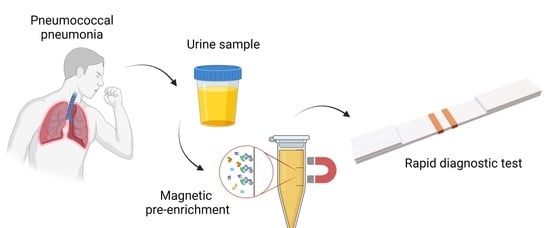
Magnetic Nanoclusters Increase the Sensitivity of Lateral Flow Immunoassays for Protein Detection: Application to Pneumolysin as a Biomarker for Streptococcus pneumoniae
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
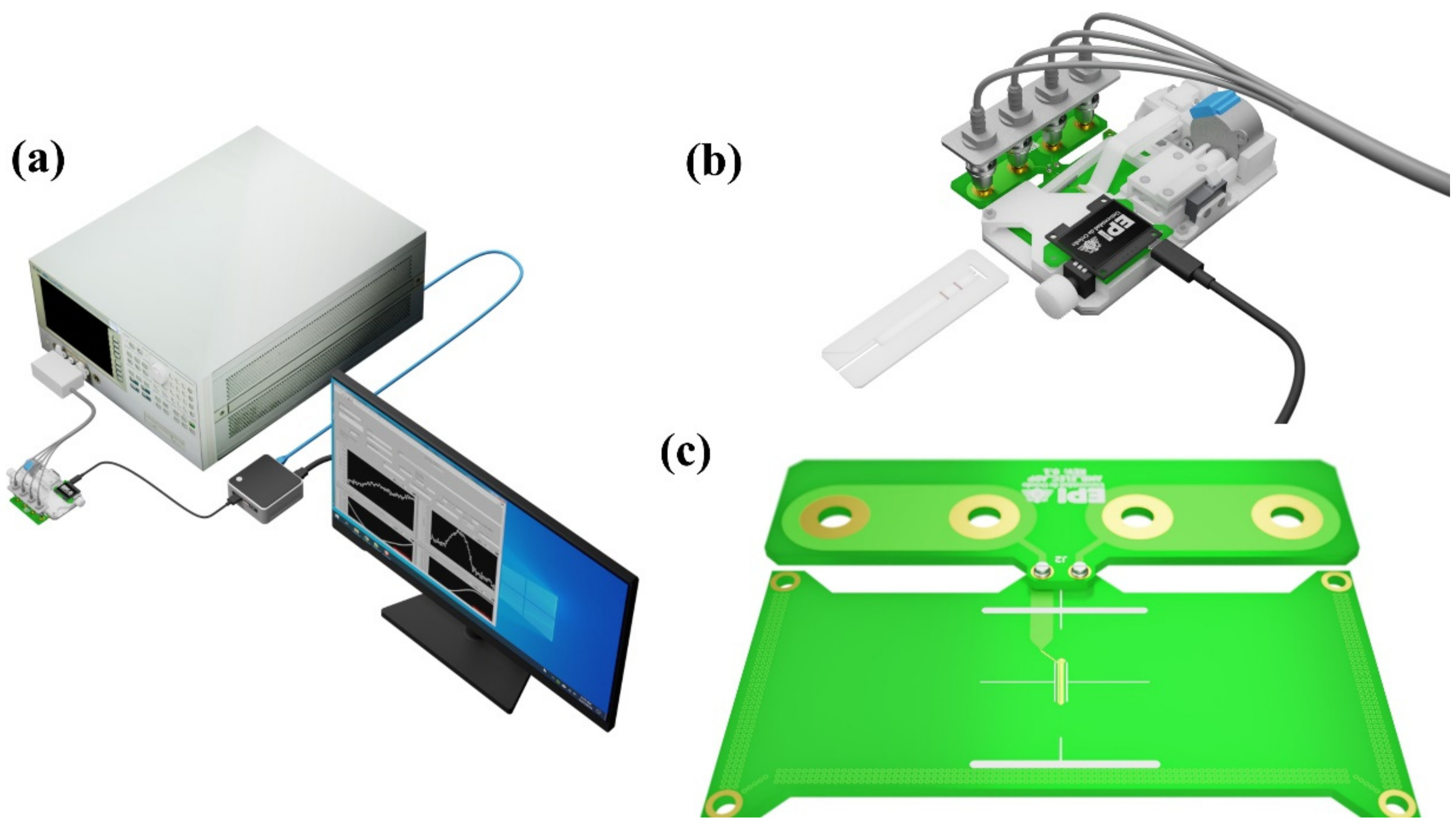
2.2. Magnetic Nanoclusters Synthesis and Characterization
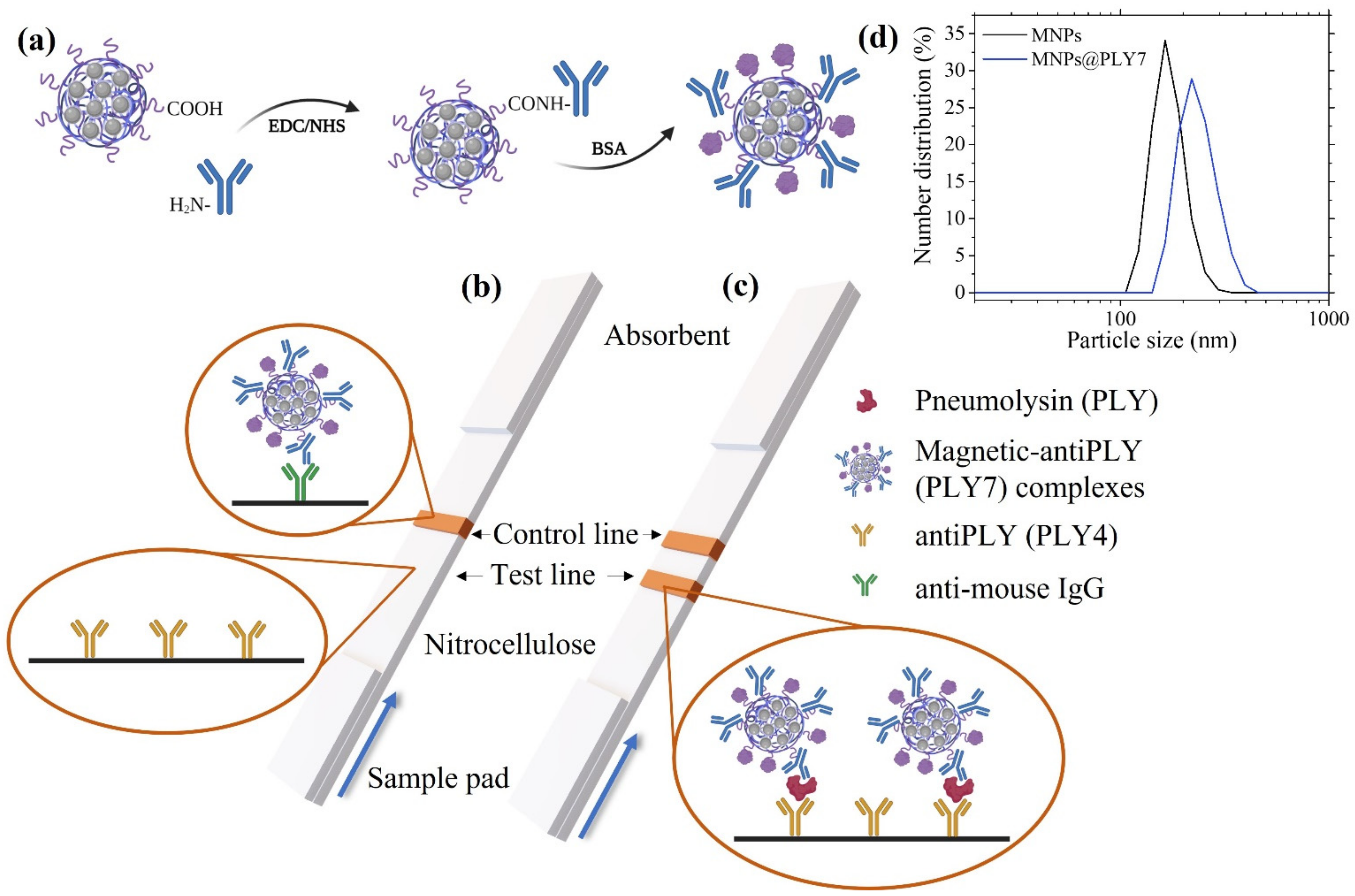
2.3. Bioconjugation of the Magnetic Clusters
2.4. Preparation of the Immuno-Strips
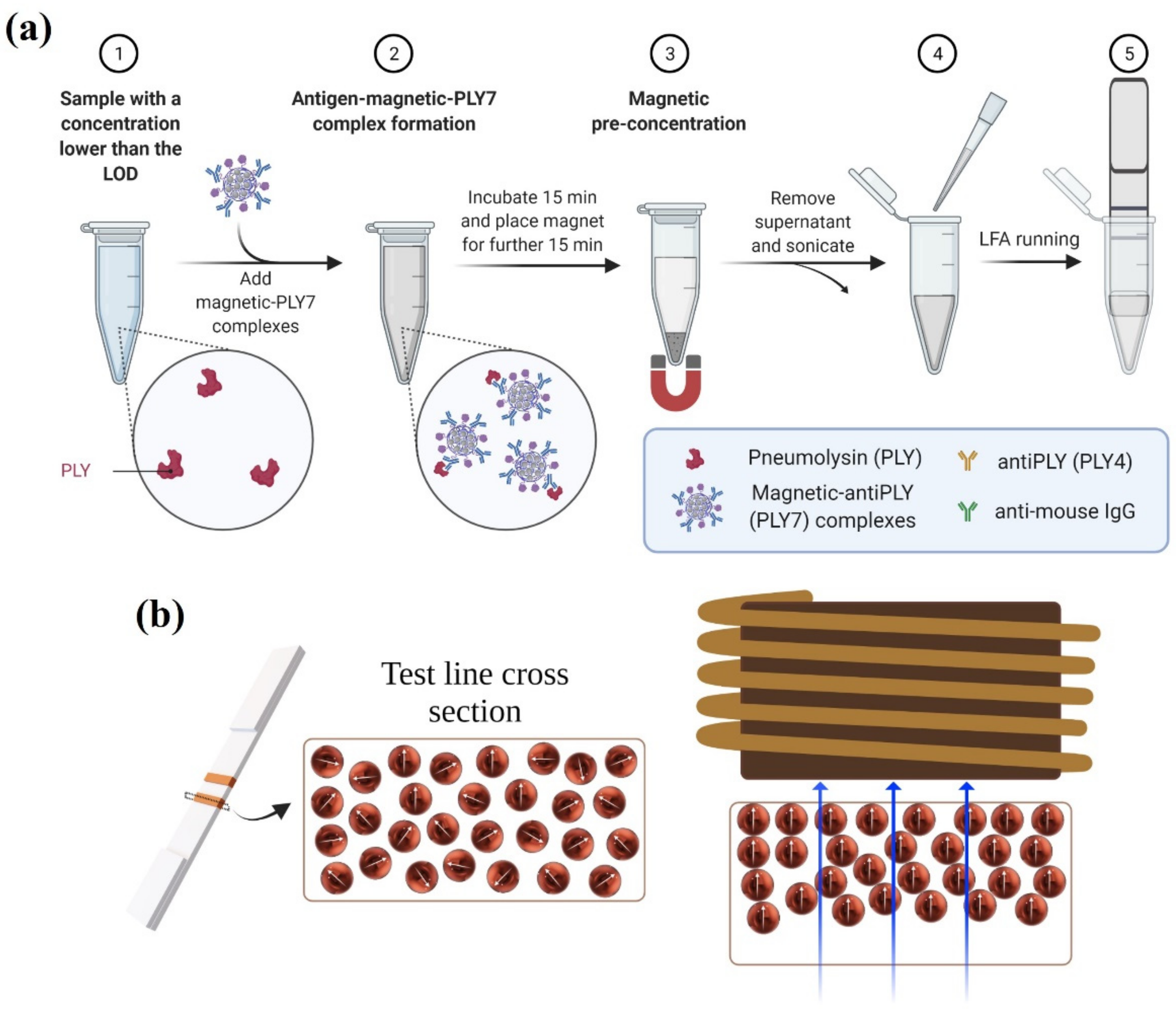
2.5. Magnetic Immuno-Concentration
2.6. Magnetic Relocation
3. Results and Discussion
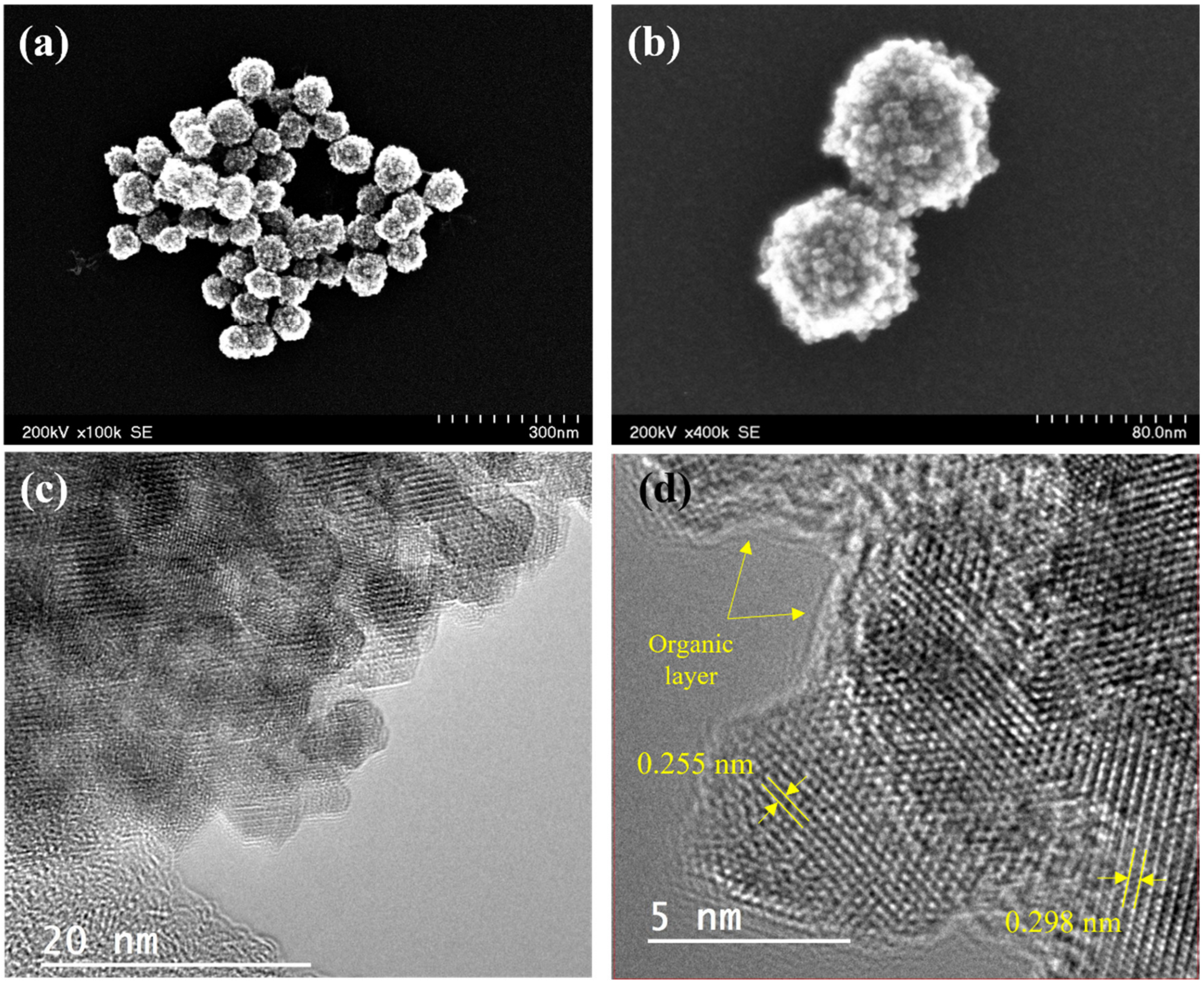
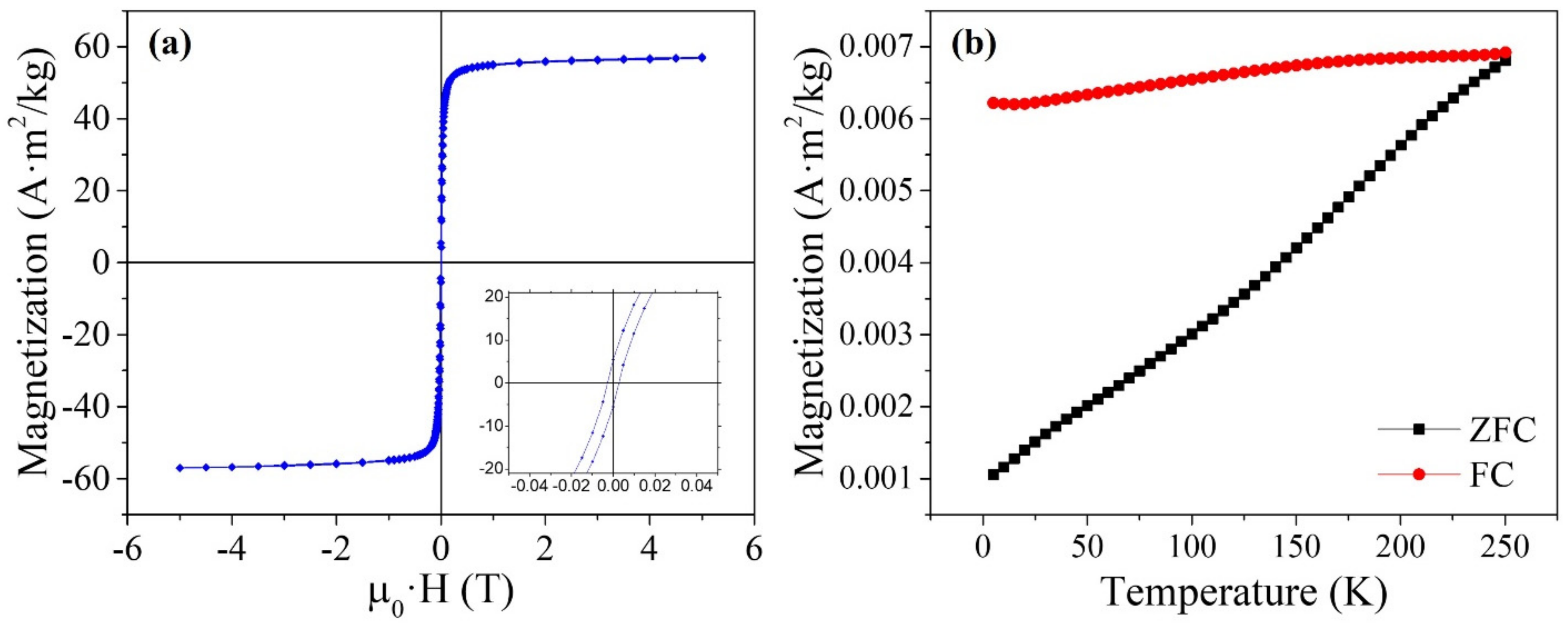
3.1. Characterization of the Magnetic Clusters
3.2. Bioconjugation of the Magnetic Clusters
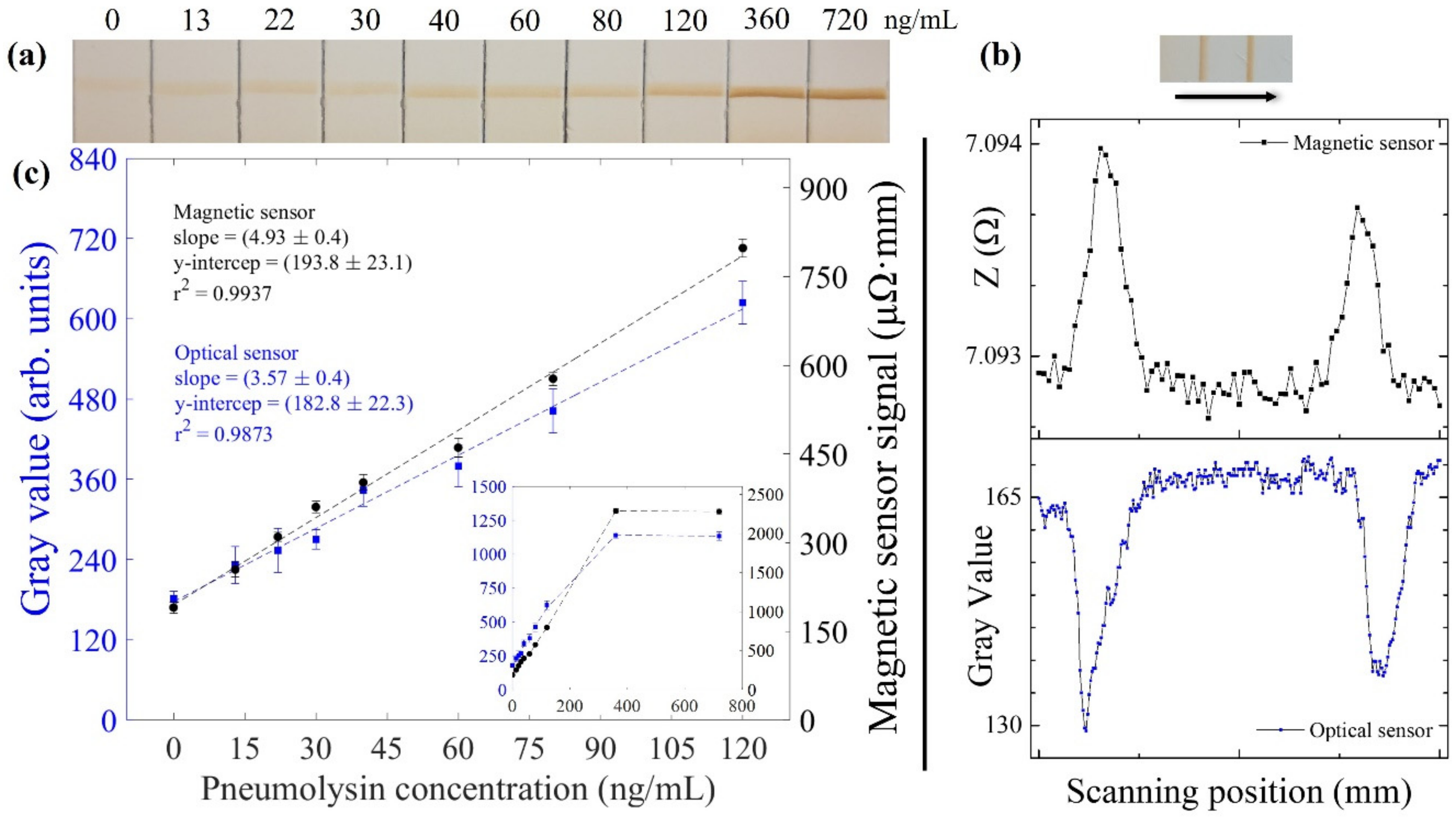
3.3. Lateral Flow Strips Reading-Out
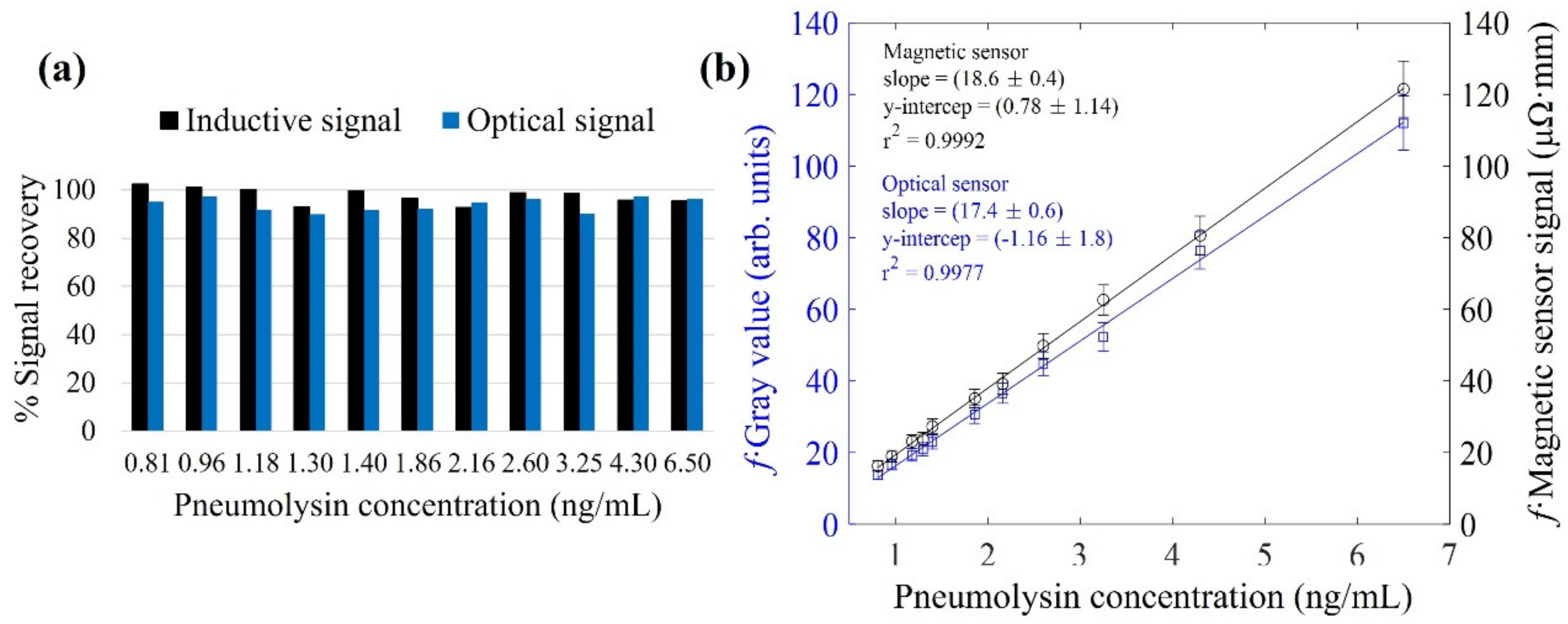
3.4. Magnetic Immuno-Concentration
3.5. Magnetic Relocation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ferreira-Coimbra, J.; Sarda, C.; Rello, J. Burden of Community-Acquired Pneumonia and Unmet Clinical Needs. Adv. Ther. 2020, 37, 1302–1318. [Google Scholar] [CrossRef] [PubMed]
- Torres, A.; Cillóniz, C.; Blasi, F.; Chalmers, J.D.; Gaillat, J.; Dartois, N.; Schmitt, H.-J.; Welte, T. Burden of Pneumococcal Community-Acquired Pneumonia in Adults across Europe: A Literature Review. Respir. Med. 2018, 137, 6–13. [Google Scholar] [CrossRef] [PubMed]
- Zar, H.J.; A Madhi, S.; Aston, S.; Gordon, S. Pneumonia in Low and Middle Income Countries: Progress and Challenges. Thorax 2013, 68, 1052–1056. [Google Scholar] [CrossRef] [PubMed]
- Guertler, C.; Wirz, B.; Christ-Crain, M.; Zimmerli, W.; Mueller, B.; Schuetz, P. Inflammatory Responses Predict Long-Term Mortality Risk in Community-Acquired Pneumonia. Eur. Respir. J. 2010, 37, 1439–1446. [Google Scholar] [CrossRef] [PubMed]
