CuxO-Modified Nanoporous Cu Foil as a Self-Supporting Electrode for Supercapacitor and Oxygen Evolution Reaction
Abstract
:1. Introduction
2. Experimental
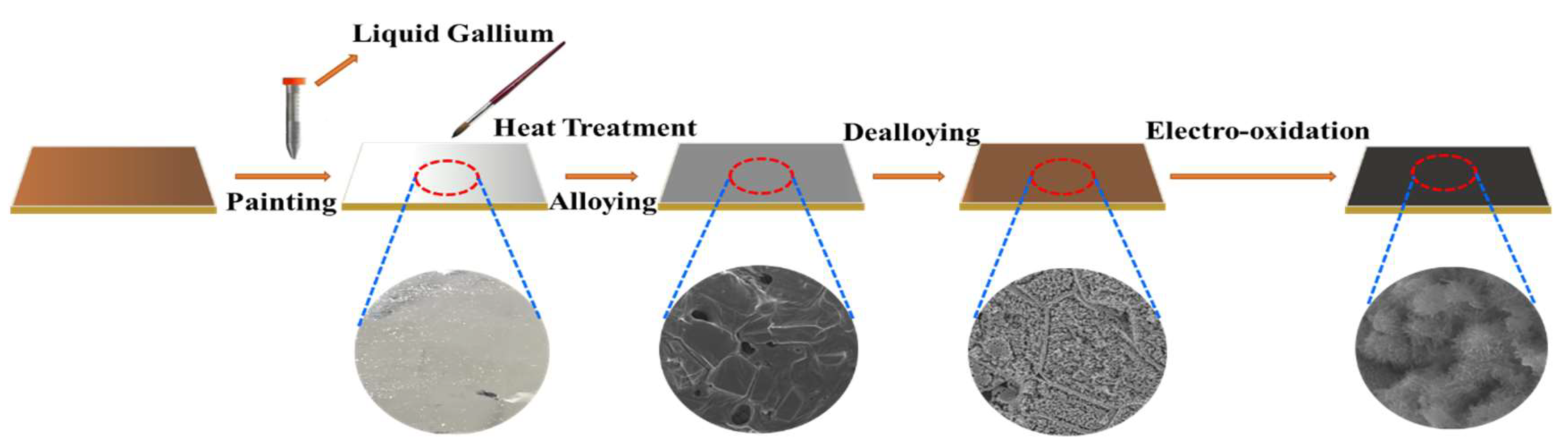
2.1. Preparation of CuxO/Cu Foils
2.2. Fabrication of Asymmetric Supercapacitor Devices
2.3. Characterizations of Catalysts
2.4. Electrochemical Measurements
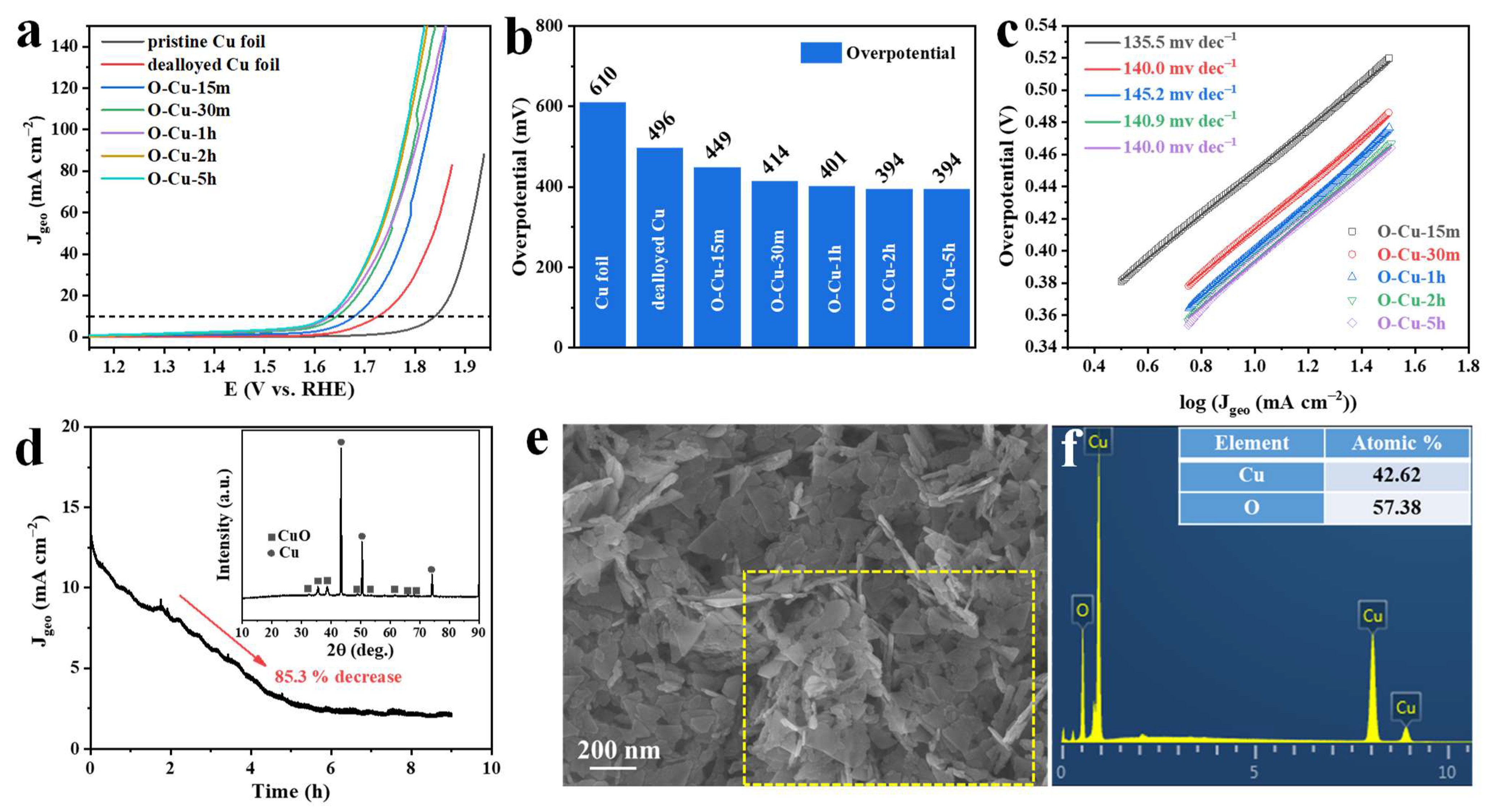
3. Results and Discussion
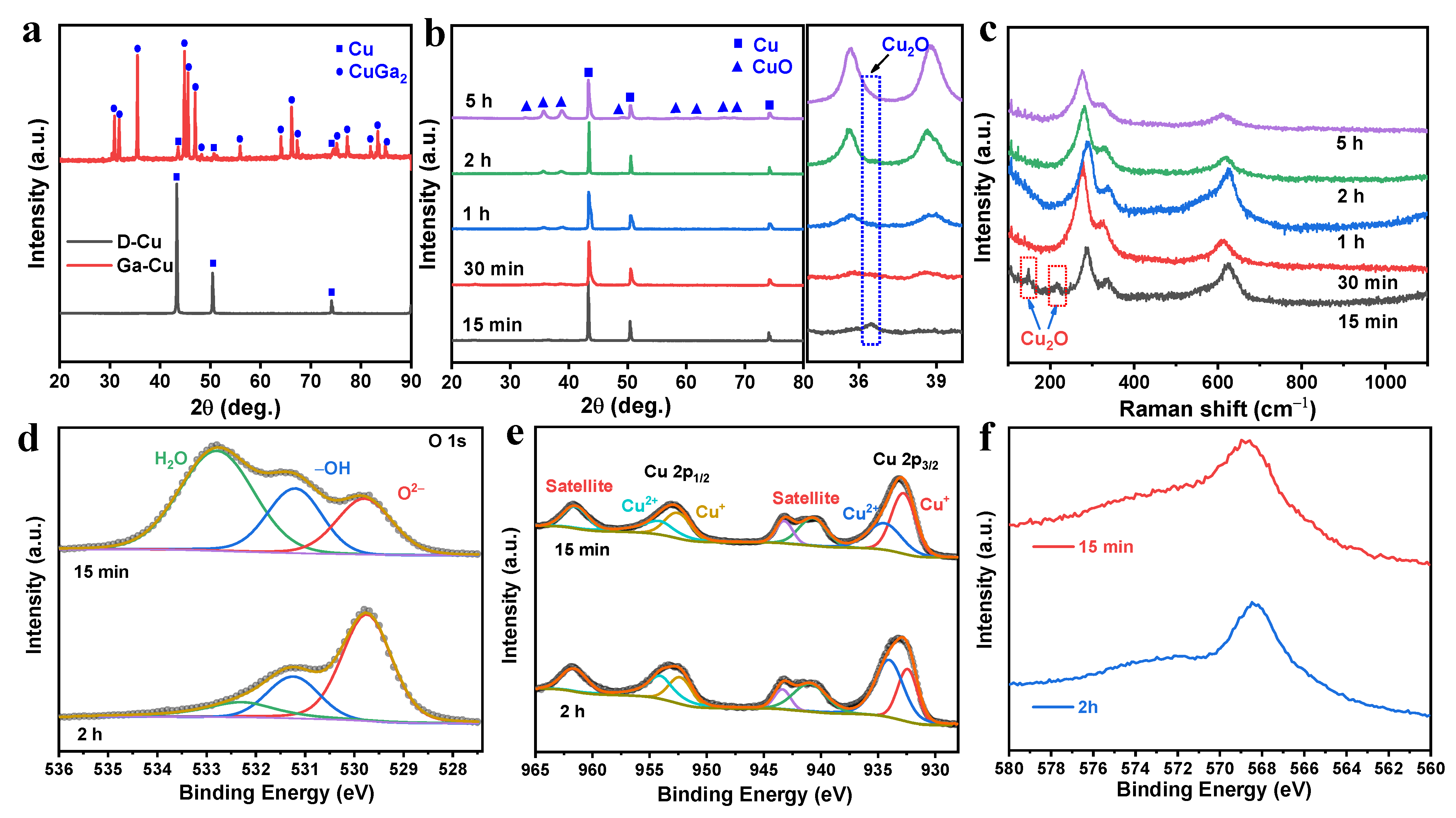
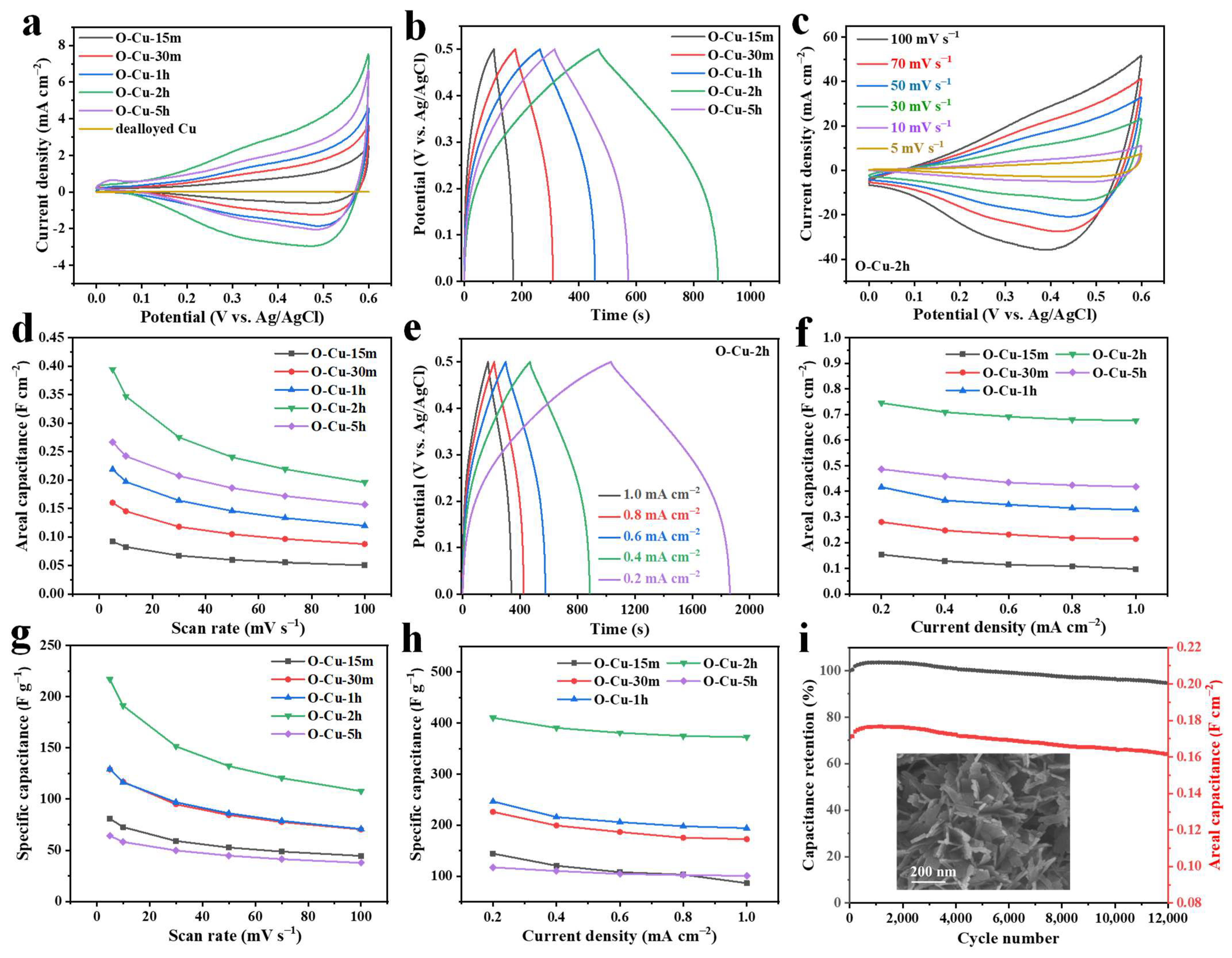
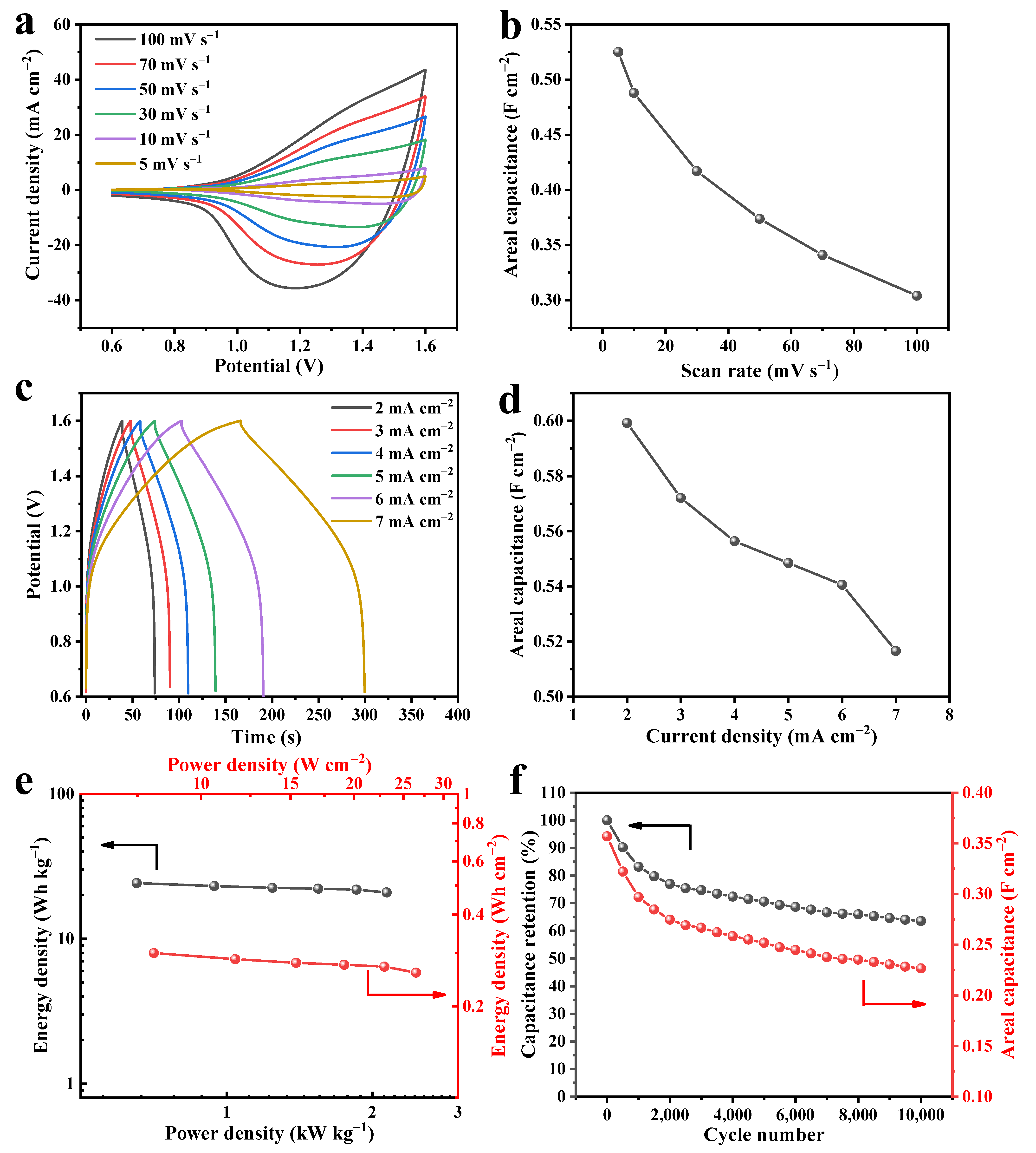
The Composition and Microstructure Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, Z.; Liu, J.; Qin, C.; Yu, H.; Xia, X.; Wang, C.; Zhang, Y.; Hu, Q.; Zhao, W. Dealloying of Cu-based metallic glasses in acidic solutions: Products and energy storage applications. Nanomaterials 2015, 5, 697–721. [Google Scholar] [CrossRef] [Green Version]
- McCue, I.; Benn, E.; Gaskey, B.; Erlebacher, J. Dealloying and dealloyed materials. Annu. Rev. Mater. Res. 2016, 46, 263–286. [Google Scholar] [CrossRef]
- Wu, X.; He, G.; Ding, Y. Dealloyed nanoporous materials for rechargeable lithium batteries. Electrochem. Energy Rev. 2020, 3, 541–580. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Y.; Yu, B.; Yin, K.; Zhang, Z. Hierarchically structured black gold film with ultrahigh porosity for solar steam generation. Adv. Mater. 2022, 34, e2200108. [Google Scholar] [CrossRef]
- Erlebacher, J.; Aziz, M.J.; Karma, A.; Dimitrov, N.; Sieradzki, K. Evolution of nanoporosity in dealloying. Nature 2001, 410, 450–453. [Google Scholar] [CrossRef]
- Erlebacher, J. An atomistic description of dealloying. J. Electrochem. Soc. 2004, 151, C614–C626. [Google Scholar] [CrossRef]
