Influence of Critical Parameters on Cytotoxicity Induced by Mesoporous Silica Nanoparticles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Criteria for Paper Selection
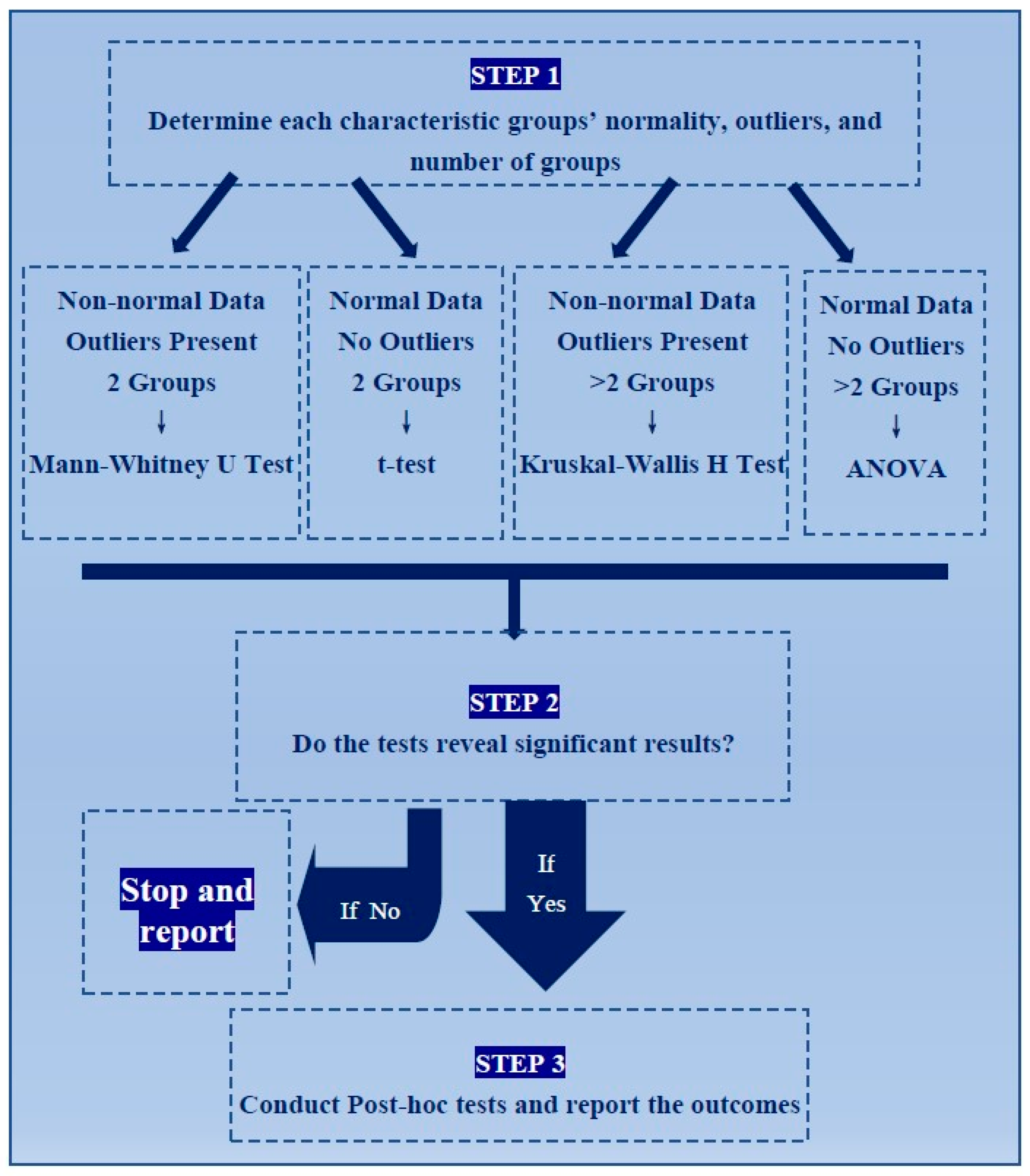
2.2. Statistical Tests
3. Results
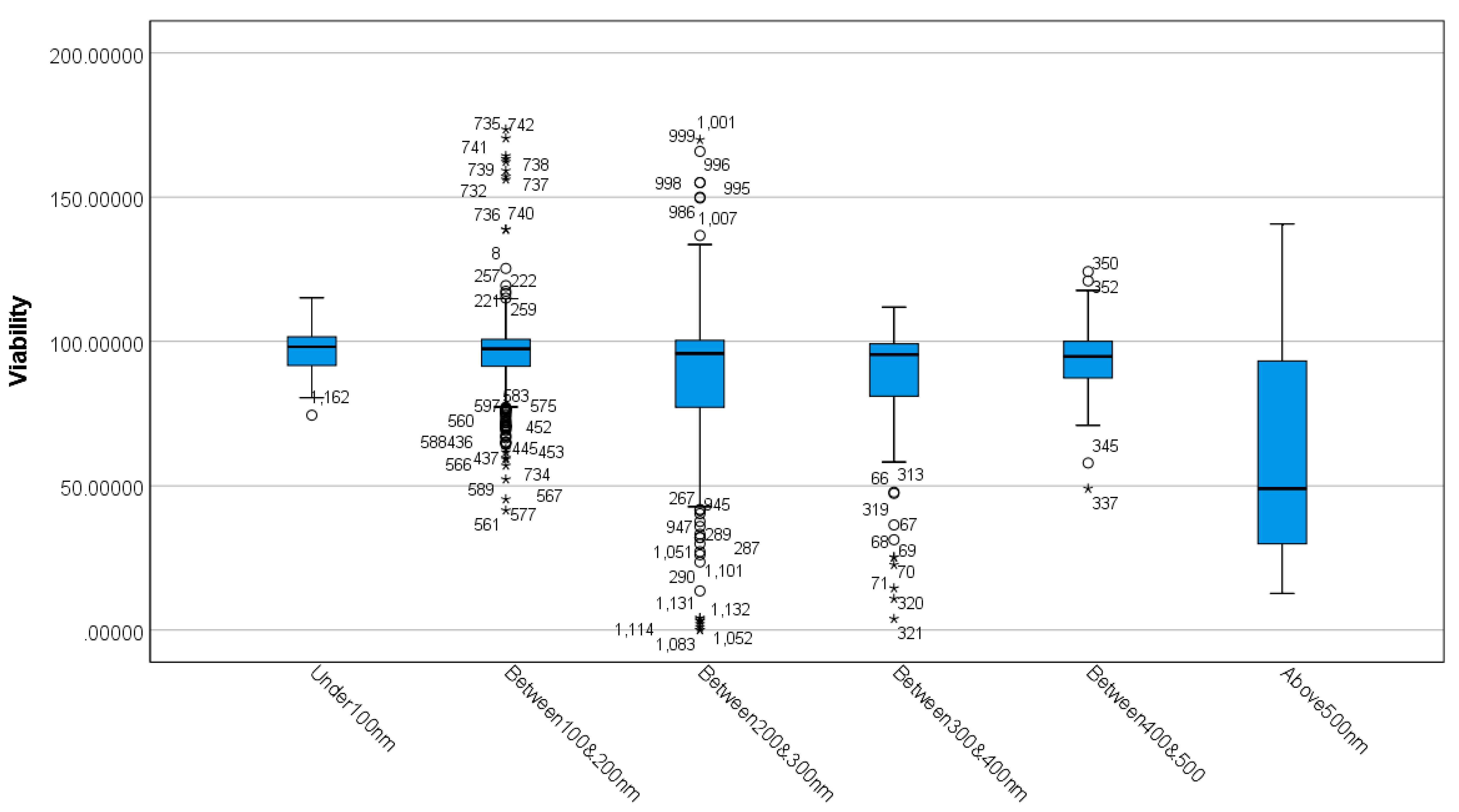
3.1. Differences between MSN Sizes Based on Viability
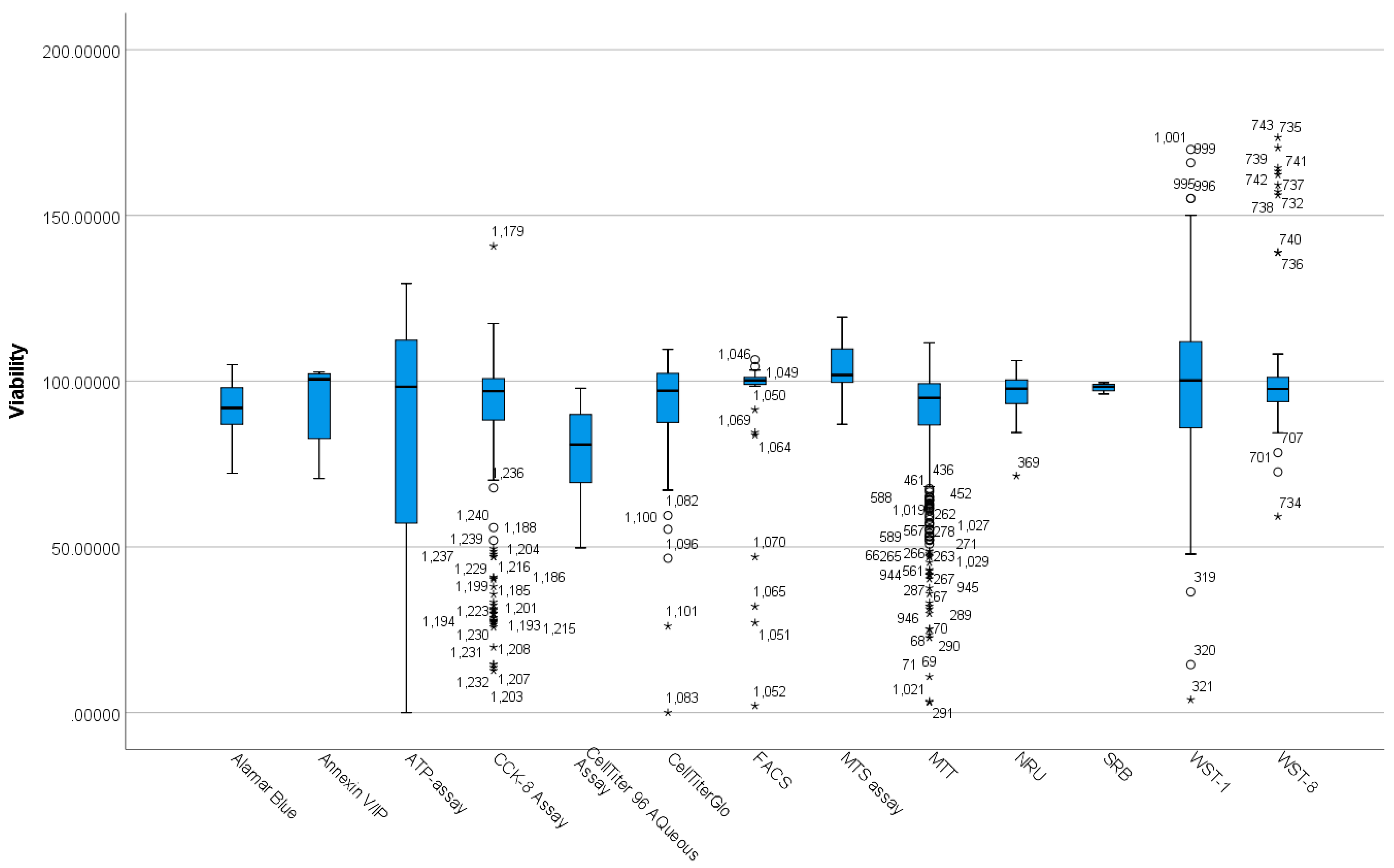
3.2. Differences between Viability Assay Methods Based on Viability
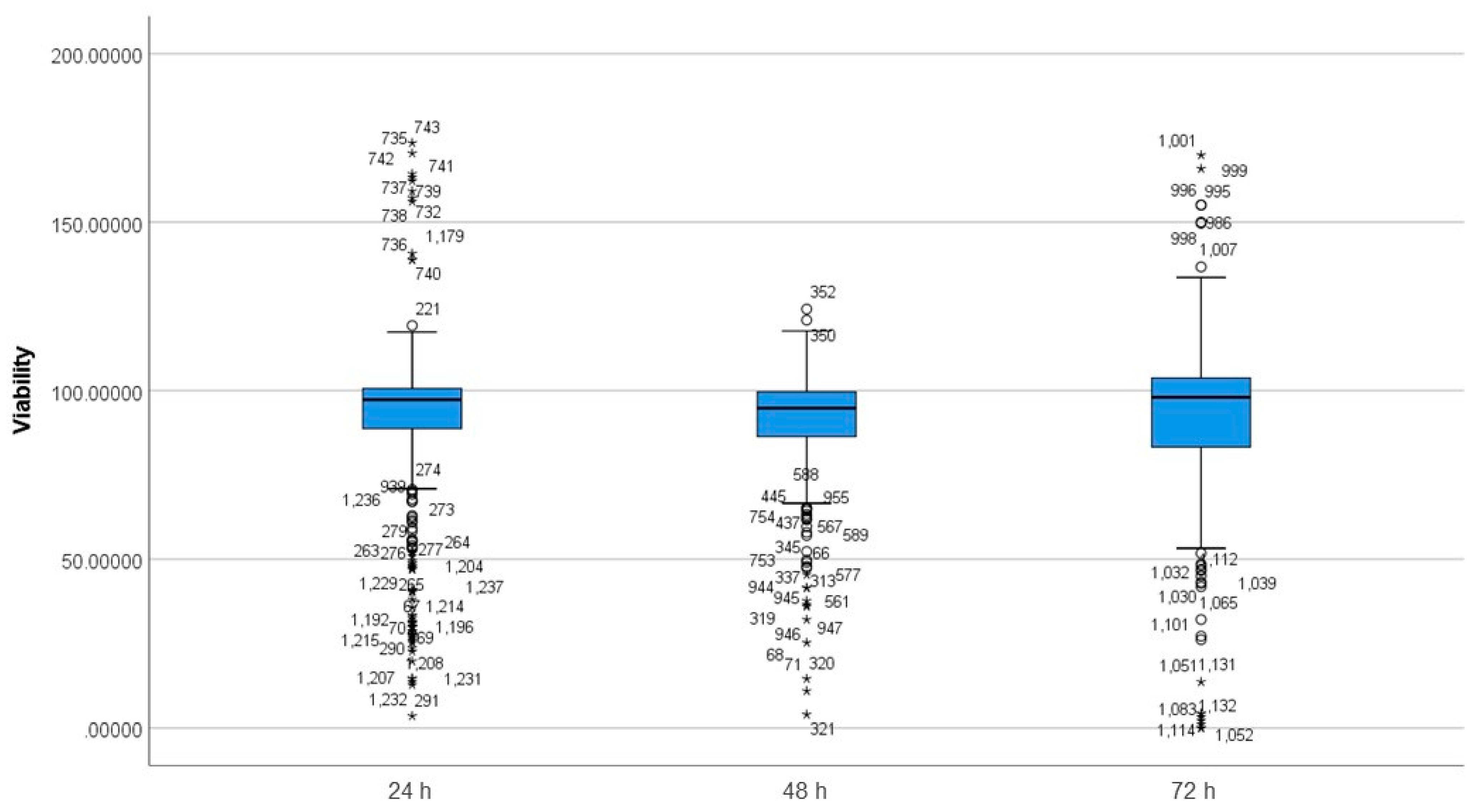
3.3. Differences between Incubation Time Groups on Viability
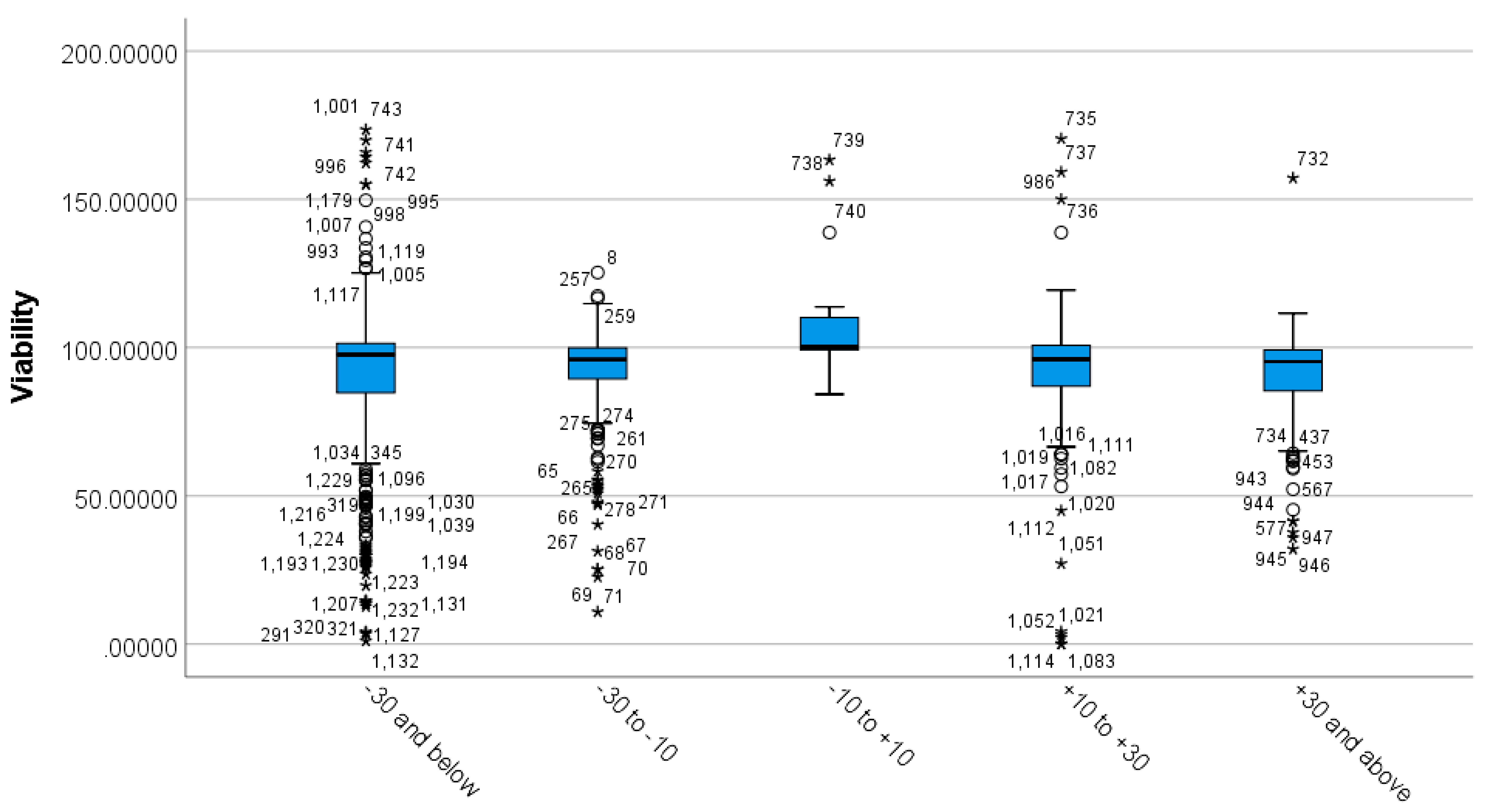
3.4. Differences between MSNs’ Zeta Potential Based on Viability
3.5. Differences in Functionalisation Based on Viability
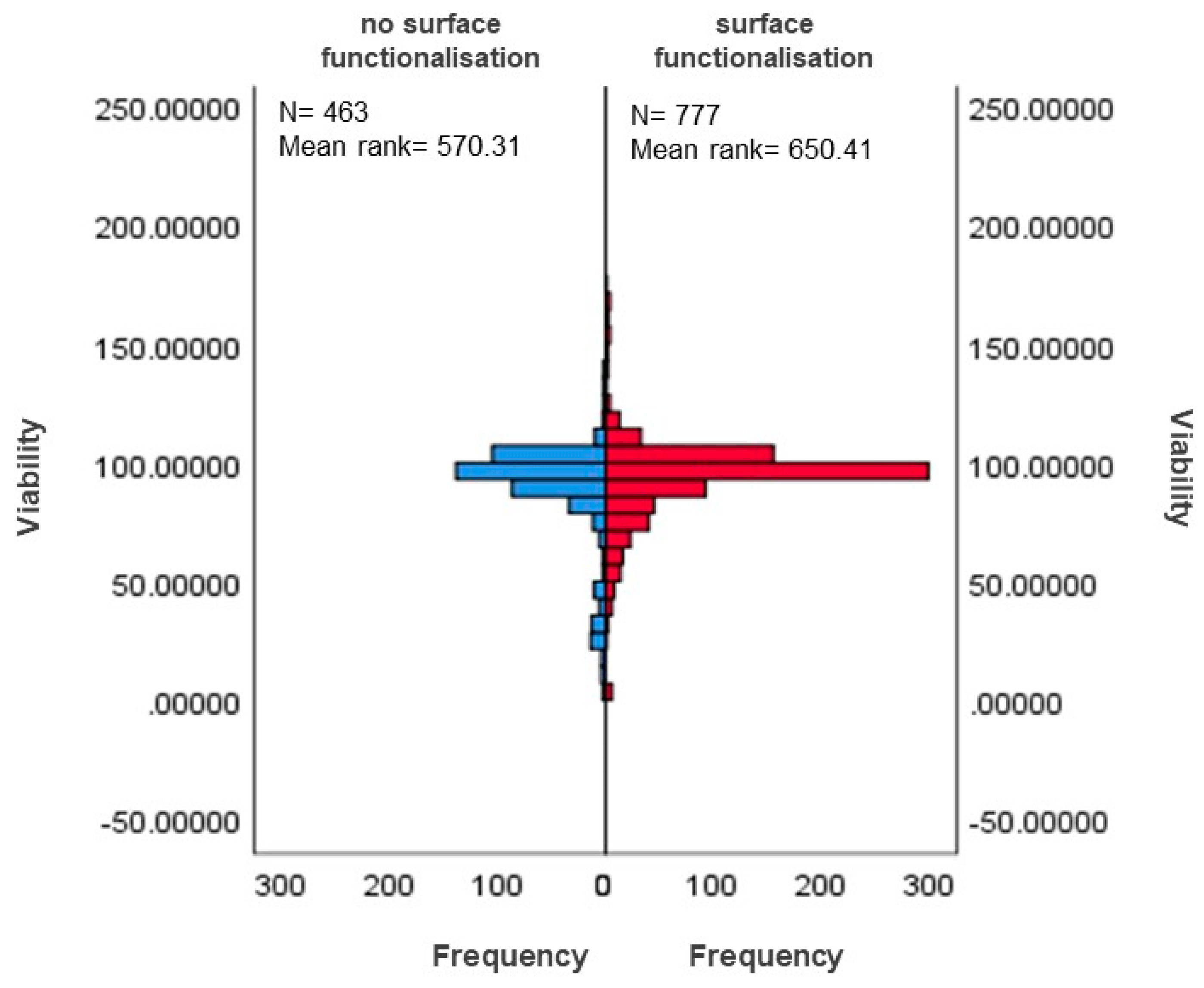
3.5.1. Differences in Surface-Functionalised and Non-Functionalised MSNs Based on Viability
3.5.2. Differences between Functionalisation Groups Based on Viability
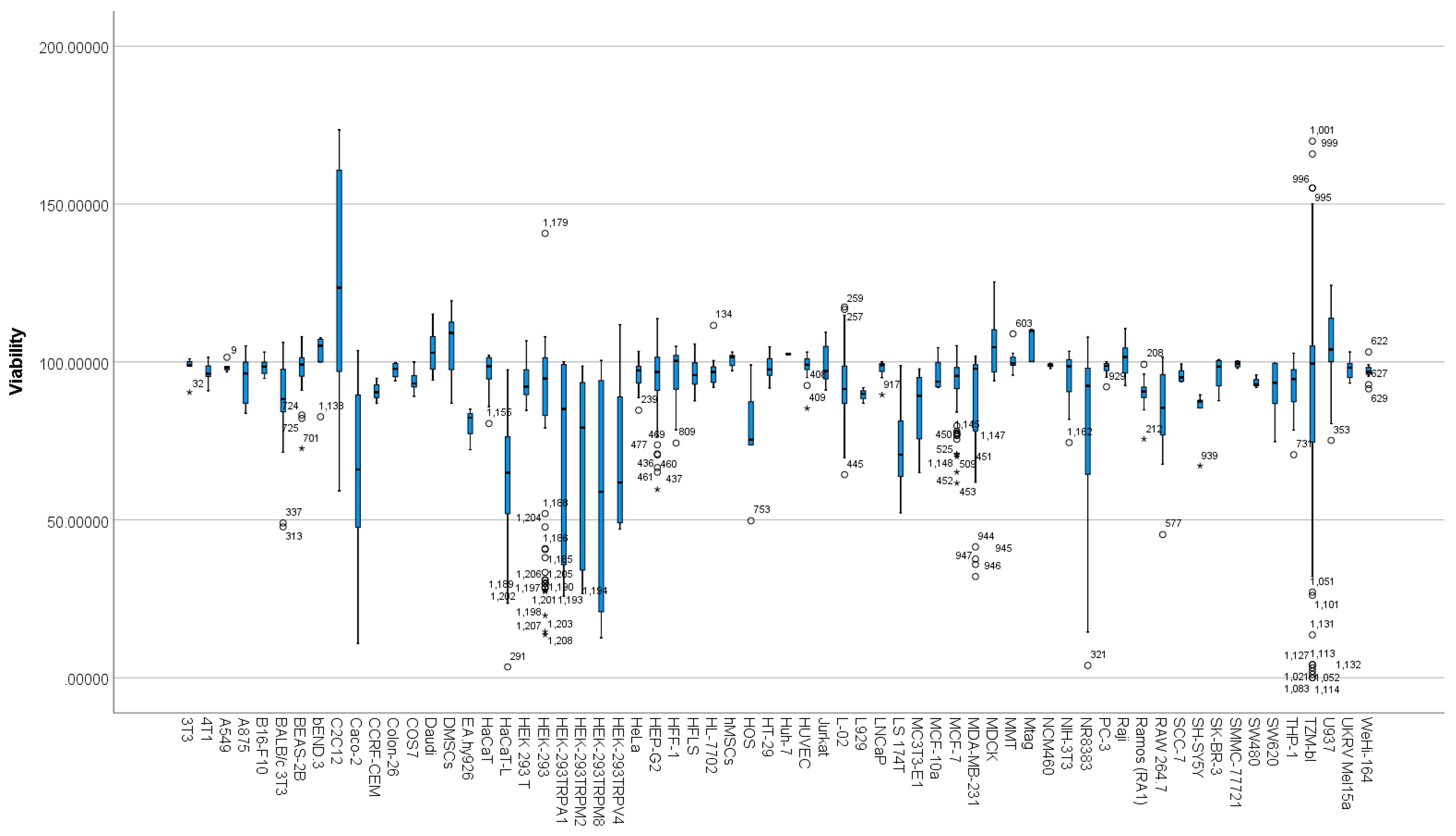
3.6. Differences between Cell Types Based on Viability
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Jin, R.; Liu, Z.; Bai, Y.; Zhou, Y.; Chen, X. Multiple-Responsive Mesoporous Silica Nanoparticles for Highly Accurate Drugs Delivery to Tumor Cells. ACS Omega 2018, 3, 4306–4315. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.-G.; Jiang, Q.; Zhang, X.-P.; Shan, K.; Liu, B.-H.; Zhao, C.; Yan, B. Mesoporous Silica Nanoparticles as a Delivery System for Improving Antiangiogenic Therapy. Int. J. Nanomed. 2019, 14, 1489–1501. [Google Scholar] [CrossRef] [PubMed]
- Wan, X.; Zhang, X.; Pan, W.; Liu, B.; Yu, L.; Wang, H.; Li, N.; Tang, B. Ratiometric Fluorescent Quantification of the Size-Dependent Cellular Toxicity of Silica Nanoparticles. Anal. Chem. 2019, 91, 6088–6096. [Google Scholar] [CrossRef] [PubMed]
