Tunable Carrier Transfer of Polymeric Carbon Nitride with Charge-Conducting CoV2O6∙2H2O for Photocatalytic O2 Evolution
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of PCN
2.2. Synthesis of Transition-Metal Vanadates (Fe, Co, Ni, Cu, Mn)
2.3. Synthesis of CoVO/PCN
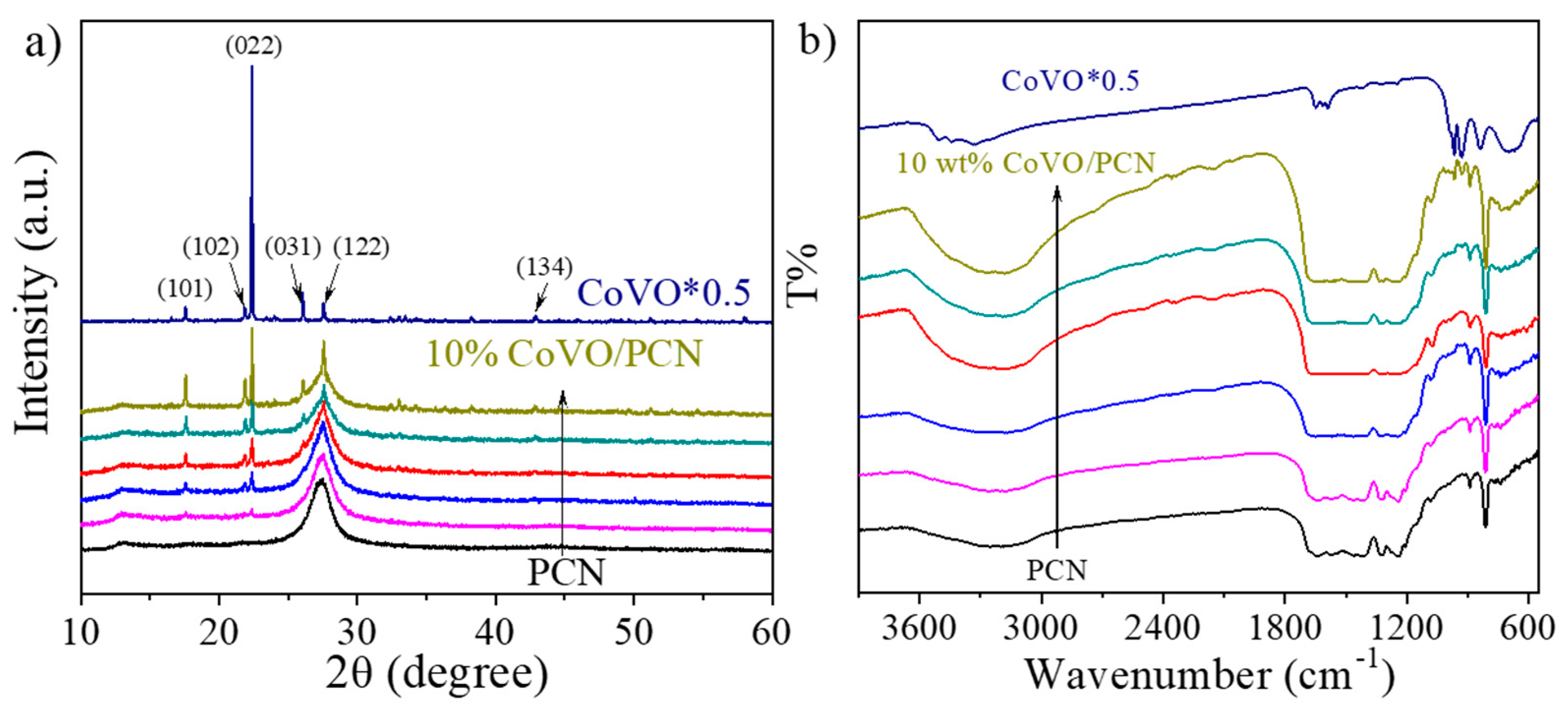
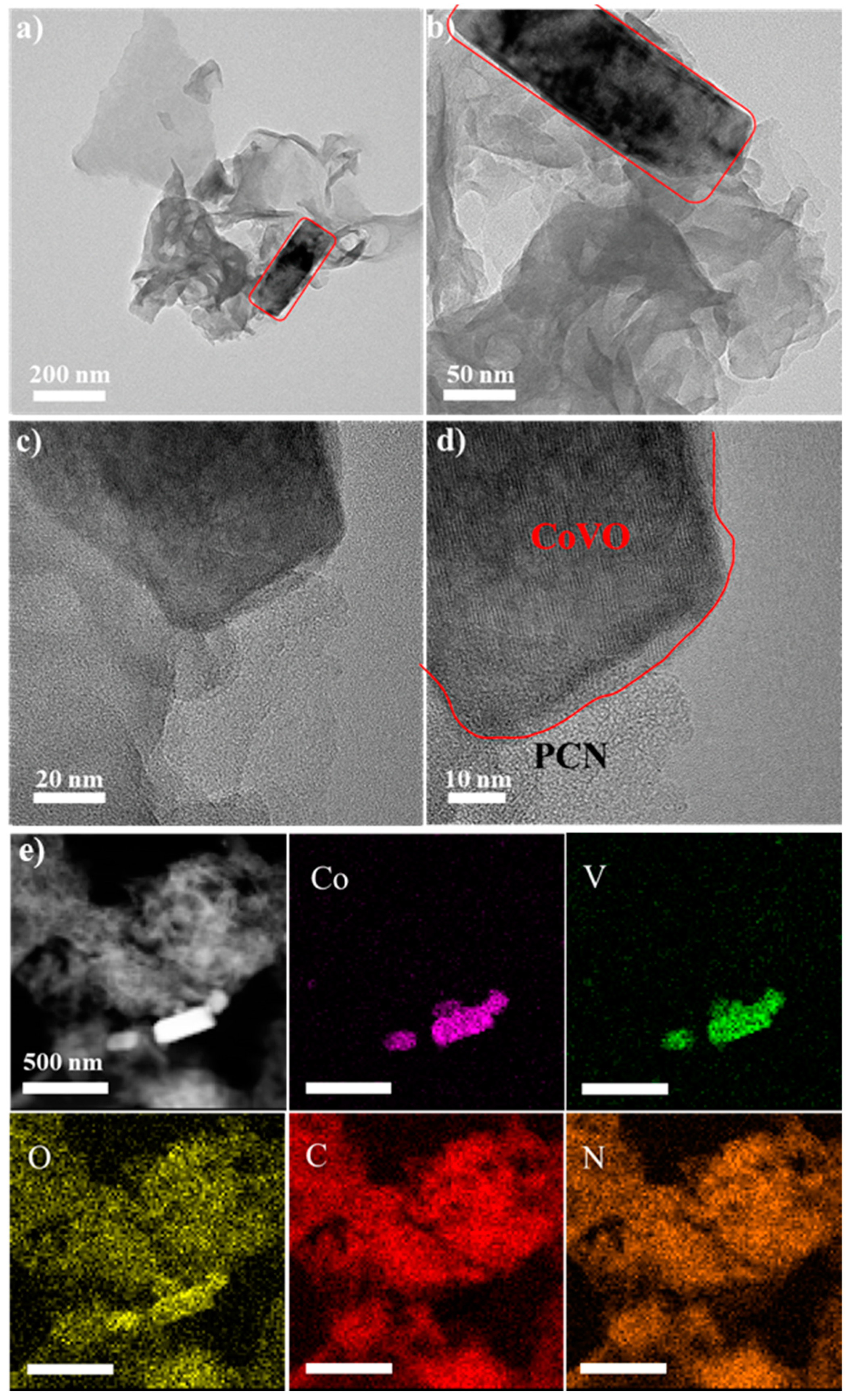
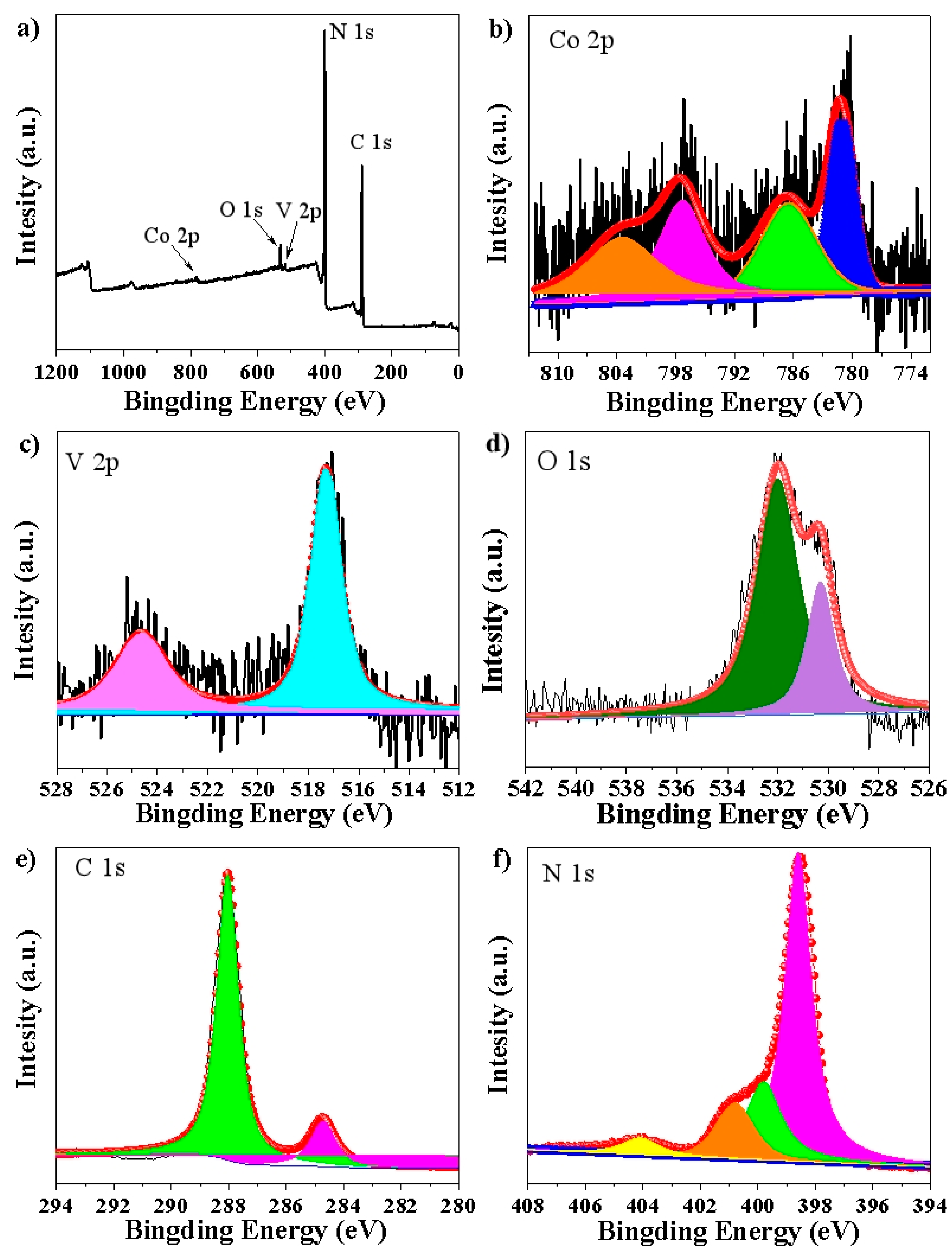
2.4. Characterization