- Li, J.; Yin, H.-M.; Li, X.-B.; Okunishi, E.; Shen, Y.-L.; He, J.; Tang, Z.-K.; Wang, W.-X.; Yücelen, E.; Li, C.; et al. Surface evolution of a Pt–Pd–Au electrocatalyst for stable oxygen reduction. Nat. Energy 2017, 2, 17111. [Google Scholar] [CrossRef]
- Xi, W.; Wang, K.; Shen, Y.; Ge, M.; Deng, Z.; Zhao, Y.; Cao, Q.; Ding, Y.; Hu, G.; Luo, J. Dynamic co-catalysis of Au single atoms and nanoporous Au for methane pyrolysis. Nat. Commun. 2020, 11, 1919. [Google Scholar] [CrossRef] [Green Version]
- Lang, X.; Hirata, A.; Fujita, T.; Chen, M. Nanoporous metal/oxide hybrid electrodes for electrochemical supercapacitors. Nat. Nanotechnol. 2011, 6, 232–236. [Google Scholar] [CrossRef]
- Jin, H.J.; Wang, X.L.; Parida, S.; Wang, K.; Seo, M.; Weissmüller, J. Nanoporous Au-Pt alloys as large strain electrochemical actuators. Nano Lett. 2010, 10, 187–194. [Google Scholar] [CrossRef]
- Jin, H.-J.; Weissmüller, J. A material with electrically tunable strength and flow stress. Science 2011, 332, 1179–1182. [Google Scholar] [CrossRef]
- Keir, D.; Pryor, M. The dealloying of copper-manganese alloys. J. Electrochem. Soc. 1980, 127, 2138–2144. [Google Scholar] [CrossRef]
- Smith, A.; Tran, T.; Wainwright, M. Kinetics and mechanism of the preparation of Raney® copper. J. Appl. Electrochem. 1999, 29, 1085–1094. [Google Scholar] [CrossRef]
- Liu, D.; Yang, Z.; Wang, P.; Li, F.; Wang, D.; He, D. Preparation of 3D nanoporous copper-supported cuprous oxide for high-performance lithium ion battery anodes. Nanoscale 2013, 5, 1917–1921. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, S.; Li, N.; An, S.; Zheng, J. Preparation and characterization of sandwich-typed three-dimensional nanoporous copper-supported tin thin-film anode for lithium ion battery. Int. J. Electrochem. Sci. 2013, 8, 347–358. [Google Scholar]
