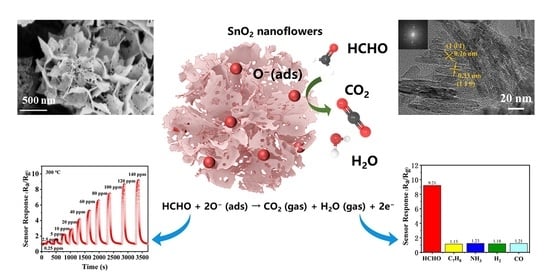
Facile Hydrothermal Synthesis of SnO2 Nanoflowers for Low-Concentration Formaldehyde Detection
Abstract
:1. Introduction
2. Experimental Section
2.1. Regents and Materials
2.2. Synthesis of SnO2 Nanoflowers
2.3. Characterization
2.4. Gas Sensing Property Measurements
3. Results and Discussion
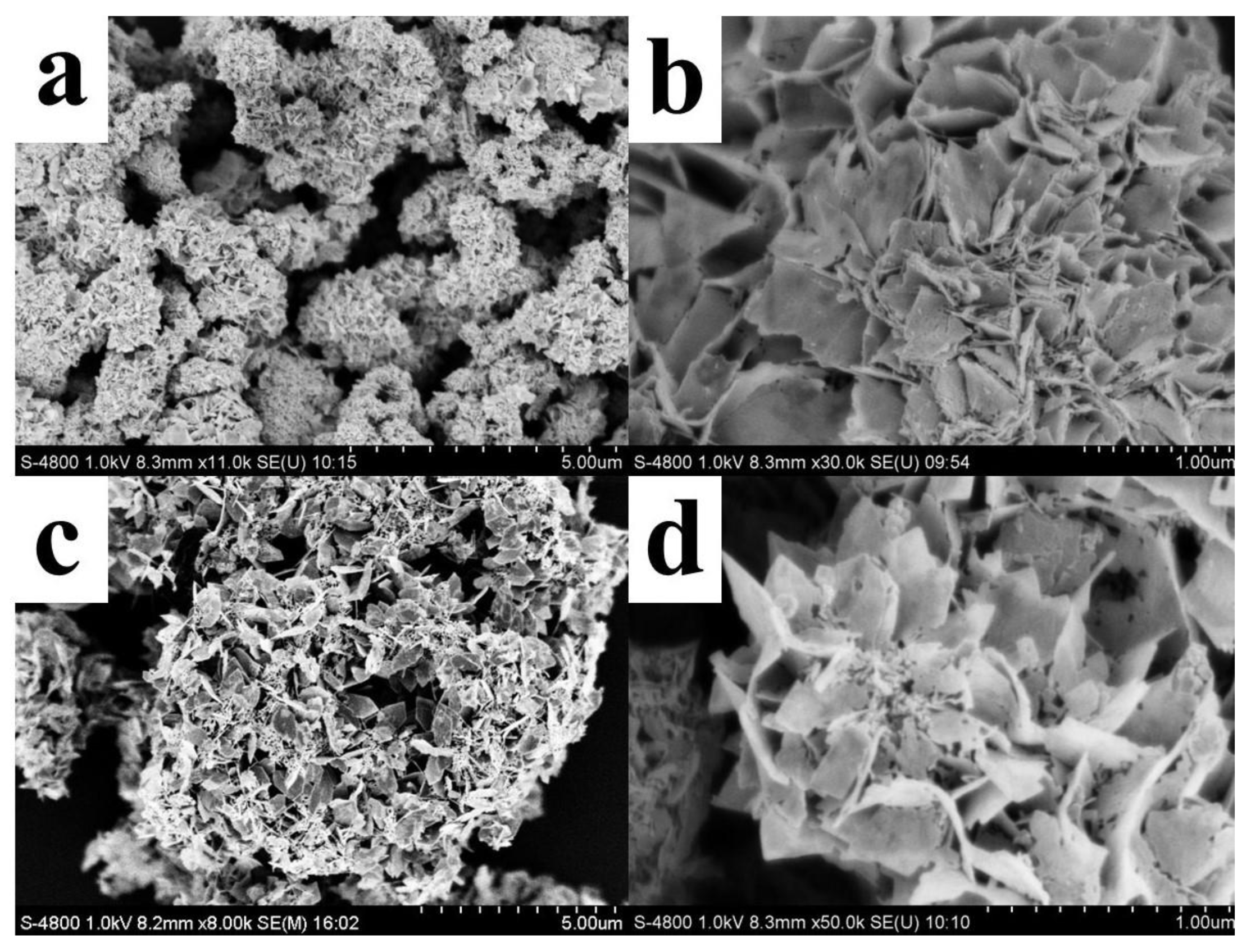
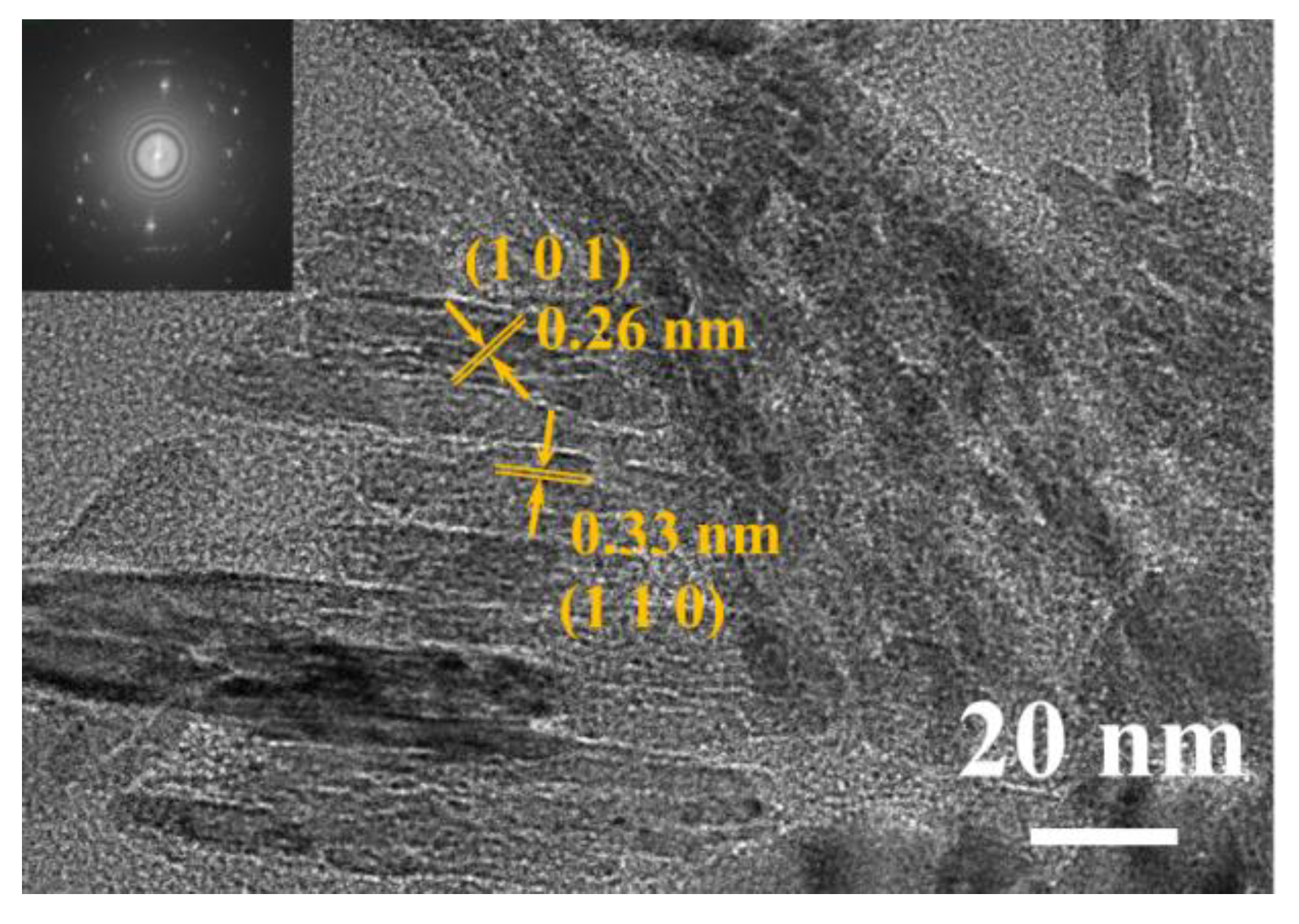
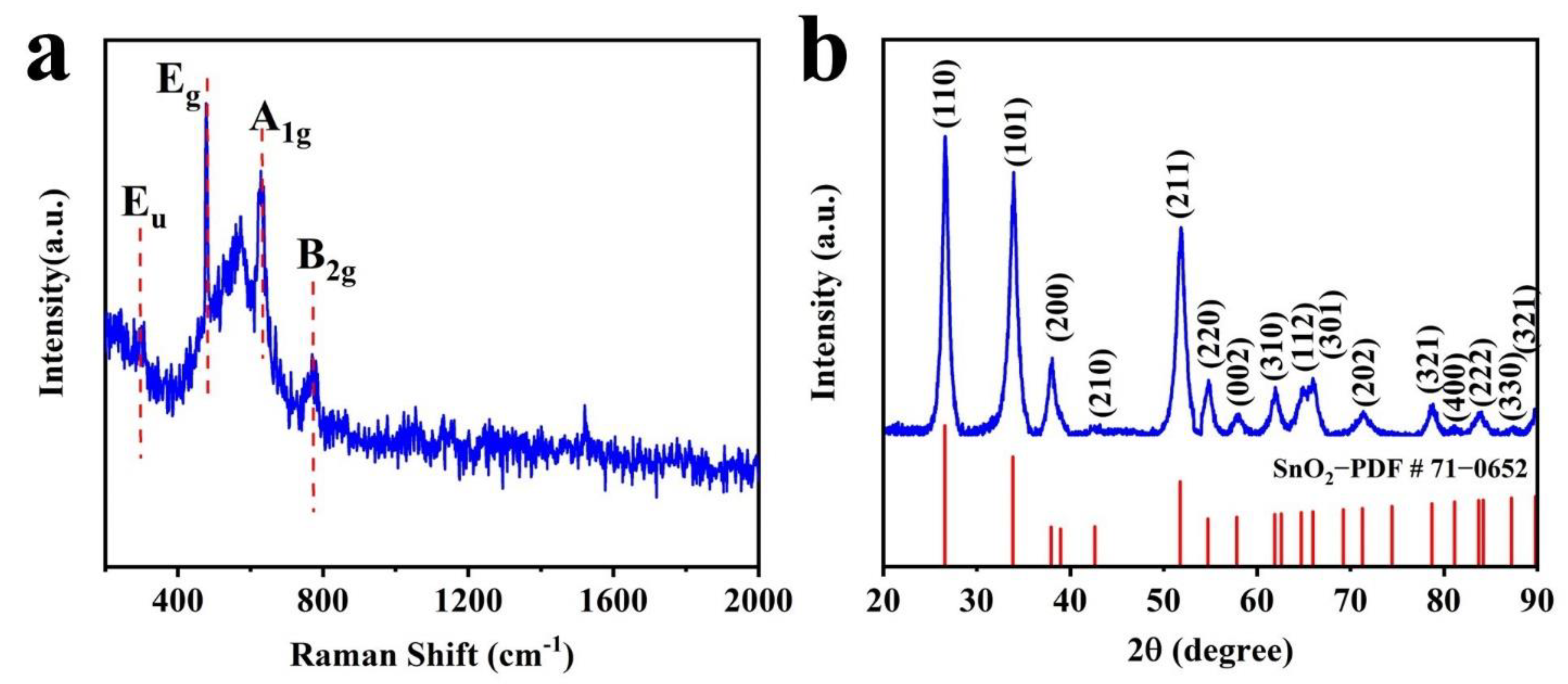
3.1. Characterization
3.2. Gas Sensing Performance
3.3. Gas Sensing Mechanisms
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, W.; Si, X.; Chen, Z.; Xu, L.; Guo, J.; Wei, L.; Cheng, G.; Du, Z. Fabrication of a humidity-resistant formaldehyde gas sensor through layering a molecular sieve on 3D ordered macroporous SnO2 decorated with Au nanoparticles. J. Alloys Compd. 2022, 919, 165788. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, D.; Wang, X.; Yang, J. Enhanced formaldehyde sensitivity of two-dimensional mesoporous SnO2 by nitrogen-doped graphene quantum dots. Rare Met. 2021, 40, 1561–1570. [Google Scholar] [CrossRef]
- Na, C.; Yoo, M.; Tsang, D.C.W.; Kim, H.W.; Kim, K. High-performance materials for effective sorptive removal of formaldehyde in air. J. Hazard. Mater. 2019, 366, 452–465. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhu, Y.; Xiang, Q.; Cheng, Z.; Chen, Y.; Xu, J. One novel humidity-resistance formaldehyde molecular probe based hydrophobic diphenyl sulfone urea dry-gel: Synthesis, sensing performance and mechanism. Sens. Actuators B Chem. 2017, 251, 590–600. [Google Scholar] [CrossRef]
