Synthesis and Characterization of 2D-WS2 Incorporated Polyaniline Nanocomposites as Photo Catalyst for Methylene Blue Degradation
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Preparation of PANI Nanotubes
2.3. Preparation of WS2-PANI Nanocomposite

2.4. Characterisation Techniques
2.5. Measurement of Photocatalytic Activities
3. Results
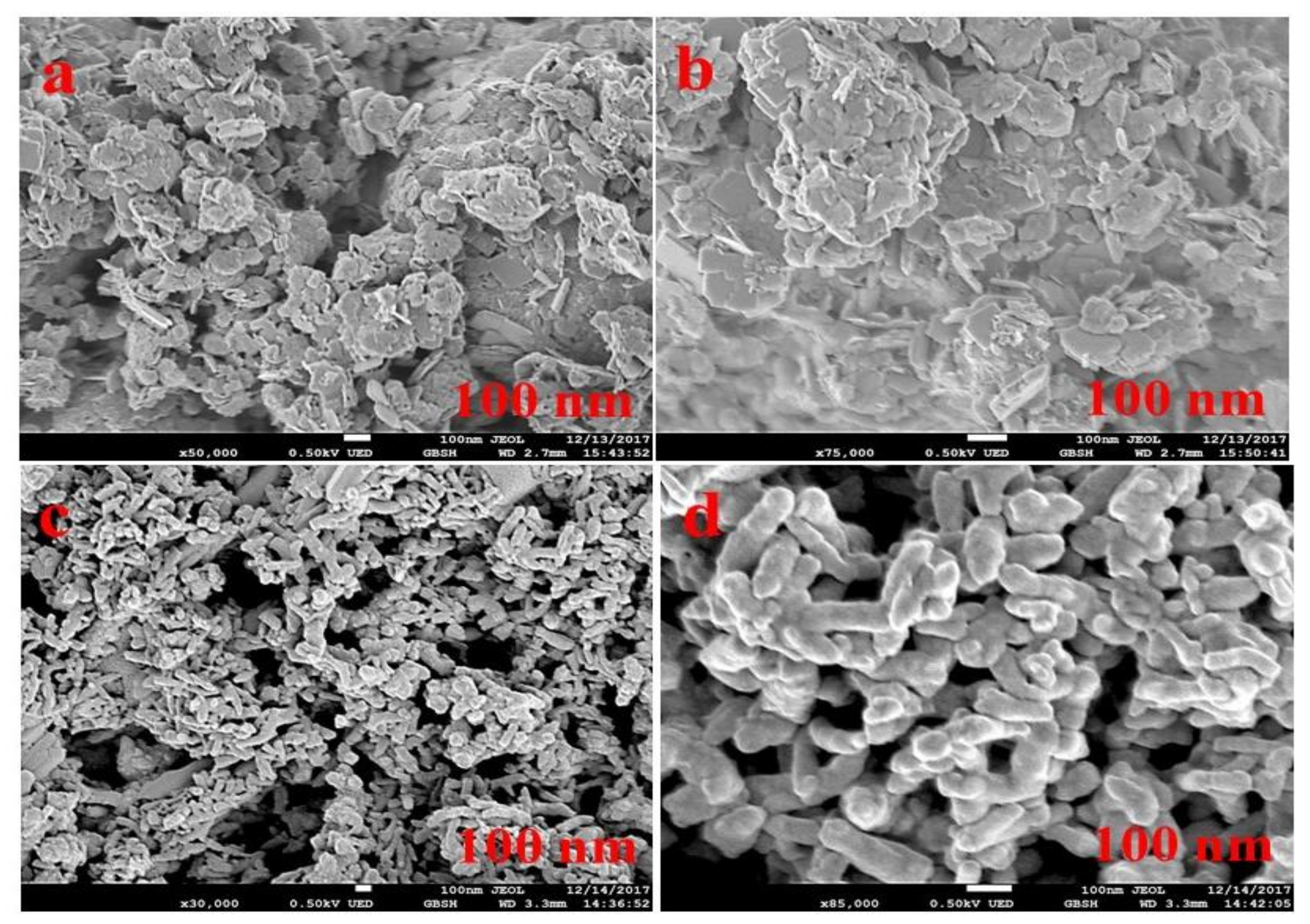
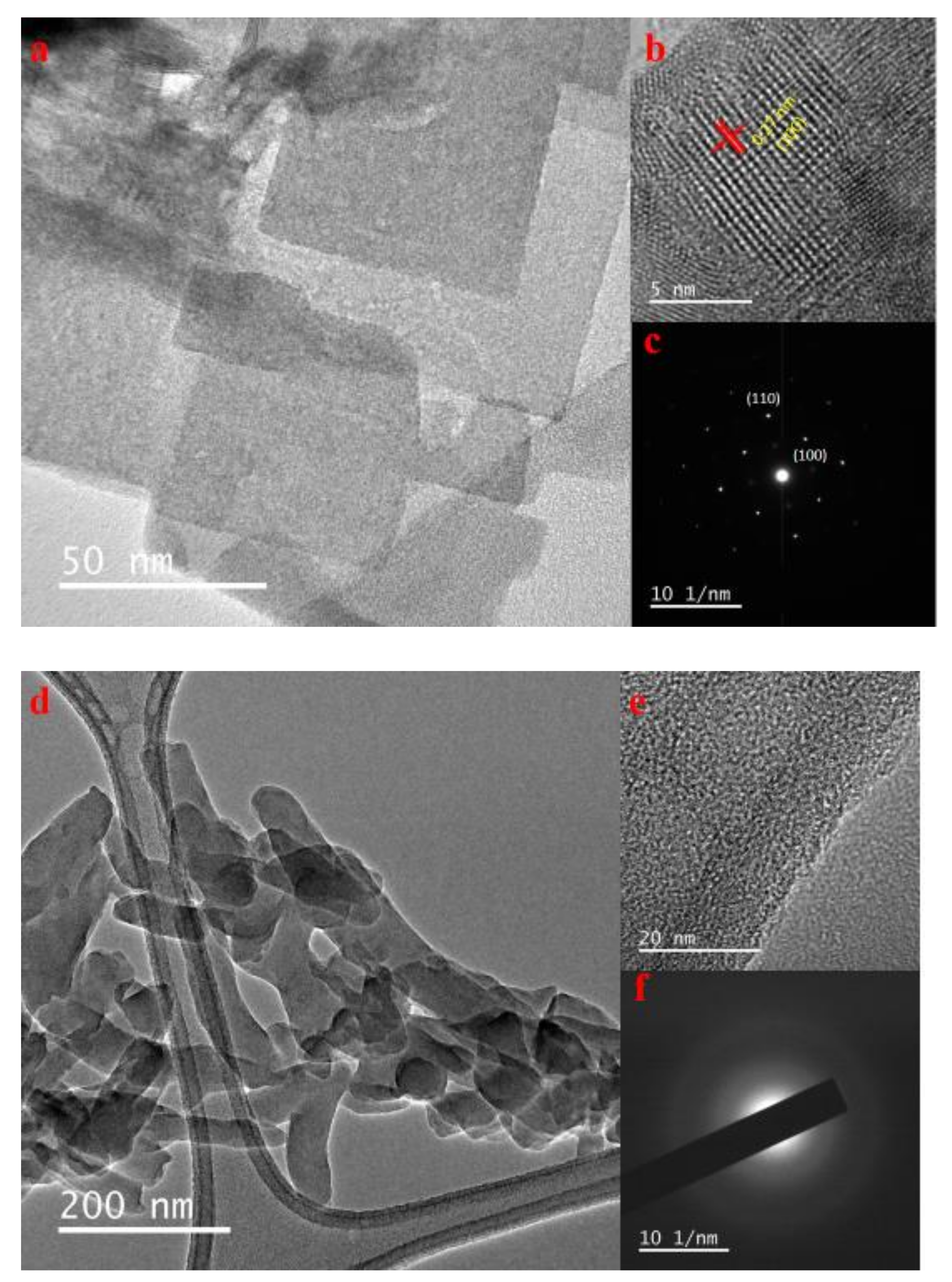
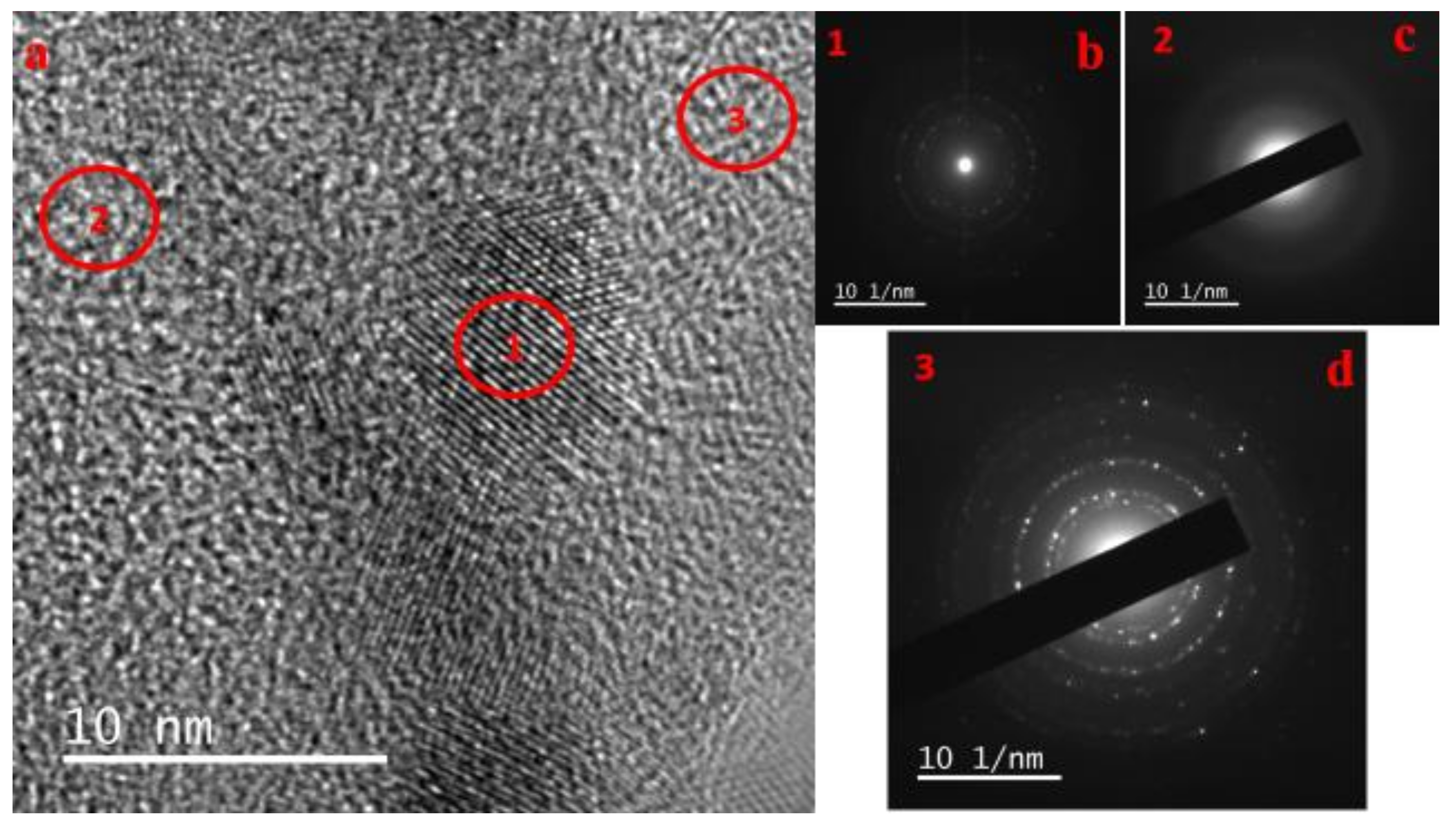
3.1. Morphological Analysis of Nanocomposites
3.2. BET Analysis
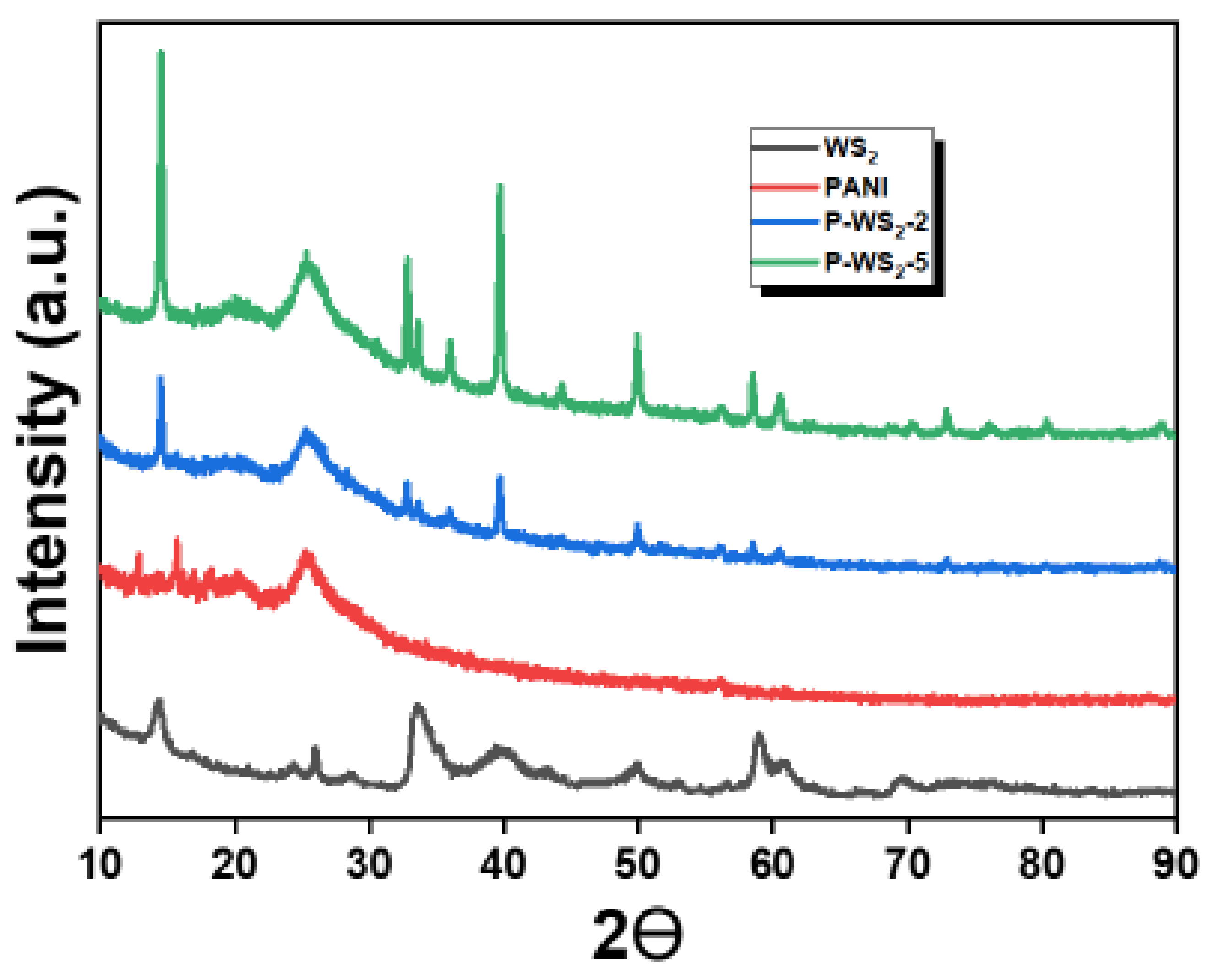
3.3. XRD Analysis
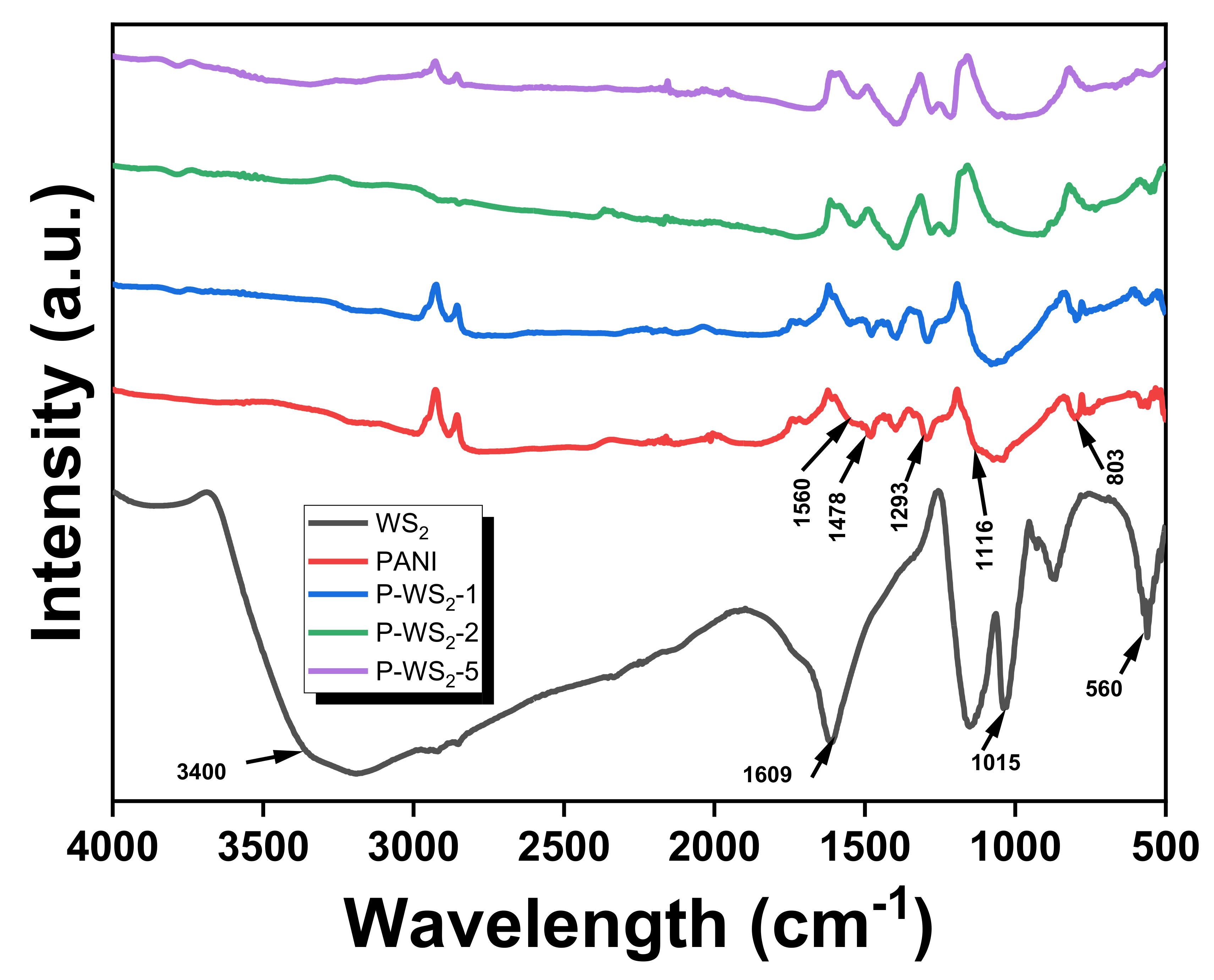
3.4. FTIR Analysis
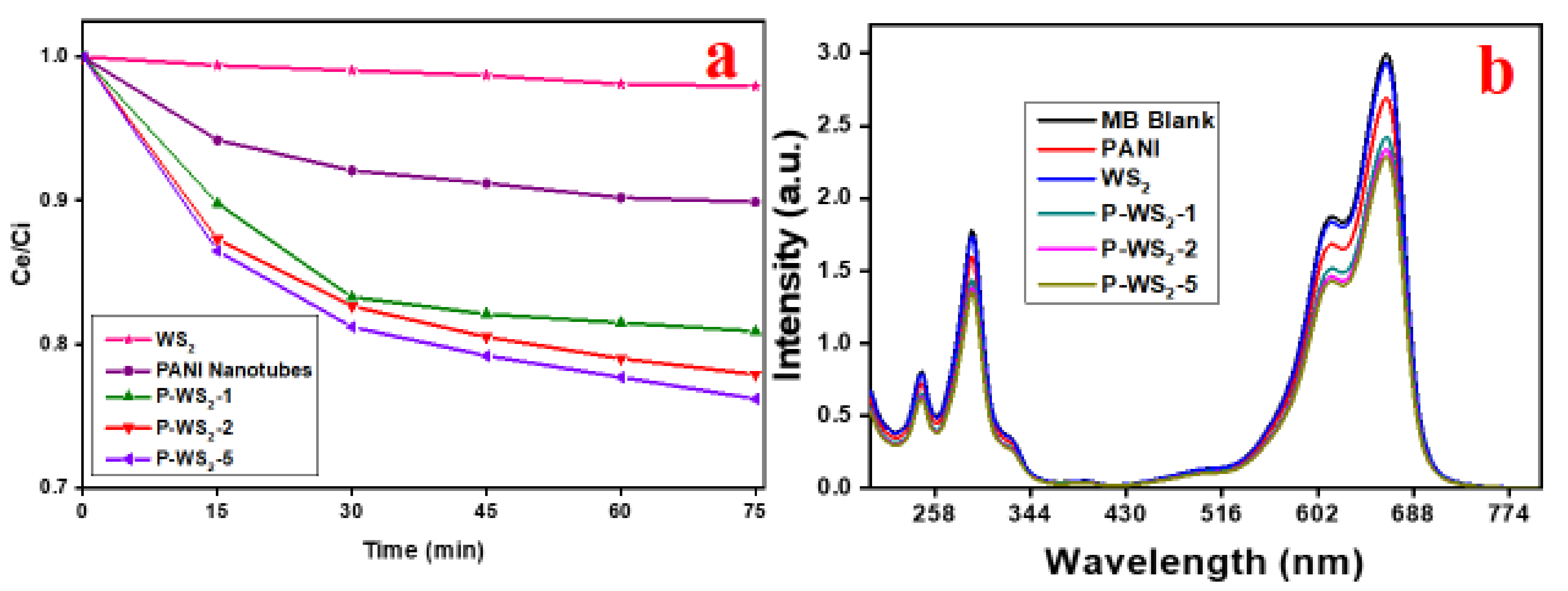
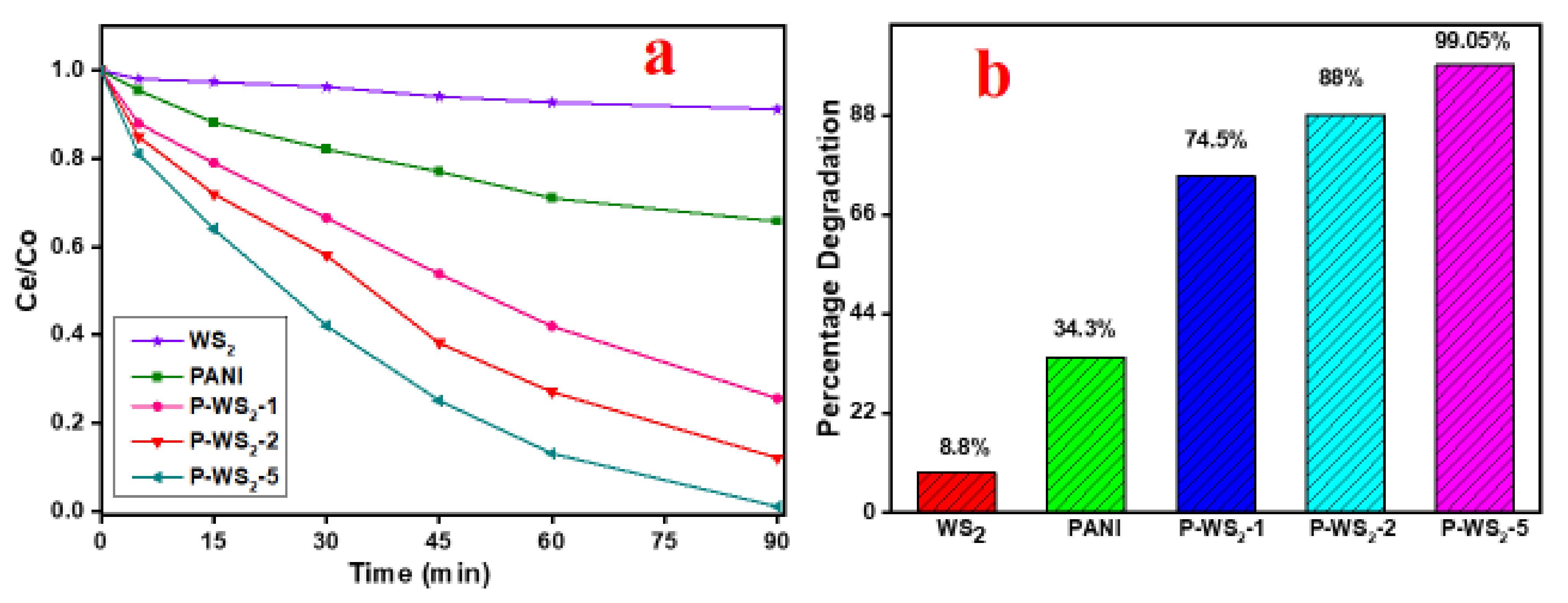
3.5. Photocatalytic Degradation of MB under UV Irradiation
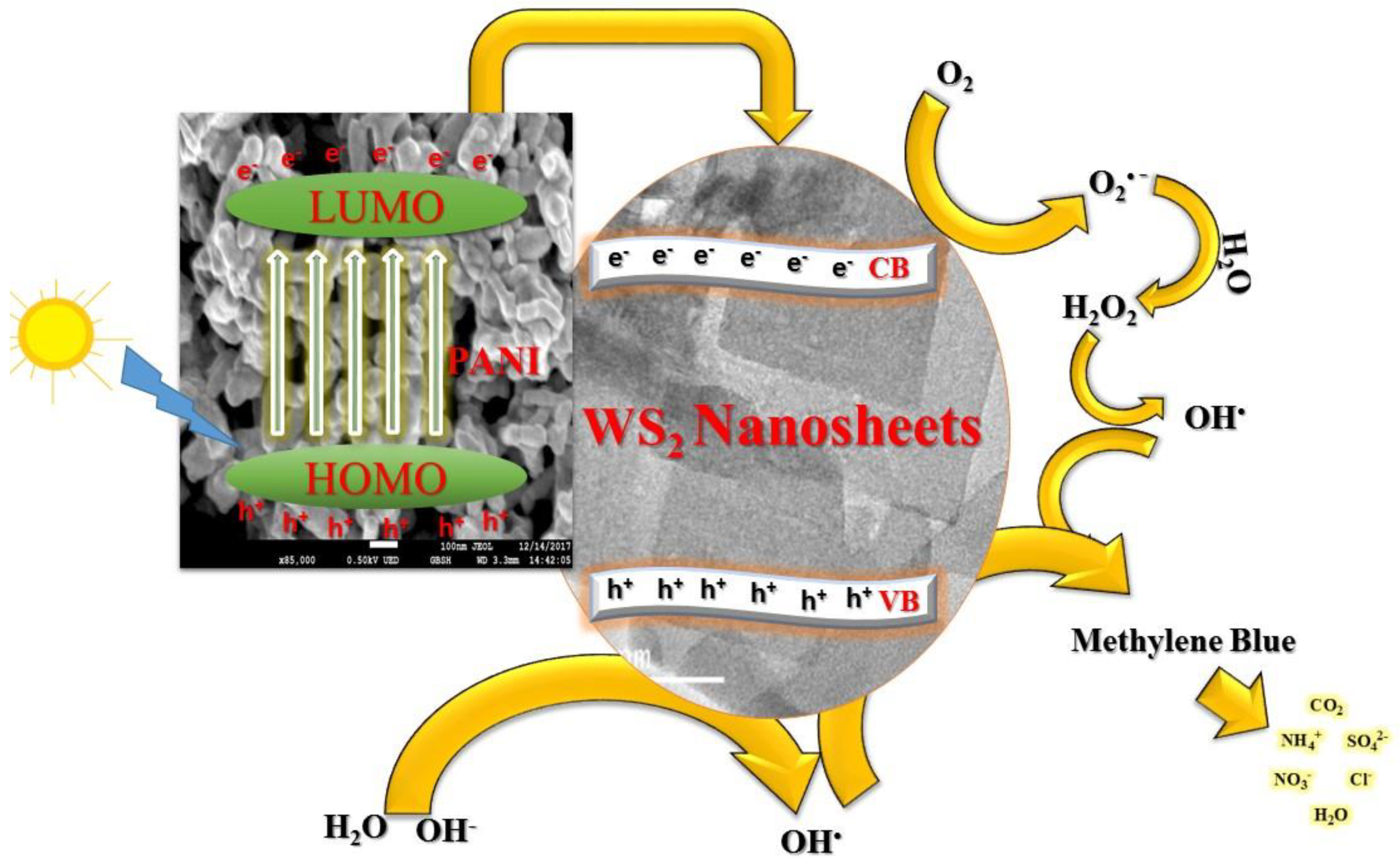
4. Mechanism of Photodegradation
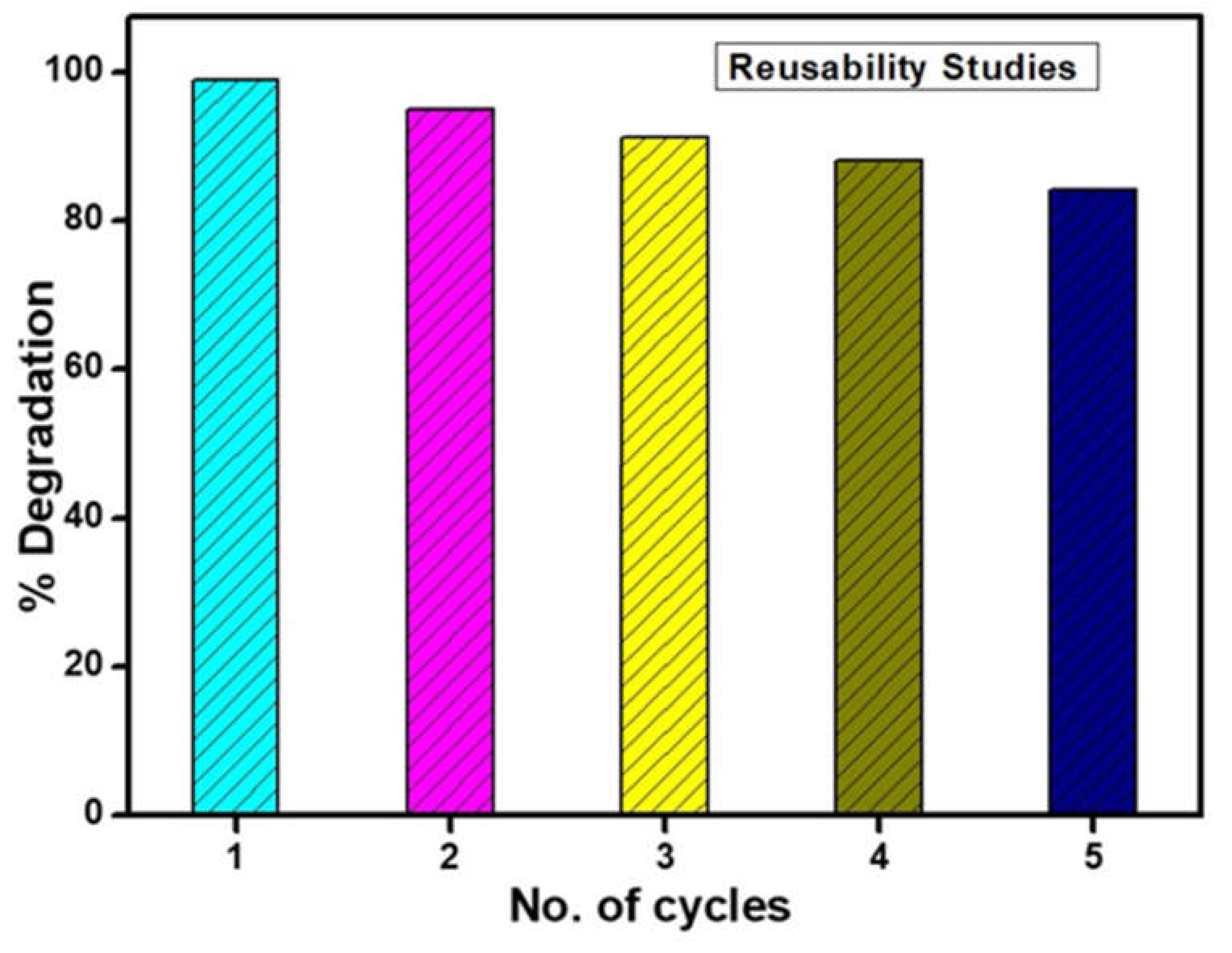
5. Reproducibility of the Photocatalysts
Comparison of Photocatalytic Efficiencies
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xing, Z.; Zhang, J.; Cui, J.; Yin, J.; Zhao, T.; Kuang, J.; Xiu, Z.; Wan, N.; Zhou, W. Recent advances in floating TiO2-based photocatalysts for environmental application. Appl. Catal. B: Environ. 2018, 225, 452–467. [Google Scholar] [CrossRef]
- Ge, M.; Cao, C.; Huang, J.; Li, S.; Chen, Z.; Zhang, K.-Q.; Al-Deyab, S.S.; Lai, Y. A review of one-dimensional TiO2nanostructured materials for environmental and energy applications. J. Mater. Chem. A 2016, 4, 6772–6801. [Google Scholar] [CrossRef]
- Shahabuddin, S.; Sarih, N.M.; Mohamad, S.; Baharin, S.N.A. Synthesis and characterization of Co3O4 nanocube-doped polyaniline nanocomposites with enhanced methyl orange adsorption from aqueous solution. RSC Adv. 2016, 6, 43388–43400. [Google Scholar] [CrossRef]