- Han, G.; Um, J.H.; Park, H.; Hong, K.; Yoon, W.-S.; Choe, H. Hierarchically structured nanoporous copper for use as lithium-ion battery anode. Scr. Mater. 2019, 163, 9–13. [Google Scholar] [CrossRef]
- Qin, C.; Zheng, D.; Hu, Q.; Zhang, X.; Wang, Z.; Li, Y.; Zhu, J.; Ou, J.Z.; Yang, C.; Wang, Y. Flexible integrated metallic glass-based sandwich electrodes for high-performance wearable all-solid-state supercapacitors. Appl. Mater. Today 2020, 19, 100539. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, M.; Wang, Z.; Qin, C.; Zhang, M.; Li, Y. Porous CuxO/Ag2O (x = 1, 2) nanowires anodized on nanoporous Cu-Ag bimetal network as a self-supported flexible electrode for glucose sensing. Appl. Surf. Sci. 2020, 515, 146062. [Google Scholar] [CrossRef]
- Li, R.; Liu, X.; Wang, H.; Wu, Y.; Lu, Z.P. Bendable nanoporous copper thin films with tunable thickness and pore features. Corros. Sci. 2016, 104, 227–235. [Google Scholar] [CrossRef]
- Diao, F.; Xiao, X.; Luo, B.; Sun, H.; Ding, F.; Ci, L.; Si, P. Two-step fabrication of nanoporous copper films with tunable morphology for SERS application. Appl. Surf. Sci. 2018, 427, 1271–1279. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Wang, Y.; Gao, H.; Niu, J.; Zhang, J.; Peng, Z.; Zhang, Z. ‘Painting’ nanostructured metals—Playing with liquid metal. Nanoscale Horiz. 2018, 3, 408–416. [Google Scholar] [CrossRef]
- Kou, T.; Wang, Y.; Zhang, C.; Sun, J.; Zhang, Z. Adsorption behavior of methyl orange onto nanoporous core-shell Cu@Cu2O nanocomposite. Chem. Eng. J. 2013, 223, 76–83. [Google Scholar] [CrossRef]
