Calcium Phosphate Nanoclusters for the Repair of Tooth Enamel Erosion
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Preparation of CaP NCs via a Facile Approach
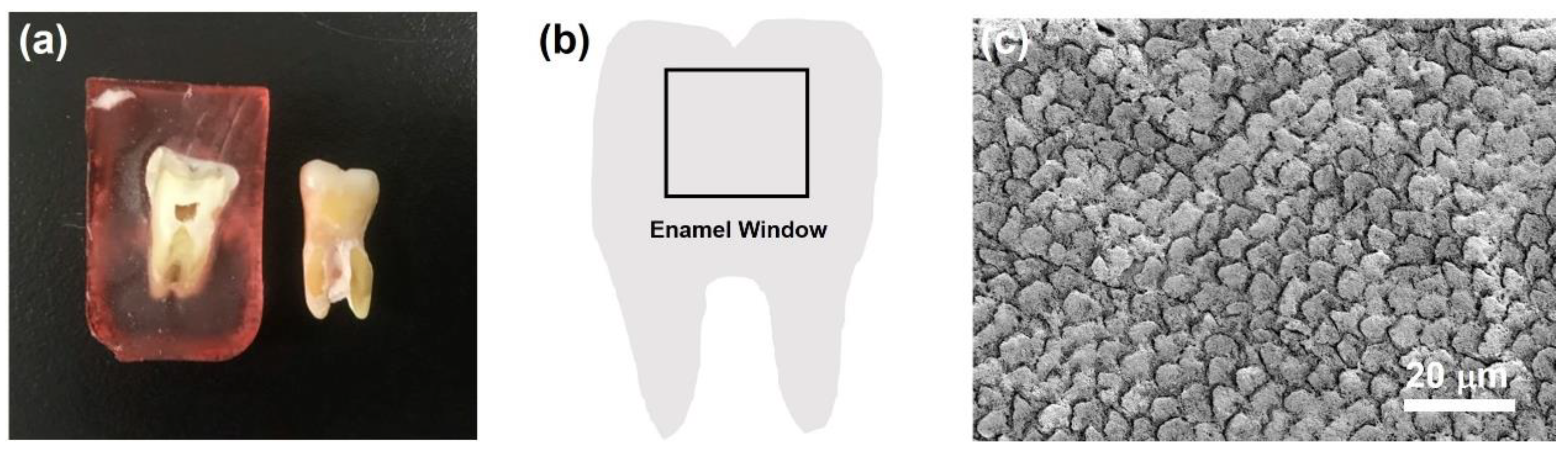
2.3. Preparation of Tooth Samples
2.4. Repair of the Tooth Enamel with CaP NCs
2.5. Material Characterization
2.6. Microhardness Test
3. Results and Discussion
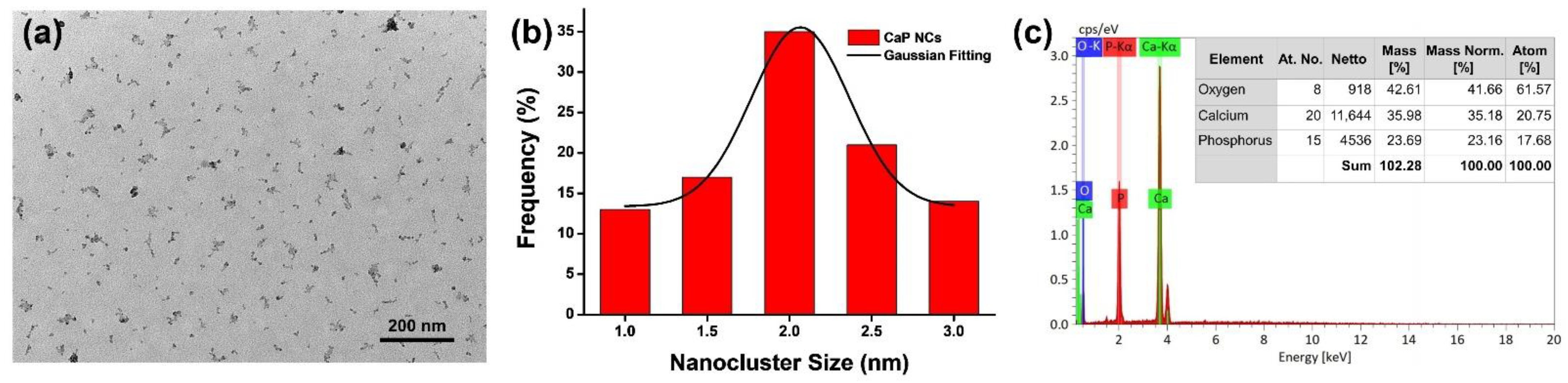
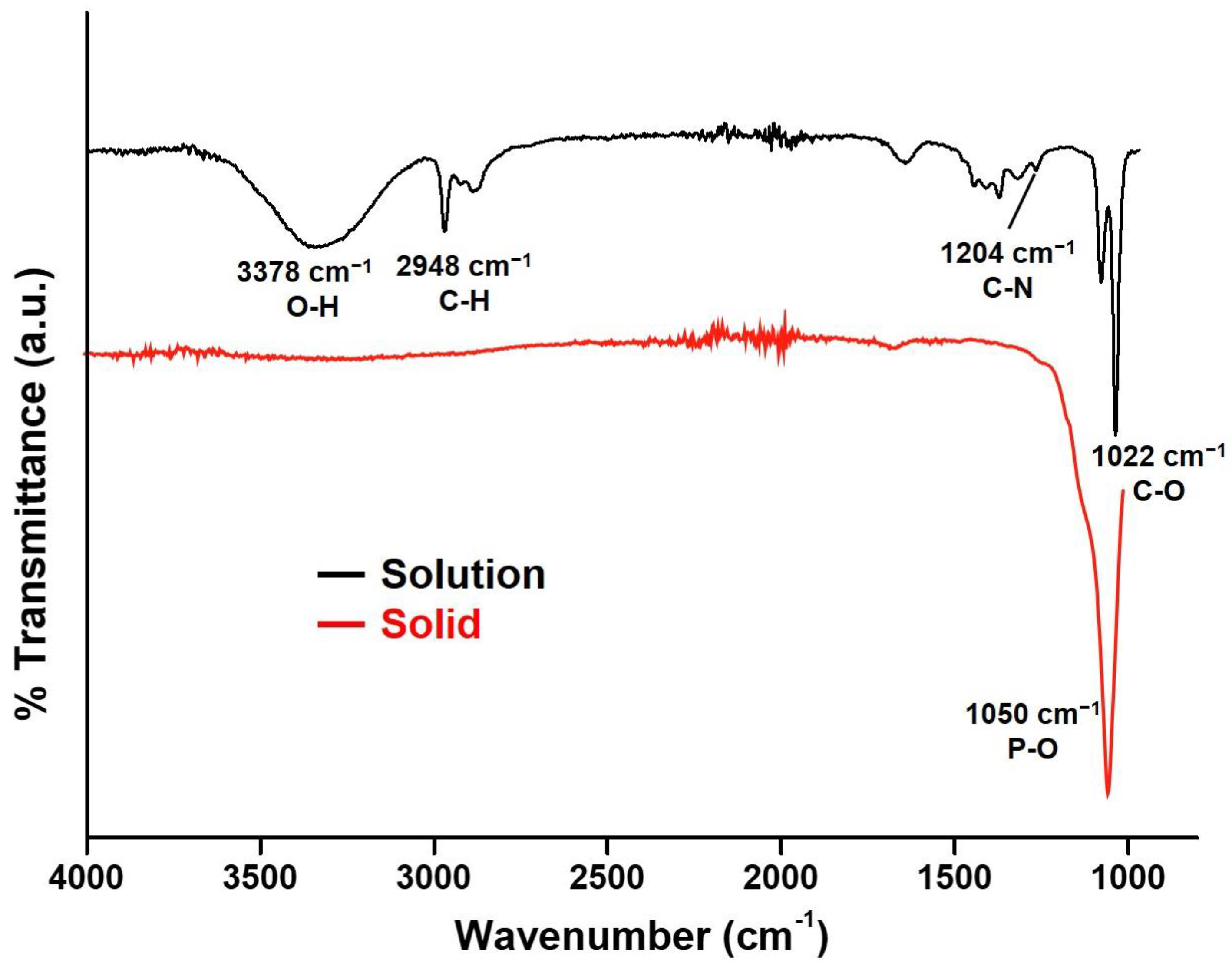
3.1. Characterizations of CaP NCs
3.2. Stability Evaluation of CaP NCs in TEA
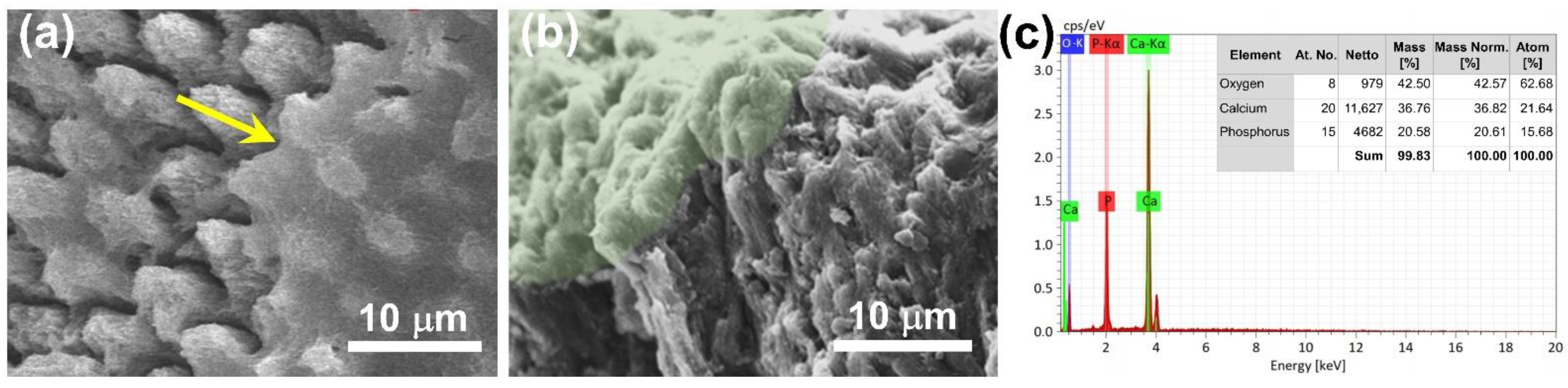
3.3. Development of CaP NCs for Repairing Tooth Enamel
3.4. Microhardness Test of the Tooth Enamel before and after Repair with CaP NCs
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cuy, J.L.; Mann, A.B.; Livi, K.J.; Teaford, M.F.; Weihs, T.P. Nanoindentation mapping of the mechanical properties of human molar tooth enamel. Arch. Oral Biol. 2002, 47, 281–291. [Google Scholar] [CrossRef]
- Mannoor, M.S.; Tao, H.; Clayton, J.D.; Sengupta, A.; Kaplan, D.L.; Naik, R.R.; Verma, N.; Omenetto, F.G.; McAlpine, M.C. Graphene-based wireless bacteria detection on tooth enamel. Nat. Commun. 2012, 3, 763. [Google Scholar] [CrossRef] [PubMed]
- Imbeni, V.; Kruzic, J.J.; Marshall, G.W.; Marshall, S.J.; Ritchie, R.O. The dentin-enamel junction and the fracture of human teeth. Nat. Mater. 2005, 4, 229–232. [Google Scholar] [CrossRef]
- Tu, Y.K.; Needleman, I.; Chambrone, L.; Lu, H.K.; Faggion, C.M. A bayesian network meta-analysis on comparisons of enamel matrix derivatives, guided tissue regeneration and their combination therapies. J. Clin. Periodontol. 2012, 39, 303–314. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.F.; Chen, J.W.; Lin, S.J.; Lu, H.K. Is coronally positioned flap procedure adjunct with enamel matrix derivative or root conditioning a relevant predictor for achieving root coverage? A systemic review. J. Periodontal Res. 2007, 42, 474–485. [Google Scholar] [CrossRef] [PubMed]