- Tzankova, V.; Aluani, D.; Yordanov, Y.; Valoti, M.; Frosini, M.; Spassova, I.; Kovacheva, D.; Tzankov, B. In Vitro Toxicity Evaluation of Lomefloxacin-Loaded MCM-41 Mesoporous Silica Nanoparticles. Drug Chem. Toxicol. 2019, 44, 238–249. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Li, N.; Yin, H.; Chen, T.; Yang, Q.; Wu, M. Thermo- and PH-Responsive, Lipid-Coated, Mesoporous Silica Nanoparticle-Based Dual Drug Delivery System To Improve the Antitumor Effect of Hydrophobic Drugs. Mol. Pharm. 2019, 16, 422–436. [Google Scholar] [CrossRef]
- Marcelo, G.; Ariana-Machado, J.; Enea, M.; Carmo, H.; Rodríguez-González, B.; Luis Capelo, J.; Lodeiro, C.; Oliveira, E. Toxicological Evaluation of Luminescent Silica Nanoparticles as New Drug Nanocarriers in Different Cancer Cell Lines. Materials 2018, 11, 1310. [Google Scholar] [CrossRef]
- Baghirov, H.; Karaman, D.; Viitala, T.; Duchanoy, A.; Lou, Y.-R.; Mamaeva, V.; Pryazhnikov, E.; Khiroug, L.; de Lange Davies, C.; Sahlgren, C.; et al. Feasibility Study of the Permeability and Uptake of Mesoporous Silica Nanoparticles across the Blood-Brain Barrier. PLoS ONE 2016, 11, e0160705. [Google Scholar] [CrossRef]
- Thi, T.T.H.; Cao, V.D.; Nguyen, T.N.Q.; Hoang, D.T.; Ngo, V.C.; Nguyen, D.H. Functionalized Mesoporous Silica Nanoparticles and Biomedical Applications. Mater. Sci. Eng. C 2019, 99, 631–656. [Google Scholar] [CrossRef]
- Niculescu, V.-C. Mesoporous Silica Nanoparticles for Bio-Applications. Front. Mater. 2020, 7, 36. [Google Scholar] [CrossRef]
