Self-Adaptive Flask-like Nanomotors Based on Fe3O4 Nanoparticles to a Physiological pH
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Synthesis of Fe3O4@OA NPs
2.3. Modification of Fe3O4@OA NPs by DMSA
2.4. Synthesis of Flask-like Carbonaceous Carriers (FCCs)
2.5. Preparation of FCNMs
2.6. Observation of the Motion of the FCNMs
2.7. Characterization
2.8. Numerical Simulation
3. Results and Discussion
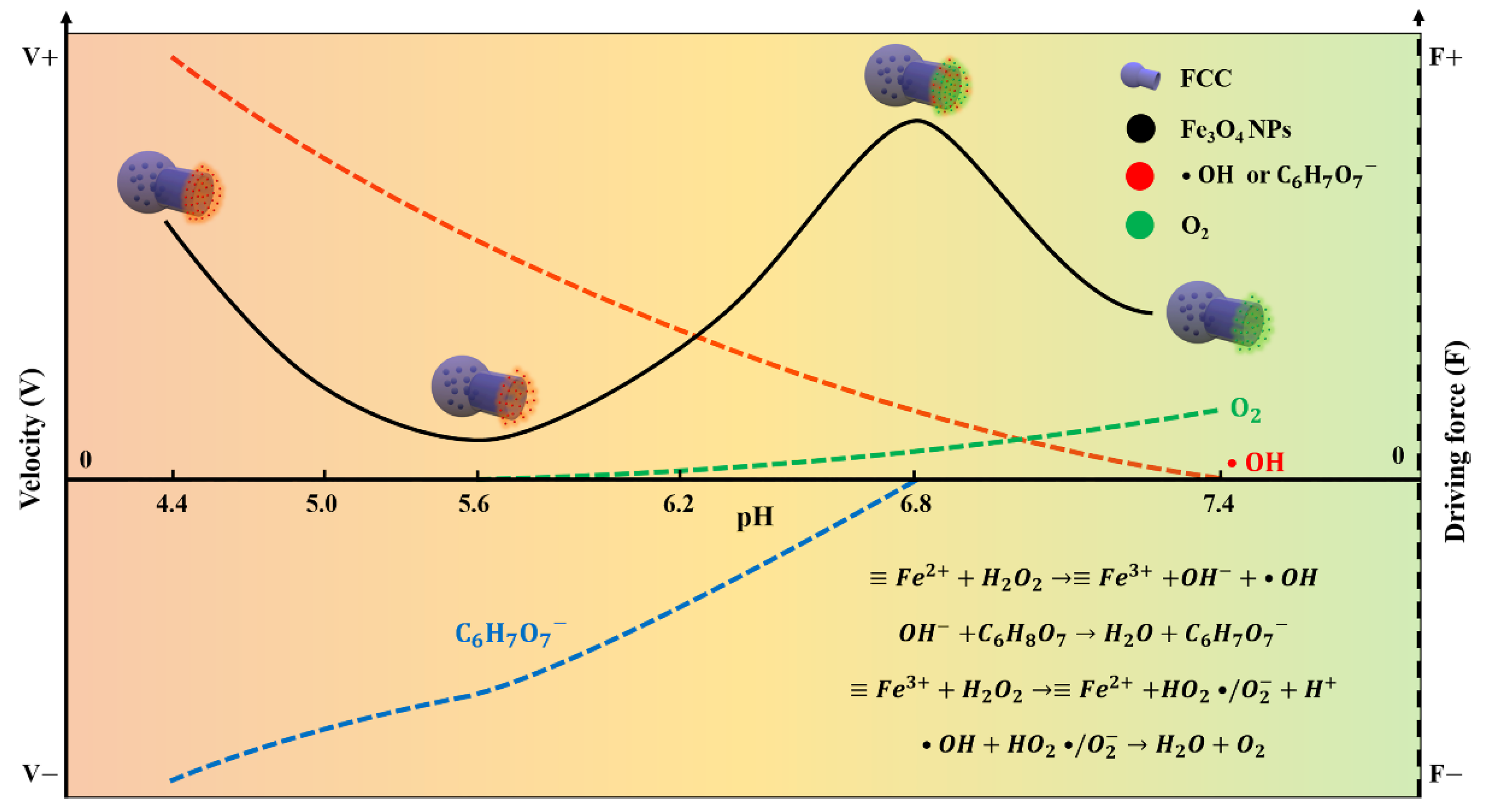
3.1. Implementation Strategy of the pH-Responsive FCNMs
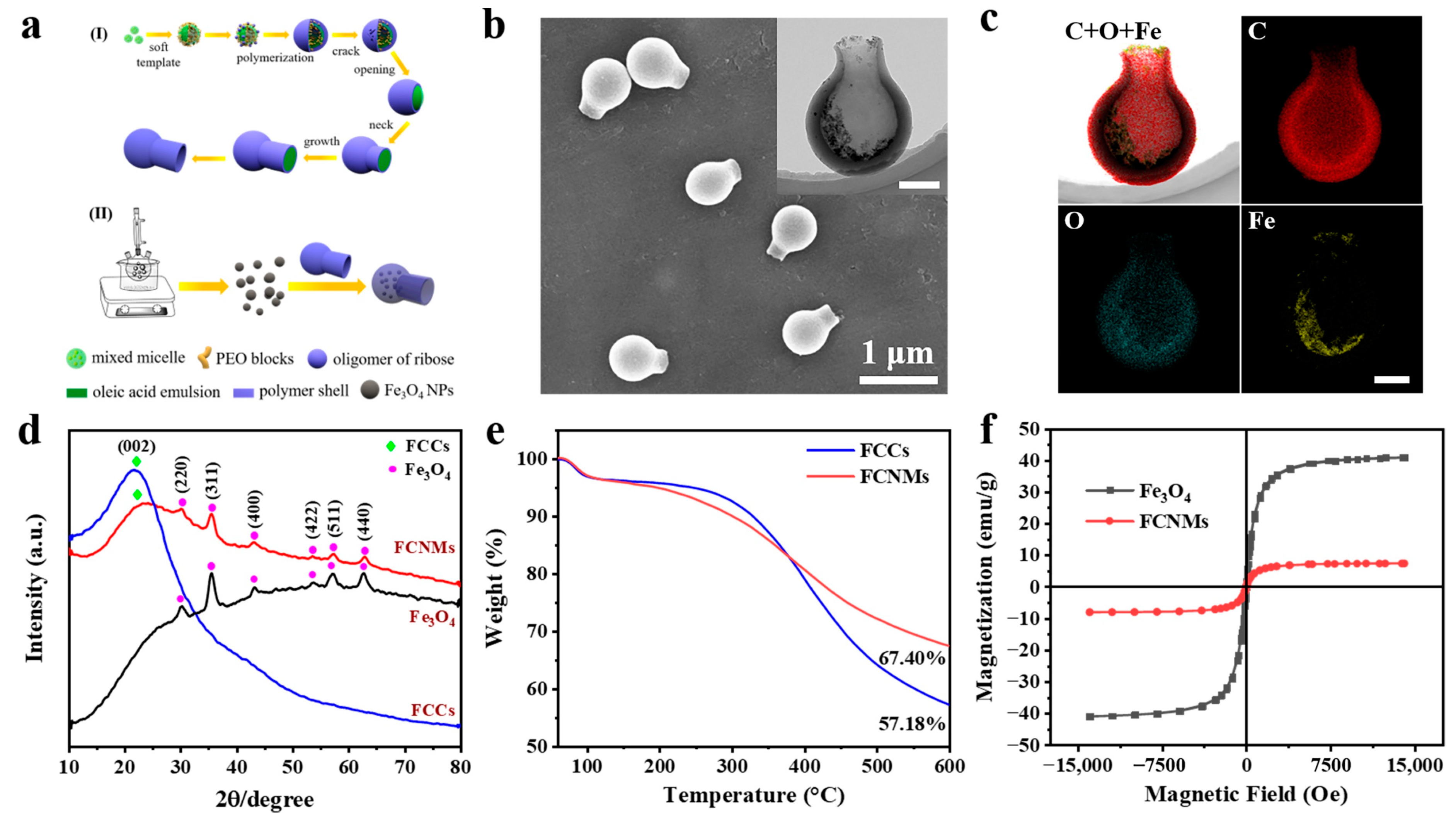
3.2. Preparation and Characterization of the FCNMs
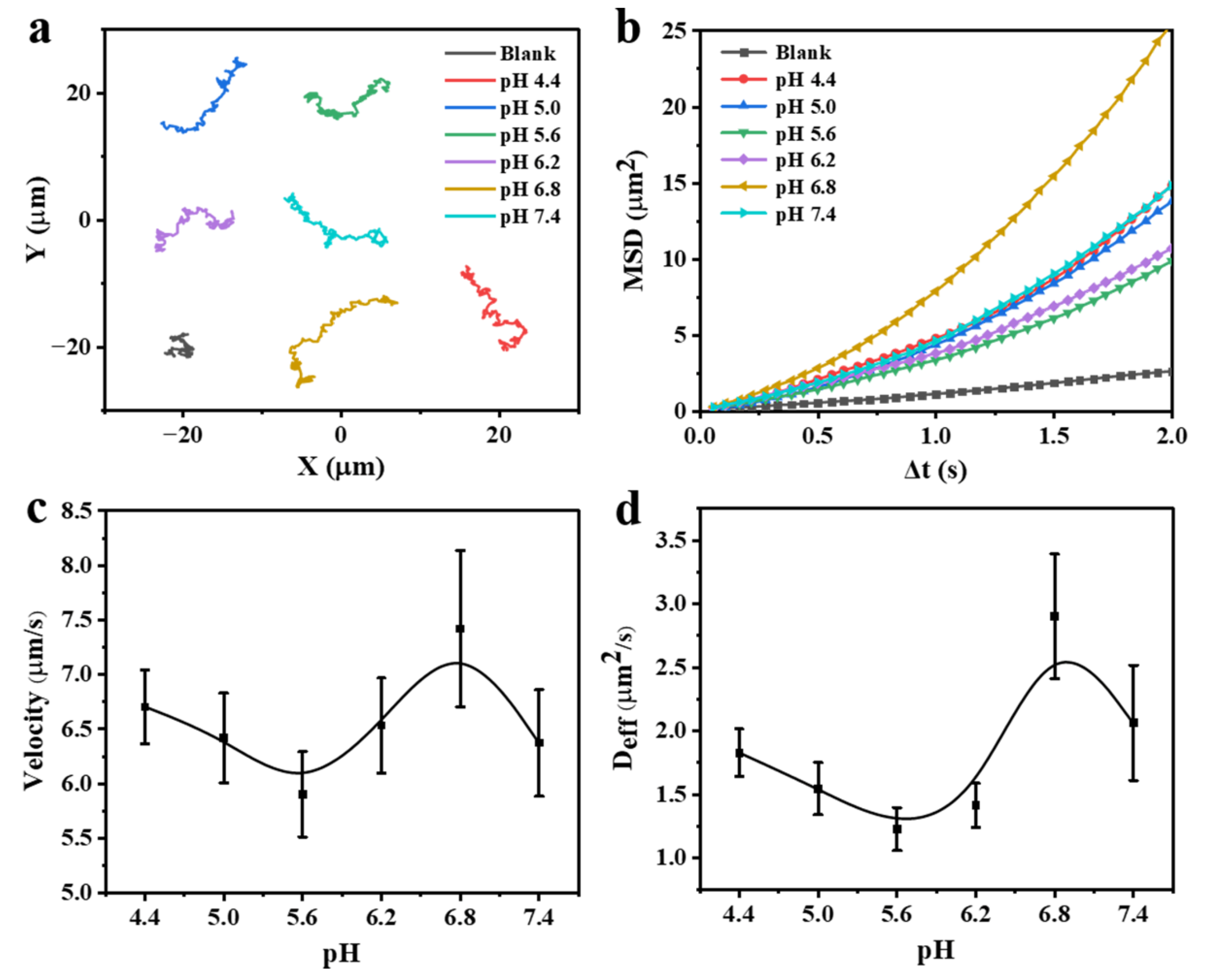
3.3. Motion Behaviors of the FCNMs at Different pH Values
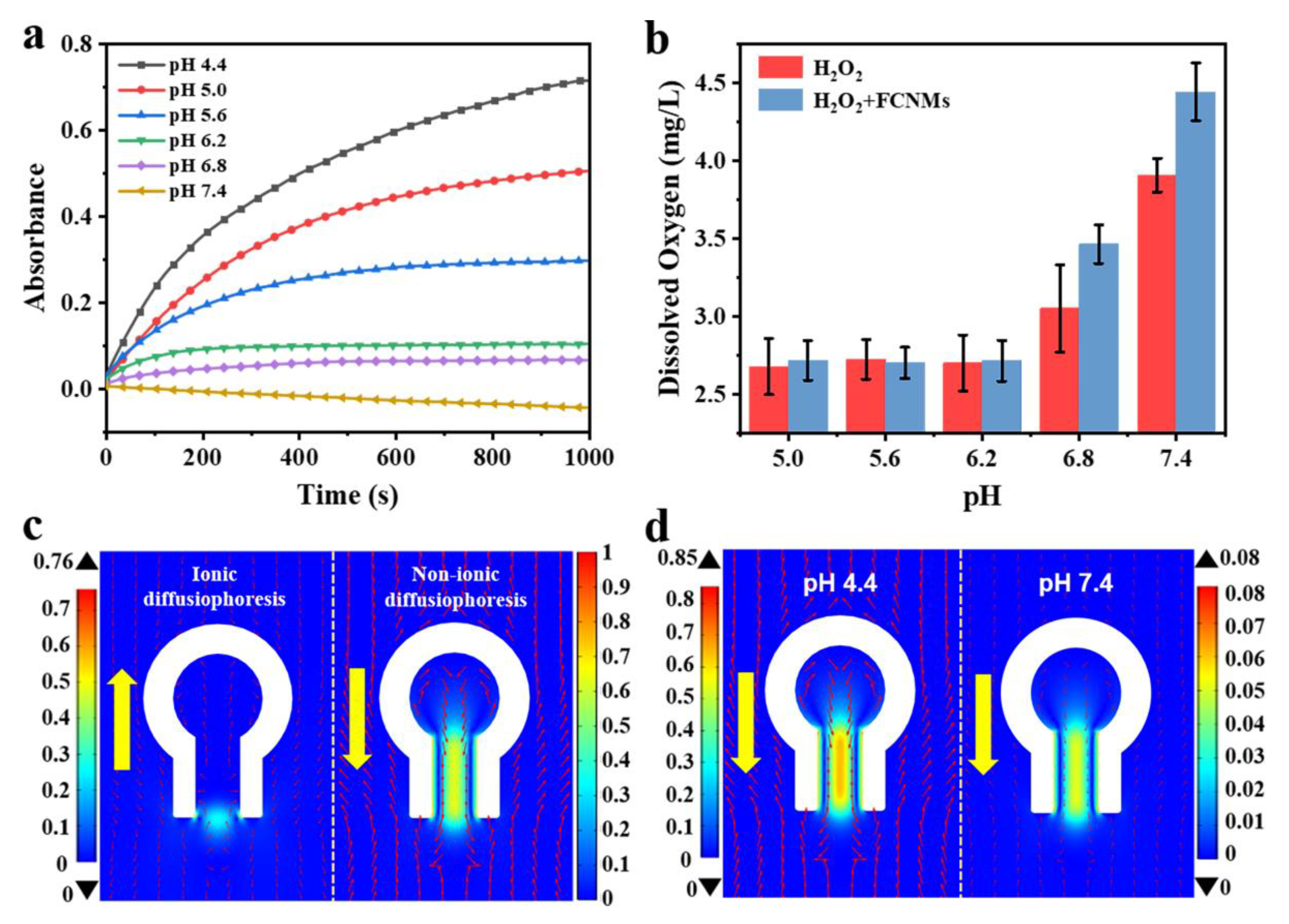
3.4. Experimental Verification and Simulation Analysis for the pH-Responsive Mechanisms
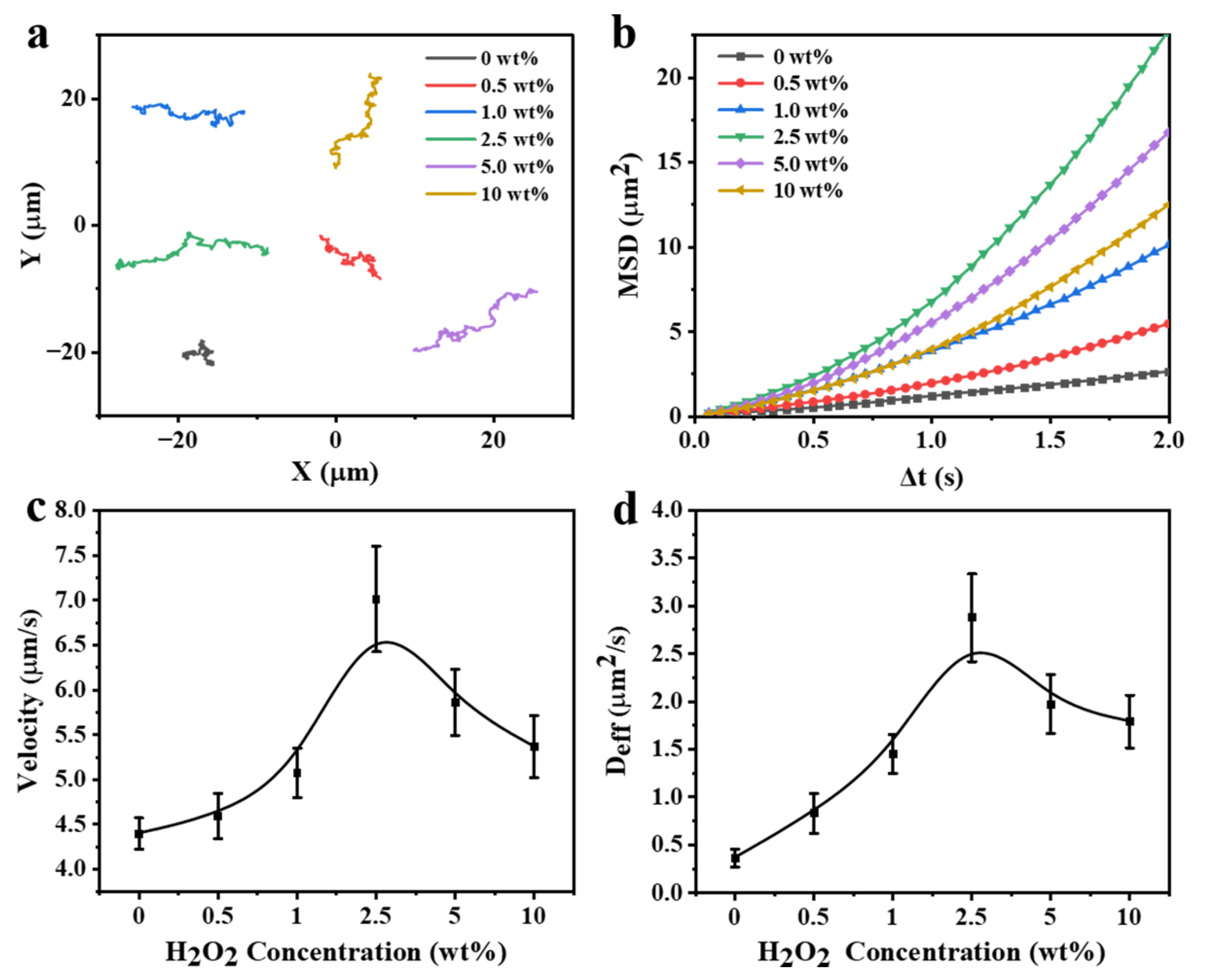
3.5. Motion Behaviors of the FCNMs at Different H2O2 Concentrations
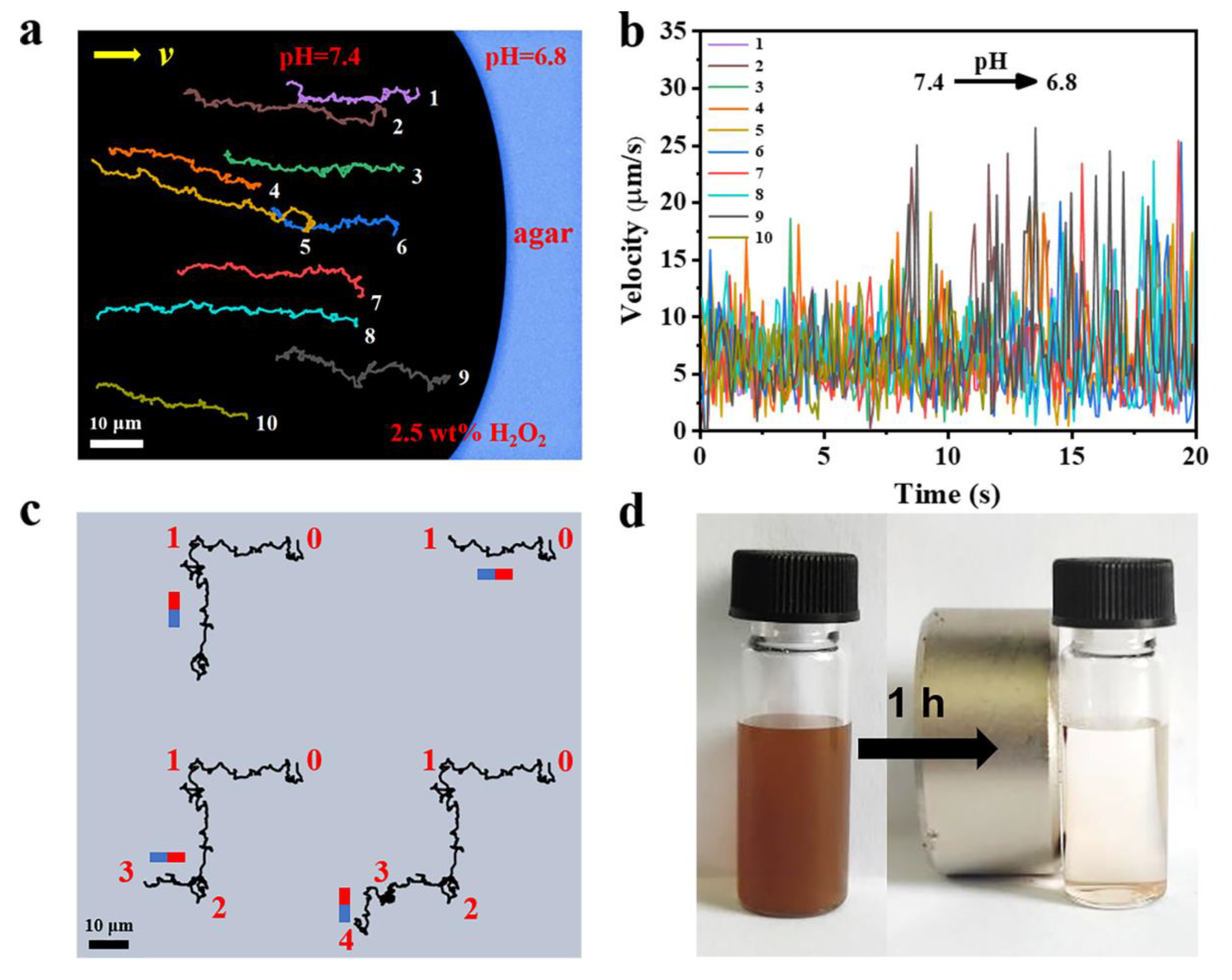
3.6. Chemotactic Motion and Magnetic Responsiveness of the FCNMs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhang, Y.; Hess, H. Chemically-powered swimming and diffusion in the microscopic world. Nat. Rev. Chem. 2021, 5, 500–510. [Google Scholar] [CrossRef]
