Construction of Core–Shell CoMoO4@γ-FeOOH Nanosheets for Efficient Oxygen Evolution Reaction
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Chemicals
2.2. Methods
2.3. Materials Characterizations
2.4. Electrochemical Measurements
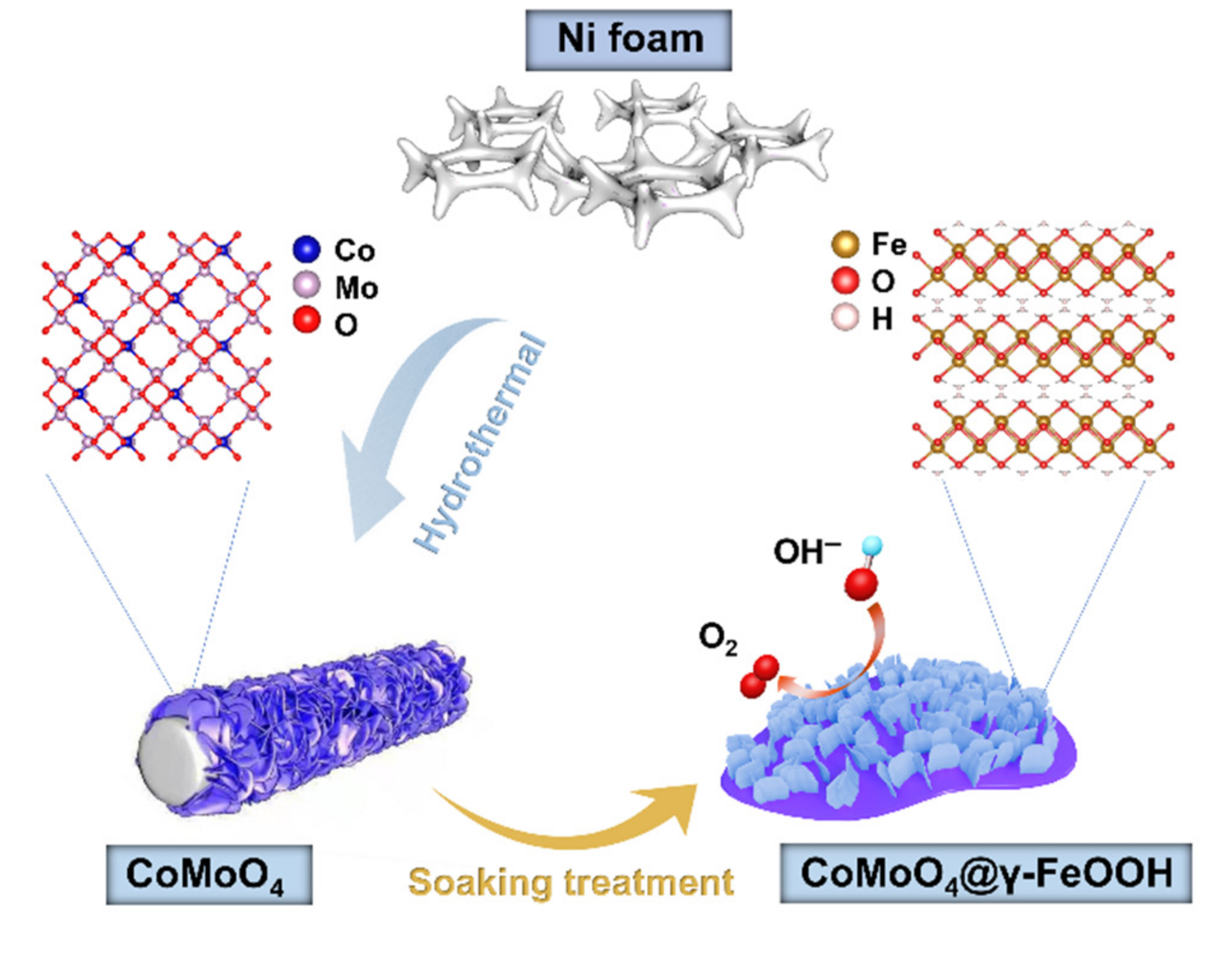
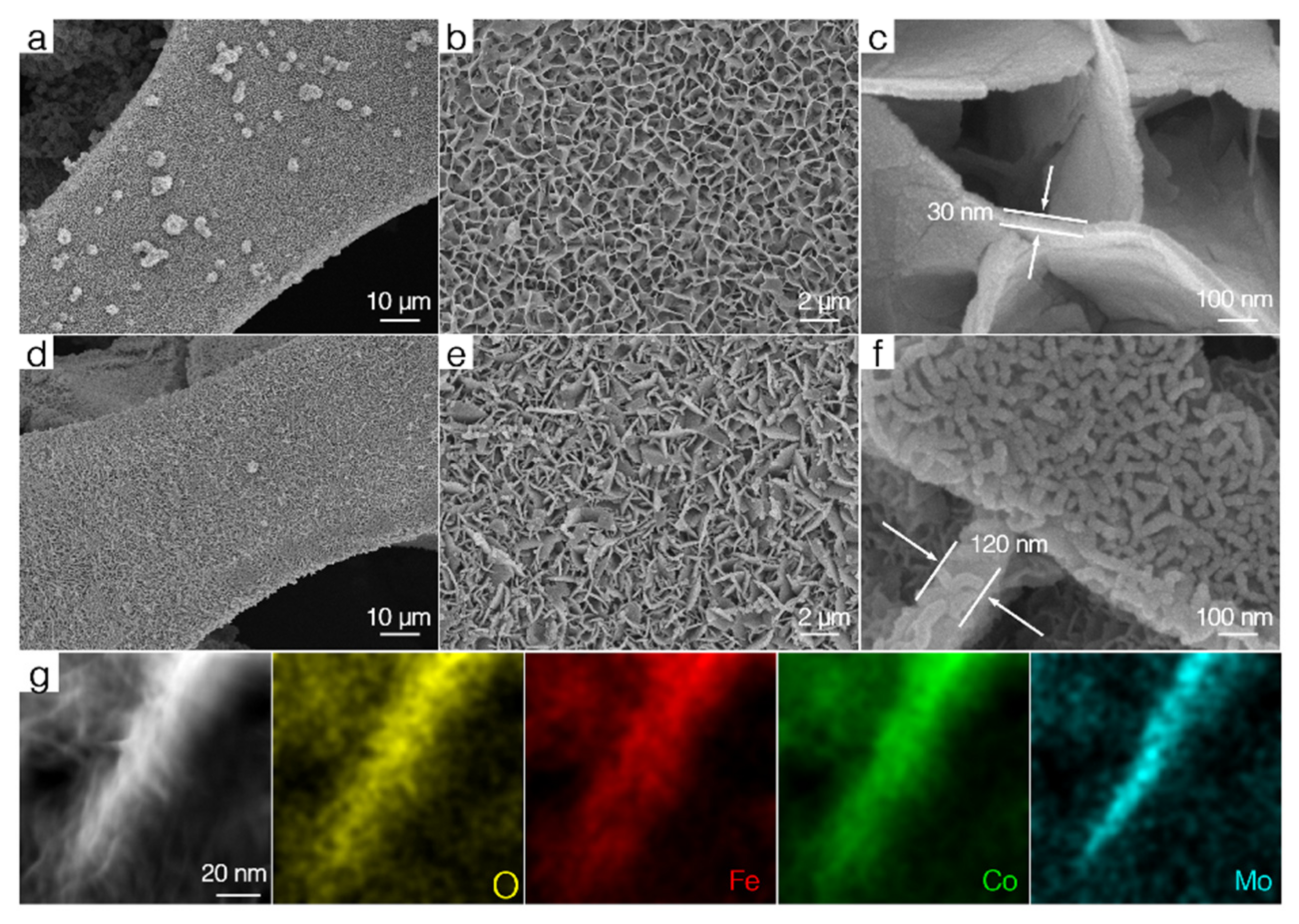
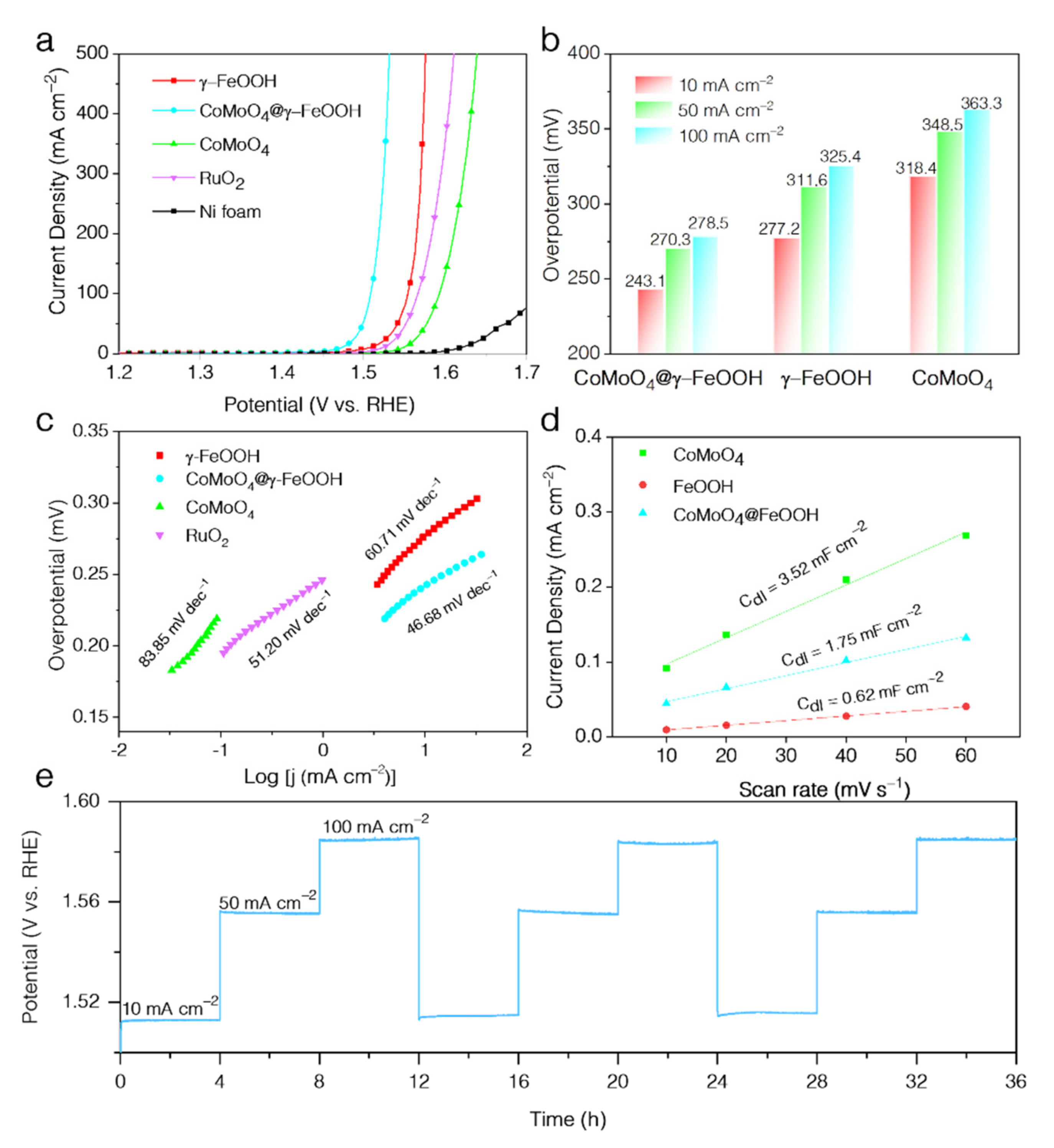
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Liu, P.; Liu, W.; Huang, Y.; Li, P.; Yan, J.; Liu, K. Mesoporous Hollow Carbon Spheres Boosted, Integrated High Performance Aqueous Zn-Ion Energy Storage. Energy Storage Mater. 2020, 25, 858–865. [Google Scholar] [CrossRef]
- Liu, P.; Zhang, Z.; Hao, R.; Huang, Y.; Liu, W.; Tan, Y.; Li, P.; Yan, J.; Liu, K. Ultra-Highly Stable Zinc Metal Anode via 3D-Printed g-C3N4 Modulating Interface for Long Life Energy Storage Systems. Chem. Eng. J. 2021, 403, 126425. [Google Scholar] [CrossRef]
- Sui, X.; Zhang, L.; Li, J.; Doyle-Davis, K.; Li, R.; Wang, Z.; Sun, X. Enhancing Metal–Support Interaction by in Situ Ion-Exchanging Strategy for High Performance Pt Catalysts in Hydrogen Evolution Reaction. J. Mater. Chem. A 2020, 8, 16582–16589. [Google Scholar] [CrossRef]
- Sui, X.; Zhang, L.; Li, J.; Doyle-Davis, K.; Li, R.; Wang, Z.; Sun, X. Advanced Support Materials and Interactions for Atomically Dispersed Noble-Metal Catalysts: From Support Effects to Design Strategies. Adv. Energy Mater. 2022, 12, 2102556. [Google Scholar] [CrossRef]
- Guan, J.; Shao, L.; Yu, L.; Wang, S.; Shi, X.; Cai, J.; Sun, Z. Two-Dimensional Mg0.2V2O5·nH2O Nanobelts Derived from V4C3 MXenes for Highly Stable Aqueous Zinc Ion Batteries. Chem. Eng. J. 2022, 443, 136502. [Google Scholar] [CrossRef]
- Yuk, S.F.; Cooper, V.R. Tuning Oxygen Electrocatalysis via Strain on LaNiO3(001). Phys. Chem. Chem. Phys. 2019, 21, 4738–4745. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, W.; Dai, J.; Sun, M.; Zhao, J.; Ji, L.; Chen, L.; Zeng, F.; Yang, F.; Huang, B.; et al. Carboxylated Carbon Nanotubes with High Electrocatalytic Activity for Oxygen Evolution in Acidic Conditions. InfoMat 2022, 4, e12273. [Google Scholar] [CrossRef]
