Synthesis, Phase Transformations and Strength Properties of Nanostructured (1 − x)ZrO2 − xCeO2 Composite Ceramics
Abstract
:1. Introduction
2. Experimental Part
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Karoutas, Z.; Brown, J.; Atwood, A.; Hallstadius, L.; Lahoda, E.; Ray, S.; Bradfute, J. The maturing of nuclear fuel: Past to Accident Tolerant Fuel. Prog. Nucl. Energy 2018, 102, 68–78. [Google Scholar] [CrossRef]
- Terrani, K.A.; Zinkle, S.J.; Snead, L.L. Advanced oxidation-resistant iron-based alloys for LWR fuel cladding. J. Nucl. Mater. 2014, 448, 420–435. [Google Scholar] [CrossRef]
- Martelli, E.; Del Nevo, A.; Lurosso, P.; Giannetti, F.; Narcisi, V.; Tarantino, M. Investigation of heat transfer in a steam generator bayonet tube for the development of PbLi technology for EU DEMO fusion reactor. Fusion Eng. Des. 2020, 159, 111772. [Google Scholar] [CrossRef]
- Hanson, S.K.; Pollington, A.D. Measuring signatures of fuel irradiation in large particle samples. J. Anal. At. Spectrom. 2021, 36, 1018–1027. [Google Scholar] [CrossRef]
- Taheranpour, N.; Talebi, S. Development of a new efficient method using genetic algorithm for increasing of fuel rod life time. Prog. Nucl. Energy 2021, 131, 103600. [Google Scholar] [CrossRef]