- Snyder, E.G.; Watkins, T.H.; Solomon, P.A.; Thoma, E.D.; Williams, R.W.; Hagler, G.S.W.; Shelow, D.; Hindin, D.A.; Kilaru, V.J.; Preuss, P.W. The Changing Paradigm of Air Pollution Monitoring. Environ. Sci. Technol. 2013, 47, 11369–11377. [Google Scholar] [CrossRef] [PubMed]
- Raffainer, I.I.; Rudolf von Rohr, P. Promoted wet oxidation of the azo dye orange II under mild conditions. Ind. Eng. Chem. Res. 2001, 40, 1083–1089. [Google Scholar] [CrossRef]
- Gupta, V.K. Suhas Application of low-cost adsorbents for dye removal—A review. J. Environ. Manag. 2009, 90, 2313–2342. [Google Scholar] [CrossRef]
- McCann, J.; Ames, B.N. Detection of carcinogens as mutagens in the Salmonella/microsome test: Assay of 300 chemicals: Discussion. Proc. Natl. Acad. Sci. 1976, 73, 950–954. [Google Scholar] [CrossRef]
- Chan, S.H.S.; Wu, T.Y.; Juan, J.C.; Teh, C.Y. Recent developments of metal oxide semiconductors as photocatalysts in advanced oxidation processes (AOPs) for treatment of dye waste-water. J. Chem. Technol. Biotechnol. 2011, 86, 1130–1158. [Google Scholar] [CrossRef]
- Huang, S.-T.; Lee, W.W.; Chang, J.-L.; Huang, W.-S.; Chou, S.-Y.; Chen, C.-C. Hydrothermal synthesis of SrTiO3 nanocubes: Characterization, photocatalytic activities, and degradation pathway. J. Taiwan Inst. Chem. Eng. 2014, 45, 1927–1936. [Google Scholar] [CrossRef]
- Shahabuddin, S.; Sarih, N.M.; Mohamad, S.; Ching, J.J. SrTiO3 Nanocube-Doped Polyaniline Nanocomposites with Enhanced Photocatalytic Degradation of Methylene Blue under Visible Light. Polymers 2016, 8, 27. [Google Scholar] [CrossRef]
- Parida, K.M.; Sahu, S.; Reddy, K.H.; Sahoo, P.C. A Kinetic, Thermodynamic, and Mechanistic Approach toward Adsorption of Methylene Blue over Water-Washed Manganese Nodule Leached Residues. Ind. Eng. Chem. Res. 2010, 50, 843–848. [Google Scholar] [CrossRef]