- Jin, H.; Guo, C.; Liu, X.; Liu, J.; Vasileff, A.; Jiao, Y.; Zheng, Y.; Qiao, S.-Z. Emerging Two-Dimensional Nanomaterials for Electrocatalysis. Chem. Rev. 2018, 118, 6337–6408. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, C.; Ma, X.; Liu, F.; Xiao, H.; Zhang, J.; Lin, Z.; Hao, Z. Engineering Ultrafine NiFe-LDH into Self-Supporting Nanosheets: Separation-and-Reunion Strategy to Expose Additional Edge Sites for Oxygen Evolution. Small 2021, 17, 2103785. [Google Scholar] [CrossRef]
- Sayler, R.I.; Hunter, B.M.; Fu, W.; Gray, H.B.; Britt, R.D. EPR Spectroscopy of Iron- and Nickel-Doped [ZnAl]-Layered Double Hydroxides: Modeling Active Sites in Heterogeneous Water Oxidation Catalysts. J. Am. Chem. Soc. 2020, 142, 1838–1845. [Google Scholar] [CrossRef] [Green Version]
- Haber, J.A.; Anzenburg, E.; Yano, J.; Kisielowski, C.; Gregoire, J.M. Multiphase Nanostructure of a Quinary Metal Oxide Electrocatalyst Reveals a New Direction for OER Electrocatalyst Design. Adv. Energy Mater. 2015, 5, 1402307. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Gao, W.; Tang, K.; Lei, M.; Yao, B.; Qi, W.; Wen, D. Structure Engineering of Ni2P by Mo Doping for Robust Electrocatalytic Water and Methanol Oxidation Reactions. Electrochimica Acta 2021, 369, 137692. [Google Scholar] [CrossRef]
- Zhou, J.; Yuan, L.; Wang, J.; Song, L.; You, Y.; Zhou, R.; Zhang, J.; Xu, J. Combinational Modulations of NiSe2 Nanodendrites by Phase Engineering and Iron-Doping towards an Efficient Oxygen Evolution Reaction. J. Mater. Chem. A 2020, 8, 8113–8120. [Google Scholar] [CrossRef]