- Voyevodin, V.N.; Tolstolutskaya, G.D.; Tikhonovsky, M.; Kuprin, A.S. Mechanisms of radiation damage and development of structural materials for operating and advanced nuclear reactors. Probl. At. Sci. Technol. 2021, 135, 3–20. [Google Scholar] [CrossRef]
- Stewart, R.; Blakely, C.; Zhang, H. Investigation of a two-year cycle pressurized water reactor core design with increased enrichment and extended burnup limits. Nucl. Eng. Des. 2021, 376, 111132. [Google Scholar] [CrossRef]
- Cheniour, A.; Pastore, G.; Harp, J.M.; Petrie, C.M.; Capps, N.A. Application of BISON to UO2 MiniFuel fission gas release analysis. J. Nucl. Mater. 2022, 565, 153686. [Google Scholar] [CrossRef]
- Gohar, Y.; Cao, Y.; Adam, R.K. ADS design concept for disposing of the US spent nuclear fuel inventory. Ann. Nucl. Energy 2021, 160, 108385. [Google Scholar] [CrossRef]
- Yun, D.; Lu, C.; Zhou, Z.; Wu, Y.; Liu, W.; Guo, S.; Shi, T.; Stubbins, J. Current state and prospect on the development of advanced nuclear fuel system materials: A review. Mater. Rep. Energy 2021, 1, 100007. [Google Scholar] [CrossRef]
- Frieß, F.; Liebert, W. Inert-matrix fuel for transmutation: Selected mid-and long-term effects on reprocessing, fuel fabrication and inventory sent to final disposal. Prog. Nucl. Energy 2022, 145, 104106. [Google Scholar] [CrossRef]