- Shahabuddin, S.; Sarih, N.M.; Ismail, F.H.; Shahid, M.M.; Huang, N.M. Synthesis of chitosan grafted-polyaniline/Co3O4 nanocube nanocomposites and their photocatalytic activity toward methylene blue dye degradation. RSC Adv. 2015, 5, 83857–83867. [Google Scholar] [CrossRef]
- Gouamid, M.; Ouahrani, M.; Bensaci, M. Adsorption Equilibrium, Kinetics and Thermodynamics of Methylene Blue from Aqueous Solutions using Date Palm Leaves. Energy Procedia 2013, 36, 898–907. [Google Scholar] [CrossRef]
- Vinothkannan, M.; Karthikeyan, C.; Kim, A.R.; Yoo, D.J. One-pot green synthesis of reduced graphene oxide (RGO)/Fe3O4 nanocomposites and its catalytic activity toward methylene blue dye degradation. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 136, 256–264. [Google Scholar] [CrossRef] [PubMed]
- Adams, V.; Marley, J.; McCarroll, C. Prilocaine induced methaemoglobinaemia in a medically compromised patient. Was this an inevitable consequence of the dose administered? Br. Dent. J. 2007, 203, 585. [Google Scholar] [CrossRef]
- Dai, K.; Lv, J.; Lu, L.; Liu, Q.; Zhu, G.; Li, D. Synthesis of micro-nano heterostructure AgBr/ZnO composite for advanced visible light photocatalysis. Mater. Lett. 2014, 130, 5–8. [Google Scholar] [CrossRef]
- Zare, E.N.; Motahari, A.; Sillanpää, M. Nanoadsorbents based on conducting polymer nanocomposites with main focus on polyaniline and its derivatives for removal of heavy metal ions/dyes: A review. Environ. Res. 2018, 162, 173–195. [Google Scholar] [CrossRef]
- Nodeh, M.K.M.; Soltani, S.; Shahabuddin, S.; Nodeh, H.R.; Sereshti, H. Equilibrium, Kinetic and Thermodynamic Study of Magnetic Polyaniline/Graphene Oxide Based Nanocomposites for Ciprofloxacin Removal from Water. J. Inorg. Organomet. Polym. Mater. 2018, 28, 1226–1234. [Google Scholar] [CrossRef]
- Jamal, R.; Zhang, L.; Wang, M.; Zhao, Q.; Abdiryim, T. Synthesis of poly (3,4-propylenedioxythiophene)/MnO2 composites and their applications in the adsorptive removal of methylene blue. Prog. Nat. Sci. Mater. Int. 2016, 26, 32–40. [Google Scholar] [CrossRef]
