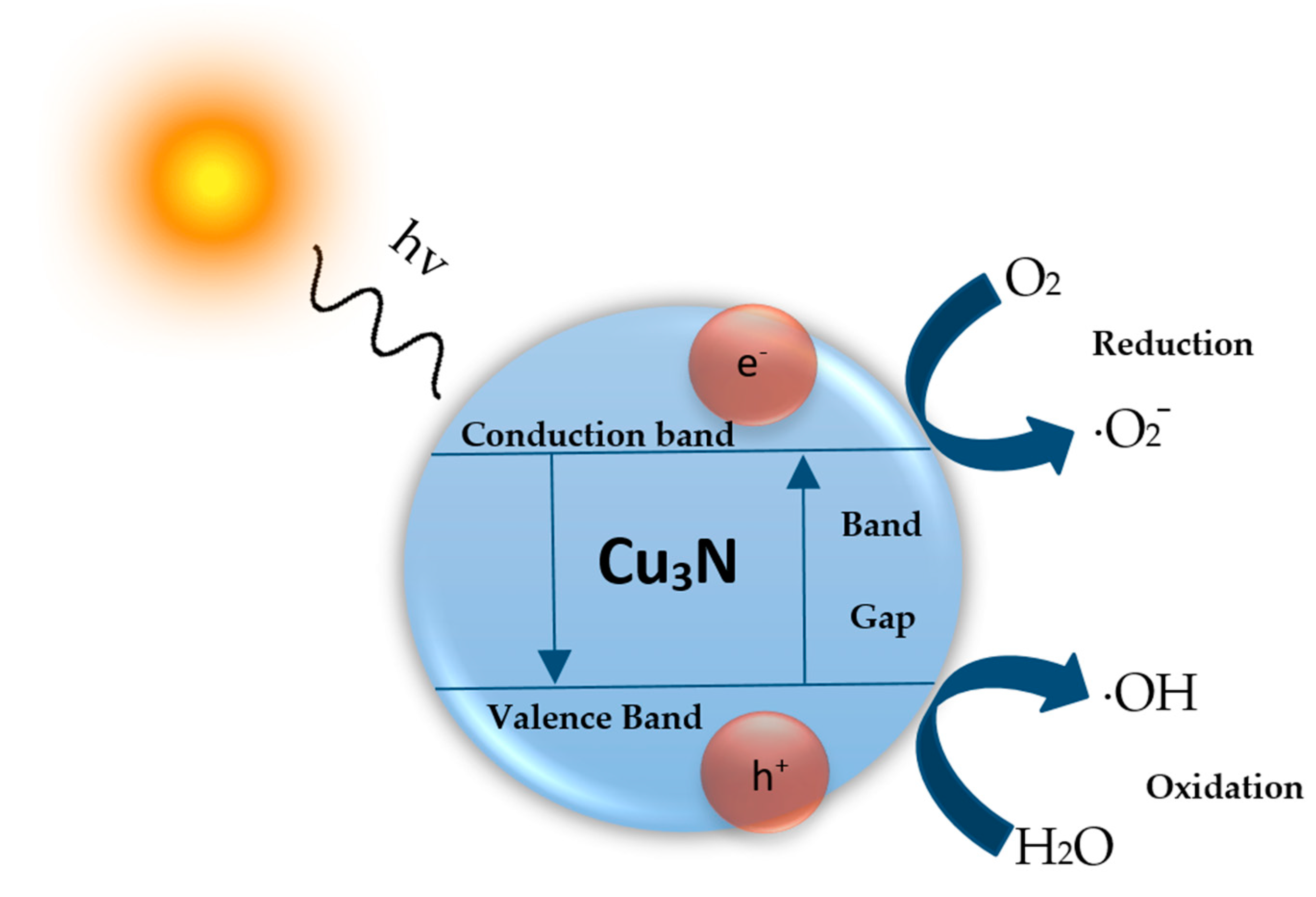
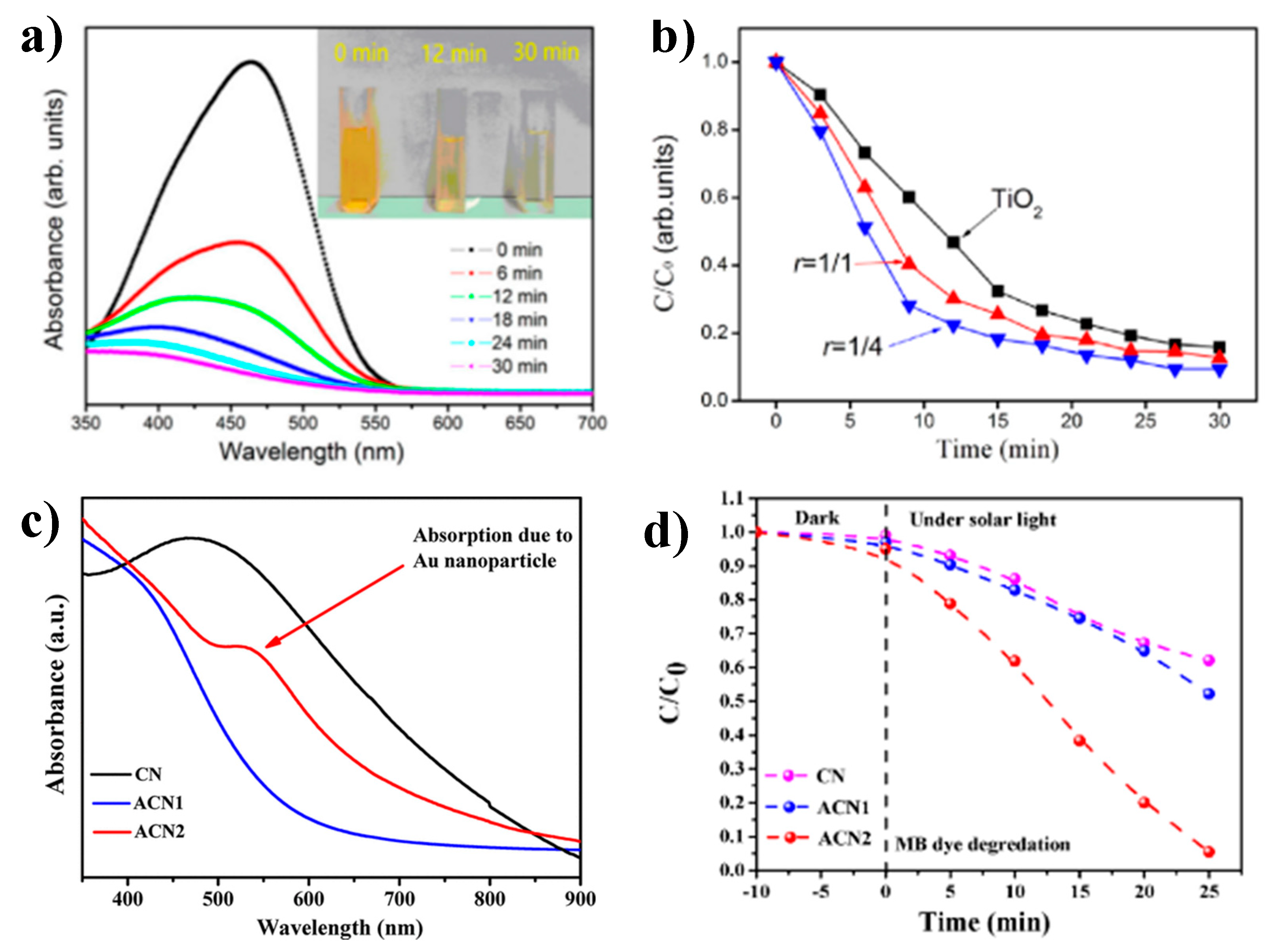
| Cu3N Morphology | Experimental Details | Band Gap (eV) | Second Phases | Ref | |||
|---|---|---|---|---|---|---|---|
| Synthesis Method | Precursors and Substrates | Conditions | Direct | Indirect | |||
| Nanocubes | Single source precursor method | Cu(NO3)2 3H2O, ODA | 1. 110 °C, 1 h 2. 260 °C, 5 min | 1.89 | - | CuO | [24] |
| Thermal decomposition | Cu(NO3)2⋅3H2O, OAm, ODE | 1. 110 °C, 1 h 2. 210 °C, 15 min | - | 1.6 eV | - | [38] | |
| Ammonolysis reaction | Cu(OAc)2, urea | 1. 300 °C, 2 h | - | - | - | [46] | |
| One-phase process | Cu(NO3)2⋅3H2O, ODA (or HAD or OAm) + ODE | 1. 150 °C, 3 h 2. 250 °C, 30 min | 1.5 | 1.04 | - | [47] | |
| One-step synthesis | Cu(NO3)2⋅3H2O, ODA | 1. 115 °C, 1 h 2. 240 °C, 5 min | 2.41 | - | - | [48] | |
| Thermal decomposition | Cu(NO3)2⋅3H2O, ODA | 1. 240 °C, 10 min | - | - | Cu/Cu2O | [49] | |
| Solvothermal synthesis method | Cu(NO3)2⋅3H2O, ODA, OAm | 1. 110 °C, 1 h 2. 240 °C, 40 min | - | - | - | [50] | |
| Spherical nanoparticles | Single source precursor method | PPC, ODA | 1. 110 °C, 1 h 2. 260 °C, 5 min | 2.21 | - | Cu | [24] |
| Pyridine-based synthesis | CuI, pyridine, NH3aq, KNH2 | 1. −35 °C 2. 130 °C, 30 min | 2.0 | - | - | [51] | |
| Thermal decomposition | Cu(NO3)2⋅3H2O, HAD | 1. 110 °C, 1h 2. 230 °C, 5 min | 2.92 | - | Cu/CuO | [52] | |
| Ammonolysis reaction | Cu(CO2CH3)2 H2O, 1-nonanol, NH3 gas | 1. 190 °C, 1h 2. 170 °C 3. 185 °C | - | - | CuO | [53] | |
| Surfactant-free Solution-phase approach | Cu(OMe)2, BZA | 140 °C, 15 min | - | - | - | [54] | |