- Bhandari, K.; Grover, V.; Roy, A.; Bhatt, R.; Banerjee, J.; Tyagi, A.K. (Y1-xNdx)3Zr5O14.5 solid solutions as inert matrices: Phase evolution, order-disorder dynamics and thermophysical behavior. Mater. Today Commun. 2021, 27, 102158. [Google Scholar] [CrossRef]
- Yang, K.; Bryce, K.; Zhu, W.; Zhao, D.; Lian, J. Multicomponent pyrochlore solid solutions with uranium incorporation—A new perspective of materials design for nuclear applications. J. Eur. Ceram. Soc. 2021, 41, 2870–2882. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, H.; Wei, H.; Zhang, J.; Tang, C.; Lu, C.; Huang, C.; Ding, S.; Li, Y. Modelling of effective irradiation swelling for inert matrix fuels. Nucl. Eng. Technol. 2021, 53, 2616–2628. [Google Scholar] [CrossRef]
- Hudák, I.; Skryja, P.; Bojanovský, J.; Jegla, Z.; Krňávek, M. The Effect of Inert Fuel Compounds on Flame Characteristics. Energies 2021, 15, 262. [Google Scholar] [CrossRef]
- Wei, H.; Zhang, J.; Zhang, Y.; Li, L.; Ding, S.; Ren, Q. Modeling of irradiation-induced thermo-mechanical coupling and multi-scale behavior in a fully ceramic-microencapsulated fuel pellet. J. Nucl. Mater. 2021, 544, 152673. [Google Scholar] [CrossRef]
- Medvedev, P.G.; Lambregts, M.J.; Meyer, M.K. Thermal conductivity and acid dissolution behavior of MgO–ZrO2 ceramics for use in LWR inert matrix fuel. J. Nucl. Mater. 2006, 349, 167–177. [Google Scholar] [CrossRef]
