A Review of Cobalt-Containing Nanomaterials, Carbon Nanomaterials and Their Composites in Preparation Methods and Application
Abstract
:1. Introduction
2. Application of Cobalt-Based Nanomaterials
2.1. Co3O4
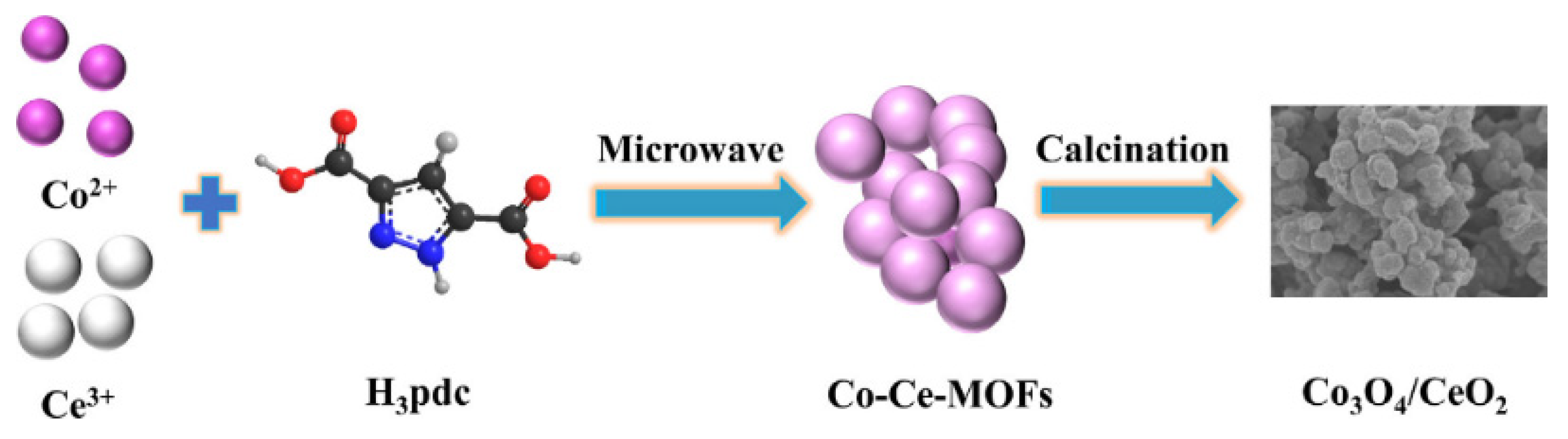
2.2. Binary Metal Oxides
2.3. Application of Cobalt Oxide Composite
2.4. Transition Metal Sulfide and Its Composites
3. Application of Carbon Nanomaterials
3.1. Application of Carbon Nanotube Materials
3.2. Application of Graphene Materials
3.3. Application of Carbon Microspheres
4. Application of Cobalt-Carbon Composites
4.1. Cobalt Oxide-Carbon Composites
4.2. Co Sulfide-Carbon Composite in LIBs Anode
5. Summary and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, R.; Zhou, A. Fundamentals, Advances and Challenges of Transition Metal Compounds-based Supercapacitors. Chem. Eng. J. 2021, 412, 128611. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, J.; Yang, S.; Yan, H.; Hong, X.; Dong, W.; Liu, Y.; Zhang, B.; Wen, Z. Interlayer space regulating of NiMn layered double hydroxides for supercapacitors by controlling hydrothermal reaction time. Electrochim. Acta 2019, 295, 1–6. [Google Scholar] [CrossRef]
- Palacin, M.R. Recent advances in rechargeable battery materials: A chemist’s perspective. Chem. Soc. Rev. 2009, 38, 2565–2575. [Google Scholar] [CrossRef] [PubMed]
- Ji, L.; Lin, Z.; Alcoutlabi, M.; Zhang, X. Recent developments in nanostructured anode materials for rechargeable lithium-ion batteries. Energy Environ. Sci. 2011, 4, 2682–2699. [Google Scholar] [CrossRef]
- Li, W.; Zhang, B. A Dendritic Nickel Cobalt Sulfide Nanostructure for Alkaline Battery Electrodes. Adv. Funct. Mater. 2018, 28, 1705937. [Google Scholar] [CrossRef] [Green Version]
- Koksbang, R.; Barker, J. Cathode materials for lithium rocking chair batteries. Solid State Ion. 1996, 3, 1–21. [Google Scholar] [CrossRef]
- Islam, M.S.; Fisher, C.A. Lithium and sodium battery cathode materials: Computational insights into voltage, diffusion and nanostructural properties. Chem. Soc. Rev. 2014, 43, 185–204. [Google Scholar] [CrossRef] [Green Version]
- Ding, Y.; Cano, Z.P. Automotive Li-Ion Batteries: Current Status and Future Perspectives. Electrochem. Energy Rev. 2019, 2, 1–28. [Google Scholar] [CrossRef]
- Andre, D.; Kim, S.J. Future generations of cathode materials: An automotive industry perspective. J. Mater. Chem. A 2015, 3, 6709–6732. [Google Scholar] [CrossRef]
- Liu, Q.; Su, X. Approaching the capacity limit of lithium cobalt oxide in lithium ion batteries via lanthanum and aluminium doping. Nat. Energy 2018, 3, 936–943. [Google Scholar] [CrossRef]
- Kang, Y.; Zhang, Y.H. Highly efficient Co3O4/CeO2 heterostructure as anode for lithium-ion batteries. J. Colloid. Interface Sci. 2021, 585, 705–715. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.; Akbar, M. A high voltage Li-ion full-cell battery with MnCo2O4/LiCoPO4 electrodes. Ceram. Int. 2020, 46, 26147–26155. [Google Scholar] [CrossRef]
- Liang, L.; Li, J. Cobalt Chalcogenides/Cobalt Phosphides/Cobaltates with Hierarchical Nanostructures for Anode Materials of Lithium-Ion Batteries: Improving the Lithiation Environment. Small 2021, 17, e1903418. [Google Scholar] [CrossRef] [PubMed]
- Aboelazm, E.A.A.; Ali, G.A.M.; Algarni, H.; Yin, H.; Zhong, Y.L.; Chong, K.F. Magnetic Electrodeposition of the Hierarchical Cobalt Oxide Nanostructure from Spent Lithium-Ion Batteries: Its Application as a Supercapacitor Electrode. J. Phys. Chem. C 2018, 122, 12200–12206. [Google Scholar] [CrossRef]
- Ali, G.A.; Fouad, O.A. Co3O4/SiO2 nanocomposites for supercapacitor application. Electrochemistry 2014, 18, 2505–2512. [Google Scholar] [CrossRef] [Green Version]
- Ali, G.; Habeeb, O.A. CaO impregnated highly porous honeycomb activated carbon from agriculture waste symmetrical supercapacitor study. J. Mater. Sci. 2019, 54, 683–692. [Google Scholar] [CrossRef]
- Lei, T.; Wang, Y.; Pan, F.; Dang, B. Nanofibrous carbon@cobaltous oxide composites for high electrochemical performance as anode materials of lithium-ion batteries. Ionics 2021, 27, 5097–5101. [Google Scholar] [CrossRef]
- Sun, H.; Sun, X.; Hu, T.; Yu, M.; Lu, F.; Lian, J. Graphene-Wrapped Mesoporous Cobalt Oxide Hollow Spheres Anode for High-Rate and Long-Life Lithium Ion Batteries. J. Phys. Chem. C 2014, 118, 2263–2272. [Google Scholar] [CrossRef]
- Choi, W.Y.; Lee, D.K. Cobalt oxide-porous carbon composite derived from CO2 for the enhanced performance of lithium-ion battery. J. CO2 Util. 2019, 30, 28–37. [Google Scholar] [CrossRef]
- Gu, F.; Liu, W. Small Co3O4/waste pinecone–derived activated porous carbon as anode materials of lithium-ion batteries. Ionics 2020, 26, 5897–5906. [Google Scholar] [CrossRef]
- Gangulibabu; Nallathamby, K. Carbonate anion controlled growth of LiCoPO4/C nanorods and its improved electrochemical behavior. Electrochim. Acta 2013, 101, 18–26. [Google Scholar] [CrossRef] [Green Version]
- Kim, D.H.; Park, G.D. Electrochemical properties of yolk-shell structured cobalt hydroxy chloride-carbon composite as an anode for lithium-ion batteries. Int. J. Energy Reseatch 2022, 46, 9761–9770. [Google Scholar] [CrossRef]
- Xu, K.; Shen, X. Cyanometallic framework-derived dual-buffer structure of Sn-Co based nanocomposites for high-performance lithium storage. J. Alloy. Compd. 2020, 830, 154680. [Google Scholar] [CrossRef]