- Hackley, V.A.; Clogston, J.D. Measuring the Hydrodynamic Size of Nanoparticles in Aqueous Media Using Batch-Mode Dynamic Light Scattering. In Characterization of Nanoparticles Intended for Drug Delivery; McNeil, S.E., Ed.; Methods in Molecular Biology; Humana Press: Totowa, NJ, USA, 2011; Volume 697, pp. 35–52. ISBN 978-1-60327-197-4. [Google Scholar]
- McNeil, S.E. Challenges for Nanoparticle Characterization. In Characterization of Nanoparticles Intended for Drug Delivery; McNeil, S.E., Ed.; Methods in Molecular Biology; Humana Press: Totowa, NJ, USA, 2011; Volume 697, pp. 9–15. ISBN 978-1-60327-197-4. [Google Scholar]
- Clogston, J.D.; Patri, A.K. Zeta Potential Measurement. In Characterization of Nanoparticles Intended for Drug Delivery; McNeil, S.E., Ed.; Methods in Molecular Biology; Humana Press: Totowa, NJ, USA, 2011; Volume 697, pp. 63–70. ISBN 978-1-60327-197-4. [Google Scholar]
- Manaia, E.B.; Abuçafy, M.P.; Chiari-Andréo, B.G.; Silva, B.L.; Oshiro-Júnior, J.A.; Chiavacci, L.A. Physicochemical Characterization of Drug Nanocarriers. Int. J. Nanomed. 2017, 12, 4991–5011. [Google Scholar] [CrossRef]
- Chauhan, S.; Manivasagam, G.; Kumar, P.; Ambasta, R.K. Cellular Toxicity of Mesoporous Silica Nanoparticle in SHSY5Y and BMMNCs Cell. Pharm. Nanotechnol. 2019, 6, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Mohammadpour, R.; Yazdimamaghani, M.; Reilly, C.A.; Ghandehari, H. Transient Receptor Potential Ion Channel–Dependent Toxicity of Silica Nanoparticles and Poly(Amido Amine) Dendrimers. J. Pharmacol. Exp. Ther. 2019, 370, 751–760. [Google Scholar] [CrossRef] [PubMed]
- Laerd Statistics One-Way ANOVA in SPSS Statistics|Laerd Statistics Premium. Available online: https://statistics.laerd.com/premium/spss/owa/one-way-anova-in-spss-28.php (accessed on 28 September 2020).