- Ivanov, I.A.; Rspayev, R.M.; Sapar, A.D.; Mustafin, D.A.; Zdorovets, M.V.; Kozlovskiy, A.L. Study of the Effect of Y2O3 Doping on the Resistance to Radiation Damage of CeO2 Microparticles under Irradiation with Heavy Xe22+ Ions. Crystals 2021, 11, 1459. [Google Scholar] [CrossRef]
- Wang, Y.; Guo, Y.; Wu, Y.; Liu, Y.; Liu, L.; Zhang, C.; Zhang, Z.; Zhang, J.; Qiu, S.; Su, G.H.; et al. Preliminary analysis on the thermal-mechanical behavior of dispersed plate-type fuel under reactivity insertion accident. Ann. Nucl. Energy 2021, 163, 108509. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, J.; Wang, H.; Wei, H.; Tang, C.; Lu, C.; Ding, S.; Li, Y. Research on the Homogenized Postirradiation Elastoplastic Constitutive Relations for Composite Nuclear Fuels. Front. Mater. 2021, 8, 207. [Google Scholar] [CrossRef]
- Tang, J.; Cheng, T.; Wang, Y.; Hu, L.; Hong, M.; Qin, W.; Cai, G.; Jiang, C.; Ren, F. Constructing high-performance radiation-resistant ternary YSZ-MgO-CNT nanocomposites via tailored nanostructures. J. Eur. Ceram. Soc. 2021, 41, 5280–5291. [Google Scholar] [CrossRef]
- Nandi, C.; Kaity, S.; Jain, D.; Grover, V.; Prakash, A.; Behere, P.G. Characterization and thermophysical properties of Zr0.8Nd0.2O1.9–MgO composite. Nucl. Eng. Technol. 2021, 53, 603–610. [Google Scholar] [CrossRef]