- Yeh, S.T.; Su, Y.; Lu, Y.C.; Lee, S.Y. Surface changes and acid dissolution of enamel after carbamide peroxide bleach treatment. Oper. Dent. 2005, 30, 507–515. [Google Scholar]
- Teng, N.C.; Wang, P.D.Y.; Chang, W.J.; Feng, S.W.; Fan, K.H.; Lin, C.T.; Hsieh, S.C.; Huang, H.M. Er:Yag laser-roughened enamel promotes osteoblastic differentiation. Photomed. Laser Surg. 2012, 30, 516–522. [Google Scholar] [CrossRef]
- Teng, N.C.; Pandey, A.; Hsu, W.H.; Huang, C.S.; Lee, W.F.; Lee, T.H.; Yang, T.C.K.; Yang, T.S.; Yang, J.C. Rehardening and the protective effect of gamma-polyglutamic acid/nano-hydroxyapatite paste on surface-etched enamel. Polymers 2021, 13, 4268. [Google Scholar] [CrossRef]
- Wu, S.M.; Chiu, H.C.; Chin, Y.T.; Lin, H.Y.; Chiang, C.Y.; Tu, H.P.; Fu, M.M.J.; Fu, E. Effects of enamel matrix derivative on the proliferation and osteogenic differentiation of human gingival mesenchymal stem cells. Stem Cell Res. Ther. 2014, 5, 52. [Google Scholar] [CrossRef] [Green Version]
- Dias, M.D.; Ventura, I. The child’s self-perception about dental decay in the change of deciduous teeth. Ann. Med. 2021, 53, S61. [Google Scholar] [CrossRef]
- Johal, B.K. Tooth decay in children: A red flag for abuse? Br. Dent. J. 2022, 232, 36. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Huang, Y.; Kim, D.; Ren, Z.; Oh, M.J.; Cormode, D.P.; Hara, A.T.; Zero, D.T.; Koo, H. Ferumoxytol nanoparticles target biofilms causing tooth decay in the human mouth. Nano Lett. 2021, 21, 9442–9449. [Google Scholar] [CrossRef] [PubMed]
- Dogan, S.; Fong, H.; Yucesoy, D.T.; Cousin, T.; Gresswell, C.; Dag, S.; Huang, G.; Sarikaya, M. Biomimetic tooth repair: Amelogenin-derived peptide enables in vitro remineralization of human enamel. ACS Biomater. Sci. Eng. 2018, 4, 1788–1796. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Yang, J.J.; Li, J.Y.; Chen, L.; Tang, B.; Chen, X.Y.; Wu, W.; Li, J.S. Hydroxyapatite-anchored dendrimer for in situ remineralization of human tooth enamel. Biomaterials 2013, 34, 5036–5047. [Google Scholar] [CrossRef]