- Hecke, T.V. Power Study of Anova versus Kruskal-Wallis Test. J. Stat. Manag. Syst. 2012, 15, 241–247. [Google Scholar] [CrossRef]
- Laerd Statistics Independent-Samples t-Test in SPSS Statistics|Laerd Statistics Premium. Available online: https://statistics.laerd.com/premium/spss/istt/independent-t-test-in-spss-24.php (accessed on 20 September 2020).
- Wright, L.; Joyce, P.; Barnes, T.J.; Lundmark, R.; Bergström, C.A.S.; Hubert, M.; Prestidge, C.A. A Comparison of Chitosan, Mesoporous Silica and Poly(Lactic-Co-Glycolic) Acid Nanocarriers for Optimising Intestinal Uptake of Oral Protein Therapeutics. J. Pharm. Sci. 2021, 110, 217–227. [Google Scholar] [CrossRef]
- Zhao, F.; Zhang, C.; Zhao, C.; Gao, W.; Fan, X.; Wu, G. A Facile Strategy to Fabricate a PH-Responsive Mesoporous Silica Nanoparticle End-Capped with Amphiphilic Peptides by Self-Assembly. Colloids Surf. B Biointerfaces 2019, 179, 352–362. [Google Scholar] [CrossRef]
- Wu, D.; Shi, X.; Zhao, F.; Chilengue, S.T.F.; Deng, L.; Dong, A.; Kong, D.; Wang, W.; Zhang, J. An Injectable and Tumor-Specific Responsive Hydrogel with Tissue-Adhesive and Nanomedicine-Releasing Abilities for Precise Locoregional Chemotherapy. Acta Biomater. 2019, 96, 123–136. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; You, Y.; Li, X.; Liu, L.; Guo, F.; Zhang, Q.; Liu, D.; Tong, Y.; Ding, S.; Wang, J. Preparation of RGD Peptide/Folate Acid Double-Targeted Mesoporous Silica Nanoparticles and Its Application in Human Breast Cancer MCF-7 Cells. Front. Pharmacol. 2020, 11, 898. [Google Scholar] [CrossRef]
- Kundu, M.; Chatterjee, S.; Ghosh, N.; Manna, P.; Das, J.; Sil, P.C. Tumor Targeted Delivery of Umbelliferone via a Smart Mesoporous Silica Nanoparticles Controlled-Release Drug Delivery System for Increased Anticancer Efficiency. Mater. Sci. Eng. C 2020, 116, 111239. [Google Scholar] [CrossRef]
- Deng, Y.-D.; Zhang, X.-D.; Yang, X.-S.; Huang, Z.-L.; Wei, X.; Yang, X.-F.; Liao, W.-Z. Subacute Toxicity of Mesoporous Silica Nanoparticles to the Intestinal Tract and the Underlying Mechanism. J. Hazard. Mater. 2021, 409, 124502. [Google Scholar] [CrossRef]
- Kim, M.-K.; Ki, D.-H.; Na, Y.-G.; Lee, H.-S.; Baek, J.-S.; Lee, J.-Y.; Lee, H.-K.; Cho, C.-W. Optimization of Mesoporous Silica Nanoparticles through Statistical Design of Experiment and the Application for the Anticancer Drug. Pharmaceutics 2021, 13, 184. [Google Scholar] [CrossRef]
- Noghreiyan, A.V.; Sazegar, M.R.; Shaegh, S.A.M.; Sazgarnia, A. Investigation of the Emission Spectra and Cytotoxicity of TiO2 and Ti-MSN/PpIX Nanoparticles to Induce Photodynamic Effects Using X-Ray. Photodiagnosis Photodyn. Ther. 2020, 30, 101770. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, X.; Wang, X.; Wang, T.; Bai, B.; Zhang, N.; Zhao, Y.; Yu, Y.; Wang, B. Efficient Delivery of Triptolide Plus a MiR-30-5p Inhibitor Through the Use of Near Infrared Laser Responsive or CADY Modified MSNs for Efficacy in Rheumatoid Arthritis Therapeutics. Front. Bioeng. Biotechnol. 2020, 8, 170. [Google Scholar] [CrossRef]
- Ali, O.M.; Bekhit, A.A.; Khattab, S.N.; Helmy, M.W.; Abdel-Ghany, Y.S.; Teleb, M.; Elzoghby, A.O. Synthesis of Lactoferrin Mesoporous Silica Nanoparticles for Pemetrexed/Ellagic Acid Synergistic Breast Cancer Therapy. Colloids Surf. B Biointerfaces 2020, 188, 110824. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, Y.; Liu, L.; Cui, Z.; Liu, X.; Wang, L.; Li, Y.; Li, Q. Combined Chemo/Photothermal Therapy Based on Mesoporous Silica-Au Core-Shell Nanoparticles for Hepatocellular Carcinoma Treatment. Drug Dev. Ind. Pharm. 2019, 45, 1487–1495. [Google Scholar] [CrossRef] [PubMed]
- Khatoon, S.; Han, H.; Jeon, J.; Rao, N.; Jeong, D.-W.; Ikram, M.; Yasin, T.; Yi, G.-R.; Park, J. Hypoxia-Responsive Mesoporous Nanoparticles for Doxorubicin Delivery. Polymers 2018, 10, 390. [Google Scholar] [CrossRef]
- Maksimović-Ivanić, D.; Bulatović, M.; Edeler, D.; Bensing, C.; Golić, I.; Korać, A.; Kaluđerović, G.N.; Mijatović, S. The Interaction between SBA-15 Derivative Loaded with Ph3Sn(CH2)6OH and Human Melanoma A375 Cell Line: Uptake and Stem Phenotype Loss. JBIC J. Biol. Inorg. Chem. 2019, 24, 223–234. [Google Scholar] [CrossRef]
- Yang, Y.; Zhao, W.; Tan, W.; Lai, Z.; Fang, D.; Jiang, L.; Zuo, C.; Yang, N.; Lai, Y. An Efficient Cell-Targeting Drug Delivery System Based on Aptamer-Modified Mesoporous Silica Nanoparticles. Nanoscale Res. Lett. 2019, 14, 390. [Google Scholar] [CrossRef]
- Xie, X.; Li, F.; Zhang, H.; Lu, Y.; Lian, S.; Lin, H.; Gao, Y.; Jia, L. EpCAM Aptamer-Functionalized Mesoporous Silica Nanoparticles for Efficient Colon Cancer Cell-Targeted Drug Delivery. Eur. J. Pharm. Sci. 2016, 83, 28–35. [Google Scholar] [CrossRef]
- Paris, J.L.; de la Torre, P.; Manzano, M.; Cabañas, M.V.; Flores, A.I.; Vallet-Regí, M. Decidua-Derived Mesenchymal Stem Cells as Carriers of Mesoporous Silica Nanoparticles. In Vitro and in Vivo Evaluation on Mammary Tumors. Acta Biomater. 2016, 33, 275–282. [Google Scholar] [CrossRef]
- Guo, C.; Hu, J.; Bains, A.; Pan, D.; Luo, K.; Li, N.; Gu, Z. The Potential of Peptide Dendron Functionalized and Gadolinium Loaded Mesoporous Silica Nanoparticles as Magnetic Resonance Imaging Contrast Agents. J. Mater. Chem. B 2016, 4, 2322–2331. [Google Scholar] [CrossRef]
- Elle, R.E.; Rahmani, S.; Lauret, C.; Morena, M.; Bidel, L.P.R.; Boulahtouf, A.; Balaguer, P.; Cristol, J.-P.; Durand, J.-O.; Charnay, C.; et al. Functionalized Mesoporous Silica Nanoparticle with Antioxidants as a New Carrier That Generates Lower Oxidative Stress Impact on Cells. Mol. Pharm. 2016, 13, 2647–2660. [Google Scholar] [CrossRef] [PubMed]
- You, Y.; Yang, L.; He, L.; Chen, T. Tailored Mesoporous Silica Nanosystem with Enhanced Permeability of the Blood–Brain Barrier to Antagonize Glioblastoma. J. Mater. Chem. B 2016, 4, 5980–5990. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Xu, C.; Wen, L.; Han, M.K.; Xiao, B.; Zhou, J.; Zhang, Y.; Zhang, Z.; Viennois, E.; Merlin, D. A Hyaluronidase-Responsive Nanoparticle-Based Drug Delivery System for Targeting Colon Cancer Cells. Cancer Res. 2016, 76, 7208–7218. [Google Scholar] [CrossRef] [PubMed]
- Mannerström, M.; Zou, J.; Toimela, T.; Pyykkö, I.; Heinonen, T. The Applicability of Conventional Cytotoxicity Assays to Predict Safety/Toxicity of Mesoporous Silica Nanoparticles, Silver and Gold Nanoparticles and Multi-Walled Carbon Nanotubes. Toxicol. Vitr. 2016, 37, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Chen, X.; Liu, Y.; Fan, L.; Lin, L.; Xu, Y.; Chen, S.; Shao, J. PH-Sensitive Mesoporous Silica Nanoparticles Anticancer Prodrugs for Sustained Release of Ursolic Acid and the Enhanced Anti-Cancer Efficacy for Hepatocellular Carcinoma Cancer. Eur. J. Pharm. Sci. 2017, 96, 456–463. [Google Scholar] [CrossRef]