- Divo, M.J.; Martinez, C.H.; Mannino, D. Ageing and the Epidemiology of Multimorbidity. Eur. Respir. J. 2014, 44, 1055–1068. [Google Scholar] [CrossRef]
- Welte, T.; Torres, A.; Nathwani, D. Clinical and Economic Burden of Community-Acquired Pneumonia among Adults in Europe. Thorax 2010, 67, 71–79. [Google Scholar] [CrossRef]
- Cilloniz, C.; Martin-Loeches, I.; Garcia-Vidal, C.; San Jose, A.; Torres, A. Microbial Etiology of Pneumonia: Epidemiology, Diagnosis and Resistance Patterns. Int. J. Mol. Sci. 2016, 17, 2120. [Google Scholar] [CrossRef]
- Hirst, R.A.; Kadioglu, A.; O’Callaghan, C.; Andrew, P.W. The Role of Pneumolysin in Pneumococcal Pneumonia and Meningitis. Clin. Exp. Immunol. 2004, 138, 195–201. [Google Scholar] [CrossRef]
- Cucchiari, D.; Pericas, J.M.; Riera, J.; Gumucio, R.; Coloma, E.; Nicolás, D. Pneumococcal Superinfection in COVID-19 Patients: A Series of 5 Cases. Med. Clin. 2020, 155, 502–505. [Google Scholar] [CrossRef]
- Pal, C.; Przydzial, P.; Chika-Nwosuh, O.; Shah, S.; Patel, P.; Madan, N. Streptococcus Pneumoniae Coinfection in COVID-19: A Series of Three Cases. Case Rep. Pulmonol. 2020, 2020, 8849068. [Google Scholar] [CrossRef]
- Mandell, L.A. Community-Acquired Pneumonia: An Overview. Postgrad. Med. 2015, 127, 607–615. [Google Scholar] [CrossRef] [PubMed]
- Tavares, D.A.; Handem, S.; Carvalho, R.J.; Paulo, A.C.; De Lencastre, H.; Hinds, J.; Sá-Leão, R. Identification of Streptococcus Pneumoniae by a Real-Time Pcr Assay Targeting SP2020. Sci. Rep. 2019, 9, 3285. [Google Scholar] [CrossRef] [PubMed]