- Zhao, J.; Liu, Y.; Sun, W.B.; Zhang, H. Amorphous calcium phosphate and its application in dentistry. Chem. Cent. J. 2011, 5, 40. [Google Scholar] [CrossRef] [Green Version]
- Lin, S.C.; Lin, W.C.; Hu, T.C.; Yan, M.; Tang, C.M. Evaluation of the bonding strength between various dental zirconia models and human teeth for dental posts through in vitro aging tests. Coatings 2021, 11, 1017. [Google Scholar] [CrossRef]
- Yang, K.C.; Chang, H.H.; Chang, M.H.; Chi, C.H.; Huang, Y.P.; Lin, F.H.; Kuo, T.F. Tooth regeneration from dental pulp stem cells: An allograft swine model. J. Tissue Eng. Regen. Med. 2012, 6, 2–3. [Google Scholar]
- Yang, K.C.; Kitamura, Y.; Wu, C.C.; Chang, H.H.; Ling, T.Y.; Kuo, T.F. Tooth germ-like construct transplantation for whole-tooth regeneration: An in vivo study in the miniature pig. Artif. Organs 2016, 40, E39–E50. [Google Scholar] [CrossRef]
- Yang, K.C.; Wang, C.H.; Chang, H.H.; Chan, W.P.; Chi, C.H.; Kuo, T.F. Fibrin glue mixed with platelet-rich fibrin as a scaffold seeded with dental bud cells for tooth regeneration. J. Tissue Eng. Regen. Med. 2012, 6, 777–785. [Google Scholar] [CrossRef]
- Breschi, L.; Mazzoni, A.; Ruggeri, A.; Cadenaro, M.; Di Lenarda, R.; Dorigo, E.D. Dental adhesion review: Aging and stability of the bonded interface. Dent. Mater. 2008, 24, 90–101. [Google Scholar] [CrossRef]
- Peumans, M.; Van Meerbeek, B.; Lambrechts, P.; Vanherle, G. Porcelain veneers: A review of the literature. J. Dent. 2000, 28, 163–177. [Google Scholar] [CrossRef]
- Sailer, I.; Makarov, N.A.; Thoma, D.S.; Zwahlen, M.; Pjetursson, B.E. All-ceramic or metal-ceramic tooth-supported fixed dental prostheses (fdps)? A systematic review of the survival and complication rates. Part i: Single crowns (scs). Dent. Mater. 2015, 31, 603–623. [Google Scholar] [CrossRef] [Green Version]
