Low-Temperature Hydrothermal Growth of ZnO Nanowires on AZO Substrates for FACsPb(IBr)3 Perovskite Solar Cells
Abstract
:1. Introduction
2. Experimental Details
2.1. Materials
2.2. ZnO Nanowire Growth
2.3. Perovskite Solar Cells Fabrication
2.4. Characterization Techniques
3. Results and Discussion
3.1. Photovoltaic Performance as a Function of Growing Substrate and ETL
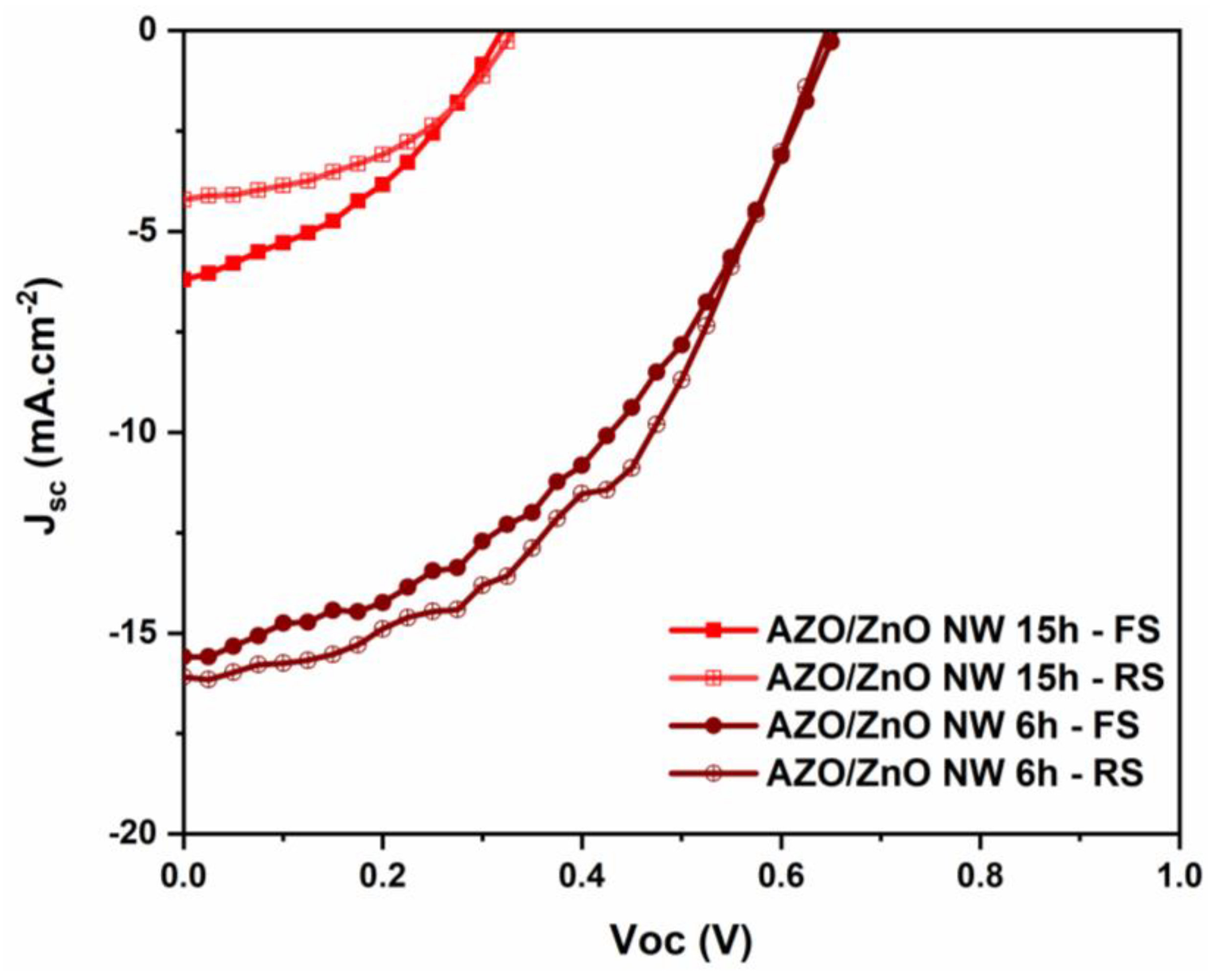
3.2. Influence of ZnO NWs’ Growing Time
3.3. Optimization of the Hydrothermal Process
3.3.1. Effect of PEI Content
3.3.2. Effect of HMTA Content
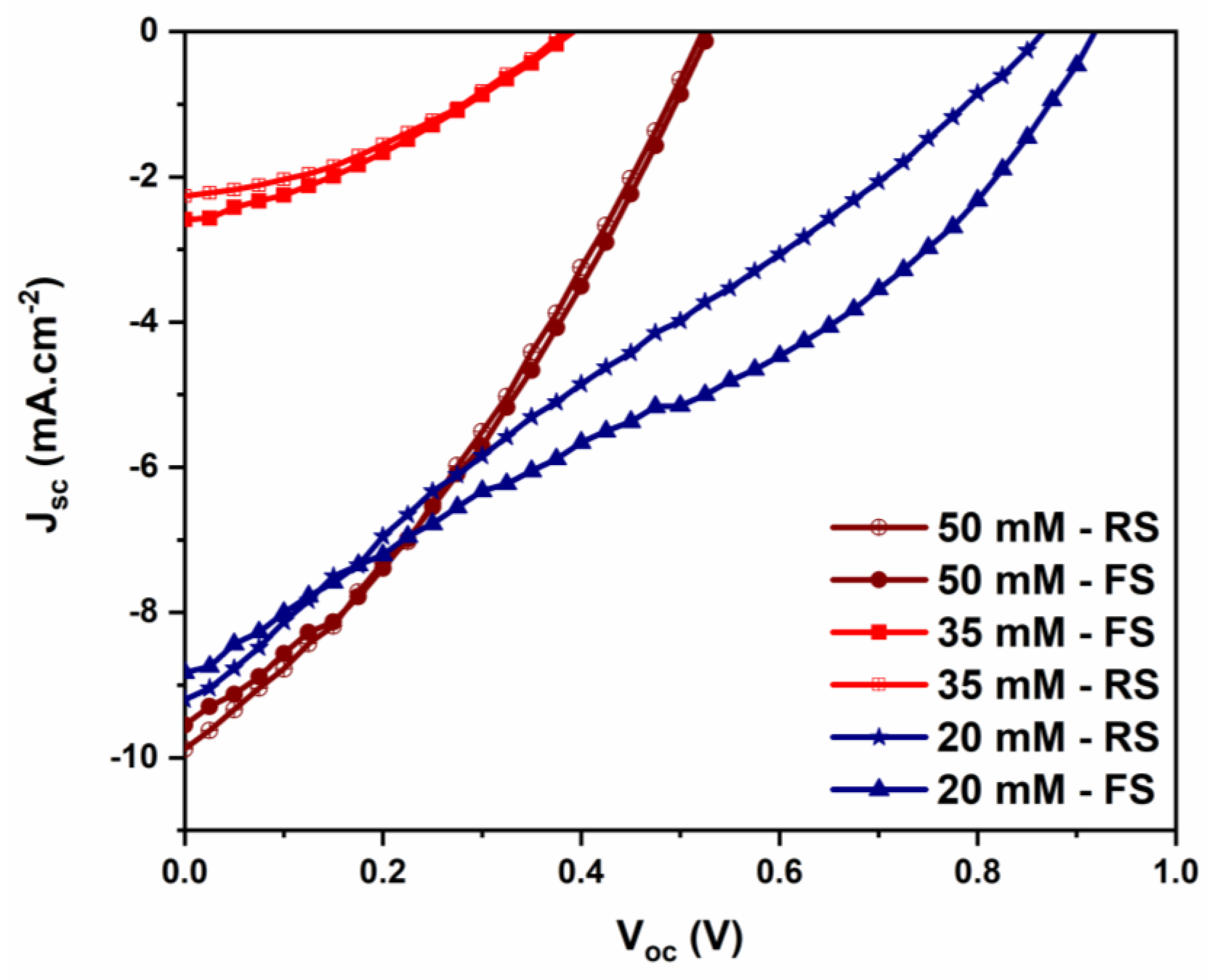
3.3.3. Effect of Zn(NO3)2 Concentration
3.4. Comparison between Spiro-OMeTAD and Cz–Pyr as HTL
| Device Structure | Jsc (mA cm−2) | Voc (V) | FF | PCE (%) | Ref |
|---|---|---|---|---|---|
| AZO/ZnO NRs/MAPbI3/Spiro-OMeTAD/Au | 16.00 | 0.80 | 0.53 | 7.00 | [74] |
| AZO/ZnO NRs/MAPbIxCl3-x/Cu | 14.87 | 0.86 | 0.28 | 3.62 | [83] |
| AZO/ZnO NRs based DSSC | 05.01 | 0.60 | 0.43 | 1.31 | [84] |
| AZO/ZnO NWs/FACsPb(IBr)3/Spiro-OMeTAD/Au | 16.10 | 0.64 | 0.47 | 4.9 | This work |
| AZO/ZnO NWs/FACsPb(IBr)3/Cz–Pyr/Au | 07.00 | 0.81 | 0.39 | 2.2 |
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kazim, S.; Nazeeruddin, M.K.; Grätzel, M.; Ahmad, S. Perovskite as light harvester: A game changer in photovoltaics. Angew. Chem. Int. Ed. 2014, 53, 2812–2824. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.M.; Teuscher, J.; Miyasaka, T.; Murakami, T.N.; Snaith, H.J. Efficient hybrid solar cells based on meso-superstructured organometal halide perovskites. Science 2012, 338, 643–647. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, H.; Lee, C.; Im, J.; Lee, K.; Moehl, T.; Marchioro, A.; Moon, S.; Humphry-Baker, R.; Yum, J.; Moser, J.E.; et al. Lead Iodide Perovskite Sensitized All-Solid-State Submicron Thin Film Mesoscopic Solar Cell with Efficiency Exceeding 9%. Science 2012, 2, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Best Research-Cell Efficiency Chart Photovoltaic Research NREL. Available online: https://www.nrel.gov/pv/cell-efficiency.html (accessed on 30 March 2021).