- Abdeldaim, G.; Herrmann, B.; Korsgaard, J.; Olcién, P.; Blomberg, J.; Strålin, K. Is Quantitative Pcr for the Pneumolysin (Ply) Gene Useful for Detection of Pneumococcal Lower Respiratory Tract Infection? Clin. Microbiol. Infect. 2009, 15, 565–570. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Molina, J.; González-Gamarra, A.; Ginel, L.; Peláez, M.; Juez, J.; Artuñedo, A.; Aldana, G.; Quesada, E.; Cabré, J.; Gómez, A.; et al. Cappric Study—Characterization of Community-Acquired Pneumonia in Spanish Adults Managed in Primary Care Settings. Microorganisms 2021, 9, 508. [Google Scholar] [CrossRef]
- Adegbola, R.A.; Obaro, S.K.; Biney, E.; Greenwood, B.M. Evaluation of Binax Now Streptococcus Pneumoniae Urinary Antigen Test in Children in a Community with a High Carriage Rate of Pneumococcus. Pediatr. Infect. Dis. J. 2001, 20, 718–719. [Google Scholar] [CrossRef]
- Navarro, D.; García-Maset, L.; Gimeno, C.; Escribano, A.; García-De-Lomas, J. Performance of the Binax Now Streptococcus Pneumoniae Urinary Antigen Assay for Diagnosis of Pneumonia in Children with Underlying Pulmonary Diseases in the Absence of Acute Pneumococcal Infection. J. Clin. Microbiol. 2004, 42, 4853–4855. [Google Scholar] [CrossRef][Green Version]
- Neill, D.R.; Mitchell, T.J.; Kadioglu, A. Pneumolysin. In Streptococcus Pneumoniae: Molecular Mechanisms of Host-Pathogen Interactions; Brown, J., Hammerschmidt, S., Orihuela, C., Eds.; Academic Press: Cambridge, MA, USA, 2015; pp. 257–275. [Google Scholar] [CrossRef]
- Mitchell, T.J.; Dalziel, C.E. The Biology of Pneumolysin. In MACPF/CDC Proteins—Agents of Defence, Attack and Invasion; Anderluh, G., Gilbert, R., Eds.; Springer: Dordrecht, The Netherlands, 2014; pp. 145–160. [Google Scholar] [CrossRef]
- Nishimoto, A.T.; Rosch, J.W.; Tuomanen, E.I. Pneumolysin: Pathogenesis and Therapeutic Target. Front. Microbiol. 2020, 11, 1543. [Google Scholar] [CrossRef]
- Maatsola, S.; Kurkinen, S.; Engström, M.T.; Nyholm, T.K.M.; Pentikäinen, O.; Salminen, J.-P.; Haataja, S. Inhibition of Pneumolysin Cytotoxicity by Hydrolysable Tannins. Antibiotics 2020, 9, 930. [Google Scholar] [CrossRef]