- Nielsen, G.D.; Larsen, S.T.; Wolkoff, P. Re-evaluation of the WHO, Formaldehyde indoor air quality guideline for cancer risk assessment. Arch. Toxicol. 2017, 91, 35–61. [Google Scholar] [CrossRef] [Green Version]
- Zhu, H.; She, J.; Zhou, M.; Fan, X. Rapid and sensitive detection of formaldehyde using portable 2-dimensional gas chromatography equipped with photoionization detectors. Sens. Actuators B Chem. 2019, 283, 182–187. [Google Scholar] [CrossRef]
- Duan, W.; Liu, A.; Li, Q.; Li, Z.; Wen, C.; Cai, Z.; Tang, S.; Li, X.; Zeng, J. Toward ultrasensitive and fast colorimetric detection of indoor formaldehyde across the visible region using cetytrimethylammonium chloride-capped bone-shaped glod nanorods as “chromophores”. Analyst 2019, 144, 4582–4588. [Google Scholar] [CrossRef]
- Kang, Z.; Zhang, D.; Li, T.; Liu, X.; Song, X. Polydopamine-modified SnO2 nanofiber composite coated QCM gas sensor for high-performance formaldehyde sensing. Sens. Actuators B Chem. 2021, 345, 130299. [Google Scholar] [CrossRef]
- Zhang, X.; Song, D.; Liu, Q.; Chen, R.; Hou, J.; Liu, J.; Zhang, H.; Yu, J.; Liu, P.; Wang, J. Designed synthesis of Ag-functionalized Ni-doped In2O3 nanorods with enhanced formaldehyde gas sensing properties. J. Mater. Chem. C 2019, 7, 7219–7229. [Google Scholar] [CrossRef]