- Li, M.; Li, Y.; Zhang, Q.; Qin, C.; Zhao, W.; Wang, Z.; Inoue, A. Ultrafine Cu2O/CuO nanosheet arrays integrated with NPC/BMG composite rod for photocatalytic degradation. Appl. Surf. Sci. 2019, 483, 285–293. [Google Scholar] [CrossRef]
- Liu, W.; Chen, L.; Cui, L.; Yan, J.; Zhang, S.; Shi, S. Freestanding 3D nanoporous Cu@1D Cu2O nanowire heterostructures: From a facile one-step protocol to robust application in Li storage. J. Mater. Chem. A 2019, 7, 15089–15100. [Google Scholar] [CrossRef]
- Wang, S.; Jiang, L.; Hu, J.; Wang, Q.; Zhan, S.; Lu, Y. Dual-functional CuxO/Cu electrodes for supercapacitors and non-enzymatic glucose sensors fabricated by femtosecond laser enhanced thermal oxidation. J. Alloys Compd. 2020, 815, 152105. [Google Scholar] [CrossRef]
- Wu, T.; Xu, L.N.; Sun, H.; Bao, Y.; Yu, H.; Guo, X.; Hu, Q.; Li, J. Hierarchical shell/core electrodes with CuO nanowires based on carbon cloths for high performance asymmetric supercapacitors. Ceram. Int. 2021, 47, 33758–33765. [Google Scholar] [CrossRef]
- Dong, C.; Wang, Y.; Xu, J.; Cheng, G.; Yang, W.; Kou, T.; Zhang, Z.; Ding, Y. 3D binder-free Cu2O@Cu nanoneedle arrays for high-performance asymmetric supercapacitors. J. Mater. Chem. A 2014, 2, 18229–18235. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, X.; Liu, H.; Li, W.; Wang, X. Synthesis and morphology control of nanoporous Cu2O/Cu and their application as electrode materials for capacitors. Nanomaterials 2019, 9, 340. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Shen, G.; Zhao, S.; He, X.; Zhang, C.; Jiang, T.; Jiang, J.; Chen, B. A one-dimensional Ag NW@NiCo/NiCo(OH)2 core–shell nanostructured electrode for a flexible and transparent asymmetric supercapacitor. J. Mater. Chem. A 2019, 7, 8184–8193. [Google Scholar] [CrossRef]