- Anderson, R.; Nel, J.G.; Feldman, C. Multifaceted Role of Pneumolysin in the Pathogenesis of Myocardial Injury in Community-Acquired Pneumonia. Int. J. Mol. Sci. 2018, 19, 1147. [Google Scholar] [CrossRef]
- García-Suárez, M.D.M.; Cima-Cabal, M.D.; Villaverde, R.; Espinosa, E.; Falguera, M.; Toyos, J.R.D.L.; Vázquez, F.; Méndez, F.J. Performance of a Pneumolysin Enzyme-Linked Immunosorbent Assay for Diagnosis of Pneumococcal Infections. J. Clin. Microbiol. 2007, 45, 3549–3554. [Google Scholar] [CrossRef][Green Version]
- Fanjul-Bolado, P.; González-García, M.B.; Costa-García, A. Voltammetric Determination of Alkaline Phosphatase and Horseradish Peroxidase Activity Using 3-Indoxyl Phosphate as Sub-Strate: Application to Enzyme Immunoassay. Talanta 2004, 64, 452–457. [Google Scholar] [CrossRef] [PubMed]
- Cima-Cabal, M.D.; Méndez, F.J.; Vazquez, F.; García-Suárez, M.D.M.; Toyos, J.R.D.L. A Specific and Ultrasensitive Chemiluminescent Sandwich Elisa Test for the Detection and Quantitation of Pneumolysin. J. Immunoass. Immunochem. 2001, 22, 99–112. [Google Scholar] [CrossRef] [PubMed]
- Leuvering, J.H.; Thal, P.J.; Van der Waart, M.; Schuurs, A.H. A SOL Particle Agglutination Assay for Human Chorionic Gonadotrophin. J. Immunol. Methods 1981, 45, 183–194. [Google Scholar] [CrossRef]
- Huang, Y.; Xu, T.; Wang, W.; Wen, Y.; Li, K.; Qian, L.; Zhang, X.; Liu, G. Lateral Flow Biosensors Based on the Use of Micro- And Nanomaterials: A Review on Recent Developments. Microchim. Acta 2019, 187, 70. [Google Scholar] [CrossRef]
- Huang, X.; Aguilar, Z.P.; Xu, H.; Lai, W.; Xiong, Y. Membrane-Based Lateral Flow Immunochromatographic Strip with Nanoparticles as Reporters for Detection: A Review. Biosens. Bioelectron. 2016, 75, 166–180. [Google Scholar] [CrossRef]