- Guo, W.; Zhao, B.; Zhou, Q.; He, Y.; Wang, Z.; Radacsi, N. Fe-doped ZnO/reduced graphene oxide nanocomposite with synergic enhanced gas sensing performance for the effective detection of formaldehyde. ACS Omega 2019, 4, 10252–10262. [Google Scholar] [CrossRef] [Green Version]
- Geng, W.; Cao, X.; Xu, S.; Yang, J.; Babar, N.; He, Z.; Zhang, Q. Synthesis of hollow spherical nickel oxide and its gas-sensing properties. Rare Met. 2021, 40, 1622–1631. [Google Scholar] [CrossRef]
- Barsan, N.; Weimar, U. Conduction model of metal oxide gas sensors. J. Electroceram. 2001, 7, 143–167. [Google Scholar] [CrossRef]
- Masuda, Y. Recent advances in SnO2 nanostrature based gas sensors. Sens. Actuators B Chem. 2022, 364, 131876. [Google Scholar] [CrossRef]
- Munnix, S.; Schmeits, M. Electronic structure of tin dioxide surfaces. Phys. Rev. B 1983, 27, 7624–7635. [Google Scholar] [CrossRef]
- Wang, D.; Chu, X.; Gong, M. Gas-sensing properties of sensors based on single-crystalline SnO2 nanorods prepared by a simple molten-salt method. Sens. Actuators B Chem. 2006, 117, 183–187. [Google Scholar] [CrossRef]
- Han, Y.; Wu, X.; Shen, G.; Dierre, B.; Gong, L.; Qu, F.; Bando, Y.; Sekiguchi, T.; Filippo, F.; Golberg, D. Solution Growth and Cathodoluminescence of Novel SnO2 Core-Shell Homogeneous Microspheres. J. Phys. Chem. C 2010, 114, 8235–8240. [Google Scholar] [CrossRef]