- Wang, L.; Hao, X.; Gao, Z.; Yang, Z.; Long, Y.; Luo, M.; Guan, J. Artificial nanomotors: Fabrication, locomotion characterization, motion manipulation, and biomedical applications. Interdiscip. Mater. 2022, 1, 256–280. [Google Scholar] [CrossRef]
- Wu, J.; Balasubramanian, S.; Kagan, D.; Manesh, K.M.; Campuzano, S.; Wang, J. Motion-based DNA detection using catalytic nanomotors. Nat. Commun. 2010, 1, 36. [Google Scholar] [CrossRef] [PubMed]
- Fu, S.; Zhang, X.; Xie, Y.; Wu, J.; Ju, H. An efficient enzyme-powered micromotor device fabricated by cyclic alternate hybridization assembly for DNA detection. Nanoscale 2017, 9, 9026–9033. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Llopis-Lorente, A.; Garcia-Fernandez, A.; Murillo-Cremaes, N.; Hortelao, A.C.; Patino, T.; Villalonga, R.; Sancenon, F.; Martinez-Manez, R.; Sanchez, S. Enzyme-Powered Gated Mesoporous Silica Nanomotors for On-Command Intracellular Payload Delivery. ACS Nano 2019, 13, 12171–12183. [Google Scholar] [CrossRef]
- Tang, S.S.; Zhang, F.Y.; Gong, H.; Wei, F.N.; Zhuang, J.; Karshalev, E.; de Avila, B.E.F.; Huang, C.Y.; Zhou, Z.D.; Li, Z.X.; et al. Enzyme-powered Janus platelet cell robots for active and targeted drug delivery. Sci. Robot. 2020, 5, eaba6137. [Google Scholar] [CrossRef]