- Umirov, N.; Seo, D.-H. Microstructure and electrochemical properties of rapidly solidified Si–Ni alloys as anode for lithium-ion batteries. J. Ind. Eng. Chem. 2019, 71, 351–360. [Google Scholar] [CrossRef]
- Wan, H.; Hu, X. Sulfur-doped honeycomb-like carbon with outstanding electrochemical performance as an anode material for lithium and sodium ion batteries. J. Colloid Interface Sci. 2020, 558, 242–250. [Google Scholar] [CrossRef]
- Zhang, H.; Hu, M. Monodisperse nitrogen-doped carbon spheres with superior rate capacities for lithium/sodium ion storage. Electrochim. Acta 2019, 297, 365–371. [Google Scholar] [CrossRef]
- Zheng, Z.; Li, P. High performance columnar-like Fe2O3@carbon composite anode via yolk@shell structural design. J. Energy Chem. 2020, 41, 126–134. [Google Scholar] [CrossRef] [Green Version]
- Shi, M.; Huang, Z. Ultralow nitrogen-doped carbon coupled carbon-doped Co3O4 microrods with tunable electron configurations for advanced Li-storage properties. Electrochim. Acta 2019, 327, 135059. [Google Scholar] [CrossRef]
- Elizabeth, I.; Singh, B.P. Electrochemical performance of Sb2S3/CNT free-standing flexible anode for Li-ion batteries. J. Mater. Sci. 2019, 54, 7110–7118. [Google Scholar] [CrossRef]
- Zhang, Q.; Liao, J. One-dimensional Fe7S8@C nanorods as anode materials for high-rate and long-life lithium-ion batteries. Appl. Surf. Sci. 2019, 473, 799–806. [Google Scholar] [CrossRef]
- Song, X.Y.; Zhang, Y.H. Lithium-Lanthanide Bimetallic Metal-Organic Frameworks towards Negative Electrode Materials for Lithium-Ion Batteries. Chemistry 2020, 26, 5654–5661. [Google Scholar] [CrossRef] [PubMed]
- Pan, G.-X.; Zhang, Y.-H. A brand-new bimetallic copper-lithium HEDP complex of fast ion migration as a promising anode for lithium ion batteries. J. Mol. Struct. 2020, 1214, 128223. [Google Scholar] [CrossRef]
- Biswal, A.; Panda, P. Facile synthesis of a nanoporous sea sponge architecture in a binary metal oxide. Nanoscale Adv. 2019, 1, 1880–1892. [Google Scholar] [CrossRef] [Green Version]
- Albohani, S.; Sundaram, M.M.; Laird, D.W. Egg shell membrane template stabilises formation of β-NiMoO4 nanowires and enhances hybrid supercapacitor behaviour. Mater. Lett. 2018, 236, 64–68. [Google Scholar] [CrossRef]
- Minakshi, M.; Mitchell, D. Phase evolution in calcium molybdate nanoparticles as a function of synthesis temperature and its electrochemical effect on energy storage. Nanoscale Adv. 2018, 1, 565–580. [Google Scholar] [CrossRef] [Green Version]
- Reddy, M.V.; Subba Rao, G.V. Metal oxides and oxysalts as anode materials for Li ion batteries. Chem. Rev. 2013, 113, 5364–5457. [Google Scholar] [CrossRef]
- Poizot, P.; Dupont, L.; Laruelle, S. Nano-sized transition-metal oxides as negative-electrode materials for lithium-ion batteries. Nature 2000, 9, 496–499. [Google Scholar] [CrossRef]
- Baji, D.S.; Nair, S.V. Highly porous disk-like shape of Co3O4 as an anode material for lithium ion batteries. J. Solid State Electrochem. 2017, 21, 2869–2875. [Google Scholar] [CrossRef]
- Li, L.; Jiang, G. Two-dimensional porous Co3O4 nanosheets for high-performance lithium ion batteries. New J. Chem. 2017, 41, 15283–15288. [Google Scholar] [CrossRef]
- Zhang, C.; Ke, F. Well-designed hollow and porous Co3O4 microspheres used as an anode for Li-ion battery. J. Solid State Electrochem. 2019, 23, 2477–2482. [Google Scholar] [CrossRef]
- Li, J.; Li, Z. Ultrathin Mesoporous Co3O4 Nanosheet Arrays for High-Performance Lithium-Ion Batteries. ACS Omega 2018, 3, 1675–1683. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Divakaran, A.M.; Minakshi, M.; Bahri, P.A.; Paul, S.; Kumari, P.; Divakaran, A.M.; Manjunatha, K.N. Rational design on materials for developing next generation lithium-ion secondary battery. Prog. Solid State Chem. 2020, 62, 100298. [Google Scholar] [CrossRef]
- Bai, J.; Li, X. Unusual Formation of ZnCo2O43D Hierarchical Twin Microspheres as a High-Rate and Ultralong-Life Lithium-Ion Battery Anode Material. Adv. Funct. Mater. 2014, 24, 3012–3020. [Google Scholar] [CrossRef]
- Sharma, Y.; Sharma, N. Studies on spinel cobaltites, FeCo2O4 and MgCo2O4 as anodes for Li-ion batteries. Solid State Ion. 2008, 179, 587–597. [Google Scholar] [CrossRef]
- Li, T.; Li, X. A novel NiCo2O4 anode morphology for lithium-ion batteries. J. Mater. Chem. A 2015, 3, 11970–11975. [Google Scholar] [CrossRef]
- Badway, F.; Plitz, I. Metal Oxides as Negative Electrode Materials in Li-Ion Cells. Electrochem. Solid-State Lett. 2002, 5, A115. [Google Scholar] [CrossRef]
- Jin, R.; Ma, Y. Manganese Cobalt Oxide (MnCo2O4) Hollow Spheres as High Capacity Anode Materials for Lithium-Ion Batteries. Energy Technol. 2017, 5, 293–299. [Google Scholar] [CrossRef]
- Jin, Y.; Wang, L. Mesoporous MnCo2O4 microflower constructed by sheets for lithium ion batteries. Mater. Lett. 2016, 177, 85–88. [Google Scholar] [CrossRef]
- Kong, X.; Zhu, T. Uniform MnCo2O4 Porous Dumbbells for Lithium-Ion Batteries and Oxygen Evolution Reactions. ACS Appl. Mater. Interfaces 2018, 10, 8730–8738. [Google Scholar] [CrossRef]