- Zhong, Y.; Chen, B.; Liang, J.; Xin, Y.; Zhang, C.; Zhang, B.; Wang, S.; He, X. Structural engineering of ultrathin, lightweight, and bendable electrodes based on a nanowire network current collector enables flexible energy-storage devices. ACS Appl. Energy Mater. 2022, 5, 5785–5796. [Google Scholar] [CrossRef]
- Yang, W.; Wang, J.; Ma, W.; Dong, C.; Cheng, G.; Zhang, Z. Free-standing CuO nanoflake arrays coated Cu foam for advanced lithium ion battery anodes. J. Power Sources 2016, 333, 88–98. [Google Scholar] [CrossRef]
- Li, M.; Wang, Z.; Zhang, Q.; Qin, C.; Inoue, A.; Guo, W. Formation and evolution of ultrathin Cu2O nanowires on NPC ribbon by anodizing for photocatalytic degradation. Appl. Surf. Sci. 2020, 506, 144819. [Google Scholar] [CrossRef]
- Xu, J.F.; Ji, W.; Shen, Z.X.; Li, W.S.; Tang, S.H.; Ye, X.R.; Jia, D.Z.; Xin, X.Q. Raman spectra of CuO nanocrystals. J. Raman Spectrosc. 1999, 30, 413–415. [Google Scholar] [CrossRef]
- Deng, Y.; Handoko, A.D.; Du, Y.; Xi, S.; Yeo, B.S. In situ Raman spectroscopy of copper and copper oxide surfaces during electrochemical oxygen evolution reaction: Identification of cuIII oxides as catalytically active species. ACS Catal. 2016, 6, 2473–2481. [Google Scholar] [CrossRef]
- Singhal, A.; Pai, M.R.; Rao, R.; Pillai, K.T.; Lieberwirth, I.; Tyagi, A.K. Copper(I) oxide nanocrystals–one step synthesis, characterization, formation mechanism, and photocatalytic properties. Eur. J. Inorg. Chem. 2013, 2013, 2640–2651. [Google Scholar] [CrossRef]