- Tschoppe, P.; Zandim, D.L.; Martus, P.; Kielbassa, A.M. Enamel and dentine remineralization by nano-hydroxyapatite toothpastes. J. Dent. 2011, 39, 430–437. [Google Scholar] [CrossRef] [Green Version]
- Mutalik, C.; Okoro, G.; Krisnawati, D.I.; Jazidie, A.; Rahmawati, E.Q.; Rahayu, D.; Hsu, W.T.; Kuo, T.R. Copper sulfide with morphology-dependent photodynamic and photothermal antibacterial activities. J. Colloid Interface Sci. 2022, 607, 1825–1835. [Google Scholar] [CrossRef]
- Mutalik, C.; Krisnawati, D.I.; Patil, S.B.; Khafid, M.; Atmojo, D.S.; Santoso, P.; Lu, S.C.; Wang, D.Y.; Kuo, S.R. Phase-dependent MoS2 nanoflowers for light-driven antibacterial application. ACS Sustain. Chem. Eng. 2021, 9, 7904–7912. [Google Scholar] [CrossRef]
- Yougbare, S.; Chou, H.L.; Yang, C.H.; Krisnawati, D.I.; Jazidie, A.; Nuh, M.; Kuo, T.R. Facet-dependent gold nanocrystals for effective photothermal killing of bacteria. J. Hazard. Mater. 2021, 407, 124617. [Google Scholar] [CrossRef]
- Zhu, Y.P.; Kuo, T.R.; Li, Y.H.; Qi, M.Y.; Chen, G.; Wang, J.L.; Xu, Y.J.; Chen, H.M. Emerging dynamic structure of electrocatalysts unveiled by in situ X-ray diffraction/absorption spectroscopy. Energy Environ. Sci. 2021, 14, 1928–1958. [Google Scholar] [CrossRef]
- Yougbare, S.; Mutalik, C.; Okoro, G.; Lin, I.H.; Krisnawati, D.I.; Jazidie, A.; Nuh, M.; Chang, C.C.; Kuo, T.R. Emerging trends in nanomaterials for antibacterial applications. Int. J. Nanomed. 2021, 16, 5831–5867. [Google Scholar] [CrossRef]
- Yougbare, S.; Mutalik, C.; Krisnawati, D.I.; Kristanto, H.; Jazidie, A.; Nuh, M.; Cheng, T.M.; Kuo, T.R. Nanomaterials for the photothermal killing of bacteria. Nanomaterials 2020, 10, 1123. [Google Scholar] [CrossRef]
- Mutalik, C.; Wang, D.Y.; Krisnawati, D.I.; Jazidie, A.; Yougbare, S.; Kuo, T.R. Light-activated heterostructured nanomaterials for antibacterial applications. Nanomaterials 2020, 10, 643. [Google Scholar] [CrossRef] [Green Version]