| Ammonolysis reaction | CuF2, NH3 gas | 1. 140 °C, 6 h 2. 300 °C, 8 h | - | - | - | [55] | |
| Ammonolysis reaction | CuCO3, pivalic acid, NH3 gas | 1. 70 °C, 30 min 2. 250 °C, 10 h | - | - | Cu | [55] | |
| Ammonolysis reaction | CuC10, 1-nonanol, NH3 gas | 1. 190 °C, 40 min | - | - | - | [56] | |
| Powders | Solid state reaction | CuO, NaNH2 | 1. 170 °C, 60 h | - | - | Cu/CuO | [57] |
| Wet processing and ammonolysis | Cu(CF3COO)2, NH3 gas | 250–350 °C, 45 min–5 h | 1.48 | - | Cu | [58] | |
| Solvothermal synthetic method | CuCl2, NaN3, Toluene/ solvent | 1. ~50 °C, 4 h 2. ~100 °C, 10–12 h 3. T ↑ for several days: ~40 °C/day | - | - | CuO | [59] | |
| Ammonolysis reaction | CuF2, NH3 gas | 250–350 °C, 6 h–18 h | - | - | - | [60] | |
| Nanocrystals | Single-step solvothermal approach | Cu(NO3)2⋅5H2O, hexamethylenetetramine (HMT) | 200 °C, 1 h | 1.6 | - | - | [26] |
| PEALD | (Cu(hfac)2), NH3 plasma gas | ALD cycle: 2 s for Cu(hfac)2 (80 °C) in 0.5 Torr, 5 s for NH3 plasma, and 5 s of N2 purge at 1 Torr. | 1.92 | - | - | [34] | |
| Solution-phase synthesis | Cu(NO3)2⋅3H2O, ODE, OAm | 1. 120 °C, 10 min 2. 240 °C, 15 min | - | - | - | [61] | |
| Ammonolysis reaction | Cu2O, NH3 gas | 250 °C, 21 h | 0.95 | - | - | [62] | |
| Solvothermal process | Cu(NO3)2⋅3H2O, ODE, OAm or HDA | 1. 150 °C, 3 h 2. 220 °C, 10 min 3. 250 °C, 10 min | - | - | Cu/Cu2O | [63] | |
| Thin films | Thermal evaporation and ammonolysis reaction | Silicon substrate and ammonia solution | 1. 120 °C, 1 h 2. 180 °C, 2 h 3. 310 °C, 4 h | 2.0 | - | Cu/Cu2O | [28] |