- Medvedev, P.G.; Frank, S.M.; O’Holleran, T.P.; Meyer, M.K. Dual phase MgO–ZrO2 ceramics for use in LWR inert matrix fuel. J. Nucl. Mater. 2005, 342, 48–62. [Google Scholar] [CrossRef]
- Nandi, C.; Grover, V.; Bhandari, K.; Bhattacharya, S.; Mishra, S.; Banerjee, J.; Prakash, A.; Tyagi, A.K. Exploring YSZ/ZrO2-PuO2 systems: Candidates for inert matrix fuel. J. Nucl. Mater. 2018, 508, 82–91. [Google Scholar] [CrossRef]
- Schneider, E.A.; Deinert, M.R.; Herring, S.T.; Cady, K.B. Burnup simulations and spent fuel characteristics of ZrO2 based inert matrix fuels. J. Nucl. Mater. 2007, 361, 41–51. [Google Scholar] [CrossRef]
- Liu, B.; Xiao, H.; Zhang, Y.; Aidhy, D.S.; Weber, W.J. Investigation of oxygen point defects in cubic ZrO2 by density functional theory. Comput. Mater. Sci. 2014, 92, 22–27. [Google Scholar] [CrossRef]
- Fridman, E.; Galperin, A.; Shwageraus, E. Dissolution, Reactor, and Environmental Behavior of ZrO2-MgO Inert Fuel Matrix: Neutronic Evaluation of ZrO2-MgO Inert Fuels; Final Report; Ben-Gurion University of the Negev: Beersheba, Israel, 2005; Volume 1, pp. 1–70. [Google Scholar]
- Schuster, B.; Lang, M.; Klein, R.; Trautmann, C.; Neumann, R.; Benyagoub, A. Structural phase transition in ZrO2 induced by swift heavy ion irradiation at high-pressure. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2009, 267, 964–968. [Google Scholar] [CrossRef]