- Ferrauto, G.; Carniato, F.; Di Gregorio, E.; Tei, L.; Botta, M.; Aime, S. Large Photoacoustic Effect Enhancement for ICG Confined inside MCM-41 Mesoporous Silica Nanoparticles. Nanoscale 2017, 9, 99–103. [Google Scholar] [CrossRef]
- Chen, L.; Zhou, X.; Nie, W.; Zhang, Q.; Wang, W.; Zhang, Y.; He, C. Multifunctional Redox-Responsive Mesoporous Silica Nanoparticles for Efficient Targeting Drug Delivery and Magnetic Resonance Imaging. ACS Appl. Mater. Interfaces 2016, 8, 33829–33841. [Google Scholar] [CrossRef]
- Cheng, Y.-J.; Zhang, A.-Q.; Hu, J.-J.; He, F.; Zeng, X.; Zhang, X.-Z. Multifunctional Peptide-Amphiphile End-Capped Mesoporous Silica Nanoparticles for Tumor Targeting Drug Delivery. ACS Appl. Mater. Interfaces 2017, 9, 2093–2103. [Google Scholar] [CrossRef]
- Fei, W.; Zhang, Y.; Han, S.; Tao, J.; Zheng, H.; Wei, Y.; Zhu, J.; Li, F.; Wang, X. RGD Conjugated Liposome-Hollow Silica Hybrid Nanovehicles for Targeted and Controlled Delivery of Arsenic Trioxide against Hepatic Carcinoma. Int. J. Pharm. 2017, 519, 250–262. [Google Scholar] [CrossRef]
- Zhou, S.; Wu, D.; Yin, X.; Jin, X.; Zhang, X.; Zheng, S.; Wang, C.; Liu, Y. Intracellular PH-Responsive and Rituximab-Conjugated Mesoporous Silica Nanoparticles for Targeted Drug Delivery to Lymphoma B Cells. J. Exp. Clin. Cancer Res. 2017, 36, 24. [Google Scholar] [CrossRef]
- Nguyen, C.T.H.; Webb, R.I.; Lambert, L.K.; Strounina, E.; Lee, E.C.; Parat, M.-O.; McGuckin, M.A.; Popat, A.; Cabot, P.J.; Ross, B.P. Bifunctional Succinylated ε-Polylysine-Coated Mesoporous Silica Nanoparticles for PH-Responsive and Intracellular Drug Delivery Targeting the Colon. ACS Appl. Mater. Interfaces 2017, 9, 9470–9483. [Google Scholar] [CrossRef] [PubMed]
- Dréau, D.; Moore, L.J.; Alvarez-Berrios, M.P.; Tarannum, M.; Mukherjee, P.; Vivero-Escoto, J.L. Mucin-1-Antibody-Conjugated Mesoporous Silica Nanoparticles for Selective Breast Cancer Detection in a Mucin-1 Transgenic Murine Mouse Model. J. Biomed. Nanotechnol. 2016, 12, 2172–2184. [Google Scholar] [CrossRef] [PubMed]
- Kienzle, A.; Kurch, S.; Schlöder, J.; Berges, C.; Ose, R.; Schupp, J.; Tuettenberg, A.; Weiss, H.; Schultze, J.; Winzen, S.; et al. Dendritic Mesoporous Silica Nanoparticles for PH-Stimuli-Responsive Drug Delivery of TNF-Alpha. Adv. Healthc. Mater. 2017, 6, 1700012. [Google Scholar] [CrossRef]
- Liu, Q.; Xu, N.; Liu, L.; Li, J.; Zhang, Y.; Shen, C.; Shezad, K.; Zhang, L.; Zhu, J.; Tao, J. Dacarbazine-Loaded Hollow Mesoporous Silica Nanoparticles Grafted with Folic Acid for Enhancing Antimetastatic Melanoma Response. ACS Appl. Mater. Interfaces 2017, 9, 21673–21687. [Google Scholar] [CrossRef]
- Chou, C.-C.; Chen, W.; Hung, Y.; Mou, C.-Y. Molecular Elucidation of Biological Response to Mesoporous Silica Nanoparticles in Vitro and in Vivo. ACS Appl. Mater. Interfaces 2017, 9, 22235–22251. [Google Scholar] [CrossRef] [PubMed]
- Paatero, I.; Casals, E.; Niemi, R.; Özliseli, E.; Rosenholm, J.M.; Sahlgren, C. Analyses in Zebrafish Embryos Reveal That Nanotoxicity Profiles Are Dependent on Surface-Functionalization Controlled Penetrance of Biological Membranes. Sci. Rep. 2017, 7, 8423. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Duo, Y.; Bao, S.; He, L.; Ling, K.; Luo, J.; Zhang, Y.; Huang, H.; Zhang, H.; Yu, X. EpCAM Aptamer-Functionalized Polydopamine-Coated Mesoporous Silica Nanoparticles Loaded with DM1 for Targeted Therapy in Colorectal Cancer. Int. J. Nanomed. 2017, 12, 6239–6257. [Google Scholar] [CrossRef]
- Martínez-Carmona, M.; Lozano, D.; Colilla, M.; Vallet-Regí, M. Lectin-Conjugated PH-Responsive Mesoporous Silica Nanoparticles for Targeted Bone Cancer Treatment. Acta Biomater. 2018, 65, 393–404. [Google Scholar] [CrossRef]
- Hei, M.; Wang, J.; Wang, K.; Zhu, W.; Ma, P.X. Dually Responsive Mesoporous Silica Nanoparticles Regulated by Upper Critical Solution Temperature Polymers for Intracellular Drug Delivery. J. Mater. Chem. B 2017, 5, 9497–9501. [Google Scholar] [CrossRef]
- Gounani, Z.; Asadollahi, M.A.; Meyer, R.L.; Arpanaei, A. Loading of Polymyxin B onto Anionic Mesoporous Silica Nanoparticles Retains Antibacterial Activity and Enhances Biocompatibility. Int. J. Pharm. 2018, 537, 148–161. [Google Scholar] [CrossRef]
- Saroj, S.; Rajput, S.J. Tailor-Made PH-Sensitive Polyacrylic Acid Functionalized Mesoporous Silica Nanoparticles for Efficient and Controlled Delivery of Anti-Cancer Drug Etoposide. Drug Dev. Ind. Pharm. 2018, 44, 1198–1211. [Google Scholar] [CrossRef] [PubMed]
- Tran, A.-V.; Shim, K.; Thi, T.-T.V.; Kook, J.-K.; An, S.S.A.; Lee, S.-W. Targeted and Controlled Drug Delivery by Multifunctional Mesoporous Silica Nanoparticles with Internal Fluorescent Conjugates and External Polydopamine and Graphene Oxide Layers. Acta Biomater. 2018, 74, 397–413. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Wang, Z.; Zhang, Y.; Zhang, K.; Xie, J.; Liu, Y.; Li, W.; Feng, N. Curcumin-Loaded Redox-Responsive Mesoporous Silica Nanoparticles for Targeted Breast Cancer Therapy. Artif. Cells Nanomed. Biotechnol. 2018, 46, 921–935. [Google Scholar] [CrossRef]
- Hou, L.; Zheng, Y.; Wang, Y.; Hu, Y.; Shi, J.; Liu, Q.; Zhang, H.; Zhang, Z. Self-Regulated Carboxyphenylboronic Acid-Modified Mesoporous Silica Nanoparticles with “Touch Switch” Releasing Property for Insulin Delivery. ACS Appl. Mater. Interfaces 2018, 10, 21927–21938. [Google Scholar] [CrossRef] [PubMed]
- Braun, K.; Stürzel, C.M.; Biskupek, J.; Kaiser, U.; Kirchhoff, F.; Lindén, M. Comparison of Different Cytotoxicity Assays for in Vitro Evaluation of Mesoporous Silica Nanoparticles. Toxicol. Vitr. 2018, 52, 214–221. [Google Scholar] [CrossRef]
- Guo, Q.; Chang, Z.; Khan, N.U.; Miao, T.; Ju, X.; Feng, H.; Zhang, L.; Sun, Z.; Li, H.; Han, L. Nanosizing Noncrystalline and Porous Silica Material—Naturally Occurring Opal Shale for Systemic Tumor Targeting Drug Delivery. ACS Appl. Mater. Interfaces 2018, 10, 25994–26004. [Google Scholar] [CrossRef]
- Lu, M.; Bai, J.; Shao, D.; Qiu, J.; Li, M.; Zheng, X.; Xiao, Y.; Wang, Z.; Chang, Z.; Chen, L.; et al. Antibacterial and Biodegradable Tissue Nano-Adhesives for Rapid Wound Closure. Int. J. Nanomed. 2018, 13, 5849–5863. [Google Scholar] [CrossRef]
- Breznan, D.; Das, D.D.; MacKinnon-Roy, C.; Bernatchez, S.; Sayari, A.; Hill, M.; Vincent, R.; Kumarathasan, P. Physicochemical Properties Can Be Key Determinants of Mesoporous Silica Nanoparticle Potency in Vitro. ACS Nano 2018, 12, 12062–12079. [Google Scholar] [CrossRef]
- Jafari, S.; Derakhshankhah, H.; Alaei, L.; Fattahi, A.; Varnamkhasti, B.S.; Saboury, A.A. Mesoporous Silica Nanoparticles for Therapeutic/Diagnostic Applications. Biomed. Pharmacother. 2019, 109, 1100–1111. [Google Scholar] [CrossRef]
- Dong, X.; Wu, Z.; Li, X.; Xiao, L.; Yang, M.; Li, Y.; Duan, J.; Sun, Z. The Size-dependent Cytotoxicity of Amorphous Silica Nanoparticles: A Systematic Review of in vitro Studies. Int. J. Nanomed. 2020, 15, 9089–9113. [Google Scholar] [CrossRef]