- Almora, O.; Baran, D.; Bazan, G.C.; Berger, C.; Cabrera, C.I.; Catchpole, K.R.; Erten-Ela, S.; Guo, F.; Hauch, J.; Ho-Baillie, A.W.Y.; et al. Device Performance of Emerging Photovoltaic Materials (Version 1). Adv. Energy Mater. 2020, 11, 2002774. [Google Scholar] [CrossRef]
- Chen, L.-C.; Tseng, Z.-L. ZnO-Based Electron Transporting Layer for Perovskite Solar Cells. Nanostruct. Sol. Cells 2017, 10, 203–215. [Google Scholar] [CrossRef] [Green Version]
- Grätzel, M. The light and shade of perovskite solar cells. Nat. Mater. 2014, 13, 838–842. [Google Scholar] [CrossRef]
- Li, G.; Zhu, R.; Yang, Y. Polymer solar cells. Nat. Photonics 2012, 6, 153–161. [Google Scholar] [CrossRef]
- Green, M.A.; Ho-Baillie, A.; Snaith, H.J. The emergence of perovskite solar cells. Nat. Photonics 2014, 8, 506–514. [Google Scholar] [CrossRef]
- Han, G.S.; Chung, H.S.; Kim, B.J.; Kim, D.H.; Lee, J.W.; Swain, B.S.; Mahmood, K.; Yoo, J.S.; Park, N.G.; Lee, J.H.; et al. Retarding charge recombination in perovskite solar cells using ultrathin MgO-coated TiO2 nanoparticulate films. J. Mater. Chem. A 2015, 3, 9160–9164. [Google Scholar] [CrossRef]
- Luo, J.; Wang, Y.; Zhang, Q. Progress in perovskite solar cells based on ZnO nanostructures. Sol. Energy 2018, 163, 289–306. [Google Scholar] [CrossRef]
- Mahmood, K.; Swain, B.S.; Amassian, A. 16.1% Efficient Hysteresis-Free Mesostructured Perovskite Solar Cells Based on Synergistically Improved ZnO Nanorod Arrays. Adv. Energy Mater. 2015, 5, 1500568. [Google Scholar] [CrossRef]
- Wang, P.; Zhao, J.; Liu, J.; Wei, L.; Liu, Z.; Guan, L.; Cao, G. Stabilization of organometal halide perovskite films by SnO2 coating with inactive surface hydroxyl groups on ZnO nanorods. J. Power Sources 2017, 339, 51–60. [Google Scholar] [CrossRef]
- Zhang, P.; Wu, J.; Zhang, T.; Wang, Y.; Liu, D.; Chen, H.; Ji, L.; Liu, C.; Ahmad, W.; Chen, Z.D.; et al. Perovskite Solar Cells with ZnO Electron-Transporting Materials. Adv. Mater. 2018, 30, 1703737. [Google Scholar] [CrossRef]
- Mahmood, K.; Swain, B.S.; Jung, H.S. Controlling the surface nanostructure of ZnO and Al-doped ZnO thin films using electrostatic spraying for their application in 12% efficient perovskite solar cells. Nanoscale 2014, 6, 9127–9138. [Google Scholar] [CrossRef] [PubMed]
- Son, D.Y.; Im, J.H.; Kim, H.S.; Park, N.G. 11% efficient perovskite solar cell based on ZnO nanorods: An effective charge collection system. J. Phys. Chem. C 2014, 118, 16567–16573. [Google Scholar] [CrossRef]
- Dong, J.; Zhao, Y.; Shi, J.; Wei, H.; Xiao, J.; Xu, X.; Luo, J.; Xu, J.; Li, D.; Luo, Y.; et al. Impressive enhancement in the cell performance of ZnO nanorod-based perovskite solar cells with Al-doped ZnO interfacial modification. Chem. Commun. 2014, 50, 13381–13384. [Google Scholar] [CrossRef]
- Mahmood, K.; Hameed, M.; Rehman, F.; Khalid, A.; Imran, M.; Mehran, M.T. A multifunctional blade-coated ZnO seed layer for high-efficiency perovskite solar cells. Appl. Phys. A Mater. Sci. Process. 2019, 125, 83. [Google Scholar] [CrossRef]
- Wang, Z.L. Zinc oxide nanostructures: Growth, properties and applications. J. Phys. Condens. Matter 2004, 16, 829–858. [Google Scholar] [CrossRef]
- Greene, L.E.; Yuhas, B.D.; Law, M.; Zitoun, D.; Yang, P. Solution-grown zinc oxide nanowires. Inorg. Chem. 2006, 45, 7535–7543. [Google Scholar] [CrossRef]
- Özgür, Ü.; Alivov, Y.I.; Liu, C.; Teke, A.; Reshchikov, M.A.; Doğan, S.; Avrutin, V.; Cho, S.; Morkoç, H. A comprehensive review of ZnO materials and devices. J. Appl. Phys. Appl. Phys. 2005, 98, 041301. [Google Scholar] [CrossRef] [Green Version]
- Zhou, H.; Shi, Y.; Wang, K.; Dong, Q.; Bai, X.; Xing, Y.; Du, Y.; Ma, T. Low-temperature processed and carbon-based ZnO/CH3NH3PbI3/C planar heterojunction perovskite solar cells. J. Phys. Chem. C 2015, 119, 4600–4605. [Google Scholar] [CrossRef]