- Chen, C.; Liu, B.; Ru, Q.; Ma, S.; An, B.; Hou, X.; Hu, S. Fabrication of cubic spinel MnCo2O4 nanoparticles embedded in graphene sheets with their improved lithium-ion and sodium-ion storage properties. J. Power Sources 2016, 326, 252–263. [Google Scholar] [CrossRef]
- Ding, R.; Li, Z.; Wang, C.; Chen, M. Porous MnCo2O4-TiO2 microspheres with a yolk-shell structure for lithium-ion battery applications. J. Alloy. Compd. 2017, 726, 445–452. [Google Scholar] [CrossRef]
- Fu, C.; Li, G. One-step calcination-free synthesis of multicomponent spinel assembled microspheres for high-performance anodes of li-ion batteries: A case study of MnCo2O4. ACS Appl. Mater. Interfaces 2014, 6, 2439–2449. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.; Xu, S.; Xu, Z.; Sun, H.; Li, L. Core-shell ellipsoidal MnCo2O4 anode with micro-/nano-structure and concentration gradient for lithium-ion batteries. ACS Appl. Mater. Interfaces 2014, 6, 21325–21334. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, X. Facile preparation and performance of hierarchical self-assembly MnCo2O4 nanoflakes as anode active material for lithium ion batteries. Electrochim. Acta 2015, 180, 866–872. [Google Scholar] [CrossRef]
- Zhou, S.; Luo, X. MnCo2O4 nanospheres for improved lithium storage performance. Ceram. Int. 2018, 44, 17858–17863. [Google Scholar] [CrossRef]
- Yun, Y.J.; Kim, J.K. A morphology, porosity and surface conductive layer optimized MnCo2O4 microsphere for compatible superior Li+ ion/air rechargeable battery electrode materials. Dalton Trans. 2016, 45, 5064–5070. [Google Scholar] [CrossRef]
- Li, G.; Xu, L.; Zhai, Y.; Hou, Y. Fabrication of hierarchical porous MnCo2O4 and CoMn2O4 microspheres composed of polyhedral nanoparticles as promising anodes for long-life LIBs. J. Mater. Chem. A 2015, 3, 14298–14306. [Google Scholar] [CrossRef]
- Xu, H.; Shen, H.; Song, X.; Kong, X.; Zhang, Y.; Qin, Z. Hydrothermal synthesis of porous hydrangea-like MnCo2O4 as anode materials for high performance lithium ion batteries. J. Electroanal. Chem. 2019, 851, 113455. [Google Scholar] [CrossRef]
- Zeng, P.; Wang, X. Excellent lithium ion storage property of porous MnCo2O4 nanorods. RSC Adv. 2016, 6, 23074–23084. [Google Scholar] [CrossRef]
- Zhang, L.; Tang, Q. Self-assembled synthesis of diamond-like MnCo2O4 as anode active material for lithium-ion batteries with high cycling stability. J. Alloy. Compd. 2017, 722, 387–393. [Google Scholar] [CrossRef]
- Wu, W.; Wang, K. Synthesis and electrochemical performance of flower-like MnCo2O4 as an anode material for sodium ion batteries. Mater. Lett. 2015, 147, 85–87. [Google Scholar] [CrossRef]
- Li, J.; Wang, J.; Liang, X.; Zhang, Z.; Liu, H.; Qian, Y.; Xiong, S. Hollow MnCo2O4 Submicrospheres with Multilevel Interiors: From Mesoporous Spheres to Yolk-in-Double-Shell Structures. ACS Appl. Mater. Interfaces 2013, 6, 24–30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, X.; Cao, K. 3D hierarchical porous ZnO/ZnCo2O4 nanosheets as high-rate anode material for lithium-ion batteries. J. Mater. Chem. A 2016, 4, 6042–6047. [Google Scholar] [CrossRef]
- Wu, M.; Leung, D.Y.C. Toluene degradation over Mn-TiO2/CeO2 composite catalyst under vacuum ultraviolet (VUV) irradiation. Chem. Eng. Sci. 2019, 195, 985–994. [Google Scholar] [CrossRef]
- Yang, X.; Wang, Y. Interior Supported Hierarchical TiO2@Co3O4 Derived from MOF-on-MOF Architecture with Enhanced Electrochemical Properties for Lithium Storage. ChemElectroChem 2019, 6, 3657–3666. [Google Scholar] [CrossRef]
- Zhao, J.; Yao, S. Porous ZnO/Co3O4/CoO/Co composite derived from Zn-Co-ZIF as improved performance anodes for lithium-ion batteries. Mater. Lett. 2019, 250, 75–78. [Google Scholar] [CrossRef]
- Liu, H.; Wu, X. Tailored Synthesis of Coral-Like CoTiO3/Co3O4/TiO2 Nanobelts with Superior Lithium Storage Capability. Energy Technol. 2019, 8, 1900774. [Google Scholar] [CrossRef]
- Liu, H.; Cao, K. Constructing hierarchical MnO2/Co3O4 heterostructure hollow spheres for high-performance Li-Ion batteries. J. Power Sources 2019, 437, 226904. [Google Scholar] [CrossRef]
- Zhong, M.; Yang, D.H. Bimetallic metal-organic framework derived Co3O4-CoFe2O4 composites with different Fe/Co molar ratios as anode materials for lithium ion batteries. Dalton Trans. 2017, 46, 15947–15953. [Google Scholar] [CrossRef]
- Ding, H.; Zhang, X.K. MOF-Templated Synthesis of Co3O4@TiO2 Hollow Dodecahedrons for High-Storage-Density Lithium-Ion Batteries. ACS Omega 2019, 4, 13241–13249. [Google Scholar] [CrossRef] [Green Version]
- Wang, P.; Zhang, P. Constructing MoS2/CoMo2S4/Co3S4 nanostructures supported by graphene layers as the anode for lithium-ion batteries. Dalton Trans. 2020, 49, 1167–1172. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Ma, W. Shape evolution and electrochemical properties of cobalt sulfide via a biomolecule-assisted solvothermal route. Solid State Sci. 2013, 17, 102–106. [Google Scholar] [CrossRef]
- Zhou, Y.; Yan, D. Hollow nanospheres of mesoporous Co9S8 as a high-capacity and long-life anode for advanced lithium ion batteries. Nano Energy 2015, 12, 528–537. [Google Scholar] [CrossRef]
- Zhang, X.; Sun, Y. Nanosized SnO2-CoS constructed porous cubeas advanced lithium-ion batteries anode. Ceram. Int. 2018, 44, 5569–5571. [Google Scholar] [CrossRef]