- de Avila, B.E.; Angsantikul, P.; Li, J.; Angel Lopez-Ramirez, M.; Ramirez-Herrera, D.E.; Thamphiwatana, S.; Chen, C.; Delezuk, J.; Samakapiruk, R.; Ramez, V.; et al. Micromotor-enabled active drug delivery for in vivo treatment of stomach infection. Nat. Commun. 2017, 8, 272. [Google Scholar] [CrossRef] [Green Version]
- Vilela, D.; Blanco-Cabra, N.; Eguskiza, A.; Hortelao, A.C.; Torrents, E.; Sanchez, S. Drug-Free Enzyme-Based Bactericidal Nanomotors against Pathogenic Bacteria. ACS Appl. Mater. Interfaces 2021, 13, 14964–14973. [Google Scholar] [CrossRef]
- Wang, L.; Hortelao, A.C.; Huang, X.; Sanchez, S. Lipase-Powered Mesoporous Silica Nanomotors for Triglyceride Degradation. Angew. Chem. Int. Ed. 2019, 58, 7992–7996. [Google Scholar] [CrossRef]
- Hortelao, A.C.; Simo, C.; Guix, M.; Guallar-Garrido, S.; Julian, E.; Vilela, D.; Rejc, L.; Ramos-Cabrer, P.; Cossio, U.; Gomez-Vallejo, V.; et al. Swarming behavior and in vivo monitoring of enzymatic nanomotors within the bladder. Sci. Robot. 2021, 6, eabd2823. [Google Scholar] [CrossRef]