- Blanco-Covián, L.; Montes-García, V.; Girard, A.; Fernández-Abedul, M.T.; Pérez-Juste, J.; Pastoriza-Santos, I.; Faulds, K.; Graham, D.; Blanco-López, M.C. Au@Ag SERRS Tags Coupled to a Lateral Flow Immunoassay for the Sensitive Detection of Pneumolysin. Nanoscale 2017, 9, 2051–2058. [Google Scholar] [CrossRef]
- Dutta, S. Point of Care Sensing and Biosensing Using Ambient Light Sensor of Smartphone: Critical Review. TrAC Trends Anal. Chem. 2018, 110, 393–400. [Google Scholar] [CrossRef]
- Chun, P. Colloidal Gold and Other Labels for Lateral Flow Immunoassays. In Lateral Flow Immunoassay; Humana Press: Totowa, NJ, USA, 2009; pp. 1–19. [Google Scholar]
- Moyano, A.; Serrano-Pertierra, E.; Salvador, M.; Martínez-García, J.C.; Rivas, M.; Blanco-López, M.C. Magnetic Lateral Flow Immunoassays. Diagnostics 2020, 10, 288. [Google Scholar] [CrossRef]
- Panferov, V.G.; Safenkova, I.V.; Zherdev, A.V.; Dzantiev, B.B. Setting up the Cut-off Level of a Sensitive Barcode Lateral Flow Assay with Magnetic Nanoparticles. Talanta 2017, 164, 69–76. [Google Scholar] [CrossRef]
- Huang, Z.; Hu, S.; Xiong, Y.; Wei, H.; Xu, H.; Duan, H.; Lai, W. Application and Development of Superparamagnetic Nanoparticles in Sample Pretreatment and Immunochromatographic As-Say. TrAC Trends Anal. Chem. 2019, 114, 151–170. [Google Scholar] [CrossRef]
- Lago-Cachón, D.; Rivas, M.; Martínez-García, J.; Oliveira-Rodríguez, M.; Blanco-López, M. High Frequency Lateral Flow Affinity Assay Using Superparamagnetic Nanoparticles. J. Magn. Magn. Mater. 2017, 423, 436–440. [Google Scholar] [CrossRef]
- Rivas, M.; Lago-Cachón, D.; Martínez-García, J.; Calleja, A. Eddy-Current Sensing of Superparamagnetic Nanoparticles with Spiral-like Copper Circuits. Sensors Actuators A Phys. 2014, 216, 123–127. [Google Scholar] [CrossRef]
