MoO3@MoS2 Core-Shell Structured Hybrid Anode Materials for Lithium-Ion Batteries
Abstract
:1. Introduction
2. Experimental Section
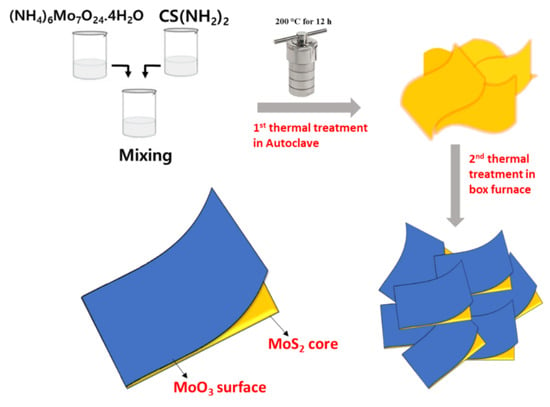
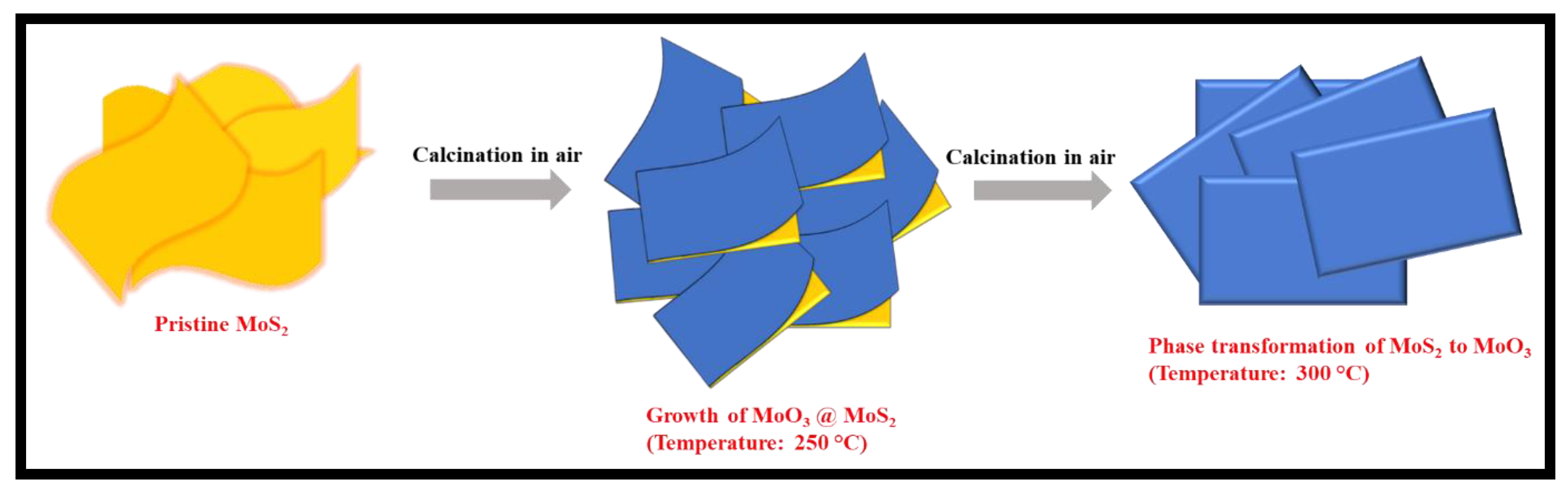
2.1. Synthesis of MoS2, MoO3@MoS2, and MoO3
2.2. Material Characterizations
2.3. Electrochemical Measurements
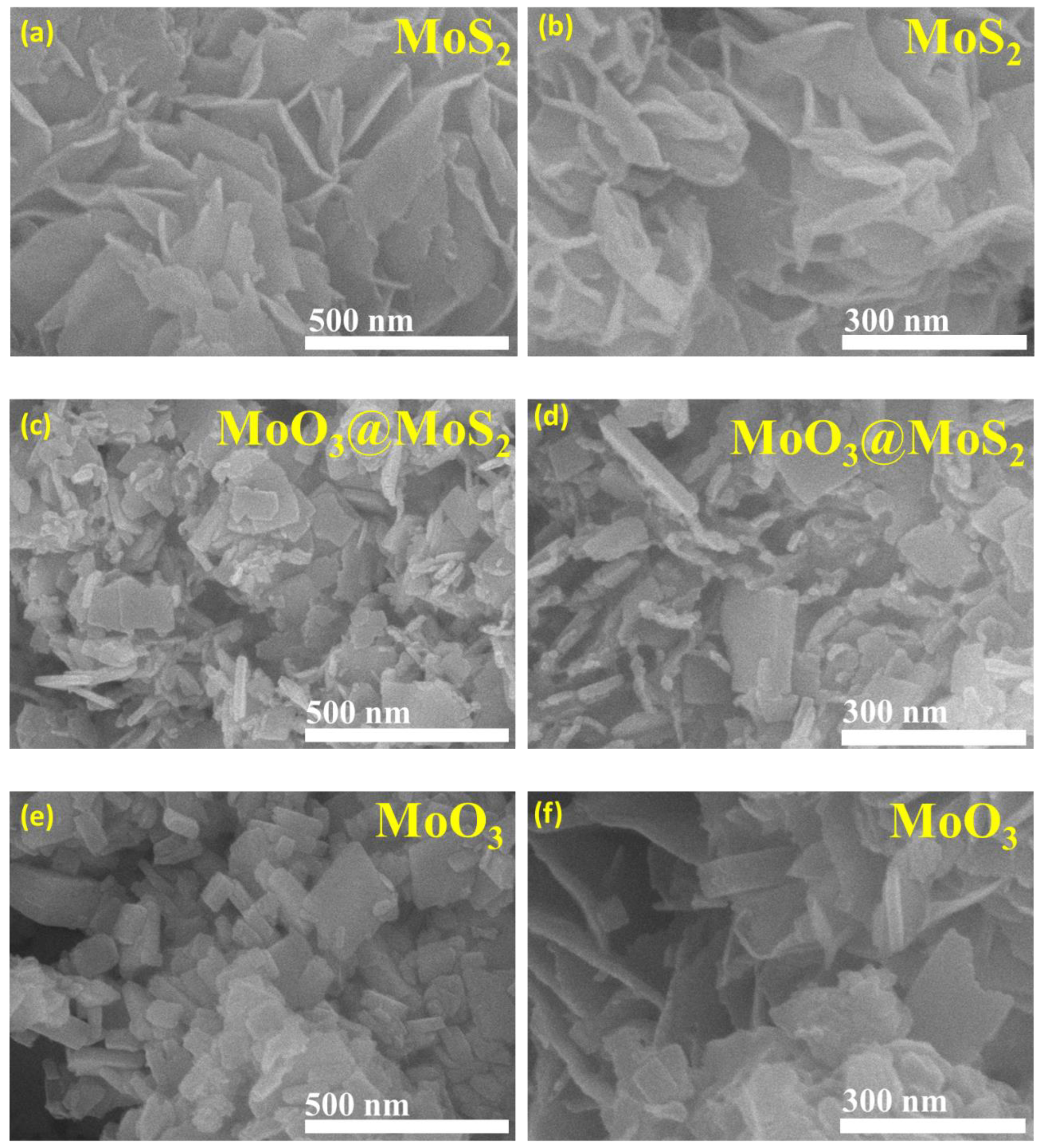
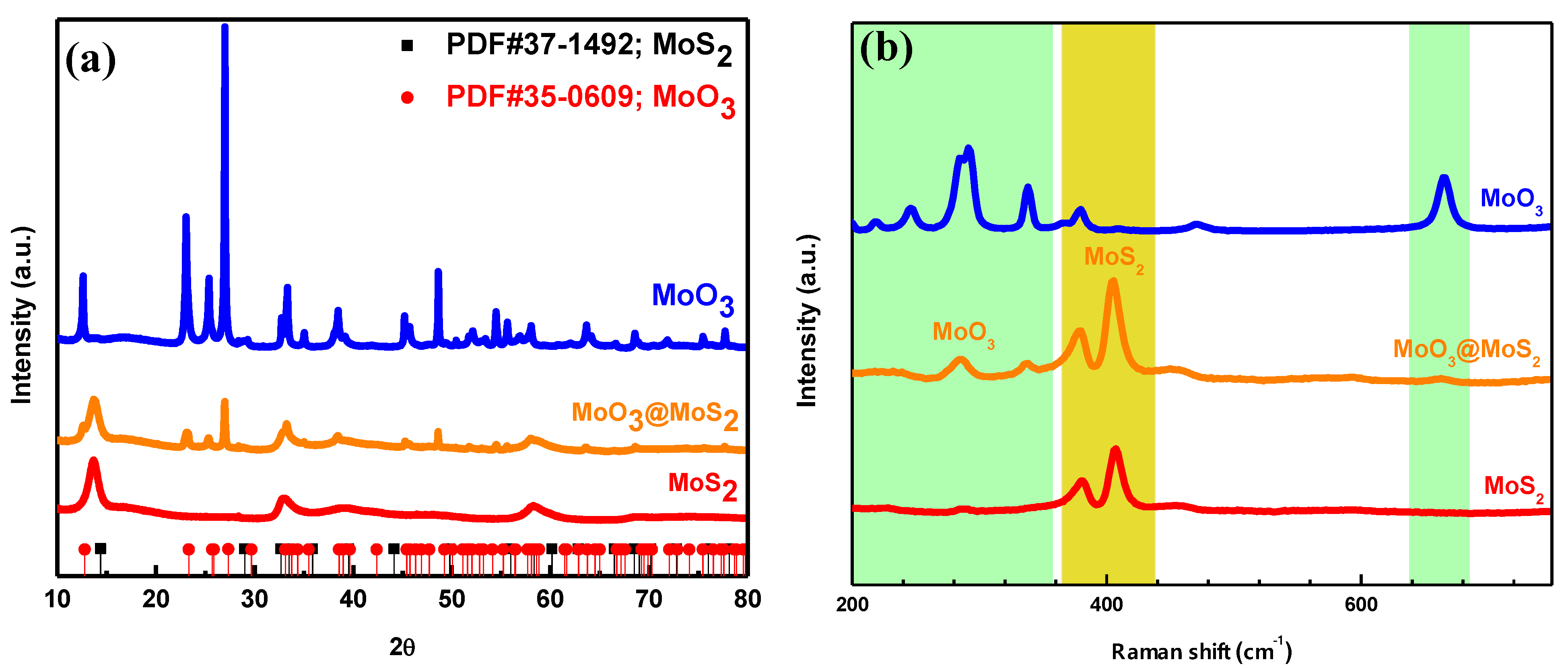
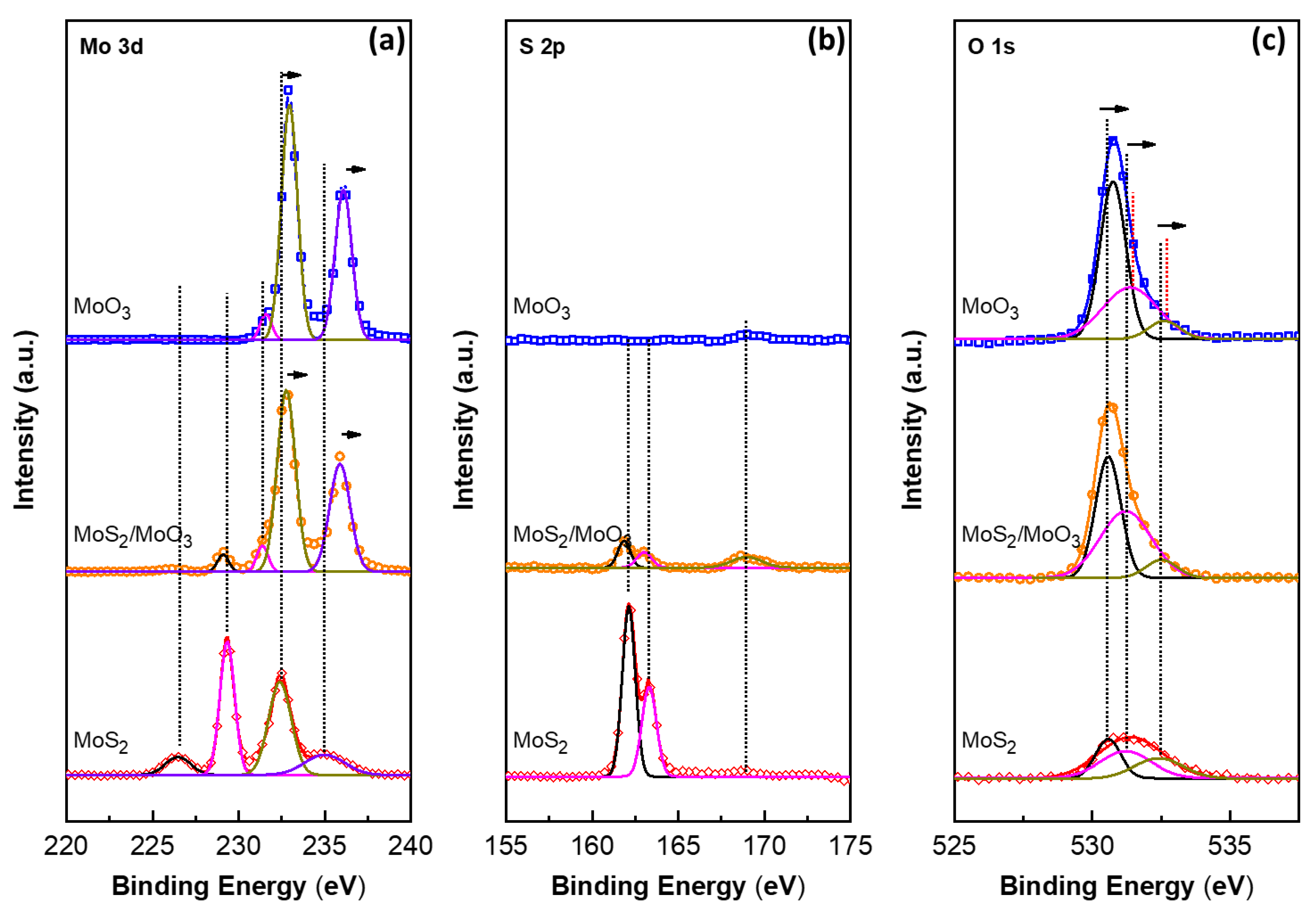
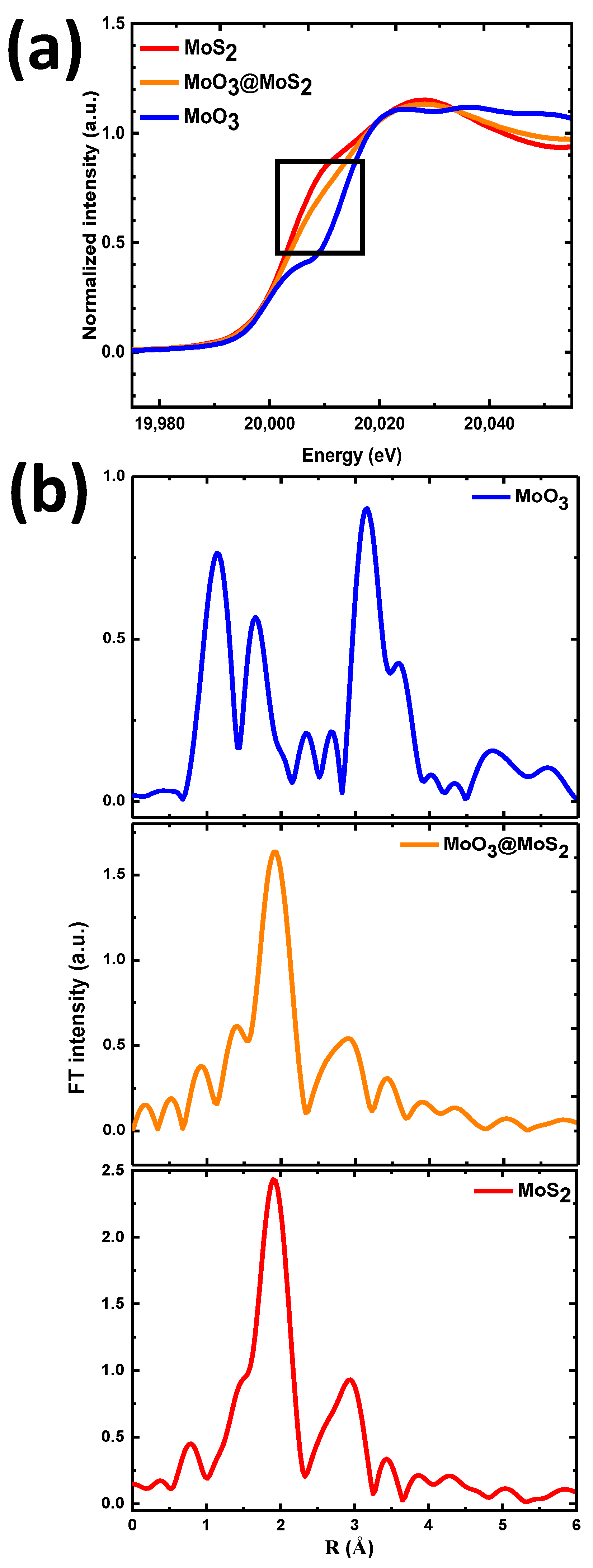
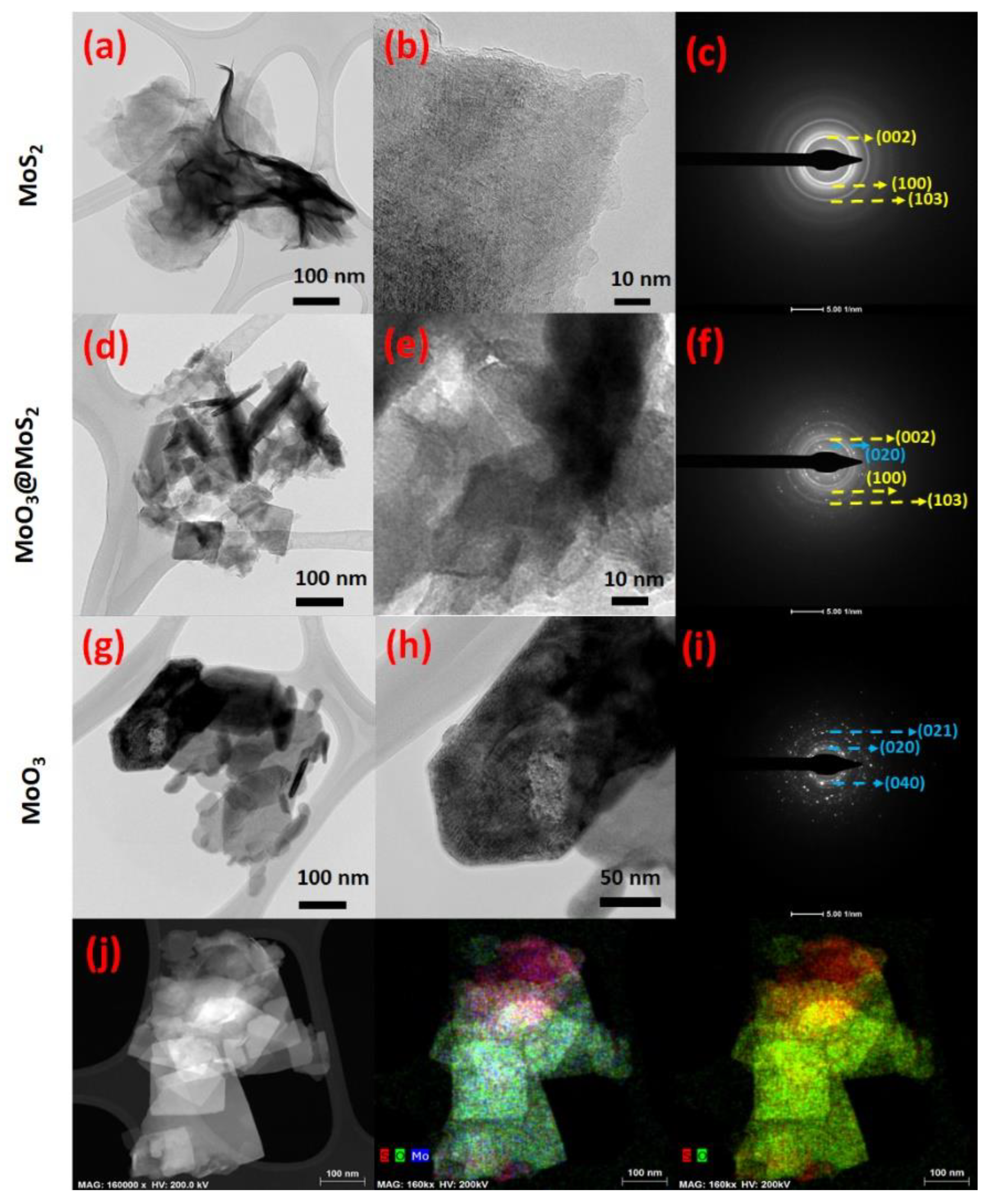
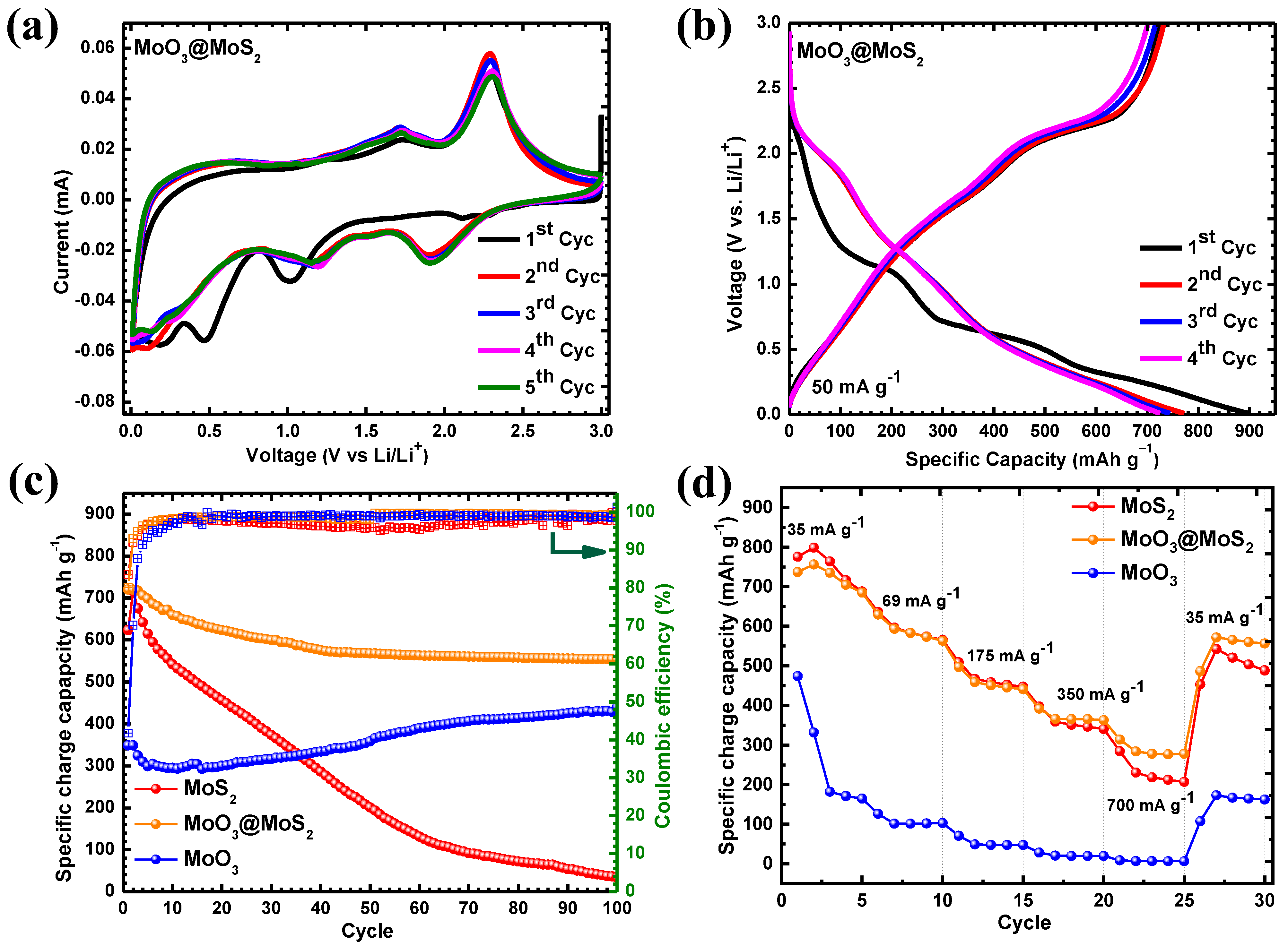
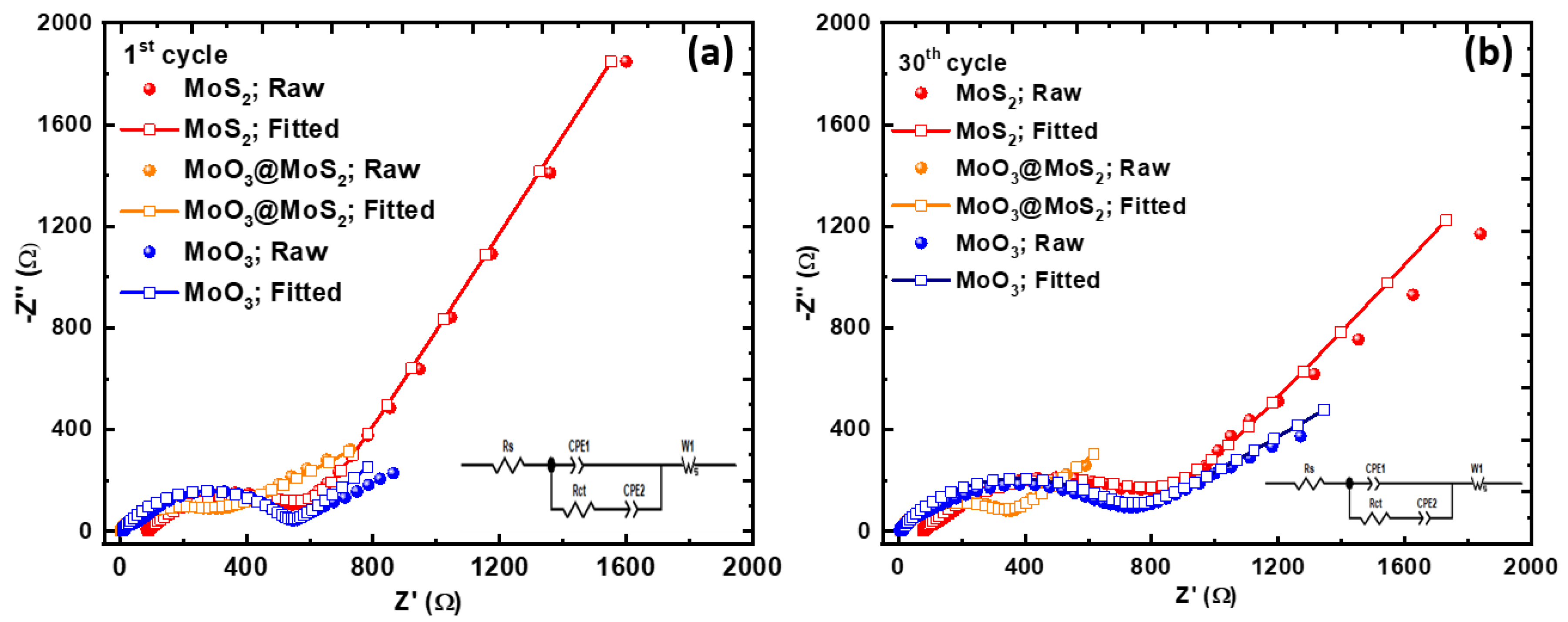
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Luo, W.; Shen, F.; Bommier, C.; Zhu, H.; Ji, X.; Hu, L. Na-Ion Battery Anodes: Materials and Electrochemistry. Acc. Chem. Res. 2016, 49, 231–240. [Google Scholar] [CrossRef] [PubMed]
- Chu, S.; Majumdar, A. Opportunities and challenges for a sustainable energy future. Nature 2012, 488, 294–303. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Cano, Z.P.; Yu, A.; Lu, J.; Chen, Z. Automotive Li-Ion Batteries: Current Status and Future Perspectives. Electrochem. Energy Rev. 2019, 2, 1–28. [Google Scholar] [CrossRef]
- Liang, Y.; Zhao, C.Z.; Yuan, H.; Chen, Y.; Zhang, W.; Huang, J.Q.; Yu, D.; Liu, Y.; Titirici, M.M.; Chueh, Y.L.; et al. A review of rechargeable batteries for portable electronic devices. InfoMat 2019, 1, 6–32. [Google Scholar] [CrossRef] [Green Version]
- Buqa, H.; Goers, D.; Holzapfel, M.; Spahr, M.E.; Novák, P. High Rate Capability of Graphite Negative Electrodes for Lithium-Ion Batteries. J. Electrochem. Soc. 2005, 152, A474. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Z.; Chen, Y.; Qu, Y.; Lai, Y.; Li, J. Morphology-controlled synthesis of MoS2 nanostructures with different lithium storage properties. J. Alloys Compd. 2014, 600, 84–90. [Google Scholar] [CrossRef]