- Giniyatova, S.G.; Sailaukhanov, N.A.; Nesterov, E.; Zdorovets, M.V. Research of Structural, Strength and Thermal Properties of ZrO2—CeO2 Ceramics Doped with Yttrium. Crystals 2022, 12, 242. [Google Scholar] [CrossRef]
- Grover, V.; Tyagi, A.K. Sub-solidus phase equilibria in the CeO2–ThO2–ZrO2 system. J. Nucl. Mater. 2002, 305, 83–89. [Google Scholar] [CrossRef]
- Agarkov, D.A.; Borik, M.A.; Bublik, V.T.; Bredikhin, S.I.; Chislov, A.S.; Kulebyakin, A.V.; Kuritsyna, I.E.; Lomonova, E.E.; Milovich, F.O.; Myzina, V.A.; et al. Structure and transport properties of melt grown Sc2O3 and CeO2 doped ZrO2 crystals. Solid State Ion. 2018, 322, 24–29. [Google Scholar] [CrossRef]
- Lee, D.-S.; Kim, W.S.; Choi, S.H.; Kim, J.; Lee, H.-W.; Lee, J.-H. Characterization of ZrO2 co-doped with Sc2O3 and CeO2 electrolyte for the application of intermediate temperature SOFCs. Solid State Ion. 2005, 176, 33–39. [Google Scholar] [CrossRef]
- Wu, X.; Wu, X.; Liang, Q.; Fan, J. Structure and oxygen storage capacity of Pr/Nd doped CeO2–ZrO2 mixed oxides. Solid State Sci. 2007, 9, 636–643. [Google Scholar] [CrossRef]
- Alin, M.; Kozlovskiy, A.L.; Zdorovets, M.V.; Uglov, V.V. Study of the mechanisms of the t-ZrO2→c-ZrO2 type polymorphic transformations in ceramics as a result of irradiation with heavy Xe22+ ions. Solid State Sci. 2022, 123, 106791. [Google Scholar] [CrossRef]