- Wang, D.; Tian, L.; Li, H.; Wan, K.; Yu, X.; Wang, P.; Chen, A.; Wang, X.; Yang, J. Mesoporous ultrathin SnO2 nanosheets in situ modified by graphene oxide for extraordinary formaldehyde detection at low temperatures. ACS Appl. Mater. Interfaces 2019, 11, 12808–12818. [Google Scholar] [CrossRef]
- Jeong, Y.J.; Kim, D.; Jang, J.; Kang, J.; Kim, R.; Kim, I. Bio-inspired heterogeneous sensitization of bimetal oxides on SnO2 scaffolds for unparalleled formaldehyde detection. Chem. Commun. 2019, 55, 3622–3625. [Google Scholar] [CrossRef]
- Wang, D.; Wan, K.; Zhang, M.; Li, H.; Wang, P.; Wang, X.; Yang, J. Constructing hierarchical SnO2 nanofiber/nanosheets for efficient formaldehyde detection. Sens. Actuators B Chem. 2019, 283, 714–723. [Google Scholar] [CrossRef]
- Guo, J.; Zhang, J.; Ju, D.; Xu, H.; Cao, B. Three-dimensional SnO2 microstructures assembled by porous nanosheets and their superior performance for gas sensing. Power Technol. 2013, 250, 40–45. [Google Scholar] [CrossRef]
- Li, G.; Cheng, Z.; Xiang, Q.; Yan, L.; Wang, X.; Xu, J. Bimetal PdAu decorated SnO2 nanosheets based gas sensor with temperature-dependent dual selectivity for detecting formaldehyde and acetone. Sens. Actuators B Chem. 2019, 283, 590–601. [Google Scholar] [CrossRef]