- Zhang, S.; Chowdari, B.V.R.; Wen, Z.; Jin, J.; Yang, J. Constructing Highly Oriented Configuration by Few-Layer MoS2: Toward High-Performance Lithium-Ion Batteries and Hydrogen Evolution Reactions. ACS Nano 2015, 9, 12464–12472. [Google Scholar] [CrossRef]
- Radovsky, G.; Popovitz-Biro, R.; Staiger, M.; Gartsman, K.; Thomsen, C.; Lorenz, T.; Seifert, G. Tenne, Synthesis of copious amounts of SnS2 and SnS2/SnS nanotubes with ordered superstructures. Angew. Chem. 2011, 123, 12524–12528. [Google Scholar] [CrossRef]
- Vikraman, D.; Hussain, S.; Prasanna, K.; Karuppasamy, K.; Jung, J.; Kim, H.-S. Facile method to synthesis hybrid phase 1T@2H MoSe2 nanostructures for rechargeable lithium ion batteries. J. Electroanal. Chem. 2019, 833, 333–339. [Google Scholar] [CrossRef]
- Hussain, S.; Faizan, M.; Vikraman, D.; Rabani, I.; Ali, B.; Kim, H.-S.; Jung, J.; Nam, K.-W. Eutectoid WxC embedded WS2 nanosheets as a hybrid composite anode for lithium-ion batteries. Ceram. Int. 2021, 47, 18646–18655. [Google Scholar] [CrossRef]
- Islam, M.; Jeong, M.-G.; Ali, G.; Oh, I.-H.; Chung, K.Y.; Sun, Y.-K.; Jung, H.-G. A 4 V Li-ion battery using all-spinel-based electrodes. ChemSusChem 2018, 11, 2165–2170. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.; Ali, G.; Akbar, M.; Ali, B.; Jeong, M.; Kim, J.; Chung, K.Y.; Nam, K.; Jung, H. Investigating the energy storage performance of the ZnMn2O4 anode for its potential application in lithium-ion batteries. Int. J. Energy Res. 2022, 46, 6444–6456. [Google Scholar] [CrossRef]
- Feng, Y.; Liu, H.; Liu, Y.; Li, J. Tunable oxygen deficient in MoO3−x/MoO2 heterostructure for enhanced lithium storage properties. Int. J. Energy Res. 2022, 46, 5789–5799. [Google Scholar] [CrossRef]
- Madian, M.; Eychmüller, A.; Giebeler, L. Current Advances in TiO2-Based Nanostructure Electrodes for High Performance Lithium Ion Batteries. Batteries 2018, 4, 7. [Google Scholar] [CrossRef] [Green Version]
- Chen, Z.; Belharouak, I.; Sun, Y.-K.; Amine, K. Titanium-Based Anode Materials for Safe Lithium-Ion Batteries. Adv. Funct. Mater. 2013, 13, 959–969. [Google Scholar] [CrossRef]