- Kagan, D.; Benchimol, M.J.; Claussen, J.C.; Chuluun-Erdene, E.; Esener, S.; Wang, J. Acoustic Droplet Vaporization and Propulsion of Perfluorocarbon-Loaded Microbullets for Targeted Tissue Penetration and Deformation. Angew. Chem. Int. Ed. 2012, 51, 7519–7522. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kong, L.; Rohaizad, N.; Nasir, M.Z.M.; Guan, J.; Pumera, M. Micromotor-Assisted Human Serum Glucose Biosensing. Anal. Chem. 2019, 91, 5660–5666. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Yuan, Y.; Wan, J.; Yang, C.; Hao, X.; Gao, Z.; Luo, M.; Guan, J. Self-adaptive enzyme-powered micromotors with switchable propulsion mechanism and motion directionality. Appl. Phys. Rev. 2021, 8, 011406. [Google Scholar] [CrossRef]
- Tu, Y.; Peng, F.; Sui, X.; Men, Y.; White, P.B.; van Hest, J.C.M.; Wilson, D.A. Self-propelled supramolecular nanomotors with temperature-responsive speed regulation. Nat. Chem. 2017, 9, 480–486. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ji, Y.; Lin, X.; Zhang, H.; Wu, Y.; Li, J.; He, Q. Thermoresponsive Polymer Brush Modulation on the Direction of Motion of Phoretically Driven Janus Micromotors. Angew. Chem. Int. Ed. 2019, 58, 4184–4188. [Google Scholar] [CrossRef] [PubMed]
- Neri, D.; Supuran, C.T. Interfering with pH regulation in tumours as a therapeutic strategy. Nat. Rev. Drug. Discov. 2011, 10, 767–777. [Google Scholar] [CrossRef] [Green Version]