- Yang, S.H.; Park, S.-K. A MOF-mediated strategy for constructing human backbone-like CoMoS3@N-doped carbon nanostructures with multiple voids as a superior anode for sodium-ion batteries. J. Mater. Chem. A 2019, 7, 13751–13761. [Google Scholar] [CrossRef]
- Yu, D.J.; Yuan, Y.F. Nickel cobalt sulfide Nanotube Array on Nickel Foam as Anode Material for Advanced Lithium-Ion Batteries. Electrochim. Acta 2016, 198, 280–286. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Y. Physical confinement and chemical adsorption of porous C/CNT micro/nano-spheres for CoS and Co9S8 as advanced lithium batteries anodes. Electrochim. Acta 2019, 299, 489–499. [Google Scholar] [CrossRef]
- Wang, H.; Ma, J. CoS/CNTs hybrid structure for improved performance lithium ion battery. J. Alloy. Compd. 2016, 676, 551–556. [Google Scholar] [CrossRef]
- Li, X.; Fu, N. Ultrafine Cobalt Sulfide Nanoparticles Encapsulated Hierarchical N-doped Carbon Nanotubes for High-performance Lithium Storage. Electrochim. Acta 2017, 225, 137–142. [Google Scholar] [CrossRef]
- Yao, Y.; Zhu, Y. Porous CoS nanosheets coated by N and S doped carbon shell on graphene foams for free-standing and flexible lithium ion battery anodes: Influence of void spaces, shell and porous nanosheet. Electrochim. Acta 2018, 271, 242–251. [Google Scholar] [CrossRef]
- Gu, Y.; Xu, Y. Graphene-wrapped CoS nanoparticles for high-capacity lithium-ion storage. ACS Appl. Mater. Interfaces 2013, 5, 801–806. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Ding, X. Embedding cobalt sulfide in reduced graphene oxide for superior lithium-ion storage. Mater. Lett. 2019, 253, 22–25. [Google Scholar] [CrossRef]
- Ni, J.; Li, Y. Carbon Nanomaterials in Different Dimensions for Electrochemical Energy Storage. Adv. Energy Mater. 2016, 6, 1600278. [Google Scholar] [CrossRef]
- Goriparti, S.; Miele, E. Review on recent progress of nanostructured anode materials for Li-ion batteries. J. Power Sources 2014, 257, 421–443. [Google Scholar] [CrossRef] [Green Version]
- Gu, J.; Du, Z. Pyridinic Nitrogen-Enriched Carbon Nanogears with Thin Teeth for Superior Lithium Storage. Adv. Energy Mater. 2016, 6, 1600917. [Google Scholar] [CrossRef]
- Lee, S.W.; Yabuuchi, N. High-power lithium batteries from functionalized carbon-nanotube electrodes. Nat. Nanotechnol. 2010, 5, 531–537. [Google Scholar] [CrossRef]
- Fan, Z.; Yan, J. A Three-Dimensional Carbon Nanotube/Graphene Sandwich and Its Application as Electrode in Supercapacitors. Adv. Mater. 2010, 22, 3723. [Google Scholar] [CrossRef]
- Qie, L.; Chen, W.-M. Nitrogen-Doped Porous Carbon Nanofiber Webs as Anodes for Lithium Ion Batteries with a Superhigh Capacity and Rate Capability. Adv. Mater. 2012, 24, 2047–2050. [Google Scholar] [CrossRef]
- Zheng, G.; Yang, Y. Hollow Carbon Nanofiber-Encapsulated Sulfur Cathodes for High Specific Capacity Rechargeable Lithium Batteries. Nano Lett. 2011, 11, 4462–4467. [Google Scholar] [CrossRef]
- Lian, P.; Zhu, X. Large reversible capacity of high quality graphene sheets as an anode material for lithium-ion batteries. Electrochim. Acta 2010, 55, 3909–3914. [Google Scholar] [CrossRef]
- Liu, F.; Song, S. Folded Structured Graphene Paper for High Performance Electrode Materials. Adv. Mater. 2012, 24, 1089–1094. [Google Scholar] [CrossRef] [PubMed]
- Lee, R.S.; Kim, H.J. Conductivity enhancement in single-walled carbon nanotube bundles doped with K and Br. Nature 1997, 388, 255–257. [Google Scholar] [CrossRef]
- Yang, S.; Song, H. Electrochemical performance of arc-produced carbon nanotubes as anode material for lithium-ion batteries. Electrochim. Acta 2007, 52, 5286–5293. [Google Scholar] [CrossRef]
- Eom, J.; Kim, D. Effects of ball-milling on lithium insertion into multi-walled carbon nanotubes synthesized by thermal chemical vapour deposition. J. Power Sources 2006, 157, 507–514. [Google Scholar] [CrossRef]
- Gao, B.; Bower, C. Enhanced saturation lithium composition in ball-milled single-walled carbon nanotubes. Chem. Phys. Lett. 2000, 327, 69–75. [Google Scholar] [CrossRef]
- Yang, S.; Huo, J. A comparative study of electrochemical properties of two kinds of carbon nanotubes as anode materials for lithium ion batteries. Electrochim. Acta 2008, 53, 2238–2244. [Google Scholar] [CrossRef]
- Bulusheva, L.; Arkhipov, V.; Fedorovskaya, E.; Zhang, S.; Kurenya, A.; Kanygin, M.; Asanov, I.; Tsygankova, A.; Chen, X.; Song, H.; et al. Fabrication of free-standing aligned multiwalled carbon nanotube array for Li-ion batteries. J. Power Sources 2016, 311, 42–48. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Geim, A.K. Two-Dimensional Gas of Massless Dirac Fermions in Graphene. Nature 2005, 438, 197–200. [Google Scholar] [CrossRef]
- Kyun, K.D.; Kim, T. A Review: Comparison of Fabrication and Characteristics of Flexible ReRAM and Multi-Insulating Graphene Oxide Layer ReRAM. Trans. Korean Inst. Electr. Eng. 2016, 65, 1369–1375. [Google Scholar]
- Song, N.; Gao, X. A review of graphene-based separation membrane: Materials, characteristics, preparation and applications. Desalination 2018, 437, 59–72. [Google Scholar] [CrossRef]
- Gangu, K.K.; Maddila, S. Characteristics of MOF, MWCNT and graphene containing materials for hydrogen storage: A review. J. Energy Chem. 2019, 30, 132–144. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Lin, Y. Three-dimensional hierarchical nanocomposites of NiSnO3/graphene encapsulated in carbon matrix as long-life anode for lithium-ion batteries. J. Alloy. Compd. 2019, 793, 492–498. [Google Scholar] [CrossRef]