- Ding, R.; Huang, Y.; Li, G.; Liao, Q.; Wei, T.; Liu, Y.; Huang, Y.; He, H. Carbon Anode Materials for Rechargeable Alkali Metal Ion Batteries and in-situ Characterization Techniques. Front. Chem. 2020, 8, 607504. [Google Scholar] [CrossRef]
- Xiong, D.; Li, X.; Bai, Z.; Shan, H.; Fan, L.; Wu, C.; Li, D.; Lu, S. Superior Cathode Performance of Nitrogen-Doped Graphene Frameworks for Lithium Ion Batteries. ACS Appl. Mater. Interfaces 2017, 9, 10643–10651. [Google Scholar] [CrossRef]
- Chen, Y.M.; Yu, X.Y.; Li, Z.; Paik, U.; Lou, X.W. Hierarchical MoS 2 tubular structures internally wired by carbon nanotubes as a highly stable anode material for lithium-ion batteries. Sci. Adv. 2016, 2, e1600021. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Wang, Y.; Yang, Y.; Lv, W.; Lian, G.; Golberg, D.; Wang, X.; Zhao, X.; Ding, Y. A MoS2/carbon hybrid anode for high-performance li-ion batteries at low temperature. Nano Energy 2020, 70, 104550. [Google Scholar] [CrossRef]
- Nzereogu, P.; Omah, A.; Ezema, F.; Iwuoha, E.; Nwanya, A. Anode materials for lithium-ion batteries: A review. Appl. Surf. Sci. Adv. 2022, 9, 100233. [Google Scholar] [CrossRef]
- Yan, Y.; Liu, Y.; Zhang, Y.; Qin, C.; Yu, H.; Bakenov, Z.; Wang, Z. Sn modified nanoporous Ge for improved lithium storage performance. J. Colloid Interface Sci. 2021, 602, 563–572. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Yamaguchi, T.; Matsumoto, S.; Hoshikawa, H.; Kumagai, T.; Okamoto, N.L.; Ichitsubo, T. Circumventing huge volume strain in alloy anodes of lithium batteries. Nat. Commun. 2020, 11, 1584. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fang, S.; Bresser, D.; Passerini, S. Transition Metal Oxide Anodes for Electrochemical Energy Storage in Lithium- and Sodium-Ion Batteries. Adv. Energy Mater. 2020, 10, 1902485. [Google Scholar] [CrossRef]