- Liu, H.; He, Q.; Jiang, H.; Lin, Y.; Zhang, Y.; Habib, M.; Chen, S.; Song, L. Electronic Structure Reconfiguration toward Pyrite NiS2 via Engineered Heteroatom Defect Boosting Overall Water Splitting. ACS Nano 2017, 11, 11574–11583. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Adimi, S.; Guo, X.; Thomas, T.; Zhu, Y.; Guo, H.; Priyanga, G.S.; Yoo, P.; Wang, J.; Chen, J.; et al. A Surface-Oxide-Rich Activation Layer (SOAL) on Ni2Mo3N for a Rapid and Durable Oxygen Evolution Reaction. Angew. Chem. Int. Ed. 2020, 59, 18036–18041. [Google Scholar] [CrossRef]
- Liu, X.; Meng, J.; Zhu, J.; Huang, M.; Wen, B.; Guo, R.; Mai, L. Comprehensive Understandings into Complete Reconstruction of Precatalysts: Synthesis, Applications, and Characterizations. Adv. Mater. 2021, 33, 2007344. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, Y.; Zhao, S.; She, S.; Zhang, F.; Chen, Y.; Williams, T.; Gengenbach, T.; Zu, L.; Mao, H.; et al. Anion Etching for Accessing Rapid and Deep Self-Reconstruction of Precatalysts for Water Oxidation. Matter 2020, 3, 2124–2137. [Google Scholar] [CrossRef]
- Wang, K.; Du, H.; He, S.; Liu, L.; Yang, K.; Sun, J.; Liu, Y.; Du, Z.; Xie, L.; Ai, W.; et al. Kinetically Controlled, Scalable Synthesis of γ-FeOOH Nanosheet Arrays on Nickel Foam toward Efficient Oxygen Evolution: The Key Role of In-Situ-Generated γ-NiOOH. Adv. Mater. 2021, 33, 2005587. [Google Scholar] [CrossRef]
- Zhou, X.; Zi, Y.; Xu, L.; Li, T.; Yang, J.; Tang, J. Core–Shell-Structured Prussian Blue Analogues Ternary Metal Phosphides as Efficient Bifunctional Electrocatalysts for OER and HER. Inorg. Chem. 2021, 60, 11661–11671. [Google Scholar] [CrossRef]
- Lv, Y.; Duan, S.; Zhu, Y.; Yin, P.; Wang, R. Enhanced OER Performances of Au@NiCo2S4 Core-Shell Heterostructure. Nanomaterials 2020, 10, 611. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Li, Y.; Wu, Q.; Su, Z.; Wang, B.; Chen, Y.; Wang, S. Hollow CoP/FeP4 Heterostructural Nanorods Interwoven by CNT as a Highly Efficient Electrocatalyst for Oxygen Evolution Reactions. Nanomaterials 2021, 11, 1450. [Google Scholar] [CrossRef] [PubMed]