- Ju, J.Y.; Ji, S. Rational design of electrochemically active polymorphic MnOx/rGO composites for Li+-rechargeable battery electrodes. Ceram. Int. 2019, 45, 9522–9528. [Google Scholar] [CrossRef]
- Shenouda, A.Y.; Momchilov, A.A. A study on graphene/tin oxide performance as negative electrode compound for lithium battery application. J. Mater. Sci. Mater. Electron. 2019, 30, 79–90. [Google Scholar] [CrossRef]
- Wu, Q.; Jiang, R. Carbon layer encapsulated Fe3O4@Reduced graphene oxide lithium battery anodes with long cycle performance. Ceram. Int. 2020, 46, 12732–12739. [Google Scholar] [CrossRef]
- Lochala, J.A.; Zhang, H. Practical Challenges in Employing Graphene for Lithium-Ion Batteries and Beyond. Small Methods 2017, 1, 1700099. [Google Scholar] [CrossRef]
- Mei, R.; Song, X. Hollow reduced graphene oxide microspheres as a high-performance anode material for Li-ion batteries. Electrochim. Acta 2015, 153, 540–545. [Google Scholar] [CrossRef]
- Wei, T.; Wang, F. Microspheres composed of multilayer graphene as anode material for lithium-ion batteries. J. Electroanal. Chem. 2011, 653, 45–49. [Google Scholar] [CrossRef]
- Guo, P.; Song, H. Electrochemical performance of graphene nanosheets as anode material for lithium-ion batteries. Electrochem. Commun. 2009, 11, 1320–1324. [Google Scholar] [CrossRef]
- Song, R.; Cao, B. A simple preparation of porous graphene nanosheets containing onion-like nano-holes with favorable high-rate Li-storage performance. RSC Adv. 2016, 6, 63373–63377. [Google Scholar] [CrossRef]
- Rish, S.K.; Tahmasebi, A. Novel composite nano-materials with 3D multilayer-graphene structures from biomass-based activated-carbon for ultrahigh Li-ion battery performance. Electrochim. Acta 2021, 390, 138839. [Google Scholar] [CrossRef]
- Luo, W.-B.; Chou, S.-L. Self-assembled graphene and LiFePO4 composites with superior high rate capability for lithium ion batteries. J. Mater. Chem. A 2014, 2, 4927–4931. [Google Scholar] [CrossRef]
- Zhou, X.; Wang, F. Graphene modified LiFePO4 cathode materials for high power lithium ion batteries. J. Mater. Chem. 2011, 21, 3353–3358. [Google Scholar] [CrossRef]
- Xu, Z.; Gao, L. Review-Recent Developments in the Doped LiFePO4 Cathode Materials for Power Lithium Ion Batteries. J. Electrochem. Soc. 2016, 163, A2600–A2610. [Google Scholar] [CrossRef]
- Li, L.; Wu, L. Review-Recent Research Progress in Surface Modification of LiFePO4 Cathode Materials. J. Electrochem. Soc. 2017, 164, A2138–A2150. [Google Scholar] [CrossRef]
- Zhu, H.; Wu, X. Three-Dimensional Macroporous Graphene-Li2FeSiO4 Composite as Cathode Material for Lithium-Ion Batteries with Superior Electrochemical Performances. ACS Appl. Mater. Interfaces 2014, 6, 11724–11733. [Google Scholar] [CrossRef]
- Liu, J.; Wickramaratne, N.P. Molecular-based design and emerging applications of nanoporous carbon spheres. Nat. Mater. 2015, 14, 763–774. [Google Scholar] [CrossRef]
- Park, S.-H.; Kim, H.-K. Spray-Assisted Deep-Frying Process for the In Situ Spherical Assembly of Graphene for Energy-Storage Devices. Chem. Mater. 2015, 27, 457–465. [Google Scholar] [CrossRef]
- Shi, J.-L.; Peng, H.-J.; Zhu, L.; Zhu, W.; Zhang, Q. Template growth of porous graphene microspheres on layered double oxide catalysts and their applications in lithium–sulfur batteries. Carbon 2000, 92, 96–105. [Google Scholar] [CrossRef]
- Li, D.; Chen, H. Porous nitrogen doped carbon sphere as high performance anode of sodium-ion battery. Carbon 2015, 94, 888–894. [Google Scholar] [CrossRef]
- Xu, S.; Zhang, Z.; Wu, T.; Xue, Y. Nanoporous carbon microspheres as anode material for enhanced capacity of lithium ion batteries. Ionics 2018, 24, 99–109. [Google Scholar] [CrossRef]
- Yi, R.; Dai, F. Micro-sized Si-C Composite with Interconnected Nanoscale Building Blocks as High-Performance Anodes for Practical Application in Lithium-Ion Batteries. Adv. Energy Mater. 2013, 3, 295–300. [Google Scholar] [CrossRef]
- Chen, T.; Pan, L. Porous nitrogen-doped carbon microspheres as anode materials for lithium ion batteries. Dalton Trans. 2014, 43, 14931–14935. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Li, H. Novel spherical microporous carbon as anode material for Li-ion batteries. Solid State Ion. 2002, 152, 43–50. [Google Scholar] [CrossRef]
- Hu, Y.-S.; Adelhelm, P. Synthesis of hierarchically porous carbon monoliths with highly ordered microstructure and their application in rechargeable lithium batteries with high-rate capability. Adv. Funct. Mater. 2007, 17, 1873–1878. [Google Scholar] [CrossRef]
- Zhang, F.; Wang, K.-X. Hierarchical porous carbon derived from rice straw for lithium ion batteries with high-rate performance. Electrochem. Commun. 2009, 11, 130–133. [Google Scholar] [CrossRef]
- Wang, F.; Song, R.; Song, H.; Chen, X.; Zhou, J.; Ma, Z.; Li, M.; Lei, Q. Simple synthesis of novel hierarchical porous carbon microspheres and their application to rechargeable lithium-ion batteries. Carbon 2015, 81, 314–321. [Google Scholar] [CrossRef]