- Salvador, M.; Gallo-Cordova, Á.; Moyano, A.; Martínez-García, J.C.; Blanco-López, M.C.; Morales, M.P.; Rivas, M. Improved Magnetic Lateral Flow Assays with Optimized Nanotags for Point-Of-Use Inductive Biosensing. Analyst 2020, 145, 5905–5914. [Google Scholar] [CrossRef] [PubMed]
- Cima-Cabal, M.D.; Vázquez, F.; Toyos, J.R.D.L.; Méndez, F.J. Rapid and Reliable Identification of Streptococcus Pneumoniae Isolates by Pneumolysin-Mediated Agglutination. J. Clin. Microbiol. 1999, 37, 1964–1966. [Google Scholar] [CrossRef]
- Toyos, J.R.D.L.; Méndez, F.J.; Aparicio, J.F.; Vázquez, F.; Suárez, M.D.M.G.; Fleites, A.; Hardisson, C.; Morgan, P.J.; Andrew, P.W.; Mitchell, T.J. Functional Analysis of Pneumolysin by Use of Monoclonal Antibodies. Infect. Immun. 1996, 64, 480–484. [Google Scholar] [CrossRef]
- Bunge, A.; Porav, A.S.; Borodi, G.; Radu, T.; Pîrnău, A.; Berghian-Grosan, C.; Turcu, R. Correlation between Synthesis Parameters and Properties of Magnetite Clusters Prepared by Solvothermal Polyol Method. J. Mater. Sci. 2018, 54, 2853–2875. [Google Scholar] [CrossRef]
- Lago-Cachón, D.; Oliveira-Rodriguez, M.; Rivas, M.; Blanco-Lopez, M.C.; Martinez-Garcia, J.C.; Moyano, A.; Salvador, M. Scanning Magneto-Inductive Sensor for Quantitative Assay of Prostate-Specific Antigen. IEEE Magn. Lett. 2017, 8, 1506305. [Google Scholar] [CrossRef]
- Moyano, A.; Serrano-Pertierra, E.; Salvador, M.; Martínez-García, J.; Piñeiro, Y.; Yañez-Vilar, S.; Gónzalez-Gómez, M.; Rivas, J.; Rivas, M.; Blanco-López, M. Carbon-Coated Superparamagnetic Nanoflowers for Biosensors Based on Lateral Flow Immunoassays. Biosensors 2020, 10, 80. [Google Scholar] [CrossRef]
- Jones, K.D. Troubleshooting Protein Binding in Nitrocellulose Membranes. IVD Technol. 1999, 5, 32–41. [Google Scholar]
- Mansfield, M.A. Nitrocellulose Membranes for Lateral Flow Immunoassays: A Technical Treatise. In Lateral Flow Immunoass; Humana Press: Totowa, NJ, USA, 2009; pp. 1–19. [Google Scholar] [CrossRef]