- Srivastava, V.; Maydannik, P.; Sharma, Y.; Sillanpää, M. Synthesis and application of polypyrrole coated tenorite nanoparticles (PPy@ TN) for the removal of the anionic food dye ‘tartrazine’and divalent metallic ions viz. Pb (II), Cd (II), Zn (II), Co (II), Mn (II) from synthetic wastewater. RSC Adv. 2015, 5, 80829–80843. [Google Scholar] [CrossRef]
- Baharin, S.N.A.; Sarih, N.M.; Mohamad, S.; Shahabuddin, S.; Sulaiman, K.; Ma’amor, A. Removal of endocrine disruptor di-(2-ethylhexyl) phthalate by modified polythiophene-coated magnetic nanoparticles: Characterization, adsorption isotherm, kinetic study, thermodynamics. RSC Adv. 2016, 6, 44655–44667. [Google Scholar] [CrossRef]
- Shahabuddin, S.; Sarih, N.M.; Afzal Kamboh, M.; Rashidi Nodeh, H.; Mohamad, S. Synthesis of polyaniline-Coated graphene oxide@ SrTiO3 nanocube nanocomposites for enhanced removal of carcinogenic dyes from aqueous solution. Polymers 2016, 8, 305. [Google Scholar] [CrossRef] [PubMed]
- Gustafsson, G.; Cao, Y.; Treacy, G.; Klavetter, F.; Colaneri, N.; Heeger, A.J. Flexible light-emitting diodes made from soluble conducting polymers. Nature 1992, 357, 477–479. [Google Scholar] [CrossRef]
- Jang, S.; Han, M.; Im, S. Preparation and characterization of conductive polyaniline/silica hybrid composites prepared by sol–gel process. Synth. Met. 2000, 110, 17–23. [Google Scholar] [CrossRef]
- Wang, F.; Min, S.; Han, Y.; Feng, L. Visible-light-induced photocatalytic degradation of methylene blue with polyaniline-sensitized TiO2 composite photocatalysts. Superlattices Microstruct. 2010, 48, 170–180. [Google Scholar] [CrossRef]
- Golsheikh, A.M.; Lim, H.N.; Zakaria, R.; Huang, N.M. Sonochemical synthesis of reduced graphene oxide uniformly decorated with hierarchical ZnS nanospheres and its enhanced photocatalytic activities. RSC Adv. 2015, 5, 12726–12735. [Google Scholar] [CrossRef]