- Reddy, M.V.; Rao, G.V.S.; Chowdari, B.V.R. Metal Oxides and Oxysalts as Anode Materials for Li Ion Batteries. Chem. Rev. 2013, 113, 5364–5457. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Zhang, Y.; Wang, Y.; Li, H.; Peng, Y. The application of nanostructured transition metal sulfides as anodes for lithium ion batteries. J. Energy Chem. 2018, 27, 1536–1554. [Google Scholar] [CrossRef] [Green Version]
- Xu, X.; Liu, W.; Kim, Y.; Cho, J. Nanostructured transition metal sulfides for lithium ion batteries: Progress and challenges. Nano Today 2014, 9, 604–630. [Google Scholar] [CrossRef]
- Zhang, G.; Liu, H.; Qu, J.; Li, J. Two-dimensional layered MoS2: Rational design, properties and electrochemical applications. Energy Environ. Sci. 2016, 9, 1190–1209. [Google Scholar] [CrossRef]
- Wu, J.; Ihsan-Ul-Haq, M.; Ciucci, F.; Huang, B.; Kim, J.-K. Rationally designed nanostructured metal chalcogenides for advanced sodium-ion batteries. Energy Storage Mater. 2021, 34, 582–628. [Google Scholar] [CrossRef]
- Kumar, V.P.; Panda, D.K. Review˗next generation 2D material molybdenum disulfide (MoS2): Properties, applications and challenges. ECS J. Solid State Sci. Technol. 2022, 11, 033012. [Google Scholar] [CrossRef]
- Zuo, J.-H.; Zhai, P.-B.; He, Q.-Q.; Wang, L.; Chen, Q.; Gu, X.-K.; Yang, Z.-L.; Gong, Y.-J. In-situ constructed three-dimensional MoS2–MoN heterostructure as the cathode of lithium–sulfur battery. Rare Met. 2022, 41, 1743–1752. [Google Scholar] [CrossRef]
- Zhang, L.; Sun, D.; Kang, J.; Feng, J.; Bechtel, H.A.; Wang, L.-W.; Cairns, E.J.; Guo, J. Electrochemical Reaction Mechanism of the MoS2 Electrode in a Lithium-Ion Cell Revealed by in Situ and Operando X-ray Absorption Spectroscopy. Nano Lett. 2018, 18, 1466–1475. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liao, J.-Y.; de Luna, B.; Manthiram, A. TiO2-B nanowire arrays coated with layered MoS2 nanosheets for lithium and sodium storage. J. Mater. Chem. A 2016, 4, 801–806. [Google Scholar] [CrossRef]