- Li, Y.; Sun, L.; Jin, M.; Du, Z.; Liu, X.; Guo, C.; Li, Y.; Huang, P.; Sun, Z. Size-Dependent Cytotoxicity of Amorphous Silica Nanoparticles in Human Hepatoma HepG2 Cells. Toxicol. Vitr. Int. J. Publ. Assoc. BIBRA 2011, 25, 1343–1352. [Google Scholar] [CrossRef] [PubMed]
- Nabeshi, H.; Yoshikawa, T.; Matsuyama, K.; Nakazato, Y.; Arimori, A.; Isobe, M.; Tochigi, S.; Kondoh, S.; Hirai, T.; Akase, T.; et al. Size-Dependent Cytotoxic Effects of Amorphous Silica Nanoparticles on Langerhans Cells. Pharmazie 2010, 65, 199–201. [Google Scholar] [PubMed]
- Napierska, D.; Thomassen, L.C.J.; Rabolli, V.; Lison, D.; Gonzalez, L.; Kirsch-Volders, M.; Martens, J.A.; Hoet, P.H. Size-Dependent Cytotoxicity of Monodisperse Silica Nanoparticles in Human Endothelial Cells. Small 2009, 5, 846–853. [Google Scholar] [CrossRef]
- Al-Rawi, M.; Diabaté, S.; Weiss, C. Uptake and Intracellular Localization of Submicron and Nano-Sized SiO₂ Particles in HeLa Cells. Arch. Toxicol. 2011, 85, 813–826. [Google Scholar] [CrossRef] [PubMed]
- Albers, C.E.; Hofstetter, W.; Siebenrock, K.A.; Landmann, R.; Klenke, F.M. In Vitro Cytotoxicity of Silver Nanoparticles on Osteoblasts and Osteoclasts at Antibacterial Concentrations. Nanotoxicology 2013, 7, 30–36. [Google Scholar] [CrossRef]
- Patil, G.; Khan, M.I.; Patel, D.K.; Sultana, S.; Prasad, R.; Ahmad, I. Evaluation of Cytotoxic, Oxidative Stress, Proinflammatory and Genotoxic Responses of Micro- and Nano-Particles of Dolomite on Human Lung Epithelial Cells A(549). Environ. Toxicol. Pharmacol. 2012, 34, 436–445. [Google Scholar] [CrossRef]
- Pietruska, J.R.; Liu, X.; Smith, A.; McNeil, K.; Weston, P.; Zhitkovich, A.; Hurt, R.; Kane, A.B. Bioavailability, Intracellular Mobilization of Nickel, and HIF-1α Activation in Human Lung Epithelial Cells Exposed to Metallic Nickel and Nickel Oxide Nanoparticles. Toxicol. Sci. J. Soc. Toxicol. 2011, 124, 138–148. [Google Scholar] [CrossRef]
- Kipen, H.M.; Laskin, D.L. Smaller Is Not Always Better: Nanotechnology Yields Nanotoxicology. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2005, 289, L696–L697. [Google Scholar] [CrossRef]
- Oberdörster, G.; Oberdörster, E.; Oberdörster, J. Nanotoxicology: An Emerging Discipline Evolving from Studies of Ultrafine Particles. Environ. Health Perspect. 2005, 113, 823–839. [Google Scholar] [CrossRef]
- Nel, A.; Xia, T.; Mädler, L.; Li, N. Toxic Potential of Materials at the Nanolevel. Science 2006, 311, 622–627. [Google Scholar] [CrossRef]
- Morishige, T.; Yoshioka, Y.; Inakura, H.; Tanabe, A.; Yao, X.; Tsunoda, S.; Tsutsumi, Y.; Mukai, Y.; Okada, N.; Nakagawa, S. Cytotoxicity of Amorphous Silica Particles against Macrophage-like THP-1 Cells Depends on Particle-Size and Surface Properties. Die Pharm-An Int. J. Pharm. Sci. 2010, 65, 596–599. [Google Scholar]
- Cha, K.E.; Myung, H. Cytotoxic Effects of Nanoparticles Assessed in Vitro and in Vivo. J. Microbiol. Biotechnol. 2007, 17, 1573–1578. [Google Scholar] [PubMed]
- Fröhlich, E. The Role of Surface Charge in Cellular Uptake and Cytotoxicity of Medical Nanoparticles. Int. J. Nanomed. 2012, 7, 5577. [Google Scholar] [CrossRef]
- Lin, W.; Huang, Y.-W.; Zhou, X.-D.; Ma, Y. In Vitro Toxicity of Silica Nanoparticles in Human Lung Cancer Cells. Toxicol. Appl. Pharmacol. 2006, 217, 252–259. [Google Scholar] [CrossRef]
- Zhao, Y.; Sun, X.; Zhang, G.; Trewyn, B.G.; Slowing, I.I.; Lin, V.S.-Y. Interaction of Mesoporous Silica Nanoparticles with Human Red Blood Cell Membranes: Size and Surface Effects. ACS Nano 2011, 5, 1366–1375. [Google Scholar] [CrossRef]
- Kettiger, H.; Karaman, D.S.; Schiesser, L.; Rosenholm, J.M.; Huwyler, J. Comparative Safety Evaluation of Silica-Based Particles. Toxicol. Vitr. 2015, 30, 355–363. [Google Scholar] [CrossRef]
- Abbasi, F.; Hashemi, H.; Samaei, M.R.; SavarDashtaki, A.; Azhdarpoor, A.; Fallahi, M.J. The Synergistic Interference Effect of Silica Nanoparticles Concentration and the Wavelength of ELISA on the Colorimetric Assay of Cell Toxicity. Sci. Rep. 2021, 11, 15133. [Google Scholar] [CrossRef] [PubMed]
- Scarcello, E.; Lambremont, A.; Vanbever, R.; Jacques, P.J.; Lison, D. Mind Your Assays: Misleading Cytotoxicity with the WST-1 Assay in the Presence of Manganese. PLoS ONE 2020, 15, e0231634. [Google Scholar] [CrossRef]
- Fisichella, M.; Dabboue, H.; Bhattacharyya, S.; Saboungi, M.-L.; Salvetat, J.-P.; Hevor, T.; Guerin, M. Mesoporous Silica Nanoparticles Enhance MTT Formazan Exocytosis in HeLa Cells and Astrocytes. Toxicol. Vitr. 2009, 23, 697–703. [Google Scholar] [CrossRef]
- Laaksonen, T.; Santos, H.; Vihola, H.; Salonen, J.; Riikonen, J.; Heikkilä, T.; Peltonen, L.; Kumar, N.; Murzin, D.; Lehto, V.-P.; et al. Failure of MTT as a Toxicity Testing Agent for Mesoporous Silicon Microparticles. Chem. Res. Toxicol. 2007, 20, 1913–1918. [Google Scholar] [CrossRef]
- Yazdimamaghani, M.; Moos, P.J.; Ghandehari, H. Time- and Dose-Dependent Gene Expression Analysis of Macrophage Response as a Function of Porosity of Silica Nanoparticles. Nanomed. Nanotechnol. Biol. Med. 2019, 21, 102041. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.; Liu, J.; Xu, J.; Sun, L.; Chen, M.; Lan, M. Nano-SiO2 induces apoptosis via activation of p53 and Bax mediated by oxidative stress in human hepatic cell line. Toxicol. Vitr. 2010, 24, 751–758. [Google Scholar] [CrossRef] [PubMed]
- Yazdimamaghani, M.; Moos, P.J.; Ghandehari, H. Global Gene Expression Analysis of Macrophage Response Induced by Nonporous and Porous Silica Nanoparticles. Nanomed. Nanotechnol. Biol. Med. 2018, 14, 533–545. [Google Scholar] [CrossRef] [PubMed]
- Jeon, S.; Clavadetscher, J.; Lee, D.-K.; Chankeshwara, S.V.; Bradley, M.; Cho, W.-S. Surface Charge-Dependent Cellular Uptake of Polystyrene Nanoparticles. Nanomaterials 2018, 8, E1028. [Google Scholar] [CrossRef]
- Li, X.; Chen, Y.; Wang, M.; Ma, Y.; Xia, W.; Gu, H. A Mesoporous Silica Nanoparticle--PEI--Fusogenic Peptide System for SiRNA Delivery in Cancer Therapy. Biomaterials 2013, 34, 1391–1401. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Quan, G.; Wu, Q.; Zhang, X.; Niu, B.; Wu, B.; Huang, Y.; Pan, X.; Wu, C. Mesoporous Silica Nanoparticles for Drug and Gene Delivery. Acta Pharm. Sin. B 2018, 8, 165–177. [Google Scholar] [CrossRef]
- Douroumis, D.; Onyesom, I.; Maniruzzaman, M.; Mitchell, J. Mesoporous Silica Nanoparticles in Nanotechnology. Crit. Rev. Biotechnol. 2013, 33, 229–245. [Google Scholar] [CrossRef] [PubMed]
- Panyam, J.; Zhou, W.-Z.; Prabha, S.; Sahoo, S.K.; Labhasetwar, V. Rapid Endo-Lysosomal Escape of Poly(DL-Lactide-Coglycolide) Nanoparticles: Implications for Drug and Gene Delivery. FASEB J. 2002, 16, 1217–1226. [Google Scholar] [CrossRef]
- Iturrioz-Rodríguez, N.; Correa-Duarte, M.Á.; Valiente, R.; Fanarraga, M.L. Engineering Sub-Cellular Targeting Strategies to Enhance Safe Cytosolic Silica Particle Dissolution in Cells. Pharmaceutics 2020, 12, 487. [Google Scholar] [CrossRef]