- Dymshits, A.; Iagher, L.; Etgar, L. Parameters influencing the growth of ZnO nanowires as efficient low temperature flexible perovskite-based solar cells. Materials 2016, 9, 60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, J.; Siempelkamp, B.D.; Mosconi, E.; De Angelis, F.; Kelly, T.L. Origin of the Thermal Instability in CH3NH3PbI3 Thin Films Deposited on ZnO. Chem. Mater. 2015, 27, 4229–4236. [Google Scholar] [CrossRef]
- Wang, Q.; Shao, Y.; Dong, Q.; Xiao, Z.; Yuan, Y.; Huang, J. Large fill-factor bilayer iodine perovskite solar cells fabricated by a low-temperature solution-process. Energy Environ. Sci. 2014, 7, 2359–2365. [Google Scholar] [CrossRef]
- Law, M.; Greene, L.E.; Johnson, J.C.; Saykally, R.; Yang, P. Nanowire dye-sensitized solar cells. Nat. Mater. 2005, 4, 455–459. [Google Scholar] [CrossRef]
- Léonard, F.; Talin, A.A. Electrical contacts to one- and two-dimensional nanomaterials. Nat. Nanotechnol. 2011, 6, 773–783. [Google Scholar] [CrossRef]
- Susner, M.A.; Carnevale, S.D.; Kent, T.F.; Gerber, L.M.; Phillips, P.J.; Sumption, M.D.; Myers, R.C. Catalyst-free ZnO nanowires on silicon by pulsed laser deposition with tunable density and aspect ratio. Phys. E Low-Dimens. Syst. Nanostruct. 2014, 62, 95–103. [Google Scholar] [CrossRef]
- Faraji, N.; Ulrich, C.; Wolff, N.; Kienle, L.; Adelung, R.; Mishra, Y.K.; Seidel, J. Visible-Light Driven Nanoscale Photoconductivity of Grain Boundaries in Self-Supported ZnO Nano- and Microstructured Platelets. Adv. Electron. Mater. 2016, 2, 1600138. [Google Scholar] [CrossRef]
- Dahiya, A.S.; Opoku, C.; Alquier, D.; Poulin-Vittrant, G.; Cayrel, F.; Graton, O.; Huu Hue, L.P.T.; Camara, N. Controlled growth of 1D and 2D ZnO nanostructures on 4H-SiC using Au catalyst. Nanoscale Res. Lett. 2014, 9, 379. [Google Scholar] [CrossRef] [Green Version]
- Boubenia, S.; Dahiya, A.S.; Morini, F.; Nadaud, K.; Alquier, D. A facile hydrothermal approach for the density tunable growth of ZnO nanowires and their electrical characterizations. Sci. Rep. 2017, 7, 15187. [Google Scholar] [CrossRef] [Green Version]
- Dahiya, A.S.; Boubenia, S.; Franzo, G.; Poulin-Vittrant, G.; Mirabella, S.; Alquier, D. Photoluminescence Study of the Influence of Additive Ammonium Hydroxide in Hydrothermally Grown ZnO Nanowires. Nanoscale Res. Lett. 2018, 13, 249. [Google Scholar] [CrossRef] [PubMed]
- Tlemcani, T.S.; Justeau, C.; Nadaud, K.; Poulin-vittrant, G.; Alquier, D. Deposition Time and Annealing Effects of ZnO Seed Layer on Enhancing Vertical Alignment of Piezoelectric ZnO Nanowires. Chemosensors 2019, 7, 7. [Google Scholar] [CrossRef] [Green Version]
- Zheng, N.; Huang, Y.; Sun, W.; Du, X.; Liu, H. In-situ pull-off of ZnO nanowire from carbon fi ber and improvement of interlaminar toughness of hierarchical ZnO nanowire/carbon fi ber hydrid composite laminates. Carbon N. Y. 2016, 110, 69–78. [Google Scholar] [CrossRef] [Green Version]
- Demes, T.; Ternon, C.; Morisot, F.; Riassetto, D.; Legallais, M.; Roussel, H.; Langlet, M. Mechanisms involved in the hydrothermal growth of ultra-thin and high aspect ratio ZnO nanowires. Appl. Surf. Sci. 2017, 410, 423–431. [Google Scholar] [CrossRef]
- Alshehri, N.A.; Lewis, A.R.; Pleydell-Pearce, C.; Maffeis, T.G.G. Investigation of the growth parameters of hydrothermal ZnO nanowires for scale up applications. J. Saudi Chem. Soc. 2018, 22, 538–545. [Google Scholar] [CrossRef] [Green Version]
- Yoo, K.; Lee, W.; Kang, K.; Kim, I.; Kang, D.; Oh, D.K.; Kim, M.C.; Choi, H.; Kim, K.; Kim, M.; et al. Low-temperature large-area fabrication of ZnO nanowires on flexible plastic substrates by solution-processible metal-seeded hydrothermal growth. Nano Converg. 2020, 7, 24. [Google Scholar] [CrossRef]