- Etacheri, V.; Wang, C.; O’Connell, M.J.; Chan, C.K.; Pol, V. GPorous carbon sphere anodes for enhanced lithium-ion storage. J. Mater. Chem. A 2015, 3, 9861–9868. [Google Scholar] [CrossRef]
- Shi, L.; Chen, Y. Fabrication of hierarchical porous carbon microspheres using porous layered double oxide templates for high-performance lithium ion batteries. Carbon 2017, 123, 186–192. [Google Scholar] [CrossRef]
- Zhang, L.; Dou, Y.; Guo, H.; Zhang, B.; Liu, X.; Wan, M.; Li, W.; Hu, X.; Dou, S.; Huang, Y.; et al. A facile way to fabricate double-shell pomegranate-like porous carbon microspheres for high-performance Li-ion batteries. J. Mater. Chem. A 2017, 5, 12073–12079. [Google Scholar] [CrossRef]
- Zhang, C.; Wu, Z.; Jan, S.; Wang, Z.; Bennaceur, S.; Kim, H.-K.; Jin, X. Glucose-derived hollow microsphere graphite with a nanosheets-constructed porous shell for improved lithium storage. Electrochim. Acta 2021, 390, 138800. [Google Scholar] [CrossRef]
- Bagri, P.; Thapaliya, B.P. Electrochemically induced crystallization of amorphous materials in molten MgCl2: Boron nitride and hard carbon. Chem. Commun. 2020, 56, 2783–2786. [Google Scholar] [CrossRef] [PubMed]
- Tu, J.; Wang, J. High-efficiency transformation of amorphous carbon into graphite nanoflakes for stable aluminum-ion battery cathodes. Nanoscale 2019, 11, 12537–12546. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Zuo, H. A green electrochemical transformation of inferior coals to crystalline graphite for stable Li-ion storage. J. Mater. Chem. A 2019, 7, 7533–7540. [Google Scholar] [CrossRef]
- Song, W.-L.; Li, S. Cellulose-derived flake graphite as positive electrodes for Al-ion batteries. Sustain. Energy Fuels 2019, 3, 3561–3568. [Google Scholar] [CrossRef]
- Zhang, C.; He, R. Amorphous Carbon-Derived Nanosheet-Bricked Porous Graphite as High-Performance Cathode for Aluminum-Ion Batteries. ACS Appl. Mater. Interfaces 2018, 10, 26510–26516. [Google Scholar] [CrossRef]
- Jin, X.; He, R.; Dai, S. Frontispiece: Electrochemical Graphitization: An Efficient Conversion of Amorphous Carbons to Nanostructured Graphites. Chem. A Eur. J. 2017, 23, 11455–11459. [Google Scholar] [CrossRef]
- Peng, J.; Chen, N. Electrochemically Driven Transformation of Amorphous Carbons to Crystalline Graphite Nanoflakes: A Facile and Mild Graphitization Method. Angew. Chem. Int. Ed. 2017, 56, 1751–1755. [Google Scholar] [CrossRef]
- Xie, Q.; Qu, S. Nitrogen-enriched graphene-like carbon architecture with tunable porosity derived from coffee ground as high performance anodes for lithium ion batteries. Appl. Surf. Sci. 2021, 537, 148092. [Google Scholar] [CrossRef]
- Zhang, X.; Zhou, J.; Liu, C.; Chen, X.; Song, H. A universal strategy to prepare porous graphene films: Binder-free anodes for high-rate lithium-ion and sodium-ion batteries. J. Mater. Chem. A 2016, 4, 8837–8843. [Google Scholar] [CrossRef]
- Ahn, W.; Song, H.S.; Park, S.-H.; Kim, K.-B.; Shin, K.-H.; Lim, S.N.; Yeon, S.-H. Morphology-controlled graphene nanosheets as anode material for lithium-ion batteries. Electrochim. Acta 2014, 132, 172–179. [Google Scholar] [CrossRef]
- Shan, H.; Xiong, D.; Li, X.; Sun, Y.; Yan, B.; Li, D.; Lawes, S.; Cui, Y.; Sun, X. Tailored lithium storage performance of graphene aerogel anodes with controlled surface defects for lithium-ion batteries. Appl. Surf. Sci. 2016, 364, 651–659. [Google Scholar] [CrossRef]
- Zhang, D.; Qian, A.; Chen, J.; Wen, J.; Wang, L.; Chen, C. Electrochemical performances of nano-Co3O4 with different morphologies as anode materials for Li-ion batteries. Ionics 2012, 18, 591–597. [Google Scholar] [CrossRef]
- Zhang, B.; Zhang, Y.; Miao, Z.; Wu, T.; Zhang, Z.; Yang, X. Micro/nano-structure Co3O4 as high capacity anode materials for lithium-ion batteries and the effect of the void volume on electrochemical performance. J. Power Sources 2014, 248, 289–295. [Google Scholar] [CrossRef]
- Yan, X.; Tian, L. Three-Dimensional Crystalline/Amorphous Co/Co3O4 Core/Shell Nanosheets as Efficient Electrocatalysts for the Hydrogen Evolution Reaction. Nano Lett. 2015, 15, 6015–6021. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Yu, Y. Template-Free Synthesis of Hollow-Structured Co3O4 Nanoparticles as High-Performance Anodes for Lithium-Ion Batteries. ACS Nano 2015, 9, 1775–1781. [Google Scholar] [CrossRef]
- Zhou, X.; Shi, J. Microwave irradiation synthesis of Co3O4 quantum dots/graphene composite as anode materials for Li-ion battery. Electrochim. Acta 2014, 143, 175–179. [Google Scholar] [CrossRef]
- Wenelska, K.; Neef, C. Carbon nanotubes decorated by mesoporous cobalt oxide as electrode material for lithium-ion batteries. Chem. Phys. Lett. 2015, 635, 185–189. [Google Scholar] [CrossRef]
- Wang, H.; Liang, Y. Advanced asymmetrical supercapacitors based on graphene hybrid materials. Nano Res. 2011, 4, 729–736. [Google Scholar] [CrossRef]
- Guo, C.X.; Wang, M. A Hierarchically Nanostructured Composite of MnO2/Conjugated Polymer/Graphene for High-Performance Lithium Ion Batteries. Adv. Energy Mater. 2011, 1, 736–741. [Google Scholar] [CrossRef]
- Gong, Y.; Yang, S. Graphene-Network-Backboned Architectures for High-Performance Lithium Storage. Adv. Mater. 2013, 25, 3979–3984. [Google Scholar] [CrossRef]