| RF and magnetron sputtering | On silicon slice and quartz plate substrate | P = 1.0 Pa, N2 gas flow 40 sccm, RF power 300 W | 1.23 −1.91 | - | [25] | ||
| DC magnetron sputtering | Quartz glass substrates | P = 1.0 Pa, N2 gas flow 3.5–4.0 sccm, RF power 100–130 W. | - | 1.44 | - | [64] | |
| Magnetron sputtering | Single-crystal silicon and quartz substrates | P = 1.0 Pa, N2 gas flow 40 sccm, RF power 250 W. | 2.0 | - | Cu/Cu2O | [65] | |
| Modified activated reactive evaporation | Borosilicate glass substrate | P = 10 mTorr, N2 gas flow 40 sccm, RF power 50 W | 2.15 | 1.60 | - | [66] | |
| Thermal evaporation method | Glass substrate | 1. P = 10−2 Torr, 1000 °C 2. N2 gas flow 100 sccm, 300 °C | - | - | Cu | [67] | |
| RF reactive sputtering | Glass substrate | P = 10 mTorr of nitrogen balanced by 10 mTorr of argon, 140–280 °C | - | - | - | [68] | |
| Doped Cu3N and their nanocomposites | |||||||
| Cu3N:Pd films | RF and DC magnetron sputtering | Single-crystal silicon substrate | P = 2 × 10−3 Pa, Ar gas flow 10 sccm, N2 gas flow 30 sccm, RF power 200 W, DC power 0–7 W for Pb | - | - | - | [30] |
| Cu3N:Ag Thin film | RF and DC magnetron sputtering | Monocrystalline silicon and glass substrate | P = 10 × 10−3 Pa, N2 gas flow 40 sccm, RF power 200 W | - | 1.59 | - | [69] |
| Cu3N nanocrystals on CNTs | PEALD | (Cu(hfac)2), NH3 gas | P = 1 torr, plasma power 100–400 W, NH3 gas 250 °C | 1.9 | - | - | [70] |
| Cu3N@SiO2 spheres | Ammonolysis | CuSiO3, NH3 gas | 350 °C, 1 h | - | - | - | [71] |
| Semiconductor Composite | Band Gap (eV) | Dye Concentration | Condition | Degradation Time | Efficiency | Particle Size | Ref |