- Ghyngazov, S.A.; Boltueva, V.A.; O’Connell, J.H.; Vershinina, T.N.; Kirilkin, N.S.; Rymzhanov, R.A.; Skuratov, V.A.; Surzhikov, A.P. Swift heavy ion induced phase transformations in partially stabilized ZrO2. Radiat. Phys. Chem. 2022, 192, 109917. [Google Scholar] [CrossRef]
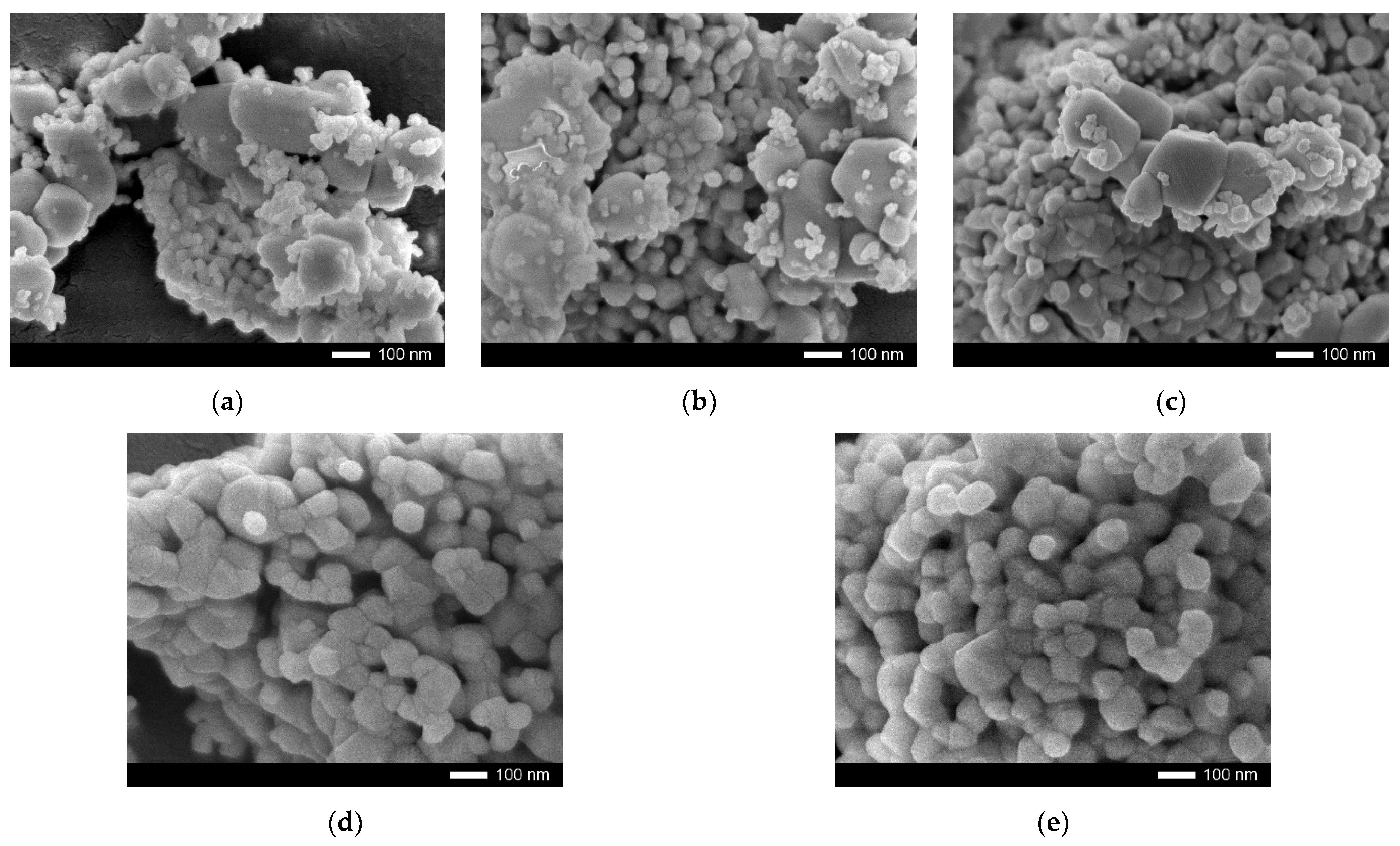
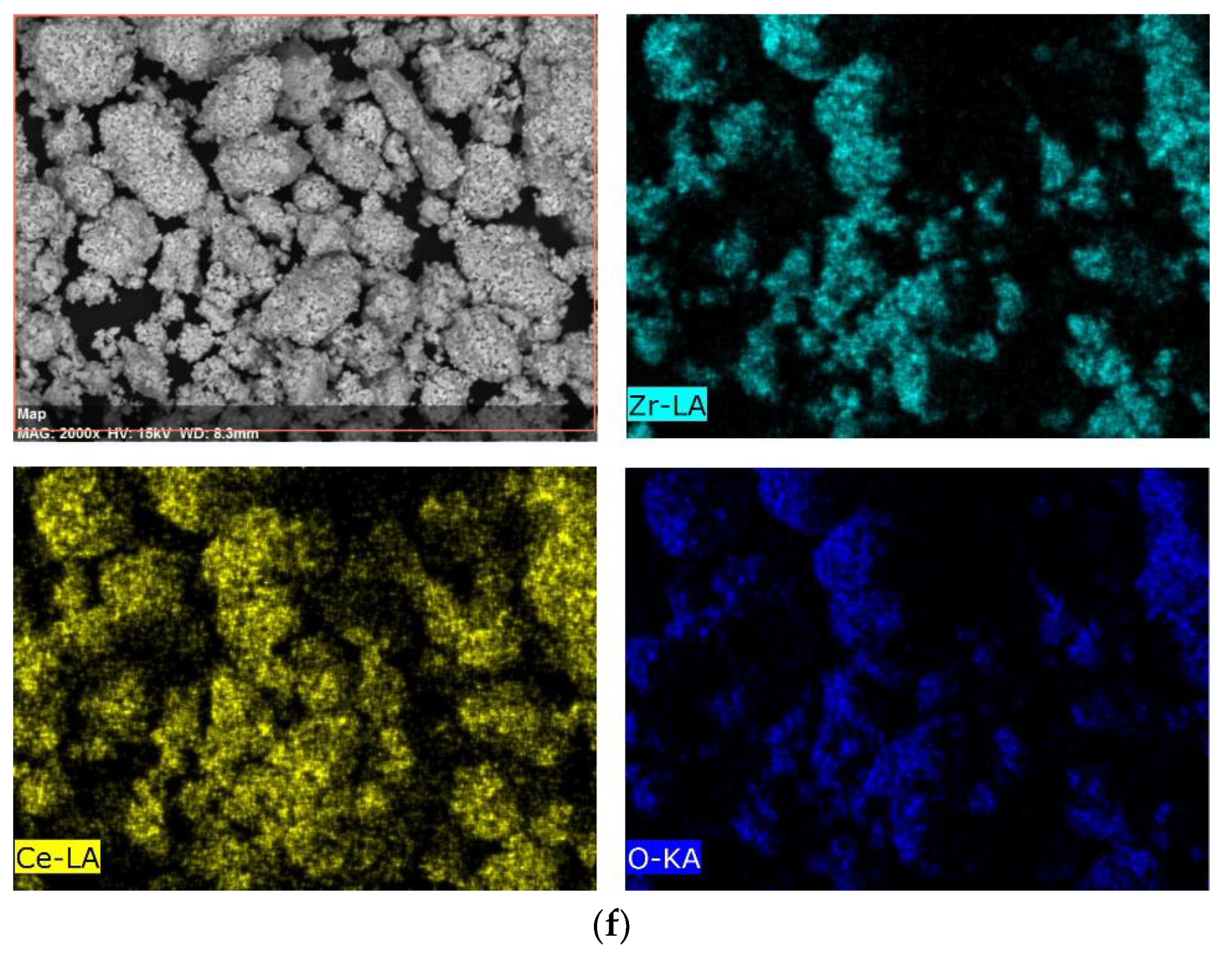
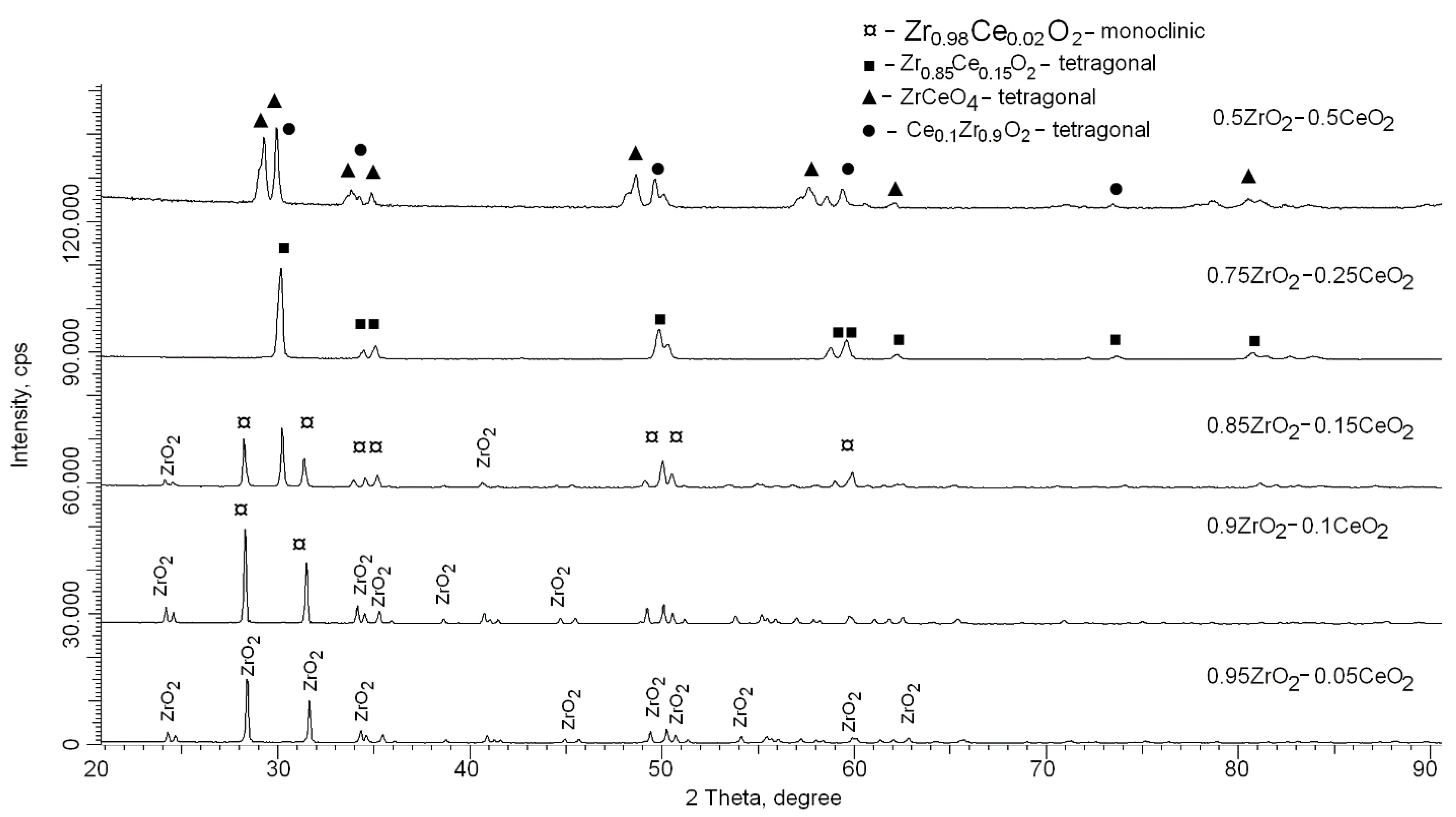
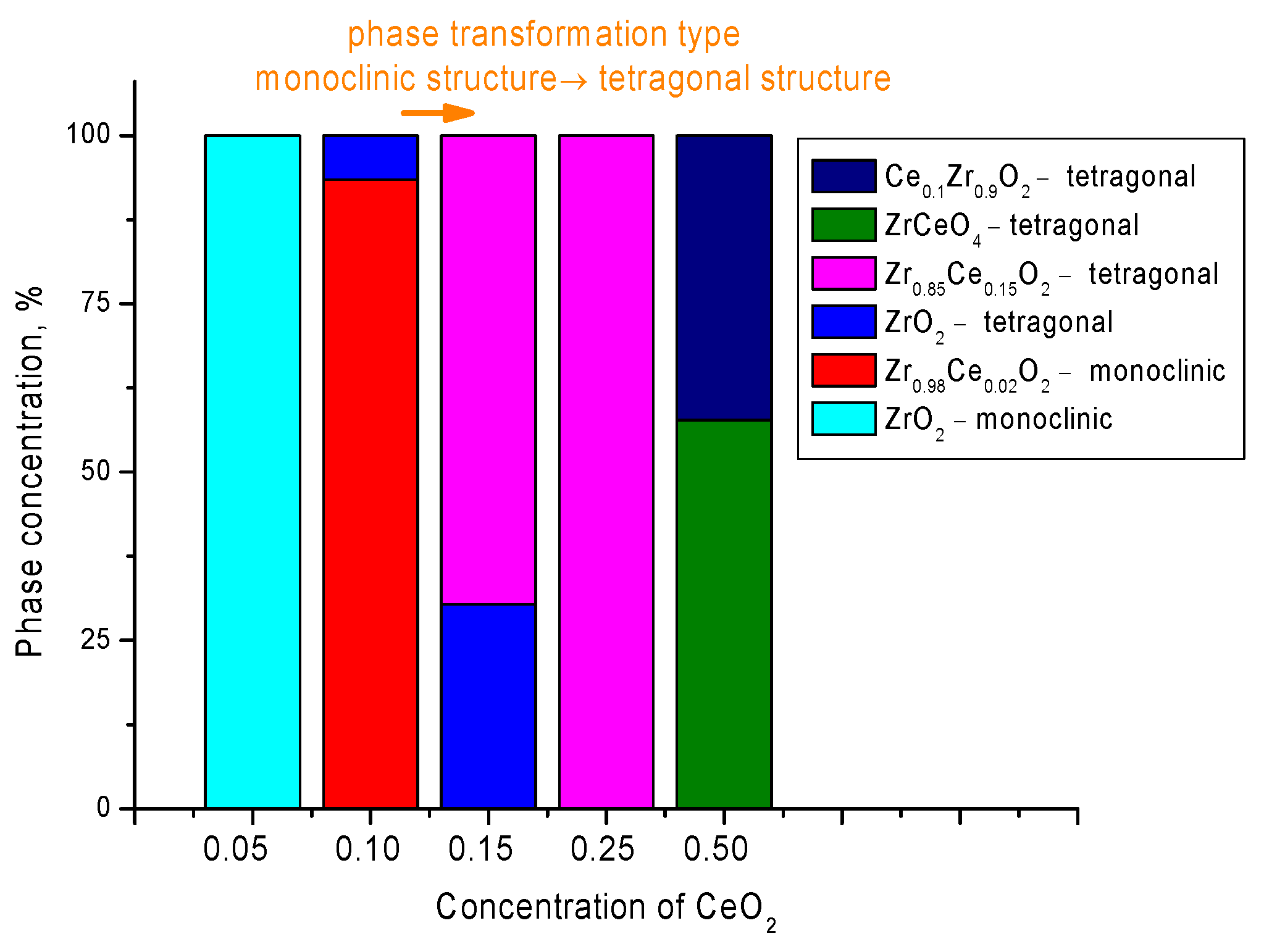
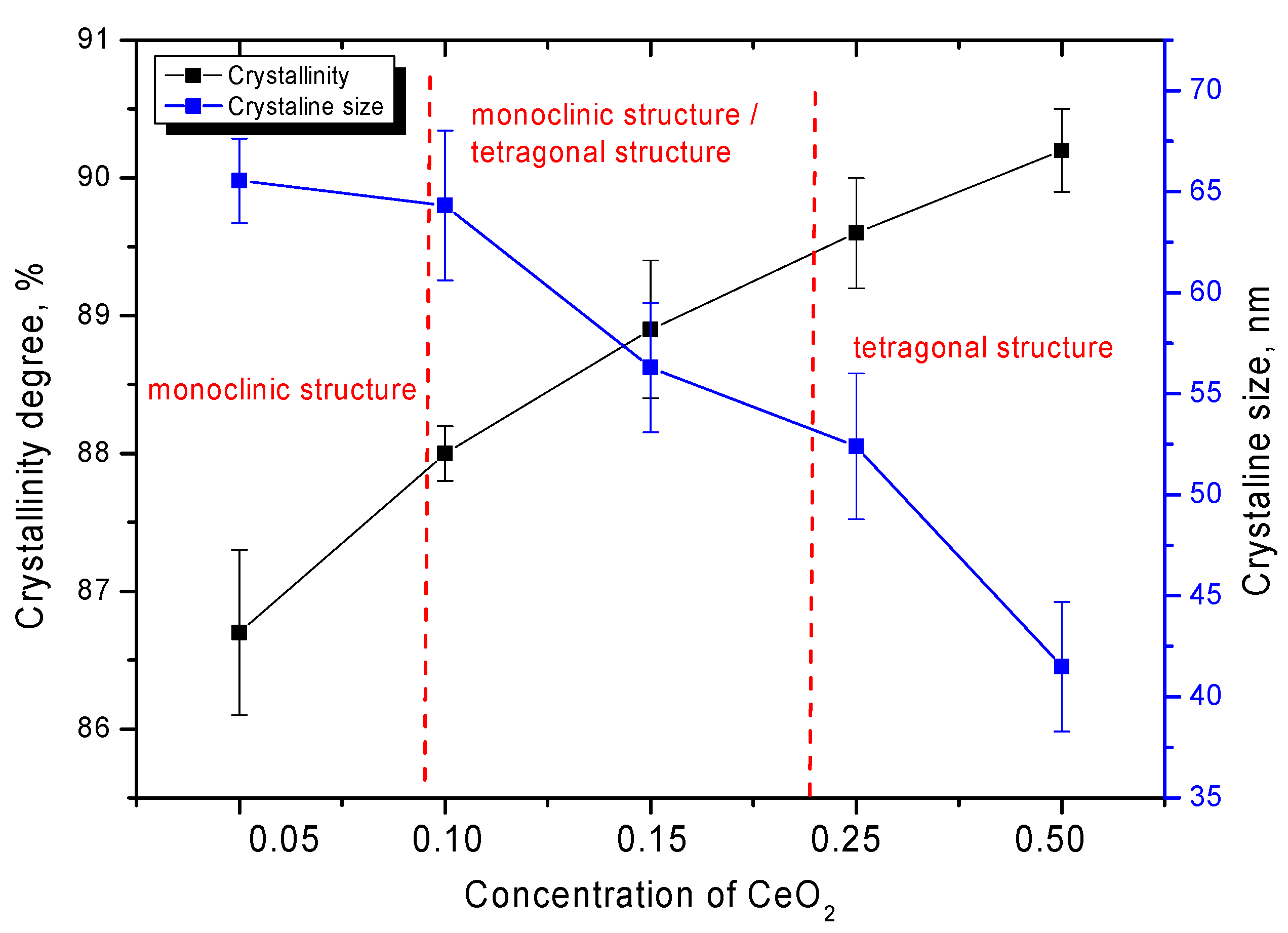
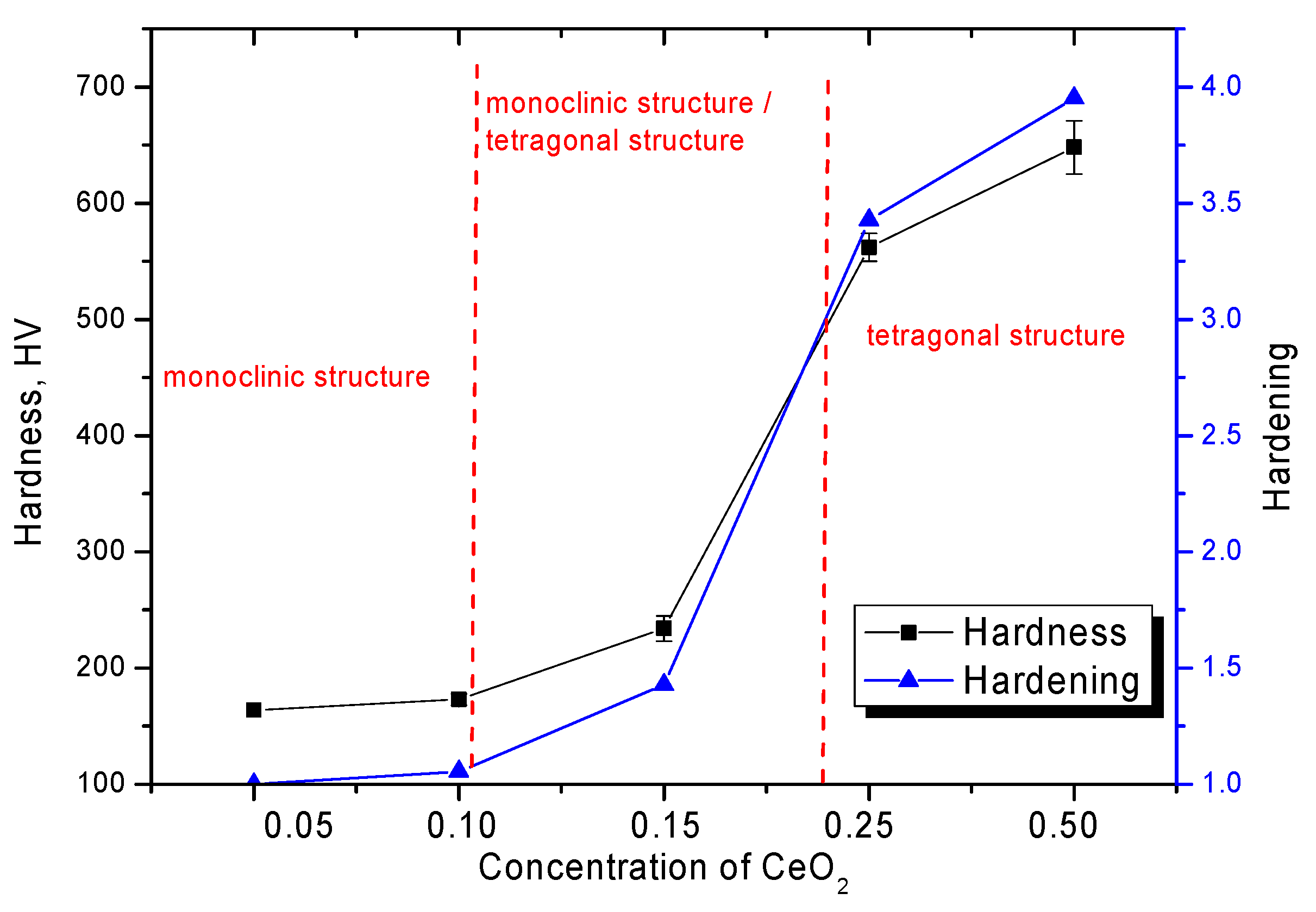

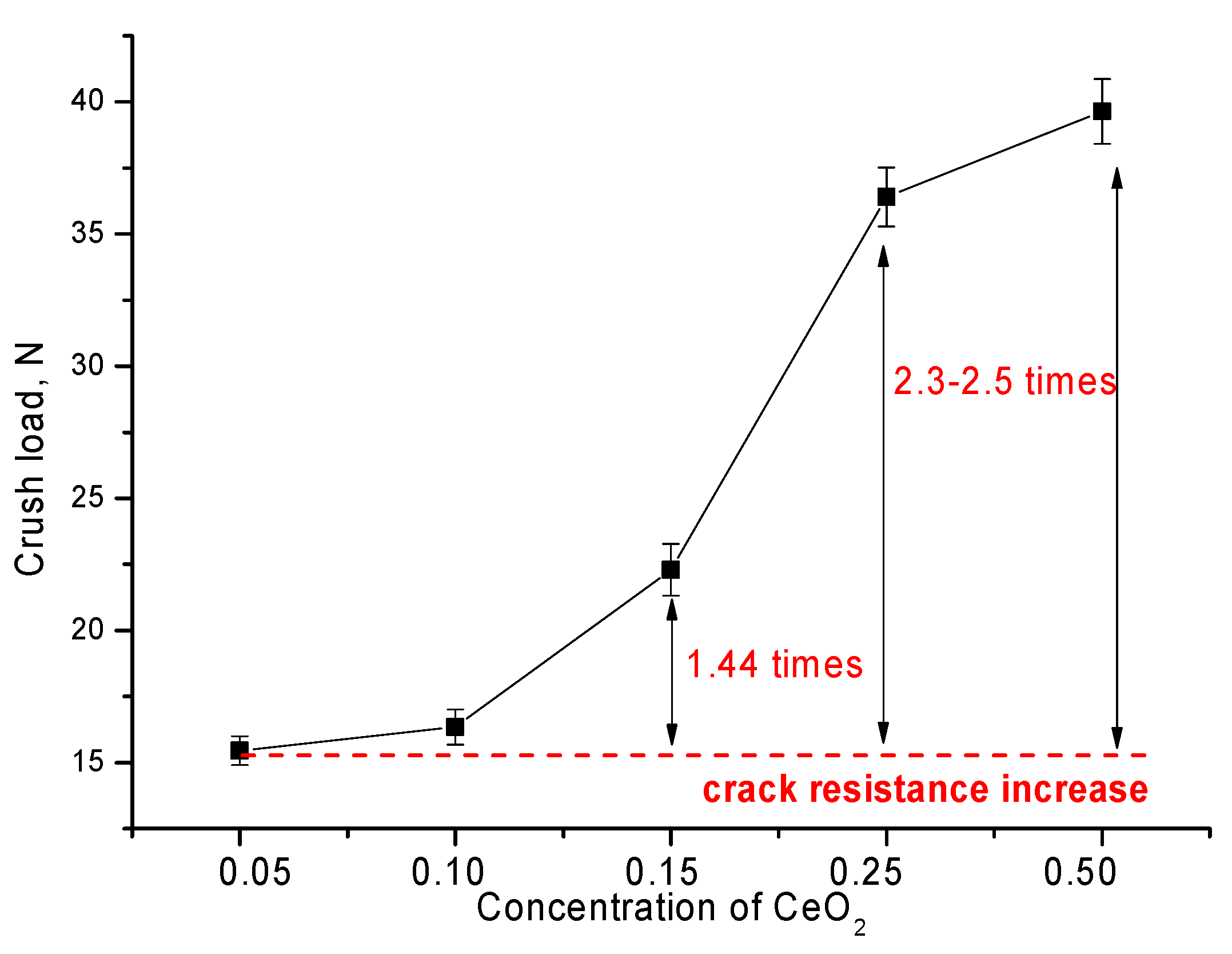
| Lattice Parameter | Concentration of CeO2 | ||||
|---|---|---|---|---|---|
| Phase | 0.05 | 0.10 | 0.15 | 0.25 | 0.50 |
| ZrO2—monoclinic P21/c(14) | a = 5.10953 ± 0.00012 Å, b = 5.18829 ± 0.00015 Å, c = 5.29281 ± 0.00016 Å, β = 98.950° | - | - | - | - |
| Zr0.98Ce0.02O2—monoclinic P21/c(14) | - | a = 5.14495 ± 0.00021 Å, b = 5.20584 ± 0.00016 Å, c = 5.30924 ± 0.00017 Å, β = 98,852° | a = 5.15503 ± 0.00015 Å, b = 5.20990 ± 0.00027 Å, c = 5.30404 ± 0.00023 Å, β = 98.773° | - | - |
| ZrO2—tetragonal P42/nmc(137) | - | a = 3.58742 ± 0.00015 Å, c = 5.17585 ± 0.00017 Å | a = 3.59234 ± 0.00011 Å, c = 5.18498 ± 0.00016 Å | - | - |
| Zr0.85Ce0.15O2—tetragonal, P42/nmc(137) | - | - | - | a = 3.61188 ± 0.00026 Å, c = 5.18693 ± 0.00017 Å | - |