- Bhuyan, T.; Singh, A.K.; Dutta, D.; Unal, A.; Ghosh, S.S.; Bandyopadhyay, D. Magnetic Field Guided Chemotaxis of iMushbots for Targeted Anticancer Therapeutics. ACS Biomater. Sci. Eng. 2017, 3, 1627–1640. [Google Scholar] [CrossRef]
- Dey, K.K.; Bhandari, S.; Bandyopadhyay, D.; Basu, S.; Chattopadhyay, A. The pH Taxis of an Intelligent Catalytic Microbot. Small 2013, 9, 1916–1920. [Google Scholar] [CrossRef]
- Singh, A.K.; Dey, K.K.; Chattopadhyay, A.; Mandal, T.K.; Bandyopadhyay, D. Multimodal chemo-magnetic control of self-propelling microbots. Nanoscale 2014, 6, 1398–1405. [Google Scholar] [CrossRef]
- Rayaroth, M.P.; Oh, D.; Lee, C.S.; Kumari, N.; Lee, I.S.; Chang, Y.S. Carbon-nitride-based micromotor driven by chromate-hydrogen peroxide redox system: Application for removal of sulfamethaxazole. J. Colloid Interface Sci. 2021, 597, 94–103. [Google Scholar] [CrossRef]
- Su, Y.; Ge, Y.; Liu, L.; Zhang, L.; Liu, M.; Sun, Y.; Zhang, H.; Dong, B. Motion-Based pH Sensing Based on the Cartridge-Case-like Micromotor. ACS Appl. Mater. Interfaces 2016, 8, 4250–4257. [Google Scholar] [CrossRef] [PubMed]