- Wang, H.; Xu, Z. Hybrid Device Employing Three-Dimensional Arrays of MnO in Carbon Nanosheets Bridges Battery-Supercapacitor Divide. Nano Lett. 2014, 14, 1987–1994. [Google Scholar] [CrossRef]
- Hou, X.; Jiang, H. In Situ Deposition of Hierarchical Architecture Assembly from Sn-Filled CNTs for Lithium-Ion Batteries. ACS Appl. Mater. Interfaces 2013, 5, 6672–6677. [Google Scholar] [CrossRef]
- Lee, S.-H.; Sridhar, V. Graphene-Nanotube-Iron Hierarchical Nanostructure as Lithium Ion Battery Anode. ACS Nano 2013, 7, 4242–4251. [Google Scholar] [CrossRef]
- Yan, C.; Chen, G. Template-Based Engineering of Carbon-Doped Co3O4 Hollow Nanofibers as Anode Materials for Lithium-Ion Batteries. Adv. Funct. Mater. 2016, 26, 1428–1436. [Google Scholar] [CrossRef]
- Wang, H.; Mao, N. Cobalt Oxide-Carbon Nanosheet Nanoarchitecture as an Anode for High-Performance Lithium-Ion Battery. ACS Appl. Mater. Interfaces 2015, 7, 2882–2890. [Google Scholar] [CrossRef]
- Wang, Y.; Yan, F. Onion-like carbon matrix supported Co3O4 nanocomposites: A highly reversible anode material for lithium ion batteries with excellent cycling stability. J. Mater. Chem. A 2013, 1, 5212–5216. [Google Scholar] [CrossRef]
- Wang, G.; Zhu, F. Preparation of Co3O4/Carbon Derived from Ionic Liquid and Its Application in Lithium-ion Batteries. Electrochim. Acta 2017, 257, 138–145. [Google Scholar] [CrossRef]
- Han, Y.; Dong, L. Cobalt oxide modified porous carbon anode enhancing electrochemical performance for Li-ion batteries. Electrochim. Acta 2015, 167, 246–253. [Google Scholar] [CrossRef]
- Qu, G.; Geng, H. Porous carbon-wrapped mesoporous Co9S8 fibers as stable anode for Li-Ion Batteries. Electrochim. Acta 2016, 211, 305–312. [Google Scholar] [CrossRef]
- Huang, J.; Tang, X.; Liu, K.; Li, Z. Synthesis of porous carbon-coated polycrystalline Co9S8 for long-term lithium ion battery. Mater. Lett. 2018, 210, 88–91. [Google Scholar] [CrossRef]
- Shi, M.; Wang, Q. MOF-derived hollow Co4S3/C nanosheet arrays grown on carbon cloth as the anode for high-performance Li-ion batteries. Dalton Trans. 2020, 49, 14115–14122. [Google Scholar] [CrossRef]
- Wang, L.; Deng, F. Self-confined synthesis of CoS1-x nanoparticles embedded in carbon nanosheets for lithium-ion batteries. J. Alloy. Compd. 2021, 885, 160947. [Google Scholar] [CrossRef]
- Wu, R. In-situ formation of hollow hybrids composed of cobalt sulfides embedded within porous carbon polyhedra/carbon nanotubes for high-performance lithium-ion batteries. Adv. Mater. 2015, 27, 3038–3044. [Google Scholar] [CrossRef]
- Zhao, F. Ultrathin mesoporous Co3O4 nanosheets-constructed hierarchical clusters as high rate capability and long life anode materials for lithium-ion batteries. Appl. Surf. Sci. 2017, 406, 46–55. [Google Scholar]
- Yin, X.; Wang, Z.; Wang, J.; Yan, G.; Xiong, X.; Li, X.; Guo, H. One-step facile synthesis of porous Co3O4 microspheres as anode materials for lithium-ion batteries. Mater. Lett. 2014, 120, 73–75. [Google Scholar] [CrossRef]
- Tian, D.; Zhou, X.L.; Zhang, Y.H.; Zhou, Z.; Bu, X.H. MOF-Derived Porous Co3O4 Hollow Tetrahedra with Excellent Performance as Anode Materials for Lithium-Ion Batteries. Inorg. Chem. 2015, 54, 8159–8161. [Google Scholar] [CrossRef]
- Jin, Y.; Wang, L. Facile synthesis of monodisperse Co3O4 mesoporous microdisks as an anode material for lithium ion batteries. Electrochim. Acta 2015, 151, 109–117. [Google Scholar] [CrossRef]
- Guo, J.; Chen, L.; Zhang, X.; Chen, H. ChemInform Abstract: Porous Co3O4 Nanorods as Anode for Lithium-Ion Battery with Excellent Electrochemical Performance. J. Solid State Chem. 2014, 45, 193–197. [Google Scholar] [CrossRef]
- Wang, Q.; Jiao, L. CoS2 Hollow Spheres: Fabrication and Their Application in Lithium-Ion Batteries. J. Phys. Chem. C 2011, 115, 8300–8304. [Google Scholar] [CrossRef]
- Sennu, P.; Christy, M.; Aravindan, V.; Lee, Y.G.; Nahm, K.S.; Lee, Y.S. Two-Dimensional Mesoporous Cobalt Sulfide Nanosheets as a Superior Anode for a Li-Ion Battery and a Bifunctional Electrocatalyst for the Li–O2 System. Chem. Mater. 2015, 27, 5726–5735. [Google Scholar] [CrossRef]
- Song, R.; Dong, Y.; Zhang, D.; Sheng, J.; Yang, S.; Song, H. Nitrogen-Doped Porous Carbon Nanosheets with Ultrahigh Capacity and Quasicapacitive Energy Storage Performance for Lithium and Sodium Storage Applications. Energy Technol. 2021, 9, 2100309. [Google Scholar] [CrossRef]
- Shi, L.; Chen, Y.; Song, H.; Li, A.; Chen, X.; Zhou, J.; Ma, Z. Preparation and Lithium-Storage Performance of a Novel Hierarchical Porous Carbon from Sucrose Using Mg-Al Layered Double Hydroxides as Template. Electrochim. Acta 2017, 231, 153–161. [Google Scholar] [CrossRef]
- Xie, Q.; Zhang, Y.; Xie, D.; Zhao, P. EDTA-Fe(III) sodium complex–derived bubble-like nitrogen-enriched highly graphitic carbon nanospheres as anodes with high specific capacity for lithium-ion batteries. Ionics 2020, 26, 85–94. [Google Scholar] [CrossRef]
- Li, X.; Geng, D.; Zhang, Y.; Meng, X.; Li, R.; Sun, X. Superior cycle stability of nitrogen-doped graphene nanosheets as anodes for lithium ion batteries. Electrochem. Commun. 2011, 13, 822–825. [Google Scholar] [CrossRef]
- Gu, D.; Li, W.; Wang, F.; Bongard, H.; Spliethoff, B.; Schmidt, W.; Weidenthaler, C.; Xia, Y.; Zhao, D.; Schüth, F. Controllable Synthesis of Mesoporous Peapod-like Co3O4@Carbon Nanotube Arrays for High-Performance Lithium-Ion Batteries. Angew. Chem. Int. Ed. 2015, 54, 7060–7064. [Google Scholar] [CrossRef]