- Yang, J.; Kelly, T.L. Decomposition and Cell Failure Mechanisms in Lead Halide Perovskite Solar Cells. Inorg. Chem. 2017, 56, 92. [Google Scholar] [CrossRef]
- Cheng, Y.; Yang, Q.; Xiao, J.; Xue, Q. On the Decomposition of Organometal Halide Perovskite Films on Zinc Oxide Nanoparticles. ACS Appl. Mater. Interfaces 2015, 7, 19986. [Google Scholar] [CrossRef]
- Schutt, K.; Nayak, P.K.; Ramadan, A.J.; Wenger, B.; Lin, Y.H.; Snaith, H.J. Overcoming Zinc Oxide Interface Instability with a Methylammonium-Free Perovskite for High-Performance Solar Cells. Adv. Funct. Mater. 2019, 29, 1900466. [Google Scholar] [CrossRef]
- Nourdine, A.; Abdelli, M.; Charvin, N.; Flandin, L. Custom Synthesis of ZnO Nanowires for Efficient Ambient Air-Processed Solar Cells. ACS Omega 2021, 6, 32365–32378. [Google Scholar] [CrossRef]
- Geng, Y.; Jeronimo, K.; Bin Che Mahzan, M.A.; Lomax, P.; Mastropaolo, E.; Cheung, R. Comparison of ZnO nanowires grown on e-beam evaporated Ag and ZnO seed layers. Nanoscale Adv. 2020, 2, 2814–2823. [Google Scholar] [CrossRef]
- Maddah, M.; Unsworth, C.P.; Plank, N.O.V. Selective growth of ZnO nanowires with varied aspect ratios on an individual substrate. Mater. Res. Express 2018, 6, 015905. [Google Scholar] [CrossRef]
- Tugral Arslan, H.; Arslan, C.; Baydogan, N. The effects of the curing parameters of the hydrothermal solution on the characteristic properties of ZnO nanowires. J. Opt. 2021, 51, 79–88. [Google Scholar] [CrossRef]
- Eskandari, A.; Abdizadeh, H.; Pourshaban, E.; Golobostanfard, M.R. An investigation into the role of polyethyleneimine in chemical bath deposition of zinc oxide nanowires. AIP Conf. Proc. 2018, 1920, 020026. [Google Scholar] [CrossRef]
- Zhitao, H.; Sisi, L.; Jinkui, C.; Yong, C. Controlled growth of well-aligned ZnO nanowire arrays using the improved hydrothermal method. J. Semicond. 2013, 34, 063002. [Google Scholar] [CrossRef]
- Consonni, V.; Briscoe, J.; Kärber, E.; Li, X.; Cossuet, T. ZnO nanowires for solar cells: A comprehensive review. Nanotechnology 2019, 30, 362001. [Google Scholar] [CrossRef]
- Justeau, C.; Tlemcani, T.S.; Poulin-Vittrant, G.; Nadaud, K.; Alquier, D. A comparative study on the effects of Au, ZnO and AZO seed layers on the performance of ZnO nanowire-based piezoelectric nanogenerators. Materials 2019, 12, 2511. [Google Scholar] [CrossRef] [Green Version]
- Karthick, S.; Hawashin, H.; Parou, N.; Vedraine, S.; Velumani, S.; Bouclé, J. Copper and Bismuth incorporated mixed cation perovskite solar cells by one-step solution process. Sol. Energy 2021, 218, 226–236. [Google Scholar] [CrossRef]
- Al-Zohbi, F.; Jouane, Y.; Benhattab, S.; Faure-Vincent, J.; Tran-Van, F.; Vedraine, S.; Bouclé, J.; Berton, N.; Schmaltz, B. Simple carbazole-based hole transporting materials with fused benzene ring substituents for efficient perovskite solar cells. New J. Chem. 2019, 43, 12211–12214. [Google Scholar] [CrossRef]
- Li, Q.; Kumar, V.; Li, Y.; Zhang, H.; Marks, T.J.; Chang, R.P.H. Fabrication of ZnO Nanorods and Nanotubes in Aqueous Solutions. Chem. Mater. 2005, 17, 1001–1006. [Google Scholar] [CrossRef]
- Qiu, J.; Li, X.; He, W.; Park, S.; Kim, H.; Hwang, Y.; Lee, J. The growth mechanism and optical properties of ultralong ZnO nanorod arrays with a high aspect ratio by a preheating hydrothermal method. Nanotechnology 2009, 20, 155603. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.; Zhang, X.; You, J. SnO2: A Wonderful Electron Transport Layer for Perovskite Solar Cells. Small 2018, 14, 1801154. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.; Kim, K.; Kim, M.; Kim, J.D.; Cho, I.; Kim, I.; Chae, H.; Han, I.; Kim, H.; Seo, J.H.; et al. Solution-Processable Ag-Mediated ZnO Nanowires for Scalable Low-Temperature Fabrication of Flexible Devices. ACS Appl. Electron. Mater. 2022, 4, 910–916. [Google Scholar] [CrossRef]