- Gao, W. Water-driven-micromotors. ACS Nano 2012, 6, 8432–8438. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, W.; D’Agostino, M.; Garcia-Gradilla, V.; Orozco, J.; Wang, J. Multi-fuel driven Janus micromotors. Small 2013, 9, 467–471. [Google Scholar] [CrossRef] [Green Version]
- Jin, D.; Yu, J.; Chan, K.H.; Zhang, L. Self-propelled magnesium based micromotors: Synthesis and magnetic steering. In Proceedings of the International Symposium of Optomechatronics Technology (ISOT 2015), Neuchatel, Switzerland, 14–16 October 2015. [Google Scholar]
- Li, M.; Li, D. Self-propulsion of aluminum particle-coated Janus droplet in alkaline solution. J. Colloid Interface Sci. 2018, 532, 657–665. [Google Scholar] [CrossRef] [PubMed]
- Cui, Q.; Le, T.-H.; Lin, Y.-J.; Miao, Y.-B.; Sung, I.T.; Tsai, W.-B.; Chan, H.-Y.; Lin, Z.-H.; Sung, H.-W. A self-powered battery-driven drug delivery device that can function as a micromotor and galvanically actuate localized payload release. Nano Energy 2019, 66, 104120. [Google Scholar] [CrossRef]
- Gao, S.; Hou, J.; Zeng, J.; Richardson, J.J.; Gu, Z.; Gao, X.; Li, D.; Gao, M.; Wang, D.-W.; Chen, P.; et al. Superassembled Biocatalytic Porous Framework Micromotors with Reversible and Sensitive pH-Speed Regulation at Ultralow Physiological H2O2 Concentration. Adv. Funct. Mater. 2019, 29, 1808900. [Google Scholar] [CrossRef]