- Ray, S.S.; Biswas, M. Water-dispersible conducting nanocomposites of polyaniline and poly(N-vinylcarbazole) with nanodimensional zirconium dioxide. Synth. Met. 2000, 108, 231–236. [Google Scholar] [CrossRef]
- Autin, O.; Romelot, C.; Rust, L.; Hart, J.; Jarvis, P.; MacAdam, J.; Parsons, S.A.; Jefferson, B. Evaluation of a UV-light emitting diodes unit for the removal of micropollutants in water for low energy advanced oxidation processes. Chemosphere 2013, 92, 745–751. [Google Scholar] [CrossRef]
- Singh, D.P. Synthesis and Growth of ZnO Nanowires. Sci. Adv. Mater. 2010, 2, 245–272. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, P.; Su, Z. Preparation of PANI–TiO2 nanocomposites and their solid-phase photocatalytic degradation. Polym. Degrad. Stab. 2006, 91, 2213–2219. [Google Scholar] [CrossRef]
- Guo, C.; Dong, L.M.; Gong, S.J.; Miao, C.Y.; Zhang, X.Y. Synthesis and Characterization of Hexagonal Boron Nitride Nanosheets. Appl. Mech. Mater. 2013, 274, 411–414. [Google Scholar] [CrossRef]
- Shahabuddin, S.; Khanam, R.; Khalid, M.; Sarih, N.M.; Ching, J.J.; Mohamad, S.; Saidur, R. Synthesis of 2D boron nitride doped polyaniline hybrid nanocomposites for photocatalytic degradation of carcinogenic dyes from aqueous solution. Arab. J. Chem. 2018, 11, 1000–1016. [Google Scholar] [CrossRef]
- Fu, S.-Y.; Sun, Z.; Huang, P.; Li, Y.-Q.; Hu, N. Some basic aspects of polymer nanocomposites: A critical review. Nano Mater. Sci. 2019, 1, 2–30. [Google Scholar] [CrossRef]
- Rout, C.S.; Joshi, P.D.; Kashid, R.V.; Joag, D.S.; More, M.A.; Simbeck, A.J.; Washington, M.; Nayak, S.K.; Late, D.J. Superior Field Emission Properties of Layered WS2-RGO Nanocomposites. Sci. Rep. 2013, 3, 3282. [Google Scholar] [CrossRef] [PubMed]
- Syed, S. Polyaniline Based Nanocomposites as Adsorbents and Photocatalysts in the Removal of Organic Dyes. Ph.D. Thesis, University of Malaya, Kuala Lumpur, Malaysia, 2016. [Google Scholar]