- Lin, C.; Li, Q.; Guang, H.; An, M. Electrodeposited Zn: A promising alternative to ZnO seed layer for hydrothermal growth of ZnO nanowire array. Mater. Lett. 2022, 314, 131848. [Google Scholar] [CrossRef]
- Hezam, M.; Algarni, A.; Ghaithan, H.; Alzahrani, H.; Alshehri, A.; Alfarhoud, A.; Labis, J.; Alduraibi, M. Hydrothermal growth optimization of vertically aligned ZnO nanowire arrays and their dye-sensitized solar cell performance under air/oxygen environments. Mater. Res. Express 2021, 8, 105501. [Google Scholar] [CrossRef]
- Liu, L.; Cao, G.; Hong, K. Seed Free Growth of Aligned ZnO Nanowire Arrays on AZO Substrate. J. Wuhan Univ. -Technol.-Mater. Sci. Ed. 2018, 33, 1372–1375. [Google Scholar] [CrossRef]
- Zhang, J.; Que, W. Preparation and characterization of solgel Al-doped ZnO thin films and ZnO nanowire arrays grown on Al-doped ZnO seed layer by hydrothermal method. Sol. Energy Mater. Sol. Cells 2010, 94, 2181–2186. [Google Scholar] [CrossRef]
- Karthick, S.; Velumani, S.; Bouclé, J. Experimental and SCAPS simulated formamidinium perovskite solar cells: A comparison of device performance. Sol. Energy 2020, 205, 349–357. [Google Scholar] [CrossRef]
- Lee, S.H.; Han, S.H.; Jung, H.S.; Shin, H.; Lee, J.; Noh, J.H.; Lee, S.; Cho, I.S.; Lee, J.K.; Kim, J.; et al. Al-doped ZnO thin film: A new transparent conducting layer for ZnO nanowire-based dye-sensitized solar cells. J. Phys. Chem. C 2010, 114, 7185–7189. [Google Scholar] [CrossRef]
- Sun, J.; Li, N.; Dong, L.; Niu, X.; Zhao, M.; Xu, Z.; Zhou, H.; Shan, C.; Pan, C. Interfacial-engineering enhanced performance and stability of ZnO nanowire-based perovskite solar cells. Nanotechnology 2021, 32, 475204. [Google Scholar] [CrossRef]
- Peña Alvarez, M.; Mayorga Burrezo, P.; Iwamoto, T.; Qiu, L.; Kertesz, M.; Taravillo, M.; Baonza, V.G.; López Navarrete, J.T.; Yamago, S.; Casado, J.; et al. Flexible, low-temperature, solution processed ZnO-based perovskite solid state solar cells. Chem. Commun. 2013, 49, 11089–11091. [Google Scholar] [CrossRef]
- Weiguang, Y.; Yali, W.; Qiang, Z.; Weimin, S. Effect of growth time on morphology and photovoltaic properties of ZnO nanowire array films. Rare Met. 2011, 30, 676–680. [Google Scholar] [CrossRef]
- Qiu, J.; Li, X.; Zhuge, F.; Gan, X.; Gao, X.; He, W.; Park, S.-J.; Kim, H.-K.; Hwang, Y.-H. Solution-derived 40 µm vertically aligned ZnO nanowire arrays as photoelectrodes in dye-sensitized solar cells. Nanotechnology 2010, 21, 195602. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Fang, L.; Chen, X.; Saleem, M. Effect of polyethyleneimine on the growth of ZnO nanorod arrays and their application in dye-sensitized solar cells. J. Alloys Compd. 2011, 509, 9456–9459. [Google Scholar] [CrossRef]
- Wu, W.; Hu, G.; Cui, S.; Zhou, Y.; Wu, H. Epitaxy of Vertical ZnO Nanorod Arrays on Highly (001) -Oriented ZnO Seed Monolayer by a Hydrothermal Route. Cryst. Growth Des. 2008, 8, 4014–4020. [Google Scholar] [CrossRef]
- Tian, Z.R.; Voigt, J.A.; Liu, J.; Mckenzie, B.; Mcdermott, M.J.; Rodriguez, M.A.; Konishi, H.; Xu, H. Complex and oriented ZnO nanostructures. Nat. Mater. 2003, 2, 821–826. [Google Scholar] [CrossRef]
- Parize, R.; Garnier, J.D.; Appert, E.; Chaix-Pluchery, O.; Consonni, V. Effects of Polyethylenimine and Its Molecular Weight on the Chemical Bath Deposition of ZnO Nanowires. ACS Omega 2018, 3, 12457–12464. [Google Scholar] [CrossRef]
- Sami, A.; Ansari, A.; Idrees, M.D.; Alam, M.M.; Imtiaz, J. Solid-State Solar Cells Based on TiO2 Nanowires and CH3NH3PbI3 Perovskite. Coatings 2021, 11, 404. [Google Scholar] [CrossRef]
- Liu, K.; Wu, W.; Chen, B.; Chen, X.; Zhang, N. Continuous Growth and Improved PL Property of ZnO Nanoarrays with Assistance of Polyethylenimine. Nanoscale 2013, 5, 5986–5993. [Google Scholar] [CrossRef] [Green Version]