|---|---|---|---|---|---|---|---|
| Cu3N@MoS2 thin films | 2.05 | MO–10 mg/mL | 500 W Hg–lamp | 30 min | 98.3% | - | [35] |
| Au-decorated Cu3N nanocubes | 1.67 | MO/MB 0.0008 mM | 250 W Universal arc lamp | 25 min for MB and MO | 84% for MO 93% for MB | NC: 10 ± 5 nm Au: 10 and 5 nm | [38] |
| Cu3N nanocubes | 2.41 | MO/MB 20 ppm | Solar simulator source 100 mW/cm2 | 180 min for MO 240 min for MB | 89% for MO 61% for MB | NC: 41 ± 7 nm | [48] |
| Cu3N thin films | 2.0 | MO–20 mg/L | 500 W Hg–lamp | 30 min | 95.5% | - | [65] |
| TiO2@Cu3N thin films | 3.0 | MO–20 mg/L | 500 W Hg–lamp | 30 min | 99.2% | - | [84] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paredes, P.; Rauwel, E.; Rauwel, P. Surveying the Synthesis, Optical Properties and Photocatalytic Activity of Cu3N Nanomaterials. Nanomaterials 2022, 12, 2218. https://doi.org/10.3390/nano12132218
Paredes P, Rauwel E, Rauwel P. Surveying the Synthesis, Optical Properties and Photocatalytic Activity of Cu3N Nanomaterials. Nanomaterials. 2022; 12(13):2218. https://doi.org/10.3390/nano12132218
Chicago/Turabian StyleParedes, Patricio, Erwan Rauwel, and Protima Rauwel. 2022. "Surveying the Synthesis, Optical Properties and Photocatalytic Activity of Cu3N Nanomaterials" Nanomaterials 12, no. 13: 2218. https://doi.org/10.3390/nano12132218
APA StyleParedes, P., Rauwel, E., & Rauwel, P. (2022). Surveying the Synthesis, Optical Properties and Photocatalytic Activity of Cu3N Nanomaterials. Nanomaterials, 12(13), 2218. https://doi.org/10.3390/nano12132218