- Chang, K.; Chen, W.-X.; Li, H.; Li, H. Microwave-assisted synthesis of SnS2/SnO2 composites by l-cysteine and their electrochemical performances when used as anode materials of Li-ion batteries. Electrochim. Acta 2011, 56, 2856–2861. [Google Scholar] [CrossRef]
- Deng, Z.; Hu, Y.; Ren, D.; Lin, S.; Jiang, H.; Li, C. Reciprocal hybridization of MoO2 nanoparticles and few-layer MoS2 for stable lithium-ion batteries. Chem. Commun. 2015, 51, 13838–13841. [Google Scholar] [CrossRef]
- Villevieille, C.; Wang, X.-J.; Krumeich, F.; Nesper, R.; Novák, P. MoS2 coating on MoO3 nanobelts: A novel approach for a high specific charge electrode for rechargeable Li-ion batteries. J. Power Sources 2015, 279, 636–644. [Google Scholar] [CrossRef]
- Hu, C.; Shu, H.; Shen, Z.; Zhao, T.; Liang, P.; Chen, X. Hierarchical MoO3/SnS2 core–shell nanowires with enhanced electrochemical performance for lithium-ion batteries. Phys. Chem. Chem. Phys. 2018, 20, 17171–17179. [Google Scholar] [CrossRef]
- Qu, B.; Sun, Y.; Liu, L.; Li, C.; Yu, C.; Zhang, X.; Chen, Y. Ultrasmall Fe2O3 nanoparticles/MoS2 nanosheets composite as high-performance anode material for lithium ion batteries. Sci. Rep. 2017, 7, 42772. [Google Scholar] [CrossRef]
- Li, Z.; Sun, P.; Zhan, X.; Zheng, Q.; Feng, T.; Braun, P.V.; Qi, S. Metallic 1T phase MoS2/MnO composites with improved cyclability for lithium-ion battery anodes. J. Alloys Compd. 2019, 796, 25–32. [Google Scholar] [CrossRef]
- Ahmed, B.; Anjum, D.H.; Hedhili, M.N.; Alshareef, H.N. Mechanistic insight into the stability of HfO2-coated MoS2 nanosheet anodes for sodium ion batteries. Small 2015, 11, 4341–4350. [Google Scholar] [CrossRef]
- Ryu, W.-H.; Wilson, H.; Sohn, S.; Li, J.; Tong, X.; Shaulsky, E.; Schroers, J.; Elimelech, M.; Taylor, A.D. Heterogeneous WSx/WO3 thorn-bush nanofiber electrodes for sodium-ion batteries. ACS Nano 2016, 10, 3257–3266. [Google Scholar] [CrossRef]