- Guo, Z.; Wang, T.; Rawal, A.; Hou, J.; Cao, Z.; Zhang, H.; Xu, J.; Gu, Z.; Chen, V.; Liang, K. Biocatalytic self-propelled submarine-like metal-organic framework microparticles with pH-triggered buoyancy control for directional vertical motion. Mater. Today 2019, 28, 10–16. [Google Scholar] [CrossRef]
- Liu, L.; Dong, Y.; Sun, Y.; Liu, M.; Su, Y.; Zhang, H.; Dong, B. Motion-based pH sensing using spindle-like micromotors. Nano Res. 2016, 9, 1310–1318. [Google Scholar] [CrossRef]
- Kim, B.H.; Lee, N.; Kim, H.; An, K.; Park, Y.I.; Choi, Y.; Shin, K.; Lee, Y.; Kwon, S.G.; Na, H.B.; et al. Large-scale synthesis of uniform and extremely small-sized iron oxide nanoparticles for high-resolution T1 magnetic resonance imaging contrast agents. J. Am. Chem. Soc. 2011, 133, 12624–12631. [Google Scholar] [CrossRef]
- Chen, Z.P.; Zhang, Y.; Zhang, S.; Xia, J.G.; Liu, J.W.; Xu, K.; Gu, N. Preparation and characterization of water-soluble monodisperse magnetic iron oxide nanoparticles via surface double-exchange with DMSA. Colloids Surf. A Physicochem. Eng. Asp. 2008, 316, 210–216. [Google Scholar] [CrossRef]