- Rehman, K.U.; Airam, S.; Lin, X.; Gao, J.; Guo, Q.; Zhang, Z. In Situ Formation of Surface-Induced Oxygen Vacancies in Co9S8/CoO/NC as a Bifunctional Electrocatalyst for Improved Oxygen and Hydrogen Evolution Reactions. Nanomaterials 2021, 11, 2237. [Google Scholar] [CrossRef] [PubMed]
- Jeon, J.; Park, K.R.; Kim, K.M.; Ko, D.; Han, H.; Oh, N.; Yeo, S.; Ahn, C.; Mhin, S. CoFeS2@CoS2 Nanocubes Entangled with CNT for Efficient Bifunctional Performance for Oxygen Evolution and Oxygen Reduction Reactions. Nanomaterials 2022, 12, 983. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Xu, Y.; Xiang, W.; Guo, X.; Tong, Y.; Xu, J.; Yang, C. Amorphous Ni-P-S@FeOOH/CC Catalyst for High Oxygen Evolution Activity: Preparation, Characterization and Modeling. Chem. Eng. Sci. 2022, 258, 117761. [Google Scholar] [CrossRef]
- Tian, X.; Yi, P.; Sun, J.; Li, C.; Liu, R.; Sun, J.-K. The Scalable Solid-State Synthesis of a Ni5P4/Ni2P–FeNi Alloy Encapsulated into a Hierarchical Porous Carbon Framework for Efficient Oxygen Evolution Reactions. Nanomaterials 2022, 12, 1848. [Google Scholar] [CrossRef]
- Yu, X.; Chen, W.; Cai, J.; Lu, X.; Sun, Z. Oxygen Vacancy-Rich MnO Nanoflakes/N-Doped Carbon Nanotubes Modified Separator Enabling Chemisorption and Catalytic Conversion of Polysulfides for Li-S Batteries. J. Colloid Interface Sci. 2022, 610, 407–417. [Google Scholar] [CrossRef]
- Barmi, M.J.; Minakshi, M. Tuning the Redox Properties of the Nanostructured CoMoO4 Electrode: Effects of Surfactant Content and Synthesis Temperature. ChemPlusChem 2016, 81, 964–977. [Google Scholar] [CrossRef]
- Minakshi, M. Lithium Intercalation into Amorphous FePO4 Cathode in Aqueous Solutions. Electrochimica Acta 2010, 55, 9174–9178. [Google Scholar] [CrossRef] [Green Version]
- Xie, W.; Huang, J.; Huang, L.; Geng, S.; Song, S.; Tsiakaras, P.; Wang, Y. Novel Fluorine-Doped Cobalt Molybdate Nanosheets with Enriched Oxygen-Vacancies for Improved Oxygen Evolution Reaction Activity. Appl. Catal. B Environ. 2022, 303, 120871. [Google Scholar] [CrossRef]