- Rao, A.; Long, H.; Harley-Trochimczyk, A.; Pham, T.; Zettl, A.; Carraro, C.; Maboudian, R. In situ localized growth of ordered metal oxide hollow sphere array on microheater platform for sensitive, ultra-fast gas sensing. ACS Appl. Mater. Interfaces 2017, 9, 2634–2641. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Yang, T.; Wang, Z.; Li, Z.; Xiao, B.; Zhao, Q.; Zhang, M. Facile synthesis cedar-like SnO2 hierarchical micro-nanostructures with improved formaldehyde gas sensing characteristics. J. Alloy. Compd. 2017, 724, 121–129. [Google Scholar] [CrossRef]
- Liu, J.; Luo, T.; Meng, F.; Qian, K.; Wan, Y.; Liu, J. Porous hierarchical In2O3 micro-/nanostructures: Preparation, formation mechanism, and their application in gas sensors for noxious volatile organic compound detection. J. Phys. Chem. C 2010, 114, 4887–4894. [Google Scholar] [CrossRef]
- Chen, T.; Sun, J.; Xue, N.; Zhang, X.; Wang, H.; Jiang, K.; Zhou, T.; Quan, H.; Guo, R. Co,N-doped GQDs/SnO2 mesoporous microspheres exhibit synergistically enhanced gas sensing properties for H2S gas detection. J. Mater. Chem. A 2022, 10, 10759–10767. [Google Scholar] [CrossRef]
- Dieguez, A.; Romano-Rodrıguez, A.; Vila, A.; Morante, J.R. The complete Raman spectrum of nanometric SnO2 particles. J. Appl. Phys. 2001, 90, 1550–1557. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Zou, X.; Chen, H.; Chu, X.; Li, G. Tailoring energy level and surface basicity of metal oxide semiconductors by rare-earth incorporation for high-performance formaldehyde detection. Inorg. Chem. Front. 2019, 6, 1767–1774. [Google Scholar] [CrossRef]