- Xie, J.; Zhang, J.; Li, S.; Grote, F.; Zhang, X.; Zhang, H.; Wang, R.; Lei, Y.; Pan, B.; Xie, Y. Controllable Disordered Engineering in Oxygen-Incorporated MoS2 Ultrathin Nanosheets for Efficient Hydrogen Evolution. J. Am. Chem. Soc. 2013, 135, 17881–17888. [Google Scholar] [CrossRef] [PubMed]
- Mishra, N.; Rathore, D.; Pandey, R.K. A comparative study of conventional type II and inverted core–shell nanostructures based on CdSe and ZnS. Opt. Quantum Electron. 2018, 50, 107. [Google Scholar] [CrossRef]
- Li, H.; Zhang, Q.; Yap, C.C.R.; Tay, B.K.; Edwin, T.H.T.; Olivier, A.; Baillargeat, D. From Bulk to Monolayer MoS2: Evolution of Raman Scattering. Adv. Funct. Mater. 2012, 22, 1385–1390. [Google Scholar] [CrossRef]
- Sinaim, H.; Ham, D.J.; Lee, J.S.; Phuruangrat, A.; Thongtem, S.; Thongtem, T. Free-polymer controlling morphology of α-MoO3 nanobelts by a facile hydrothermal synthesis, their electrochemistry for hydrogen evolution reactions and optical properties. J. Alloys Compd. 2012, 516, 172–178. [Google Scholar] [CrossRef]
- Vikraman, D.; Liu, H.; Hussain, S.; Karuppasamy, K.; Youi, H.-K.; Jung, J.; Kang, J.; Kim, H.-S. Influence of morphological tuned nanostructure hybrid layers on efficient bulk heterojunction organic solar cell and X-ray detector performances. Appl. Surf. Sci. 2021, 543, 148863. [Google Scholar] [CrossRef]
- Kim, S.S.; Britcher, L.; Kumar, S.; Griesser, H.J. XPS Study of Sulfur and Phosphorus Compounds with Different Oxidation States. Sains Malays. 2018, 47, 1913–1922. [Google Scholar] [CrossRef]
- Dahl-Petersen, C.; Šarić, M.; Brorson, M.; Moses, P.G.; Rossmeisl, J.; Lauritsen, J.V.; Helveg, S. Topotactic Growth of Edge-Terminated MoS2 from MoO2 Nanocrystals. ACS Nano 2018, 12, 5351–5358. [Google Scholar] [CrossRef] [Green Version]
- Hussain, S.; Rabani, I.; Vikraman, D.; Feroze, A.; Ali, M.; Seo, Y.-S.; Song, W.; An, K.-S.; Kim, H.-S.; Chun, S.-H.; et al. MoS2@X2C (X = Mo or W) hybrids for enhanced supercapacitor and hydrogen evolution performances. Chem. Eng. J. 2020, 421, 127843. [Google Scholar] [CrossRef]