- Mansfield, M.A.; OEM Diagnostics Group. Lateral Flow Membrane Technology—Technical Note; EMD Millipore: Bedford, MA, USA, 2014. [Google Scholar]
- Peddis, D.; Jönsson, P.E.; Laureti, S.; Varvaro, G. Magnetic Interactions: A Tool to Modify the Magnetic Properties of Materials Based on Nanoparticles. In Frontiers of Nanoscience; Binns, C., Ed.; Elsevier: Amsterdam, The Netherlands, 2014; Volume 6, pp. 129–188. [Google Scholar] [CrossRef]
- Zhang, H.; Zeng, D.; Liu, Z. The Law of Approach to Saturation in Ferromagnets Originating from the Magnetocrystalline Anisotropy. J. Magn. Magn. Mater. 2010, 322, 2375–2380. [Google Scholar] [CrossRef]
- Salvador, M.; Marqués-Fernández, J.L.; Martínez-García, J.C.; Fiorani, D.; Arosio, P.; Avolio, M.; Brero, F.; Balanean, F.; Guerrini, A.; Sangregorio, C.; et al. Double-Layer Fatty Acid Nanoparticles as a Multiplatform for Diagnostics and Therapy. Nanomaterials 2022, 12, 205. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xu, H.; Wei, M.; Gu, H.; Xu, Q.; Zhu, W. Study of Superparamagnetic Nanoparticles as Labels in the Quantitative Lateral Flow Immunoassay. Mater. Sci. Eng. C 2009, 29, 714–718. [Google Scholar] [CrossRef]
- Welch, N.G.; Scoble, J.; Muir, B.; Pigram, P.J. Orientation and Characterization of Immobilized Antibodies for Improved Immunoassays (Review). Biointerphases 2017, 12, 02D301. [Google Scholar] [CrossRef] [PubMed]
- Clogston, J.D.; Patri, A.K. Zeta Potential Measurement. Methods Mol. Biol. 2011, 697, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Magnusson, B.; Örnemark, U. (Eds.) Eurachem Guide: The Fitness for Purpose of Analytical Methods—A Laboratory Guide to Method Validation and Related Topics. 2014. Available online: http://Eurachem.Org/Images/Stories/Guides/Pdf/Valid.Pdf (accessed on 17 July 2021).