- Li, Y.; Jiang, H.; Yan, X.; Zhu, Y.; Zhang, W.; Zhang, M.; Zhu, W.; Javed, M.S.; Pan, J.; Hussain, S. 3D nanostructured CuxO modified copper foam as a binder-free electrode for all-solid-state supercapacitor. Ceram. Int. 2021, 47, 31138–31148. [Google Scholar] [CrossRef]
- Zheng, M.; Xiao, X.; Li, L.; Gu, P.; Dai, X.; Tang, H.; Hu, Q.; Xue, H.; Pang, H. Hierarchically nanostructured transition metal oxides for supercapacitors. Sci. China Mater. 2017, 61, 185–209. [Google Scholar] [CrossRef] [Green Version]
- Guo, Y.; Hong, X.; Wang, Y.; Li, Q.; Meng, J.; Dai, R.; Liu, X.; He, L.; Mai, L. Multicomponent hierarchical Cu-doped NiCo-LDH/CuO double arrays for ultralong-life hybrid fiber supercapacitor. Adv. Funct. Mater. 2019, 29, 1809004. [Google Scholar] [CrossRef]
- Moosavifard, S.E.; El-Kady, M.F.; Rahmanifar, M.S.; Kaner, R.B.; Mousavi, M.F. Designing 3D highly ordered nanoporous CuO electrodes for high-performance asymmetric supercapacitors. ACS Appl. Mater. Interfaces 2015, 7, 4851–4860. [Google Scholar] [CrossRef]
- Jiang, Y.; Liu, J. Definitions of pseudocapacitive materials: A brief review. Energy Environ. Mater. 2019, 2, 30–37. [Google Scholar] [CrossRef] [Green Version]
- Wang, K.; Dong, X.; Zhao, C.; Qian, X.; Xu, Y. Facile synthesis of Cu2O/CuO/RGO nanocomposite and its superior cyclability in supercapacitor. Electrochim. Acta 2015, 152, 433–442. [Google Scholar] [CrossRef]
- Li, Y.; Wang, X.; Yang, Q.; Javed, M.S.; Liu, Q.; Xu, W.; Hu, C.; Wei, D. Ultra-fine CuO nanoparticles embedded in three-dimensional graphene network nano-structure for high-performance flexible supercapacitors. Electrochim. Acta 2017, 234, 63–70. [Google Scholar] [CrossRef]