- Mutalik, C.; Hsiao, Y.-C.; Chang, Y.-H.; Krisnawati, D.I.; Alimansur, M.; Jazidie, A.; Nuh, M.; Chang, C.-C.; Wang, D.-Y.; Kuo, T.-R. High uv-vis-nir light-induced antibacterial activity by heterostructured TiO2-FeS2 nanocomposites. Int. J. Nanomed. 2020, 15, 8911. [Google Scholar] [CrossRef]
- Tung, C.W.; Kuo, T.R.; Hsu, C.S.; Chuang, Y.; Chen, H.C.; Chang, C.K.; Chien, C.Y.; Lu, Y.J.; Chan, T.S.; Lee, J.F.; et al. Light-induced activation of adaptive junction for efficient solar-driven oxygen evolution: In situ unraveling the interfacial metal-silicon junction. Adv. Energy Mater. 2019, 9, 1901308. [Google Scholar] [CrossRef]
- Kuo, T.-R.; Liao, H.-J.; Chen, Y.-T.; Wei, C.-Y.; Chang, C.-C.; Chen, Y.-C.; Chang, Y.-H.; Lin, J.-C.; Lee, Y.-C.; Wen, C.-Y. Extended visible to near-infrared harvesting of earth-abundant FeS2-TiO2 heterostructures for highly active photocatalytic hydrogen evolution. Green Chem. 2018, 20, 1640–1647. [Google Scholar] [CrossRef]
- Chang, Y.H.; Lin, J.C.; Chen, Y.C.; Kuo, T.R.; Wang, D.Y. Facile synthesis of two-dimensional ruddlesden-popper perovskite quantum dots with fine-tunable optical properties. Nanoscale Res. Lett. 2018, 13, 247. [Google Scholar] [CrossRef]
- Kuo, T.R.; Chen, W.T.; Liao, H.J.; Yang, Y.H.; Yen, H.C.; Liao, T.W.; Wen, C.Y.; Lee, Y.C.; Chen, C.C.; Wang, D.Y. Improving hydrogen evolution activity of earth-abundant cobalt-doped iron pyrite catalysts by surface modification with phosphide. Small 2017, 13, 1603356. [Google Scholar] [CrossRef]
- Wang, J.L.; Tan, H.Y.; Kuo, T.R.; Lin, S.C.; Hsu, C.S.; Zhu, Y.P.; Chu, Y.C.; Chen, T.L.; Lee, J.F.; Chen, H.M. In situ identifying the dynamic structure behind activity of atomically dispersed platinum catalyst toward hydrogen evolution reaction. Small 2021, 17, 2005713. [Google Scholar] [CrossRef]
- Pan, X.Y.; Chen, C.H.; Chang, Y.H.; Wang, D.Y.; Lee, Y.C.; Liou, C.C.; Wang, Y.X.; Hu, C.C.; Kuo, T.R. Osteoporosis risk assessment using multilayered gold-nanoparticle thin film via saldi-ms measurement. Anal. Bioanal. Chem. 2019, 411, 2793–2802. [Google Scholar] [CrossRef]