- Calestani, D.; Mosca, R.; Zanichelli, M.; Villani, M.; Zappettini, A. Aldehyde detection by ZnO tetrapod-based gas sensors. J. Mater. Chem. 2011, 21, 15532–15536. [Google Scholar] [CrossRef]
- Zhang, W.; Cheng, X.L.; Zhang, X.; Xu, Y.; Gao, S.; Zhao, H.; Huo, L. High selectivity to ppb-level HCHO sensor based on mesoporous tubular SnO2 at low temperature. Sens. Actuators B Chem. 2017, 247, 664–672. [Google Scholar] [CrossRef]
- Dong, C.; Li, Q.; Chen, G.; Xiao, X.; Wang, Y. Enhanced formaldehyde sensing performance of 3D hierarchical porous structure Pt-functionalized NiO via a facile solution combustion synthesis. Sens. Actuators B Chem. 2015, 220, 171–179. [Google Scholar] [CrossRef]
- Wang, J.; Liu, L.; Cong, S.; Qi, J.; Xu, B. An enrichment method to detect low concentration formaldehyde. Sens. Actuators B Chem. 2008, 134, 1010–1015. [Google Scholar] [CrossRef]
- Gao, L.; Fu, H.; Zhu, J.; Wang, J.; Chen, Y.; Liu, H. Synthesis of SnO2 nanoparticles for formaldehyde detection with high sensitivity and good selectivity. J. Mater. Res. 2020, 35, 2208–2217. [Google Scholar] [CrossRef] [PubMed]