- Gao, C.; Zhou, C.; Lin, Z.; Yang, M.; He, Q. Surface Wettability-Directed Propulsion of Glucose-Powered Nanoflask Motors. ACS Nano 2019, 13, 12758–12766. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Gao, C.; Lin, Z.; Wang, D.; Li, Y.; Yuan, Y.; Zhu, B.; He, Q. Autonomous Motion of Bubble-Powered Carbonaceous Nanoflask Motors. Langmuir 2020, 36, 7039–7045. [Google Scholar] [CrossRef] [PubMed]
- Dey, K.K.; Sen, A. Chemically Propelled Molecules and Machines. J. Am. Chem. Soc. 2017, 139, 7666–7676. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, T.; Lin, J.; Xu, L.; Guan, J. Self-Adaptive Flask-like Nanomotors Based on Fe3O4 Nanoparticles to a Physiological pH. Nanomaterials 2022, 12, 2049. https://doi.org/10.3390/nano12122049
Gao T, Lin J, Xu L, Guan J. Self-Adaptive Flask-like Nanomotors Based on Fe3O4 Nanoparticles to a Physiological pH. Nanomaterials. 2022; 12(12):2049. https://doi.org/10.3390/nano12122049
Chicago/Turabian StyleGao, Tianyu, Jinwei Lin, Leilei Xu, and Jianguo Guan. 2022. "Self-Adaptive Flask-like Nanomotors Based on Fe3O4 Nanoparticles to a Physiological pH" Nanomaterials 12, no. 12: 2049. https://doi.org/10.3390/nano12122049
APA StyleGao, T., Lin, J., Xu, L., & Guan, J. (2022). Self-Adaptive Flask-like Nanomotors Based on Fe3O4 Nanoparticles to a Physiological pH. Nanomaterials, 12(12), 2049. https://doi.org/10.3390/nano12122049