- Parize, R.; Chaix-pluchery, O.; Verrier, C.; Appert, E.; Consonni, V. Effects of Hexamethylenetetramine on the Nucleation and Radial Growth of ZnO Nanowires by Chemical Bath Deposition. J. Phys. Chem. C 2016, 120, 5242–5250. [Google Scholar] [CrossRef]
- Mcpeak, K.M.; Le, T.P.; Britton, N.G.; Nickolov, Z.S.; Elabd, Y.A.; Baxter, J.B. Chemical Bath Deposition of ZnO Nanowires at Near-Neutral pH Conditions without Hexamethylenetetramine (HMTA): Understanding the Role of HMTA in ZnO Nanowire Growth. Langmuir 2011, 27, 3672–3677. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, K.; Sarwar, S.; Mehran, M.T. Current status of electron transport layers in perovskite solar cells: Materials and properties. RSC Adv. 2017, 7, 17044–17062. [Google Scholar] [CrossRef] [Green Version]
- La Ferrara, V.; De Maria, A.; Rametta, G.; Della Noce, M.; Mercaldo, L.V.; Borriello, C.; Bruno, A.; Veneri, P.D. ZnO nanorods/AZO photoanode for perovskite solar cells fabricated in ambient air. Mater. Res. Express 2017, 4, 085025. [Google Scholar] [CrossRef]
- Nakar, R.; Cho, A.-N.; Berton, N.; Faure-Vincent, J.; Tran-Van, F.; Park, N.-G.; Schmaltz, B. Triphenylamine 3, 6-carbazole derivative as hole-transporting material for mixed cation perovskite solar cells. Chem. Pap. 2018, 72, 1779–1787. [Google Scholar] [CrossRef]
- Nakar, R.; Ramos, F.J.; Dalinot, C.; Marques, P.S.; Cabanetos, C.; Leriche, P.; Sanguinet, L.; Kobeissi, M.; Blanchard, P.; Faure-Vincent, J.; et al. Cyclopentadithiophene and Fluorene Spiro-Core-Based Hole-Transporting Materials for Perovskite Solar Cells. J. Phys. Chem. C 2019, 123, 22767–22774. [Google Scholar] [CrossRef]
- Berton, N.; Nakar, R.; Schmaltz, B. DMPA-containing carbazole-based hole transporting materials for perovskite solar cells: Recent advances and perspectives. Synth. Met. 2019, 252, 91–106. [Google Scholar] [CrossRef]
- Benhattab, S.; Cho, A.N.; Nakar, R.; Berton, N.; Tran-Van, F.; Park, N.G.; Schmaltz, B. Simply designed carbazole-based hole transporting materials for efficient perovskite solar cells. Org. Electron. 2018, 56, 27–30. [Google Scholar] [CrossRef]
- Khenkin, M.V.; Katz, E.A.; Abate, A.; Bardizza, G.; Berry, J.J.; Brabec, C.; Brunetti, F.; Bulović, V.; Burlingame, Q.; Di Carlo, A.; et al. Consensus statement for stability assessment and reporting for perovskite photovoltaics based on ISOS procedures. Nat. Energy 2020, 5, 35–49. [Google Scholar] [CrossRef]
- Wei, J.; Wang, Q.; Huo, J.; Gao, F.; Gan, Z.; Zhao, Q.; Li, H. Mechanisms and Suppression of Photoinduced Degradation in Perovskite Solar Cells. Adv. Energy Mater. 2021, 11, 2002326. [Google Scholar] [CrossRef]
- Boyd, C.C.; Cheacharoen, R.; Leijtens, T.; McGehee, M.D. Understanding Degradation Mechanisms and Improving Stability of Perovskite Photovoltaics. Chem. Rev. 2019, 119, 3418–3451. [Google Scholar] [CrossRef]
- Tsai, H.; Asadpour, R.; Blancon, J.; Stoumpos, C.C.; Durand, O.; Strzalka, J.W.; Chen, B.; Verduzco, R.; Ajayan, P.M.; Tretiak, S.; et al. Light-induced lattice expansion leads to high-efficiency perovskite solar cells. Sol. Cells 2018, 70, 67–70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, H.; Yan, L.; Liu, J.; Li, J.; Wang, H. Fabrication of well-aligned ZnO nanorod photoanodes for perovskite solar cells. J. Mater. Sci. Mater. Electron. 2016, 27, 6872–6880. [Google Scholar] [CrossRef]
- Zhang, Q.; Hou, S.; Li, C. Titanium Dioxide-Coated Zinc Oxide Nanorods as an Efficient Photoelectrode in Dye-Sensitized Solar Cells. Nanomaterials 2020, 10, 1598. [Google Scholar] [CrossRef] [PubMed]
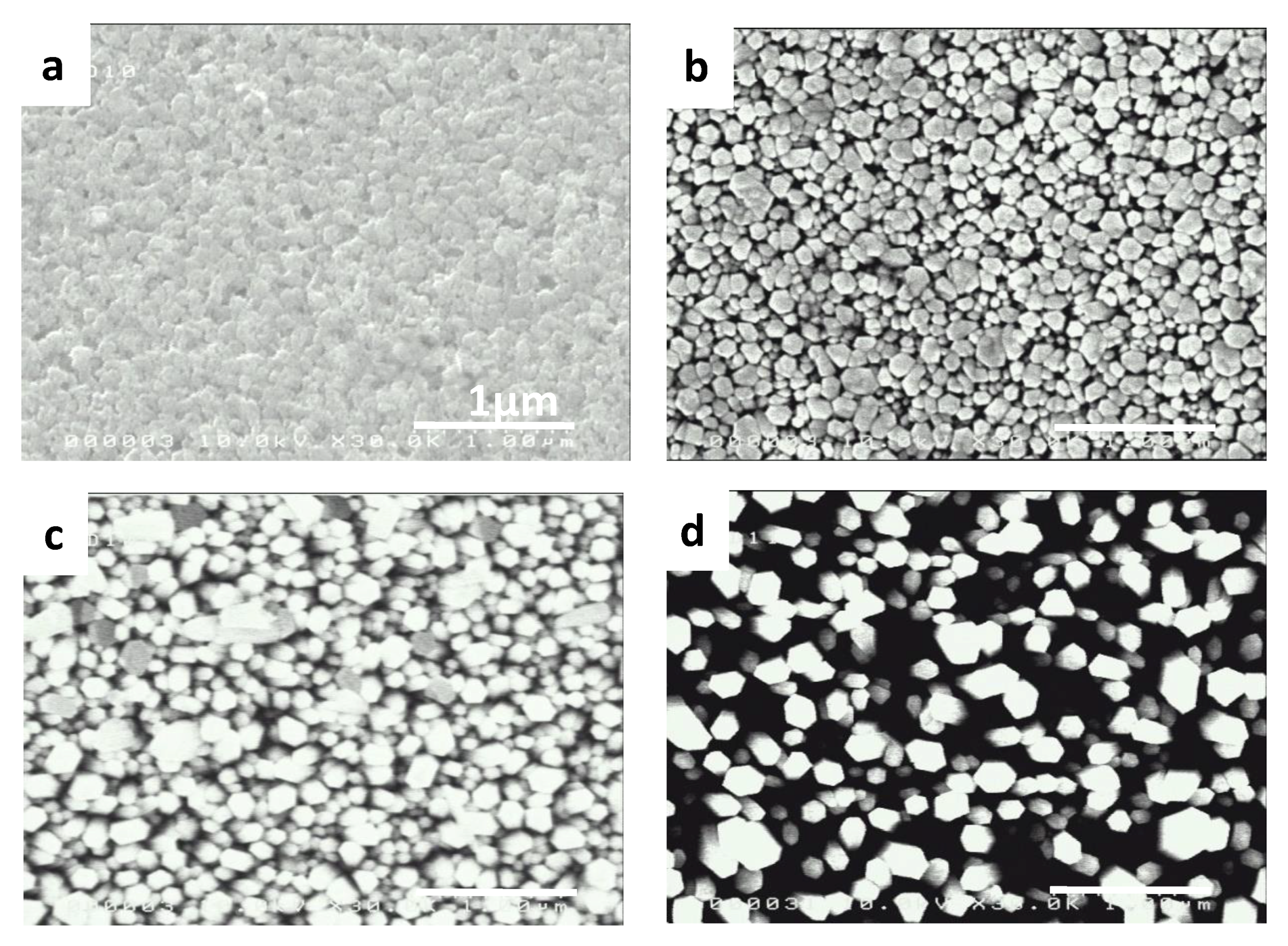
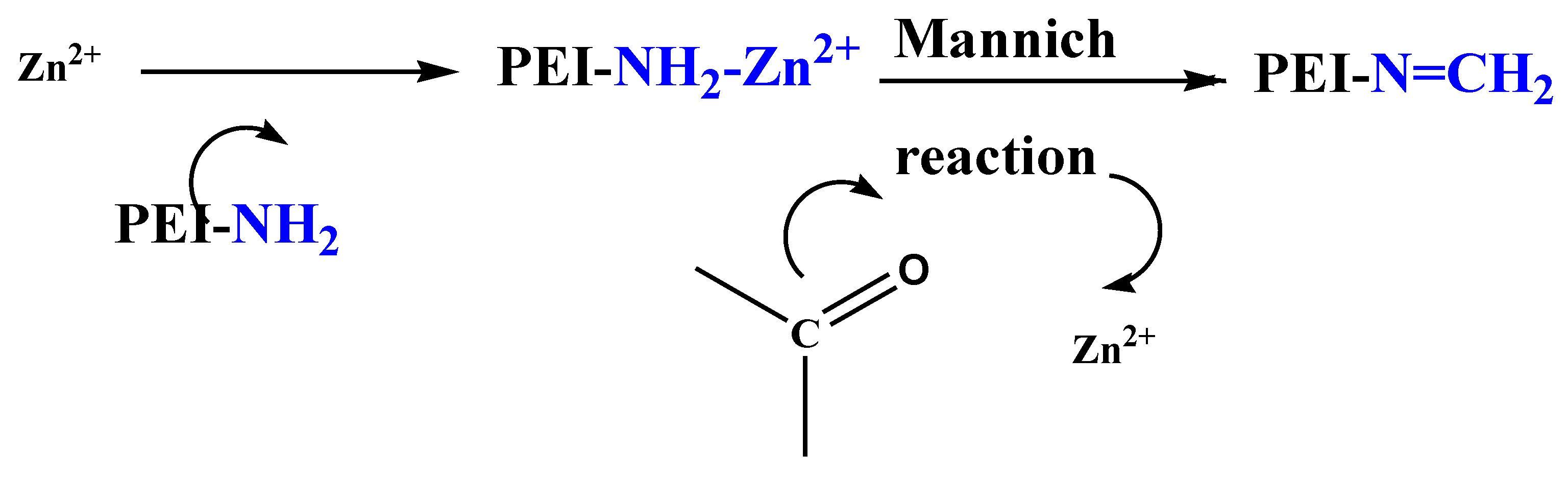
| Growing Time | Length (L) (μm) | Average Diameter (d) (nm) | Density (D) (NW/µm2) | Void Fraction (%) |
|---|---|---|---|---|
| 2 h | 0.1 | - | - | - |
| 4 h | 0.3 | - | - | - |
| 6 h | 0.4 | 127 | 53 | ~28 |