- Wu, T.; Song, E.; Zhang, S.; Luo, M.; Zhao, C.; Zhao, W.; Liu, J.; Huang, F. Engineering Metallic Heterostructure Based on Ni3N and 2M-MoS2 for Alkaline Water Electrolysis with Industry-Compatible Current Density and Stability. Adv. Mater. 2022, 34, 2108505. [Google Scholar] [CrossRef]
- Li, T.; Yin, J.; Sun, D.; Zhang, M.; Pang, H.; Xu, L.; Zhang, Y.; Yang, J.; Tang, Y.; Xue, J. Manipulation of Mott−Schottky Ni/CeO2 Heterojunctions into N-Doped Carbon Nanofibers for High-Efficiency Electrochemical Water Splitting. Small 2022, 18, 2106592. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Bi, H.; Gai, S.; He, F.; Gao, P.; Dai, Y.; Zhang, X.; Yang, D.; Zhang, M.; Yang, P. Uniformly Dispersed ZnFe2O4 Nanoparticles on Nitrogen-Modified Graphene for High-Performance Supercapacitor as Electrode. Sci. Rep. 2017, 7, 43116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gong, C.; Li, W.; Lei, Y.; He, X.; Chen, H.; Du, X.; Fang, W.; Wang, D.; Zhao, L. Interfacial Engineering of ZIF-67 Derived CoSe/Co(OH)2 Catalysts for Efficient Overall Water Splitting. Compos. Part B Eng. 2022, 236, 109823. [Google Scholar] [CrossRef]
- Wan, K.; Luo, J.; Zhou, C.; Zhang, T.; Arbiol, J.; Lu, X.; Mao, B.-W.; Zhang, X.; Fransaer, J. Hierarchical Porous Ni3S4 with Enriched High-Valence Ni Sites as a Robust Electrocatalyst for Efficient Oxygen Evolution Reaction. Adv. Funct. Mater. 2019, 29, 1900315. [Google Scholar] [CrossRef] [Green Version]
- Choi, J.; Kim, D.; Zheng, W.; Yan, B.; Li, Y.; Lee, L.Y.S.; Piao, Y. Interface Engineered NiFe2O4−x/NiMoO4 Nanowire Arrays for Electrochemical Oxygen Evolution. Appl. Catal. B Environ. 2021, 286, 119857. [Google Scholar] [CrossRef]
- Zheng, M.; Guo, K.; Jiang, W.-J.; Tang, T.; Wang, X.; Zhou, P.; Du, J.; Zhao, Y.; Xu, C.; Hu, J.-S. When MoS2 Meets FeOOH: A “One-Stone-Two-Birds’’ Heterostructure as a Bifunctional Electrocatalyst for Efficient Alkaline Water Splitting. Appl. Catal. B Environ. 2019, 244, 1004–1012. [Google Scholar] [CrossRef]