- Chhetri, K.; Dahal, B.; Mukhiya, T.; Tiwari, A.P.; Muthurasu, A.; Kim, T.; Kim, H.; Kim, H.Y. Integrated hybrid of graphitic carbon-encapsulated CuxO on multilayered mesoporous carbon from copper MOFs and polyaniline for asymmetric supercapacitor and oxygen reduction reactions. Carbon 2021, 179, 89–99. [Google Scholar] [CrossRef]
- Kamble, G.P.; Rasal, A.S.; Gaikwad, S.B.; Gurav, V.S.; Chang, J.-Y.; Kolekar, S.S.; Ling, Y.-C.; Ghule, A.V. CuCo2O4 Nanorods Coated with CuO Nanoneedles for Supercapacitor Applications. ACS Appl. Nano Mater. 2021, 4, 12702–12711. [Google Scholar] [CrossRef]
- Zhan, Y.; Bai, J.; Guo, F.; Zhou, H.; Shu, R.; Yu, Y.; Qian, L. Facile synthesis of biomass-derived porous carbons incorporated with CuO nanoparticles as promising electrode materials for high-performance supercapacitor applications. J. Alloys Compd. 2021, 885, 161014. [Google Scholar] [CrossRef]
- Stoller, M.D.; Ruoff, R.S. Best practice methods for determining an electrode material’s performance for ultracapacitors. Energy Environ. Sci. 2010, 3, 1294–1301. [Google Scholar] [CrossRef]
- Prasad Ojha, G.; Muthurasu, A.; Prasad Tiwari, A.; Pant, B.; Chhetri, K.; Mukhiya, T.; Dahal, B.; Lee, M.; Park, M.; Kim, H.-Y. Vapor solid phase grown hierarchical CuxO NWs integrated MOFs-derived CoS2 electrode for high-performance asymmetric supercapacitors and the oxygen evolution reaction. Chem. Eng. J. 2020, 399, 125532. [Google Scholar] [CrossRef]
- Pawar, S.M.; Pawar, B.S.; Hou, B.; Kim, J.; Aqueel Ahmed, A.T.; Chavan, H.S.; Jo, Y.; Cho, S.; Inamdar, A.I.; Gunjakar, J.L.; et al. Self-assembled two-dimensional copper oxide nanosheet bundles as an efficient oxygen evolution reaction (OER) electrocatalyst for water splitting applications. J. Mater. Chem. A 2017, 5, 12747–12751. [Google Scholar] [CrossRef] [Green Version]