- Omelyanchik, A.; Lamura, G.; Peddis, D.; Canepa, F. Optimization of a NdFeB Permanent Magnet Configuration for In-Vivo Drug Delivery Experiments. J. Magn. Magn. Mater. 2020, 522, 167491. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salvador, M.; Marqués-Fernández, J.L.; Bunge, A.; Martínez-García, J.C.; Turcu, R.; Peddis, D.; García-Suárez, M.d.M.; Cima-Cabal, M.D.; Rivas, M. Magnetic Nanoclusters Increase the Sensitivity of Lateral Flow Immunoassays for Protein Detection: Application to Pneumolysin as a Biomarker for Streptococcus pneumoniae. Nanomaterials 2022, 12, 2044. https://doi.org/10.3390/nano12122044
Salvador M, Marqués-Fernández JL, Bunge A, Martínez-García JC, Turcu R, Peddis D, García-Suárez MdM, Cima-Cabal MD, Rivas M. Magnetic Nanoclusters Increase the Sensitivity of Lateral Flow Immunoassays for Protein Detection: Application to Pneumolysin as a Biomarker for Streptococcus pneumoniae. Nanomaterials. 2022; 12(12):2044. https://doi.org/10.3390/nano12122044
Chicago/Turabian StyleSalvador, María, José Luis Marqués-Fernández, Alexander Bunge, José Carlos Martínez-García, Rodica Turcu, Davide Peddis, María del Mar García-Suárez, María Dolores Cima-Cabal, and Montserrat Rivas. 2022. "Magnetic Nanoclusters Increase the Sensitivity of Lateral Flow Immunoassays for Protein Detection: Application to Pneumolysin as a Biomarker for Streptococcus pneumoniae" Nanomaterials 12, no. 12: 2044. https://doi.org/10.3390/nano12122044
APA StyleSalvador, M., Marqués-Fernández, J. L., Bunge, A., Martínez-García, J. C., Turcu, R., Peddis, D., García-Suárez, M. d. M., Cima-Cabal, M. D., & Rivas, M. (2022). Magnetic Nanoclusters Increase the Sensitivity of Lateral Flow Immunoassays for Protein Detection: Application to Pneumolysin as a Biomarker for Streptococcus pneumoniae. Nanomaterials, 12(12), 2044. https://doi.org/10.3390/nano12122044