- Brezesinski, T.; Wang, J.; Tolbert, S.H.; Dunn, B. Ordered mesoporous α-MoO3 with iso-oriented nanocrystalline walls for thin-film pseudocapacitors. Nat. Mater. 2010, 9, 146–151. [Google Scholar] [CrossRef]
- Zhou, K.; Zhou, W.; Liu, X.; Sang, Y.; Ji, S.; Li, W.; Lu, J.; Li, L.; Niu, W.; Liu, H. Ultrathin MoO3 nanocrystal self-assembled on graphene nanosheets via oxygen bonding as supercapacitor electrodes of high capacitance and long cycle life. Nano Energy 2015, 12, 510–520. [Google Scholar] [CrossRef]
- Zhang, H.; Lin, H.; Zheng, Y.; Hu, Y.; MacLennan, A. Understanding of the effect of synthesis temperature on the crystallization and activity of nano-MoS2 catalyst. Appl. Catal. B Environ. 2015, 165, 537–546. [Google Scholar] [CrossRef]
- Wang, Q.; Li, J. Facilitated lithium storage in MoS2 overlayers supported on coaxial carbon nanotubes. J. Phys. Chem. C 2007, 111, 1675–1682. [Google Scholar] [CrossRef]
- Cao, D.; Dai, Y.; Xie, S.; Wang, H.; Niu, C. Pyrolytic synthesis of MoO3 nanoplates within foam-like carbon nanoflakes for enhanced lithium ion storage. J. Colloid Interface Sci. 2018, 514, 686–693. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, W.; Li, M.; Cui, P.; Chen, D.; Gengenbach, T.; Chu, L.; Liu, H.; Song, G. Glucose-assisted synthesis of the hierarchical TiO2 nanowire@MoS2 nanosheet nanocomposite and its synergistic lithium storgae performance. J. Mater. Chem. A 2015, 3, 2762–2769. [Google Scholar] [CrossRef]
- Li, X.; Fu, J.; Sun, Y.; Sun, M.; Cheng, S.; Chen, K.; Yang, X.; Lou, Q.; Xu, T.; Shang, Y.; et al. Design and understanding of core/branch-structured VS2 nanosheets@CNTs as high-performance anode materials for lithium-ion batteries. Nanoscale 2019, 11, 13343–13353. [Google Scholar] [CrossRef]