- Slowing, I.; Trewyn, B.G.; Lin, V.S.-Y. Effect of Surface Functionalization of MCM-41-Type Mesoporous Silica Nanoparticles on the Endocytosis by Human Cancer Cells. J. Am. Chem. Soc. 2006, 128, 14792–14793. [Google Scholar] [CrossRef]
- Gaidano, G.; Ballerini, P.; Gong, J.Z.; Inghirami, G.; Neri, A.; Newcomb, E.W.; Magrath, I.T.; Knowles, D.M.; Dalla-Favera, R. P53 Mutations in Human Lymphoid Malignancies: Association with Burkitt Lymphoma and Chronic Lymphocytic Leukemia. Proc. Natl. Acad. Sci. USA 1991, 88, 5413–5417. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, I.-L.; Gramatke, A.M.; Joksimovic, R.; Sokolowski, M.; Gradzielski, M.; Haase, A. Size and Cell Type Dependent Uptake of Silica Nanoparticles. J. Nanomed. Nanotechnol. 2014, 5, 248–258. [Google Scholar] [CrossRef]
- Chung, T.-H.; Wu, S.-H.; Yao, M.; Lu, C.-W.; Lin, Y.-S.; Hung, Y.; Mou, C.-Y.; Chen, Y.-C.; Huang, D.-M. The Effect of Surface Charge on the Uptake and Biological Function of Mesoporous Silica Nanoparticles in 3T3-L1 Cells and Human Mesenchymal Stem Cells. Biomaterials 2007, 28, 2959–2966. [Google Scholar] [CrossRef]
- Choi, J.Y.; Gupta, B.; Ramasamy, T.; Jeong, J.-H.; Jin, S.G.; Choi, H.-G.; Yong, C.S.; Kim, J.O. PEGylated Polyaminoacid-Capped Mesoporous Silica Nanoparticles for Mitochondria-Targeted Delivery of Celastrol in Solid Tumors. Colloids Surf. B Biointerfaces 2018, 165, 56–66. [Google Scholar] [CrossRef]
- Benezra, M.; Phillips, E.; Overholtzer, M.; Zanzonico, P.B.; Tuominen, E.; Wiesner, U.; Bradbury, M.S. Ultrasmall integrin-targeted silica nanoparticles modulate signaling events and cellular processes in a concentration-dependent manner. Small 2015, 11, 1721–1732. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhang, X.; Wang, M.; Li, X.; Mu, H.; Wang, A.; Liu, W.; Li, Y.; Wu, Z.; Sun, K. Synthesis of a Bi-Functional Dendrimer-Based Nanovehicle Co-Modified with RGDyC and TAT Peptides for Neovascular Targeting and Penetration. Int. J. Pharm. 2016, 501, 112–123. [Google Scholar] [CrossRef]
- Shinderman-Maman, E.; Cohen, K.; Weingarten, C.; Nabriski, D.; Twito, O.; Baraf, L.; Hercbergs, A.; Davis, P.J.; Werner, H.; Ellis, M.; et al. The Thyroid Hormone-Avβ3 Integrin Axis in Ovarian Cancer: Regulation of Gene Transcription and MAPK-Dependent Proliferation. Oncogene 2016, 35, 1977–1987. [Google Scholar] [CrossRef]
- Yuan, Y.; Wang, Z.; Cai, P.; Liu, J.; Liao, L.-D.; Hong, M.; Chen, X.; Thakor, N.; Liu, B. Conjugated Polymer and Drug Co-Encapsulated Nanoparticles for Chemo- and Photo-Thermal Combination Therapy with Two-Photon Regulated Fast Drug Release. Nanoscale 2015, 7, 3067–3076. [Google Scholar] [CrossRef]
- Zheng, Y.; Ji, S.; Czerwinski, A.; Valenzuela, F.; Pennington, M.; Liu, S. FITC-Conjugated Cyclic RGD Peptides as Fluorescent Probes for Staining Integrin Avβ3/Avβ5 in Tumor Tissues. Bioconjug. Chem. 2014, 25, 1925–1941. [Google Scholar] [CrossRef]
- Kim, W.; Kim, W.K.; Lee, K.; Son, M.J.; Kwak, M.; Chang, W.S.; Min, J.K.; Song, N.W.; Lee, J.; Bae, K.H. A reliable approach for assessing size-dependent effects of silica nanoparticles on cellular internalization behavior and cytotoxic mechanisms. Int. J. Nanomedicine 2019, 14, 7375–7387. [Google Scholar] [CrossRef]
- Mohamed, B.M.; Verma, N.K.; Prina-Mello, A.; Williams, Y.; Davies, A.M.; Bakos, G.; Tormey, L.; Edwards, C.; Hanrahan, J.; Salvati, A.; et al. Activation of Stress-Related Signalling Pathway in Human Cells upon SiO2 Nanoparticles Exposure as an Early Indicator of Cytotoxicity. J. Nanobiotechnol. 2011, 9, 29. [Google Scholar] [CrossRef] [PubMed]
- Shapero, K.; Fenaroli, F.; Lynch, I.; Cottell, D.C.; Salvati, A.; Dawson, K.A. Time and Space Resolved Uptake Study of Silica Nanoparticles by Human Cells. Mol. Biosyst. 2011, 7, 371–378. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Liao, L.; Zhu, L.; Zhang, P.; Guo, K.; Kong, J.; Ji, C.; Liu, B. Size-Dependent Cellular Uptake Efficiency, Mechanism, and Cytotoxicity of Silica Nanoparticles toward HeLa Cells. Talanta 2013, 107, 408–415. [Google Scholar] [CrossRef]
- Passagne, I.; Morille, M.; Rousset, M.; Pujalté, I.; L’azou, B. Implication of Oxidative Stress in Size-Dependent Toxicity of Silica Nanoparticles in Kidney Cells. Toxicology 2012, 299, 112–124. [Google Scholar] [CrossRef]
- Blechinger, J.; Bauer, A.T.; Torrano, A.A.; Gorzelanny, C.; Bräuchle, C.; Schneider, S.W. Uptake Kinetics and Nanotoxicity of Silica Nanoparticles Are Cell Type Dependent. Small Weinh. Bergstr. Ger. 2013, 9, 3970–3980. [Google Scholar] [CrossRef] [PubMed]
- Pronsato, L.; La Colla, A.; Ronda, A.C.; Milanesi, L.; Boland, R.; Vasconsuelo, A. High Passage Numbers Induce Resistance to Apoptosis in C2C12 Muscle Cells. Biocell. J. Soc. Latinoam. Microsc. Electron. Al 2013, 37, 1–9. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, L.; Yu, C.; Yang, X.-F.; Wang, H. Monocyte and Macrophage Differentiation: Circulation Inflammatory Monocyte as Biomarker for Inflammatory Diseases. Biomark. Res. 2014, 2, 1. [Google Scholar] [CrossRef]
- Song, Y.; Li, X.; Du, X. Exposure to Nanoparticles Is Related to Pleural Effusion, Pulmonary Fibrosis and Granuloma. Eur. Respir. J. 2009, 34, 559–567. [Google Scholar] [CrossRef]
- Henseleit, U.; Zhang, J.; Wanner, R.; Haase, I.; Kolde, G.; Rosenbach, T. Role of P53 in UVB-Induced Apoptosis in Human HaCaT Keratinocytes. J. Investig. Dermatol. 1997, 109, 722–727. [Google Scholar] [CrossRef]
- Chen, L.-L.; Wang, W.-J. P53 Regulates Lipid Metabolism in Cancer. Int. J. Biol. Macromol. 2021, 192, 45–54. [Google Scholar] [CrossRef]
- Ishimine, M.; Lee, H.-C.; Nakaoka, H.; Orita, H.; Kobayashi, T.; Mizuguchi, K.; Endo, M.; Inoue, I.; Sato, K.; Yokomizo, T. The Relationship between TP53 Gene Status and Carboxylesterase 2 Expression in Human Colorectal Cancer. Dis. Markers 2018, 2018, 5280736. [Google Scholar] [CrossRef] [PubMed]
- Vrana, J.A.; Decker, R.H.; Johnson, C.R.; Wang, Z.; Jarvis, W.D.; Richon, V.M.; Ehinger, M.; Fisher, P.B.; Grant, S. Induction of Apoptosis in U937 Human Leukemia Cells by Suberoylanilide Hydroxamic Acid (SAHA) Proceeds through Pathways That Are Regulated by Bcl-2/Bcl-XL, c-Jun, and P21CIP1, but Independent of P53. Oncogene 1999, 18, 7016–7025. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Bodmer, W.F. Analysis of P53 Mutations and Their Expression in 56 Colorectal Cancer Cell Lines. Proc. Natl. Acad. Sci. USA 2006, 103, 976–981. [Google Scholar] [CrossRef]
- Velletri, T.; Xie, N.; Wang, Y.; Huang, Y.; Yang, Q.; Chen, X.; Chen, Q.; Shou, P.; Gan, Y.; Cao, G.; et al. P53 Functional Abnormality in Mesenchymal Stem Cells Promotes Osteosarcoma Development. Cell Death Dis. 2016, 7, e2015. [Google Scholar] [CrossRef] [PubMed]
- Bouaoun, L.; Sonkin, D.; Ardin, M.; Hollstein, M.; Byrnes, G.; Zavadil, J.; Olivier, M. TP53 Variations in Human Cancers: New Lessons from the IARC TP53 Database and Genomics Data. Hum. Mutat. 2016, 37, 865–876. [Google Scholar] [CrossRef]