| ZrCeO4—tetragonal P42/nmc(137) | - | - | - | - | a = 3.73690 ± 0.00017 Å, c = 5.29911 ± 0.00012 Å |
| Ce0.1Zr0.9O2—tetragonal P42/nmc(137) | - | - | - | - | a = 3.62745 ± 0.00014 Å, c = 5.22967 ± 0.00018 Å |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Berguzinov, A.; Kozlovskiy, A.; Khametova, A.A.; Shlimas, D.I. Synthesis, Phase Transformations and Strength Properties of Nanostructured (1 − x)ZrO2 − xCeO2 Composite Ceramics. Nanomaterials 2022, 12, 1979. https://doi.org/10.3390/nano12121979
Berguzinov A, Kozlovskiy A, Khametova AA, Shlimas DI. Synthesis, Phase Transformations and Strength Properties of Nanostructured (1 − x)ZrO2 − xCeO2 Composite Ceramics. Nanomaterials. 2022; 12(12):1979. https://doi.org/10.3390/nano12121979
Chicago/Turabian StyleBerguzinov, Askhat, Artem Kozlovskiy, Ainagul A. Khametova, and Dmitriy I. Shlimas. 2022. "Synthesis, Phase Transformations and Strength Properties of Nanostructured (1 − x)ZrO2 − xCeO2 Composite Ceramics" Nanomaterials 12, no. 12: 1979. https://doi.org/10.3390/nano12121979
APA StyleBerguzinov, A., Kozlovskiy, A., Khametova, A. A., & Shlimas, D. I. (2022). Synthesis, Phase Transformations and Strength Properties of Nanostructured (1 − x)ZrO2 − xCeO2 Composite Ceramics. Nanomaterials, 12(12), 1979. https://doi.org/10.3390/nano12121979