- Zhu, K.; Wang, X.; Liu, J.; Li, S.; Wang, H.; Yang, L.; Liu, S.; Xie, T. Novel amorphous MoS2/MoO3/nitrogen-doped carbon composite with excellent electrochemical performance for lithium ion batteries and sodium ion batteries. ACS Sustain. Chem. Eng. 2017, 5, 8025–8034. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, Z.; Fu, Y.; Li, Q. Porous hollow carbon spheres decorated with molybdenum diselenide nanosheets as anodes for highly reversible lithium and sodium storage. Nanoscale 2015, 7, 10198–10203. [Google Scholar] [CrossRef]
- Liu, M.; Gao, H.; Hu, G.; Zhu, K.; Huang, H. Facile preparation of core-shell Si@Li4Ti5O12 nanocomposite as large-capacity lithium-ion battery anode. J. Energy Chem. 2020, 40, 89–98. [Google Scholar] [CrossRef] [Green Version]

| Serial No. | Material | Average Electrical Conductivity (µS·cm−2) |
|---|---|---|
| 01 | MoS2 | 5.2442 |
| 02 | MoO3@MoS2 | 0.6443 |
| 03 | MoO3 | 0.0282 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Faizan, M.; Hussain, S.; Islam, M.; Kim, J.-Y.; Han, D.; Bae, J.-H.; Vikraman, D.; Ali, B.; Abbas, S.; Kim, H.-S.; et al. MoO3@MoS2 Core-Shell Structured Hybrid Anode Materials for Lithium-Ion Batteries. Nanomaterials 2022, 12, 2008. https://doi.org/10.3390/nano12122008
Faizan M, Hussain S, Islam M, Kim J-Y, Han D, Bae J-H, Vikraman D, Ali B, Abbas S, Kim H-S, et al. MoO3@MoS2 Core-Shell Structured Hybrid Anode Materials for Lithium-Ion Batteries. Nanomaterials. 2022; 12(12):2008. https://doi.org/10.3390/nano12122008
Chicago/Turabian StyleFaizan, Muhammad, Sajjad Hussain, Mobinul Islam, Ji-Young Kim, Daseul Han, Jee-Hwan Bae, Dhanasekaran Vikraman, Basit Ali, Saleem Abbas, Hyun-Seok Kim, and et al. 2022. "MoO3@MoS2 Core-Shell Structured Hybrid Anode Materials for Lithium-Ion Batteries" Nanomaterials 12, no. 12: 2008. https://doi.org/10.3390/nano12122008
APA StyleFaizan, M., Hussain, S., Islam, M., Kim, J.-Y., Han, D., Bae, J.-H., Vikraman, D., Ali, B., Abbas, S., Kim, H.-S., Singh, A. N., Jung, J., & Nam, K.-W. (2022). MoO3@MoS2 Core-Shell Structured Hybrid Anode Materials for Lithium-Ion Batteries. Nanomaterials, 12(12), 2008. https://doi.org/10.3390/nano12122008