- Zhang, S.L.; Guan, B.Y.; Lu, X.F.; Xi, S.; Du, Y.; Lou, X.W.D. Metal atom-doped Co3O4 hierarchical nanoplates for electrocatalytic oxygen evolution. Adv. Mater. 2020, 32, e2002235. [Google Scholar] [CrossRef]
- Suen, N.-T.; Hung, S.-F.; Quan, Q.; Zhang, N.; Xu, Y.-J.; Chen, H.M. Electrocatalysis for the oxygen evolution reaction: Recent development and future perspectives. Chem. Soc. Rev. 2017, 46, 337–365. [Google Scholar] [CrossRef]
- Li, X.; Du, X.; Ma, X.; Wang, Z.; Hao, X.; Abudula, A.; Yoshida, A.; Guan, G. CuO nanowire@Co3O4 ultrathin nanosheet core-shell arrays: An effective catalyst for oxygen evolution reaction. Electrochim. Acta 2017, 250, 77–83. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Z.; Lin, J.; He, X.; Xin, Y.; Liang, P.; Zhang, C. CuxO-Modified Nanoporous Cu Foil as a Self-Supporting Electrode for Supercapacitor and Oxygen Evolution Reaction. Nanomaterials 2022, 12, 2121. https://doi.org/10.3390/nano12122121
Li Z, Lin J, He X, Xin Y, Liang P, Zhang C. CuxO-Modified Nanoporous Cu Foil as a Self-Supporting Electrode for Supercapacitor and Oxygen Evolution Reaction. Nanomaterials. 2022; 12(12):2121. https://doi.org/10.3390/nano12122121
Chicago/Turabian StyleLi, Zhenhan, Jianbin Lin, Xin He, Yue Xin, Ping Liang, and Chi Zhang. 2022. "CuxO-Modified Nanoporous Cu Foil as a Self-Supporting Electrode for Supercapacitor and Oxygen Evolution Reaction" Nanomaterials 12, no. 12: 2121. https://doi.org/10.3390/nano12122121
APA StyleLi, Z., Lin, J., He, X., Xin, Y., Liang, P., & Zhang, C. (2022). CuxO-Modified Nanoporous Cu Foil as a Self-Supporting Electrode for Supercapacitor and Oxygen Evolution Reaction. Nanomaterials, 12(12), 2121. https://doi.org/10.3390/nano12122121