- Kuo, T.R.; Chen, Y.C.; Wang, C.I.; Shen, T.H.; Wang, H.Y.; Pan, X.Y.; Wang, D.Y.; Liou, C.C.; Chang, Y.H.; Chen, Y.C.; et al. Highly oriented langmuir-blodgett film of silver cuboctahedra as an effective matrix-free sample plate for surface-assisted laser desorption/ionization mass spectrometry. Nanoscale 2017, 9, 11119–11125. [Google Scholar] [CrossRef]
- Chang, T.-K.; Cheng, T.-M.; Chu, H.-L.; Tan, S.-H.; Kuo, J.-C.; Hsu, P.-H.; Su, C.-Y.; Chen, H.-M.; Lee, C.-M.; Kuo, T.-R. Metabolic mechanism investigation of antibacterial active cysteine-conjugated gold nanoclusters in escherichia coli. ACS Sustain. Chem. Eng. 2019, 7, 15479–15486. [Google Scholar] [CrossRef]
- Yougbare, S.; Chang, T.-K.; Tan, S.-H.; Kuo, J.-C.; Hsu, P.-H.; Su, C.-Y.; Kuo, T.-R. Antimicrobial gold nanoclusters: Recent developments and future perspectives. Int. J. Mol. Sci. 2019, 20, 2924. [Google Scholar] [CrossRef] [Green Version]
- Cheng, T.M.; Chu, H.L.; Lee, Y.C.; Wang, D.Y.; Chang, C.C.; Chung, K.L.; Yen, H.C.; Hsiao, C.W.; Pan, X.Y.; Kuo, T.R.; et al. Quantitative analysis of glucose metabolic cleavage in glucose transporters overexpressed cancer cells by target-specific fluorescent gold nanoclusters. Anal. Chem. 2018, 90, 3974–3980. [Google Scholar] [CrossRef]
- Kaur, N.; Aditya, R.N.; Singh, A.; Kuo, T.R. Biomedical applications for gold nanoclusters: Recent developments and future perspectives. Nanoscale Res. Lett. 2018, 13, 302. [Google Scholar] [CrossRef] [Green Version]
- Li, C.-H.; Kuo, T.-R.; Su, H.-J.; Lai, W.-Y.; Yang, P.-C.; Chen, J.-S.; Wang, D.-Y.; Wu, Y.-C.; Chen, C.-C. Fluorescence-guided probes of aptamer-targeted gold nanoparticles with computed tomography imaging accesses for in vivo tumor resection. Sci. Rep. 2015, 5, 15675. [Google Scholar] [CrossRef]