- Liu, P.F.; Yang, S.; Zhang, B.; Yang, H.G. Defect-Rich Ultrathin Cobalt–Iron Layered Double Hydroxide for Electrochemical Overall Water Splitting. ACS Appl. Mater. Interfaces 2016, 8, 34474–34481. [Google Scholar] [CrossRef]
- Qiu, Z.; Ma, Y.; Edvinsson, T. In Operando Raman Investigation of Fe Doping Influence on Catalytic NiO Intermediates for Enhanced Overall Water Splitting. Nano Energy 2019, 66, 104118. [Google Scholar] [CrossRef]
- Xiong, Y.; Xu, L.; Jin, C.; Sun, Q. Interface-Engineered Atomically Thin Ni3S2/MnO2 Heterogeneous Nanoarrays for Efficient Overall Water Splitting in Alkaline Media. Appl. Catal. B Environ. 2019, 254, 329–338. [Google Scholar] [CrossRef]
- Che, Q.; Li, Q.; Chen, X.; Tan, Y.; Xu, X. Assembling Amorphous (Fe-Ni)Cox-OH/Ni3S2 Nanohybrids with S-Vacancy and Interfacial Effects as an Ultra-Highly Efficient Electrocatalyst: Inner Investigation of Mechanism for Alkaline Water-to-Hydrogen/Oxygen Conversion. Appl. Catal. B Environ. 2020, 263, 118338. [Google Scholar] [CrossRef]
- Li, Z.; Niu, W.; Zhou, L.; Yang, Y. Phosphorus and Aluminum Codoped Porous NiO Nanosheets as Highly Efficient Electrocatalysts for Overall Water Splitting. ACS Energy Lett. 2018, 3, 892–898. [Google Scholar] [CrossRef]
- Jin, Y.; Huang, S.; Yue, X.; Shu, C.; Shen, P.K. Highly Stable and Efficient Non-Precious Metal Electrocatalysts of Mo-Doped NiOOH Nanosheets for Oxygen Evolution Reaction. Int. J. Hydrog. Energy 2018, 43, 12140–12145. [Google Scholar] [CrossRef]
- Hao, S.; Chen, L.; Yu, C.; Yang, B.; Li, Z.; Hou, Y.; Lei, L.; Zhang, X. NiCoMo Hydroxide Nanosheet Arrays Synthesized via Chloride Corrosion for Overall Water Splitting. ACS Energy Lett. 2019, 4, 952–959. [Google Scholar] [CrossRef]
- Guan, C.; Xiao, W.; Wu, H.; Liu, X.; Zang, W.; Zhang, H.; Ding, J.; Feng, Y.P.; Pennycook, S.J.; Wang, J. Hollow Mo-Doped CoP Nanoarrays for Efficient Overall Water Splitting. Nano Energy 2018, 48, 73–80. [Google Scholar] [CrossRef]