- Sun, L.; Deng, Q.; Li, Y.; Mi, H.; Wang, S.; Deng, L.; Ren, X.; Zhang, P. CoO-Co3O4 heterostructure nanoribbon/RGO sandwich-like composites as anode materials for high performance lithium-ion batteries. Electrochim. Acta 2017, 241, 252–260. [Google Scholar] [CrossRef]
- Lu, H.; Qian, R.; Yao, T.; Li, C.; Li, L.; Wang, H. Synthesis of Spherical Carbon-Coated CoP Nanoparticles for High-Performance Lithium-Ion Batteries. Energy Technol. 2021, 9, 2100605. [Google Scholar] [CrossRef]
- Zhang, X.; Zhao, Y.; Zhang, C.; Wu, C.; Li, X.; Jin, M.; Lan, W.; Pan, X.; Zhou, J.; Xie, E. Cobalt sulfide embedded carbon nanofibers as a self-supporting template to improve lithium ion battery performances. Electrochim. Acta 2021, 366, 137351. [Google Scholar] [CrossRef]














| Types of Materials | Cycle Number | Current Density | Reversible Capacity (mA h g−1) | Structure | Reference |
|---|---|---|---|---|---|
| Nanoporous carbon microspheres | 500 | 200 mA g−1 | 650 | double-carbon-shell | [130] |
| NCMs | 50 | 210 mA g−1 | 926.654 | porous spherical | [121] |
| AMC | 100 | 1860 mA g−1 | 280 | hollow microsphere | [131] |
| GNSs | 30 | 0.2 mA cm−2 | 502 | disordered graphene layers | [109] |
| N-GNSs | 100 | 100 mA g−1 | 760 | porous architecture | [139] |
| Porous graphene film | 50 | 50 mA g−1 | 195 | porous structure | [140] |
| 3DMGS | 170 | 100 mA g−1 | 1513.2 | multilayer nanostructures | [111] |
| CNTs | 30 | 0.2 mA cm−2 | 266 | single-walled | [96] |
| VA-CNTs | 50 | 100 mA g−1 | 350 | multi-walled | [97] |
| Types of Materials | Current Density (A g−1) | Structure | Cycle Number | Reversible Capacity (mA h g−1) | Reference |
|---|---|---|---|---|---|
| Co3O4 | 1.8 (2C) | porous disk-like | 100 | 304 | [165] |
| Ultrathin mesoporous Co3O4 nanosheet | 2 | irregular small blocks | 50 | 479 | [166] |
| Co3O4 | 0.4 | porous microspheres | 40 | 654 | [167] |
| Co3O4 | 0.8 | mesoporous microstructure | 50 | 260 | [168] |
| Co3O4 | 2 | porous nanorod | 25 | 516 | [169] |
| Co3O4 | 2 | nanocages | 25 | 252 | [38] |
| Co3O4 microspheres | 0.1 | hollow and porous | 520 | 550 | [40] |
| Co3O4 Nanosheet Arrays | 0.1 | mesoporous | 80 | 1576.9 | [41] |
| CoS2 | 0.1 | hollow spheres | 40 | 320 | [170] |
| 2D Co3S4 | 0.7 | nano thickness sheet-like | 400 | 416 | [171] |
| MnCo2O4 | 0.4 | core-shell ellipsoidal | 150 | 620.0 | [53] |
| MnCo2O4 | 0.1 | nano sheet | 50 | 681 | [54] |
| MnCo2O4 | 1 | hierarchical porous | 1000 | 740 | [53] |
| CoMn2O4 | 1 | microspheres | 1000 | 420 | [57] |
| MnCo2O4 | 0.1 | porous hydrangea-like | 100 | 930 | [58] |
| Co9S8 | 2 | hollow nanospheres of mesoporous | 800 | 896 | [73] |
| Nitrogen-Doped Carbon Nanosheets | 1 | porous nanosheets | 1000 | 222 | [172] |
| Carbon | 0.05 | hierarchical porous | 50 | 877.9 | [173] |
| Porous graphene film | 10 | porous structure | 10,000 | 971 | [140] |
| 3D multilayer-graphene | 5 | cloud-like | 1100 | 260.3 | [111] |
| N-doped carbon nanospheres | 0.1 | nanospheres | 150 | 505 | [174] |
| N-doped graphene | 0.1 | nanosheets | 100 | 452 | [175] |
| Hierarchical porous Carbon microspheres | 1 | hierarchical porous microspheres | 70 | 200 | [127] |
| C-Co9S8 | 2 | hierarchical porous | 400 | 476 | [161] |
| MnCo2O4/C | 0.05 | quasi-hollow microspheres | 50 | 488 | [56] |
| Co3O4/APC (Activated porous carbon) | 0.5 | lotus root-like and layered porous | 200 | 625 | [20] |
| Co/CPC (CO2-derived porous carbon) | 1 | porous | 300 | 1179 | [19] |
| Co3O4@CNT | 0.01 | mesoporous peapod-like | 100 | 700 | [176] |
| Co3O4@Graphene core-shell | 1 | mesoporous hollow spheres | 200 | 700 | [18] |
| CoO-Co3O4-RGO | 0.1 | sandwich-like | 200 | 994 | [177] |
| Carbon@cobaltous oxide | 0.2 | nanofiber | 100 | 892 | [17] |
| CoP@C | 0.5 | uniform spherical morphology | 1000 | 483.4 | [178] |
| Co1-XS-CNFs | 2 | onion-like carbonaceous | 500 | 252 | [179] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, H.; Wang, W.; Yang, L.; Dong, L.; Wang, D.; Xu, X.; Wang, D.; Huang, J.; Lv, M.; Wang, H. A Review of Cobalt-Containing Nanomaterials, Carbon Nanomaterials and Their Composites in Preparation Methods and Application. Nanomaterials 2022, 12, 2042. https://doi.org/10.3390/nano12122042
Chen H, Wang W, Yang L, Dong L, Wang D, Xu X, Wang D, Huang J, Lv M, Wang H. A Review of Cobalt-Containing Nanomaterials, Carbon Nanomaterials and Their Composites in Preparation Methods and Application. Nanomaterials. 2022; 12(12):2042. https://doi.org/10.3390/nano12122042
Chicago/Turabian StyleChen, Hongfeng, Wei Wang, Lin Yang, Liang Dong, Dechen Wang, Xinkai Xu, Dijia Wang, Jingchun Huang, Mengge Lv, and Haiwang Wang. 2022. "A Review of Cobalt-Containing Nanomaterials, Carbon Nanomaterials and Their Composites in Preparation Methods and Application" Nanomaterials 12, no. 12: 2042. https://doi.org/10.3390/nano12122042
APA StyleChen, H., Wang, W., Yang, L., Dong, L., Wang, D., Xu, X., Wang, D., Huang, J., Lv, M., & Wang, H. (2022). A Review of Cobalt-Containing Nanomaterials, Carbon Nanomaterials and Their Composites in Preparation Methods and Application. Nanomaterials, 12(12), 2042. https://doi.org/10.3390/nano12122042