- Kuo, J.C.; Tan, S.H.; Hsiao, Y.C.; Mutalik, C.; Chen, H.M.; Yougbare, S.; Kuo, T.R. Unveiling the antibacterial mechanism of gold nanoclusters via in situ transmission electron microscopy. ACS Sustain. Chem. Eng. 2022, 10, 464–471. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, H.; Tian, Z.; Lu, D.; Yu, Y.; Cestellos-Blanco, S.; Sakimoto, K.K.; Yang, P. Bacteria photosensitized by intracellular gold nanoclusters for solar fuel production. Nat. Nanotech. 2018, 13, 900–905. [Google Scholar] [CrossRef]
- Galchenko, M.; Black, A.; Heymann, L.; Klinke, C. Field effect and photoconduction in Au-25 nanoclusters films. Adv. Mater. 2019, 31, 1900684. [Google Scholar] [CrossRef] [Green Version]
- Huang, Q.Q.; Hu, M.Y.; Li, Y.L.; Chen, N.N.; Li, Y.; Wei, Q.H.; Fu, F.F. Novel ultrabright luminescent copper nanoclusters and application in light-emitting devices. Chem. Commun. 2021, 57, 9890–9893. [Google Scholar] [CrossRef]
- Shao, C.Y.; Jin, B.A.; Mu, Z.; Lu, H.; Zhao, Y.Q.; Wu, Z.F.; Yan, L.M.; Zhang, Z.S.; Zhou, Y.C.; Pan, H.H.; et al. Repair of tooth enamel by a biomimetic mineralization frontier ensuring epitaxial growth. Sci. Adv. 2019, 5, eaaw9569. [Google Scholar] [CrossRef] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, C.-H.; Mutalik, C.; Yougbaré, S.; Teng, N.-C.; Kuo, T.-R. Calcium Phosphate Nanoclusters for the Repair of Tooth Enamel Erosion. Nanomaterials 2022, 12, 1997. https://doi.org/10.3390/nano12121997
Wang C-H, Mutalik C, Yougbaré S, Teng N-C, Kuo T-R. Calcium Phosphate Nanoclusters for the Repair of Tooth Enamel Erosion. Nanomaterials. 2022; 12(12):1997. https://doi.org/10.3390/nano12121997
Chicago/Turabian StyleWang, Chia-Hsien, Chinmaya Mutalik, Sibidou Yougbaré, Nai-Chia Teng, and Tsung-Rong Kuo. 2022. "Calcium Phosphate Nanoclusters for the Repair of Tooth Enamel Erosion" Nanomaterials 12, no. 12: 1997. https://doi.org/10.3390/nano12121997
APA StyleWang, C.-H., Mutalik, C., Yougbaré, S., Teng, N.-C., & Kuo, T.-R. (2022). Calcium Phosphate Nanoclusters for the Repair of Tooth Enamel Erosion. Nanomaterials, 12(12), 1997. https://doi.org/10.3390/nano12121997