- Mostafaei, A.; Zolriasatein, A. Synthesis and characterization of conducting polyaniline nanocomposites containing ZnO nanorods. Prog. Nat. Sci. 2012, 22, 273–280. [Google Scholar] [CrossRef]
- Sing, K.S.W. Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity (Recommendations 1984). Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Seo, J.W.; Jun, Y.W.; Park, S.W.; Nah, H.; Moon, T.; Park, B.; Kim, J.G.; Kim, Y.J.; Cheon, J. Two-dimensional nanosheet crystals. Angew. Chem. Int. Ed. 2007, 46, 8828–8831. [Google Scholar] [CrossRef]
- Gordon, R.; Yang, D.; Crozier, E.D.; Jiang, D.T.; Frindt, R.F. Structures of exfoliated single layers of WS2, MoS2, and MoSe2 in aqueous suspension. Phys. Rev. B 2002, 65, 125407. [Google Scholar] [CrossRef]
- Vattikuti, S.P.; Byon, C.; Reddy, C.V. Preparation and improved photocatalytic activity of mesoporous WS2 using combined hydrothermal-evaporation induced self-assembly method. Mater. Res. Bull. 2016, 75, 193–203. [Google Scholar] [CrossRef]
- Shi, L.; Wang, X.; Lu, L.; Yang, X.; Wu, X. Preparation of TiO2/polyaniline nanocomposite from a lyotropic liquid crystalline solution. Synth. Met. 2009, 159, 2525–2529. [Google Scholar] [CrossRef]
- Hazarika, S.J.; Mohanta, D. Inorganic fullerene-type WS2 nanoparticles: Processing, characterization and its photocatalytic performance on malachite green. Appl. Phys. A 2017, 123, 381. [Google Scholar] [CrossRef]
- Vattikuti, S.V.P.; Byon, C. Effect of CTAB Surfactant on Textural, Structural, and Photocatalytic Properties of Mesoporous WS2. Sci. Adv. Mater. 2015, 7, 2639–2645. [Google Scholar] [CrossRef]