| 15 h | 1.3 | 165 | 29 | ~58 |
| Growing Time | Jsc (mA cm−2) | Voc (V) | FF | PCE (%) | Rsh (Ω) | Rs (Ω) |
|---|---|---|---|---|---|---|
| 6 h | 16.1 | 0.64 | 0.47 | 4.9 | 1400 | 70 |
| 15 h | 4.2 | 0.32 | 0.46 | 0.6 | 1200 | 130 |
| [PEI] mM | [PEI]/[HMTA] Ratio | L (μm) | d (nm) | D NW/µm2 | Void Fraction% |
|---|---|---|---|---|---|
| -- | -- | 0.3 | 131 | 59 | ~36 |
| 5 | 10 | 0.3 | 135 | 59 | ~37 |
| 7 | 7.15 | 0.4 | 147 | 49 | ~44 |
| 11 | 4.50 | 0.5 | ~125 | 58 | ~45 |
| 13 | 3.85 | 0.2 | 150 | 50 | ~28 |
| [Zn(NO3)2]/[HMTA] Ratio | [HMTA] mM | L (μm) | d (nm) | D (NW/µm2) | Void fraction (%) | NWs Aspect Ratio L/d |
|---|---|---|---|---|---|---|
| 1 | 50 | 0.5 | 124.6 | 58 | ~45 | ~4.01 |
| 1.33 | 37.5 | 0.5 | 125.0 | 58 | ~36 | ~4.01 |
| 2 | 25 | 0.3 | 101.3 | 76 | ~37 | ~2.66 |
| [Zn(NO3)2] (mM) | L (μm) | d (nm) | D (NW/µm2) | Void Fraction (%) | NWs Aspect Ratio L/d |
|---|---|---|---|---|---|
| 20 mM | 0.25 | 109 | 58 | ~43 | 2.28 |
| 35 mM | 0.30 | 117 | 57 | ~46 | 2.56 |
| 50 mM | 0.50 | 125 | 58 | ~36 | 4.00 |
| ZnO(NO3)2 Concentration | Jsc (mA cm−2) | Voc (V) | FF | PCE (%) | Rsh (Ω) | Rs (Ω) |
|---|---|---|---|---|---|---|
| 20 mM | 8.9 | 0.92 | 0.33 | 2.7 | 600 | 200 |
| 35 mM | 2.2 | 0.38 | 0.37 | 0.3 | 1250 | 470 |
| 50 mM | 9.4 | 0.53 | 0.35 | 1.7 | 650 | 160 |
| Growing Time | Jsc (mA cm−2) | Voc (V) | FF | PCE (%) | Rsh (Ω) | Rs (Ω) |
|---|---|---|---|---|---|---|
| Initial set | 16.1 | 0.64 | 0.47 | 4.9 | 1400 | 70 |
| Final set | 8.9 | 0.92 | 0.33 | 2.7 | 600 | 200 |
| Jsc (mA.cm−2) | Voc (V) | FF | PCE (%) | Rsh (Ω) | Rs (Ω) | |||
|---|---|---|---|---|---|---|---|---|
| ZnO 0.40 µm/Spiro-OMeTAD | FS | Best | 16.4 | 0.66 | 0.36 | 3.8 | 420 | 100 |
| Average | 13.7 | 0.69 | 0.35 | 3.4 | 780 | 120 | ||
| RS | Best | 16.1 | 0.64 | 0.47 | 4.9 | 1400 | 70 | |
| Average | 16.7 | 0.68 | 0.43 | 4.7 | 1350 | 80 | ||
| ZnO 0.25 µm/Spiro-OMeTAD | FS | Best | 9.1 | 0.86 | 0.25 | 2.0 | 530 | 330 |
| Average | 8.2 | 0.85 | 0.25 | 1.8 | 560 | 380 | ||
| RS | Best | 8.9 | 0.92 | 0.33 | 2.7 | 600 | 200 | |
| Average | 8.5 | 0.91 | 0.32 | 2.5 | 580 | 220 | ||
| ZnO 0.40 µm/Cz–Pyr | FS | Best | 7.3 | 0.82 | 0.35 | 2.1 | 1070 | 240 |
| Average | 5.5 | 0.71 | 0.34 | 1.3 | 1350 | 400 | ||
| RS | Best | 7.0 | 0.81 | 0.39 | 2.2 | 940 | 230 | |
| Average | 5.6 | 0.70 | 0.34 | 1.3 | 1100 | 400 | ||
| ZnO 0.25 µm/Cz–Pyr | FS | Best | 5.0 | 0.62 | 0.33 | 1.0 | 800 | 330 |
| Average | 3.9 | 0.74 | 0.29 | 0.8 | 1170 | 970 | ||
| RS | Best | 4.6 | 0.62 | 0.40 | 1.1 | 1840 | 280 | |
| Average | 3.9 | 0.73 | 0.30 | 0.8 | 1550 | 1030 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sekar, K.; Nakar, R.; Bouclé, J.; Doineau, R.; Nadaud, K.; Schmaltz, B.; Poulin-Vittrant, G. Low-Temperature Hydrothermal Growth of ZnO Nanowires on AZO Substrates for FACsPb(IBr)3 Perovskite Solar Cells. Nanomaterials 2022, 12, 2093. https://doi.org/10.3390/nano12122093
Sekar K, Nakar R, Bouclé J, Doineau R, Nadaud K, Schmaltz B, Poulin-Vittrant G. Low-Temperature Hydrothermal Growth of ZnO Nanowires on AZO Substrates for FACsPb(IBr)3 Perovskite Solar Cells. Nanomaterials. 2022; 12(12):2093. https://doi.org/10.3390/nano12122093
Chicago/Turabian StyleSekar, Karthick, Rana Nakar, Johann Bouclé, Raphaël Doineau, Kevin Nadaud, Bruno Schmaltz, and Guylaine Poulin-Vittrant. 2022. "Low-Temperature Hydrothermal Growth of ZnO Nanowires on AZO Substrates for FACsPb(IBr)3 Perovskite Solar Cells" Nanomaterials 12, no. 12: 2093. https://doi.org/10.3390/nano12122093
APA StyleSekar, K., Nakar, R., Bouclé, J., Doineau, R., Nadaud, K., Schmaltz, B., & Poulin-Vittrant, G. (2022). Low-Temperature Hydrothermal Growth of ZnO Nanowires on AZO Substrates for FACsPb(IBr)3 Perovskite Solar Cells. Nanomaterials, 12(12), 2093. https://doi.org/10.3390/nano12122093