- Aliyandi, A.; Zuhorn, I.S.; Salvati, A. Disentangling Biomolecular Corona Interactions with Cell Receptors and Implications for Targeting of Nanomedicines. Front. Bioeng. Biotechnol. 2020, 8, 599454. [Google Scholar] [CrossRef]
- Ban, Z.; Yuan, P.; Yu, F.; Peng, T.; Zhou, Q.; Hu, X. Machine Learning Predicts the Functional Composition of the Protein Corona and the Cellular Recognition of Nanoparticles. Proc. Natl. Acad. Sci. USA 2020, 117, 10492–10499. [Google Scholar] [CrossRef]
- Paula, A.J.; Araujo Júnior, R.T.; Martinez, D.S.T.; Paredes-Gamero, E.J.; Nader, H.B.; Durán, N.; Justo, G.Z.; Alves, O.L. Influence of Protein Corona on the Transport of Molecules into Cells by Mesoporous Silica Nanoparticles. ACS Appl. Mater. Interfaces 2013, 5, 8387–8393. [Google Scholar] [CrossRef]
- Noureddine, A.; Paffett, M.L.; Franco, S.; Chan, A.E.; Pallikkuth, S.; Lidke, K.; Serda, R.E. Endolysosomal Mesoporous Silica Nanoparticle Trafficking along Microtubular Highways. Pharmaceutics 2021, 14, 56. [Google Scholar] [CrossRef]




| Reference | Studies | Statistic | df | Sig. |
|---|---|---|---|---|
| [19] | Wright et al., 2021 | 0.928 | 6 | 0.565 |
| [20] | Zhao et al., 2019 | 0.895 | 18 | 0.047 |
| [21] | Wu et al., 2019 | 0.95 | 12 | 0.632 |
| [22] | Yan et al., 2020 | 0.911 | 16 | 0.122 |
| [23] | Kundu et al., 2020 | 0.95 | 6 | 0.74 |
| [24] | Deng et al., 2021 | 0.933 | 12 | 0.415 |
| [25] | Kim et al., 2021 | 0.952 | 30 | 0.188 |
| [26] | Vejdani Noghreiyan et al., 2020 | 0.967 | 5 | 0.854 |
| [27] | Zhang et al., 2020 | 0.948 | 36 | 0.089 |
| [28] | Ali et al., 2020 | 0.873 | 6 | 0.238 |
| [29] | Wang et al., 2019 | 0.883 | 12 | 0.096 |
| [30] | Khatoon et al., 2018 | 0.891 | 5 | 0.364 |
| [31] | Maksimović-Ivanić et al., 2019 | 0.992 | 9 | 0.998 |
| [32] | Yang et al., 2019 | 0.975 | 32 | 0.648 |
| [33] | Xie et al., 2016 | 0.885 | 10 | 0.151 |
| [34] | Paris et al., 2016 | 0.902 | 10 | 0.228 |
| [35] | Guo et al., 2016 | 0.945 | 32 | 0.103 |
| [36] | Ebabe Elle et al., 2016 | 0.974 | 32 | 0.63 |
| [37] | You et al., 2016 | 0.952 | 6 | 0.754 |
| [38] | Zhang et al., 2016 | 0.945 | 8 | 0.664 |
| [39] | Mannerström et al., 2016 | 0.842 | 64 | 0 |
| [40] | T. Li et al., 2017 | 0.916 | 6 | 0.48 |
| [41] | Ferrauto et al., 2017 | 0.897 | 6 | 0.358 |
| [42] | Chen et al., 2016 | 0.911 | 28 | 0.021 |
| [43] | Cheng et al., 2017 | 0.955 | 20 | 0.441 |
| [44] | Fei et al., 2017 | 0.931 | 96 | 0 |
| [45] | Zhou et al., 2017 | 0.973 | 30 | 0.638 |
| [46] | Nguyen et al., 2017 | 0.932 | 44 | 0.012 |
| [47] | Dréau et al., 2016 | 0.816 | 14 | 0.008 |
| [48] | Kienzle et al., 2017 | 0.958 | 17 | 0.599 |
| [49] | Liu et al., 2017 | 0.873 | 29 | 0.002 |
| [50] | Chou et al., 2017 | 0.905 | 72 | 0 |
| [51] | Paatero et al., 2017 | 0.75 | 12 | 0.003 |
| [52] | Y. Li et al., 2017 | 0.899 | 6 | 0.371 |
| [53] | Martínez-Carmona et al., 2018 | 0.95 | 8 | 0.713 |
| [54] | Hei et al., 2017 | 0.825 | 4 | 0.155 |
| [55] | Gounani et al., 2018 | 0.865 | 144 | 0 |
| [56] | Saroj and Rajput, 2018 | 0.758 | 24 | 0 |
| [57] | Tran et al., 2018 | 0.863 | 10 | 0.084 |
| [58] | Li et al., 2018 | 0.687 | 32 | 0 |
| [59] | Hou et al., 2018 | 0.96 | 6 | 0.816 |
| [60] | Braun et al., 2018 | 0.913 | 155 | 0 |
| [61] | Guo et al., 2018 | 0.913 | 16 | 0.129 |
| [62] | Lu et al., 2018 | 0.788 | 28 | 0 |
| [15] | Mohammadpour et al., 2019 | 0.902 | 64 | 0 |
| Total N | 1240 |
| Test Statistic | 75.276 a |
| Degree Of Freedom | 5 |
| Asymptotic Sig.(2-sided test) | 0.000 |
| Total N | 1236 |
| Test Statistic | 81.539 a |
| Degree Of Freedom | 12 |
| Asymptotic Sig.(2-sided test) | 0.000 |
| Total N | 1217 |
| Test Statistic | 12.007 a |
| Degree Of Freedom | 2 |
| Asymptotic Sig. (2-sided test) | 0.002 |
| Total N | 1240 |
| Test Statistic | 29.115 a |
| Degree Of Freedom | 4 |
| Asymptotic Sig. (2-sided test) | 0.000 |
| Total N | 1240 |
| Mann–Whitney U | 203,113.500 |
| Wilcoxon W | 505,366.500 |
| Test Statistic | 203,113.500 |
| Standard Error | 6099.500 |
| Standardised Test Statistic | 3.810 |
| Asymptotic Sig.(2-sided test) | 0.000 |
| Total N | 1239 |
| Test Statistic | 189.917 a |
| Degree Of Freedom | 39 |
| Asymptotic Sig.(2-sided test) | 0.000 |
| Total N | 1240 |
| Test Statistic | 279.742 a |
| Degree Of Freedom | 63 |
| Asymptotic Sig.(2-sided test) | 0.000 |
| Parameters | Considerations |
|---|---|
| Diameter Size | Size-dependent differential agglomeration |
| Exposure/incubation time | Time-dependent decrease in cell viability due to increased cellular uptake or intracellular accumulation; Shorter incubation time could cause lysosomal pathway activation by the MSNs |
| Zeta Potential | Low zeta potential values may correlate with reduced cellular uptake and decreased cytotoxicity |
| Functionalisation | Biocompatible surface modification to control undesirable surface reactivity; Along with an additional layer of protein corona, influences zeta potential, agglomeration of MSNs, cellular uptake, and downstream toxicity; Functional groups that allow endo-lysosomal escape (e.g., cationic in the acidic endo-lysosomal compartment) of MSN-drug entity into the cytoplasm or nucleus to enhance the efficacy of the drug load to intracellular targets; Functional groups that target specific cell receptors to ensure precise and sensitive therapy and/or diagnosis |
| Viability assay | Testing under acellular conditions; Employ multiple assays for cytotoxicity assessments |
| Cell type | Differential sensitivity of specific cell types; Varied cellular uptake mechanisms e.g., clathrin-mediated or caveolin-mediated endocytosis that result in different levels of uptake and intracellular accumulation; Cell type-specific receptor-mediated selective uptake; Identify cell lines for p53 competency |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmadi, A.; Sokunbi, M.; Patel, T.; Chang, M.-W.; Ahmad, Z.; Singh, N. Influence of Critical Parameters on Cytotoxicity Induced by Mesoporous Silica Nanoparticles. Nanomaterials 2022, 12, 2016. https://doi.org/10.3390/nano12122016
Ahmadi A, Sokunbi M, Patel T, Chang M-W, Ahmad Z, Singh N. Influence of Critical Parameters on Cytotoxicity Induced by Mesoporous Silica Nanoparticles. Nanomaterials. 2022; 12(12):2016. https://doi.org/10.3390/nano12122016
Chicago/Turabian StyleAhmadi, Amirsadra, Moses Sokunbi, Trisha Patel, Ming-Wei Chang, Zeeshan Ahmad, and Neenu Singh. 2022. "Influence of Critical Parameters on Cytotoxicity Induced by Mesoporous Silica Nanoparticles" Nanomaterials 12, no. 12: 2016. https://doi.org/10.3390/nano12122016
APA StyleAhmadi, A., Sokunbi, M., Patel, T., Chang, M.-W., Ahmad, Z., & Singh, N. (2022). Influence of Critical Parameters on Cytotoxicity Induced by Mesoporous Silica Nanoparticles. Nanomaterials, 12(12), 2016. https://doi.org/10.3390/nano12122016