- Liu, Z.; Miao, Y.-E.; Liu, M.; Ding, Q.; Tjiu, W.W.; Cui, X.; Liu, T. Flexible polyaniline-coated TiO2/SiO2 nanofiber membranes with enhanced visible-light photocatalytic degradation performance. J. Colloid Interface Sci. 2014, 424, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Eskizeybek, V.; Sarı, F.; Gülce, H.; Gülce, A.; Avcı, A. Preparation of the new polyaniline/ZnO nanocomposite and its photocatalytic activity for degradation of methylene blue and malachite green dyes under UV and natural sun lights irradiations. Appl. Catal. B Environ. 2012, 119-120, 197–206. [Google Scholar] [CrossRef]
- Sboui, M.; Nsib, M.F.; Rayes, A.; Swaminathan, M.; Houas, A. TiO2–PANI/Cork composite: A new floating photocatalyst for the treatment of organic pollutants under sunlight irradiation. J. Environ. Sci. 2017, 60, 3–13. [Google Scholar] [CrossRef]
- Jeghan, S.M.N.; Kang, M. Facile synthesis and photocatalytic activity of cubic spinel urchin-like copper cobaltite architecture. Mater. Res. Bull. 2017, 91, 108–113. [Google Scholar] [CrossRef]
- Kim, K.N.; Jung, H.-R.; Lee, W.-J. Hollow cobalt ferrite–polyaniline nanofibers as magnetically separable visible-light photocatalyst for photodegradation of methyl orange. J. Photochem. Photobiol. A: Chem. 2016, 321, 257–265. [Google Scholar] [CrossRef]
- Chang, B.Y.S.; Mehmood, M.S.; Pandikumar, A.; Huang, N.M.; Lim, H.N.; Marlinda, A.R.; Yusoff, N.; Chiu, W.S. Hydrothermally prepared graphene-titania nanocomposite for the solar photocatalytic degradation of methylene blue. DESALINATION Water Treat. 2015, 1–8. [Google Scholar] [CrossRef]


| BET Analysis | ||||
|---|---|---|---|---|
| S.No. | Material | BET Surface Area (m2g−1) | Pore Size Å | Pore Volume cm3/g |
| 1 | WS2 Nanosheets | 34 | 85 | 0.07 |
| 2 | PANI Nanotubes | 30 | 152 | 0.07 |
| 3 | WS2-PANI-1 | 32 | 173 | 0.12 |
| 4 | WS2-PANI-2 | 33 | 177 | 0.13 |
| 5 | WS2-PANI-5 | 36 | 210 | 0.19 |
| Photocatalyst | Model Dye | Conc. of Dye (mgL−1) | Amount of Catalyst (mg/mL) | % Degradation | Degradation Time (min.) | Source of Light | Ref. |
|---|---|---|---|---|---|---|---|