- Jia, X.; Wang, N.; Tian, J.; Zhang, Y.; Lu, D.; Tan, J.; Qiao, R.; Chen, L.; Zhang, W.; Zhong, J. A highly sensitive gas sensor employing biomorphic SnO2 with multi-level tubes/pores structure: Bio-templated from waste of flax. RSC Adv. 2019, 9, 19993–20001. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ji, H.; Zeng, W.; Li, Y. Gas sensing mechanisms of metal oxide semiconductors: A focus review. Nanoscale 2019, 11, 22664–22684. [Google Scholar] [CrossRef] [PubMed]
- Cai, H.; Luo, N.; Hu, Q.; Xue, Z.; Wang, X.; Xu, J. Multishell SnO2 Hollow Microspheres Loaded with Bimetal PdPt Nanoparticles for Ultrasensitive and Rapid Formaldehyde MEMS Sensors. ACS Sens. 2022, 7, 1484–1494. [Google Scholar] [CrossRef] [PubMed]
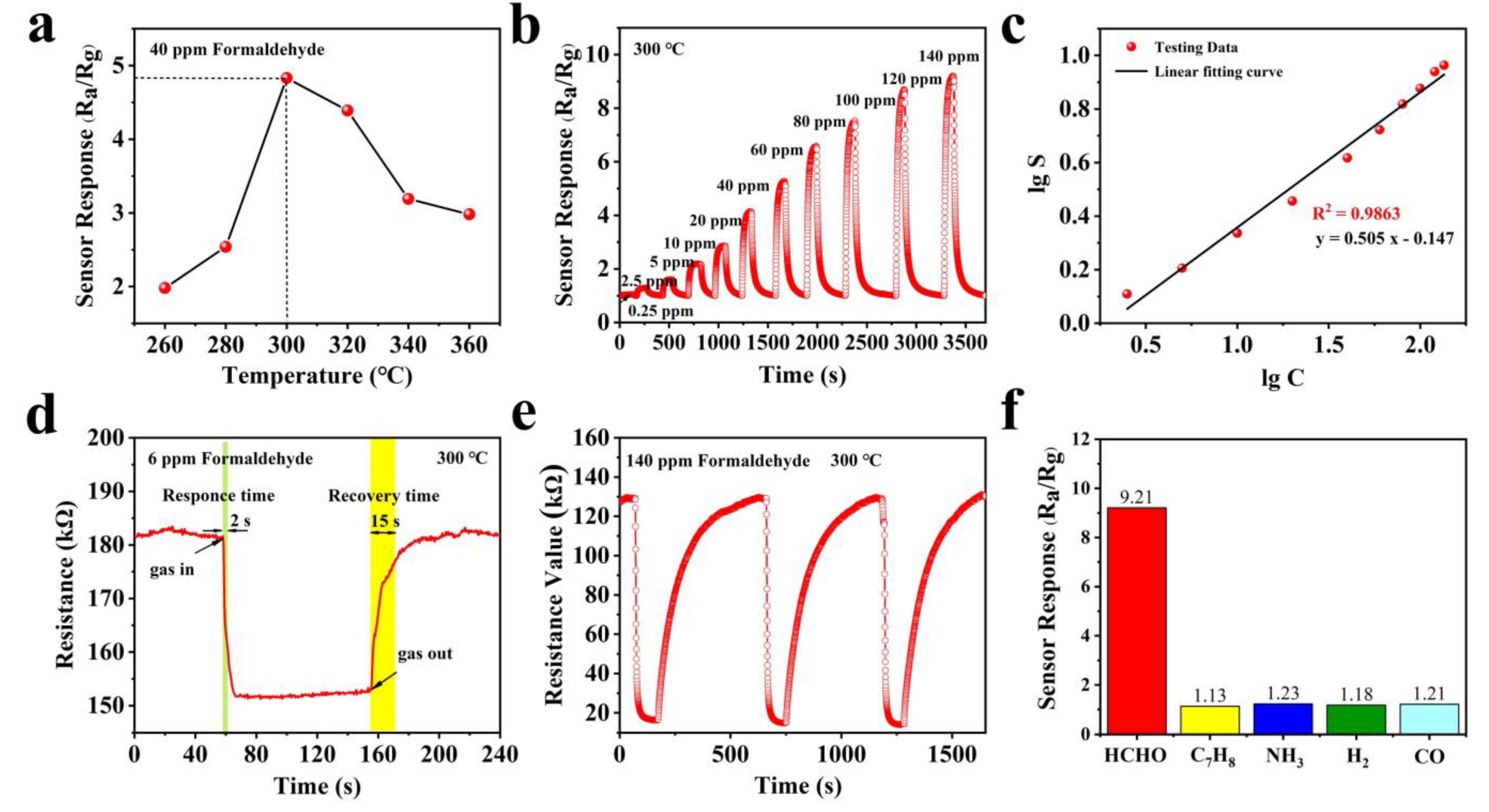
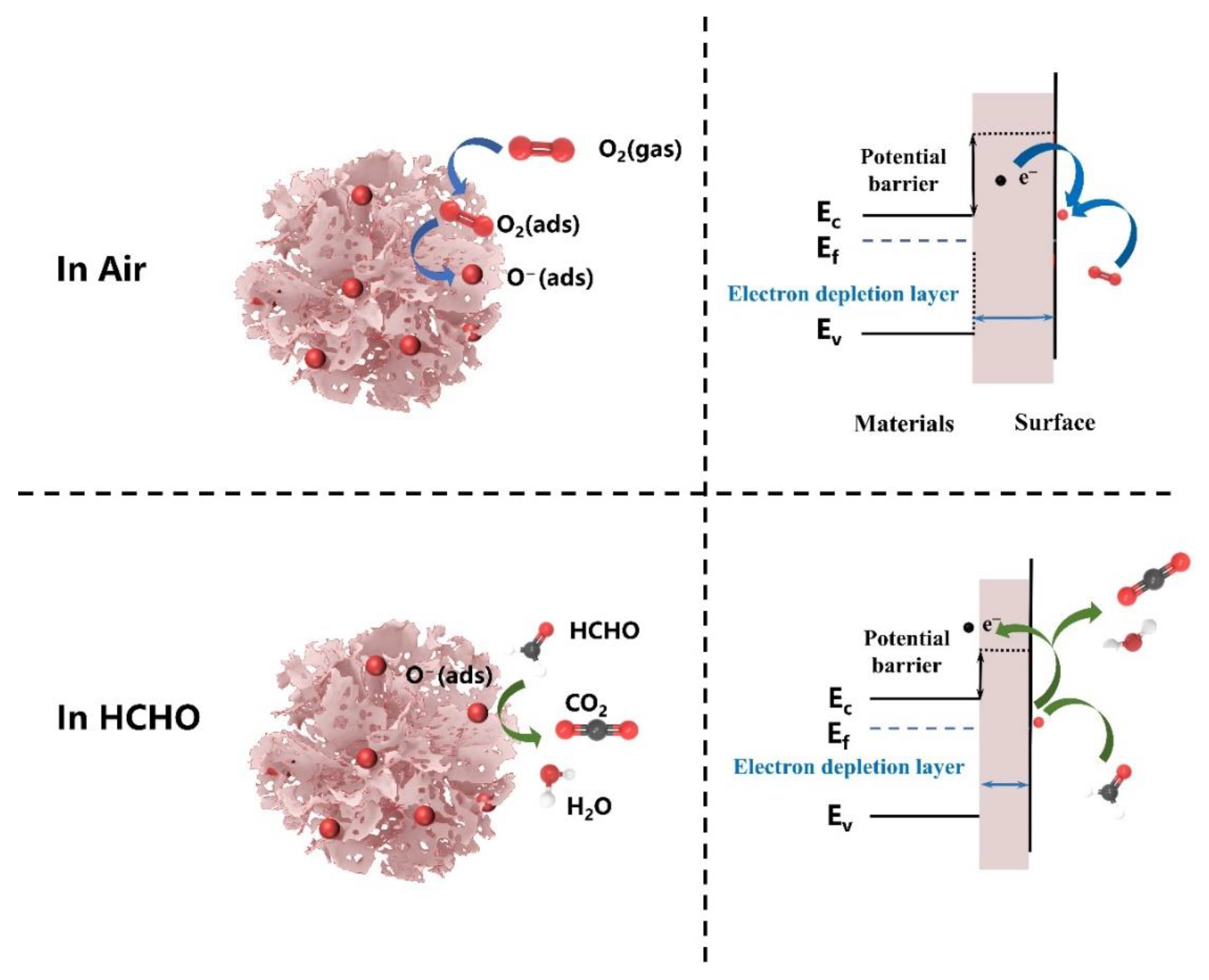
| Materials | Synthesis Route | Morphology | Temp. (°C) | Conc. (ppm) | Res. | Res./Rec. Time (s/s) | Ref. |
|---|---|---|---|---|---|---|---|
| PdAu/SnO2 | hydro-solvothermal | 3D nanosheets | 110 | 100 | 125 | 68/32 | [21] |
| SnO2 | sacrificial template | hollow sphere array | 300 | 0.5 | ~3 | 1.8/5.4 | [22] |
| SnO2 | hydrothermal | Cedar | 200 | 100 | 13.3 | <1/13 | [23] |
| SnO2 | solvothermal | mesoporous tubular | 200 | 100 | 37 | 17/25 | [29] |
| Pt/NiO | solution combustion | 3D Porous | 200 | 1000 | 8.2 | 102/70 | [30] |
| MWCNTs-doped SnO2 | sol-gel | nanometer-size powder | 250 | 50 | 3.8 | 100/90 | [31] |
| SnO2 | hydrothermal | nanoparticles | 230 | 50 | 35 | 20/23 | [32] |