- Wang, D.; Li, Q.; Han, C.; Xing, Z.; Yang, X. When NiO@Ni Meets WS2 Nanosheet Array: A Highly Efficient and Ultrastable Electrocatalyst for Overall Water Splitting. ACS Cent. Sci. 2018, 4, 112–119. [Google Scholar] [CrossRef] [Green Version]
- Luo, X.; Shao, Q.; Pi, Y.; Huang, X. Trimetallic Molybdate Nanobelts as Active and Stable Electrocatalysts for the Oxygen Evolution Reaction. ACS Catal. 2019, 9, 1013–1018. [Google Scholar] [CrossRef]
- Xu, H.; Shang, H.; Wang, C.; Jin, L.; Chen, C.; Wang, C.; Du, Y. Three-Dimensional Open CoMoOx/CoMoSx/CoSx Nanobox Electrocatalysts for Efficient Oxygen Evolution Reaction. Appl. Catal. B Environ. 2020, 265, 118605. [Google Scholar] [CrossRef]
- Park, H.; Park, B.H.; Choi, J.; Kim, S.; Kim, T.; Youn, Y.-S.; Son, N.; Kim, J.H.; Kang, M. Enhanced Electrochemical Properties and OER Performances by Cu Substitution in NiCo2O4 Spinel Structure. Nanomaterials 2020, 10, 1727. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, H.; Li, J.; Sheng, G.; Zhang, Y.; Mohamad, A.A.; Luo, J.; Zhong, Z.; Shao, W. Construction of Core–Shell CoMoO4@γ-FeOOH Nanosheets for Efficient Oxygen Evolution Reaction. Nanomaterials 2022, 12, 2215. https://doi.org/10.3390/nano12132215
Song H, Li J, Sheng G, Zhang Y, Mohamad AA, Luo J, Zhong Z, Shao W. Construction of Core–Shell CoMoO4@γ-FeOOH Nanosheets for Efficient Oxygen Evolution Reaction. Nanomaterials. 2022; 12(13):2215. https://doi.org/10.3390/nano12132215
Chicago/Turabian StyleSong, Huijun, Jingjing Li, Guan Sheng, Yinling Zhang, Ahmad Azmin Mohamad, Juan Luo, Zhangnan Zhong, and Wei Shao. 2022. "Construction of Core–Shell CoMoO4@γ-FeOOH Nanosheets for Efficient Oxygen Evolution Reaction" Nanomaterials 12, no. 13: 2215. https://doi.org/10.3390/nano12132215
APA StyleSong, H., Li, J., Sheng, G., Zhang, Y., Mohamad, A. A., Luo, J., Zhong, Z., & Shao, W. (2022). Construction of Core–Shell CoMoO4@γ-FeOOH Nanosheets for Efficient Oxygen Evolution Reaction. Nanomaterials, 12(13), 2215. https://doi.org/10.3390/nano12132215