| Graphene/ZnS | Methylene Blue | 10 | 0.2 | 95 | 180 | UV | [26] |
| Chitosan/PANI/Co3O4 | Methylene Blue | 10 | 0.3 | 88 | 180 | UV | [12] |
| PANI/TiO2/SiO2 membrane | Methyl Orange | 1.5 | - | 87 | 90 | Visible light | [44] |
| PANI/ZnO | Methylene Blue | 3.2 | 0.4 | 99 | 300 | Sunlight | [45] |
| TiO2-PANI/Cork | Methyl Orange | 15 | 1 | 95 | 210 | Natural sunlight | [46] |
| PANI/SrTiO3 | Methylene Blue | 10 | 0.3 | 97 | 90 | Visible light | [10] |
| Urachin like CuCo2O4 | Methyl Orange | 10 | 0.6 | 100 | 240 | Sunlight | [47] |
| Hollow CoFe2O4 –PANI nanofibers | Methyl Orange | 20 | 0.2 | 85 | 120 | LED | [48] |
| rGO–TiO2, | Methylene Blue | 10 | 0.125 | 100 | 180 | Sunlight | [49] |
| PANI/WS2-5 | Methylene Blue | 10 | 0.2 | 99.05 | 90 | UV | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shahabuddin, S.; Mehmood, S.; Ahmad, I.; Sridewi, N. Synthesis and Characterization of 2D-WS2 Incorporated Polyaniline Nanocomposites as Photo Catalyst for Methylene Blue Degradation. Nanomaterials 2022, 12, 2090. https://doi.org/10.3390/nano12122090
Shahabuddin S, Mehmood S, Ahmad I, Sridewi N. Synthesis and Characterization of 2D-WS2 Incorporated Polyaniline Nanocomposites as Photo Catalyst for Methylene Blue Degradation. Nanomaterials. 2022; 12(12):2090. https://doi.org/10.3390/nano12122090
Chicago/Turabian StyleShahabuddin, Syed, Shahid Mehmood, Irfan Ahmad, and Nanthini Sridewi. 2022. "Synthesis and Characterization of 2D-WS2 Incorporated Polyaniline Nanocomposites as Photo Catalyst for Methylene Blue Degradation" Nanomaterials 12, no. 12: 2090. https://doi.org/10.3390/nano12122090
APA StyleShahabuddin, S., Mehmood, S., Ahmad, I., & Sridewi, N. (2022). Synthesis and Characterization of 2D-WS2 Incorporated Polyaniline Nanocomposites as Photo Catalyst for Methylene Blue Degradation. Nanomaterials, 12(12), 2090. https://doi.org/10.3390/nano12122090