| SnO2 | hydrothermal | nanoflowers | 300 | 120 | 9.2 | 2/15 | This work |
| Test Condition | Response (Ra/Rg) | Resistance (kΩ) | Res. Time (s) | Rec. Time (s) |
|---|---|---|---|---|
| In N2 | 4.7 | 961.6 | 5.5 | 16.0 |
| In Air | 7.4 | 1667.1 | 5.0 | 18.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiang, C.; Chen, T.; Zhao, Y.; Sun, J.; Jiang, K.; Li, Y.; Zhu, X.; Zhang, X.; Zhang, N.; Guo, R. Facile Hydrothermal Synthesis of SnO2 Nanoflowers for Low-Concentration Formaldehyde Detection. Nanomaterials 2022, 12, 2133. https://doi.org/10.3390/nano12132133
Xiang C, Chen T, Zhao Y, Sun J, Jiang K, Li Y, Zhu X, Zhang X, Zhang N, Guo R. Facile Hydrothermal Synthesis of SnO2 Nanoflowers for Low-Concentration Formaldehyde Detection. Nanomaterials. 2022; 12(13):2133. https://doi.org/10.3390/nano12132133
Chicago/Turabian StyleXiang, Chao, Tingting Chen, Yan Zhao, Jianhai Sun, Kaisheng Jiang, Yongzhen Li, Xiaofeng Zhu, Xinxiao Zhang, Ning Zhang, and Ruihua Guo. 2022. "Facile Hydrothermal Synthesis of SnO2 Nanoflowers for Low-Concentration Formaldehyde Detection" Nanomaterials 12, no. 13: 2133. https://doi.org/10.3390/nano12132133
APA StyleXiang, C., Chen, T., Zhao, Y., Sun, J., Jiang, K., Li, Y., Zhu, X., Zhang, X., Zhang, N., & Guo, R. (2022). Facile Hydrothermal Synthesis of SnO2 Nanoflowers for Low-Concentration Formaldehyde Detection. Nanomaterials, 12(13), 2133. https://doi.org/10.3390/nano12132133