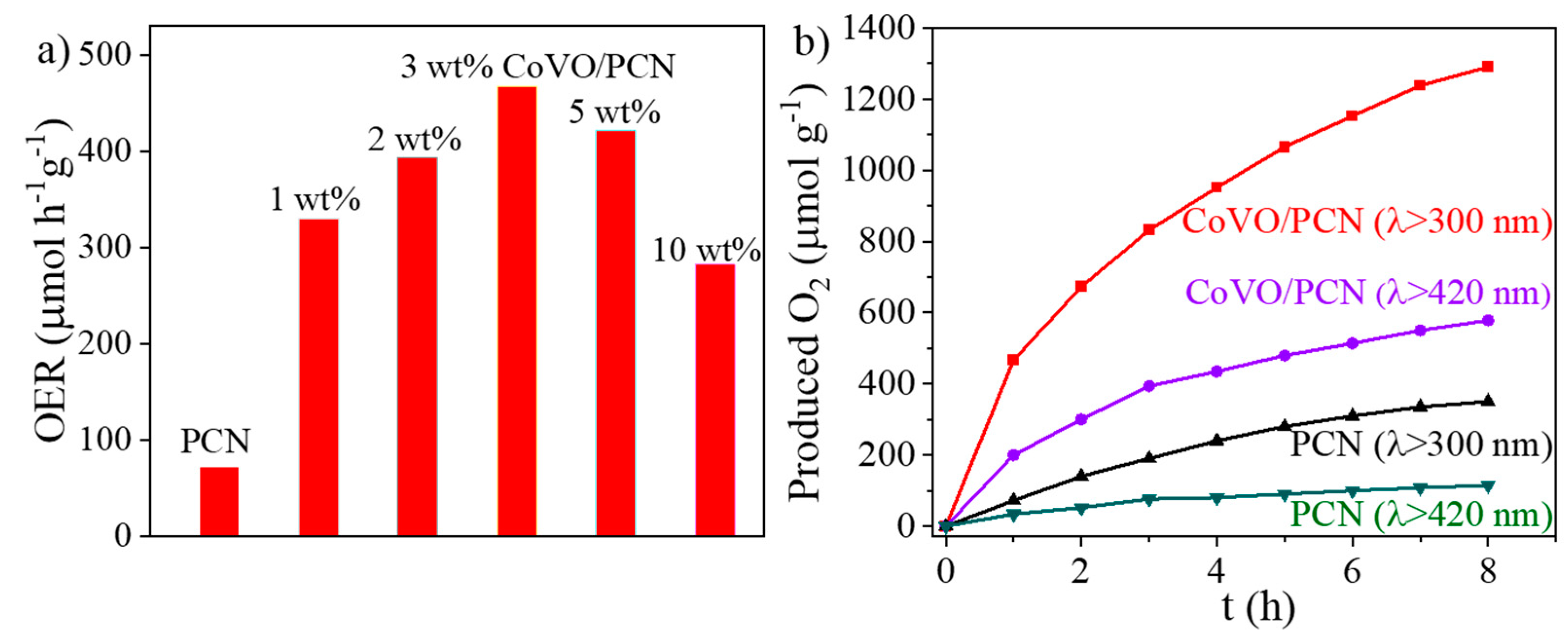
2.5. Photoactivity for Water Oxidation Reaction
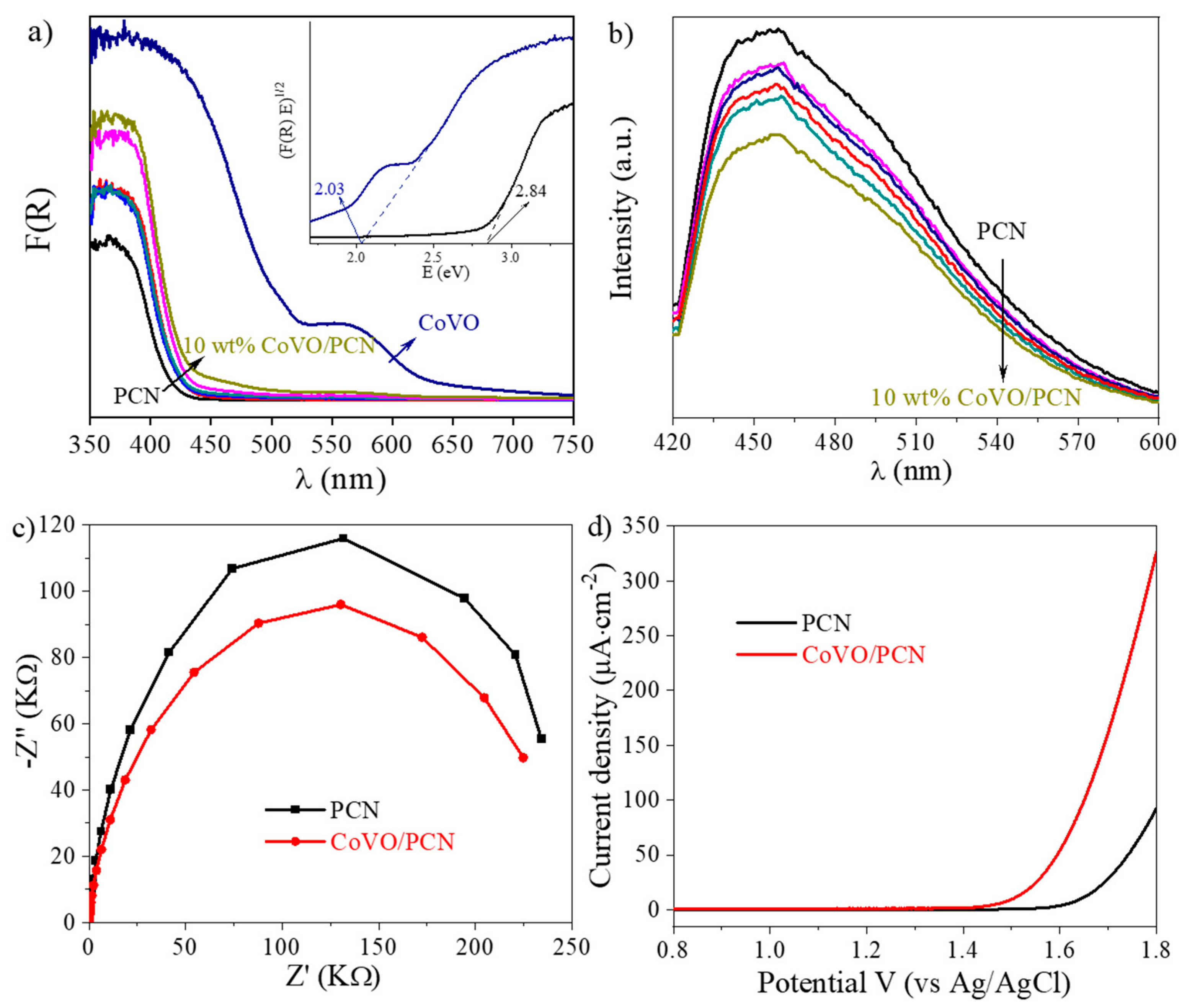
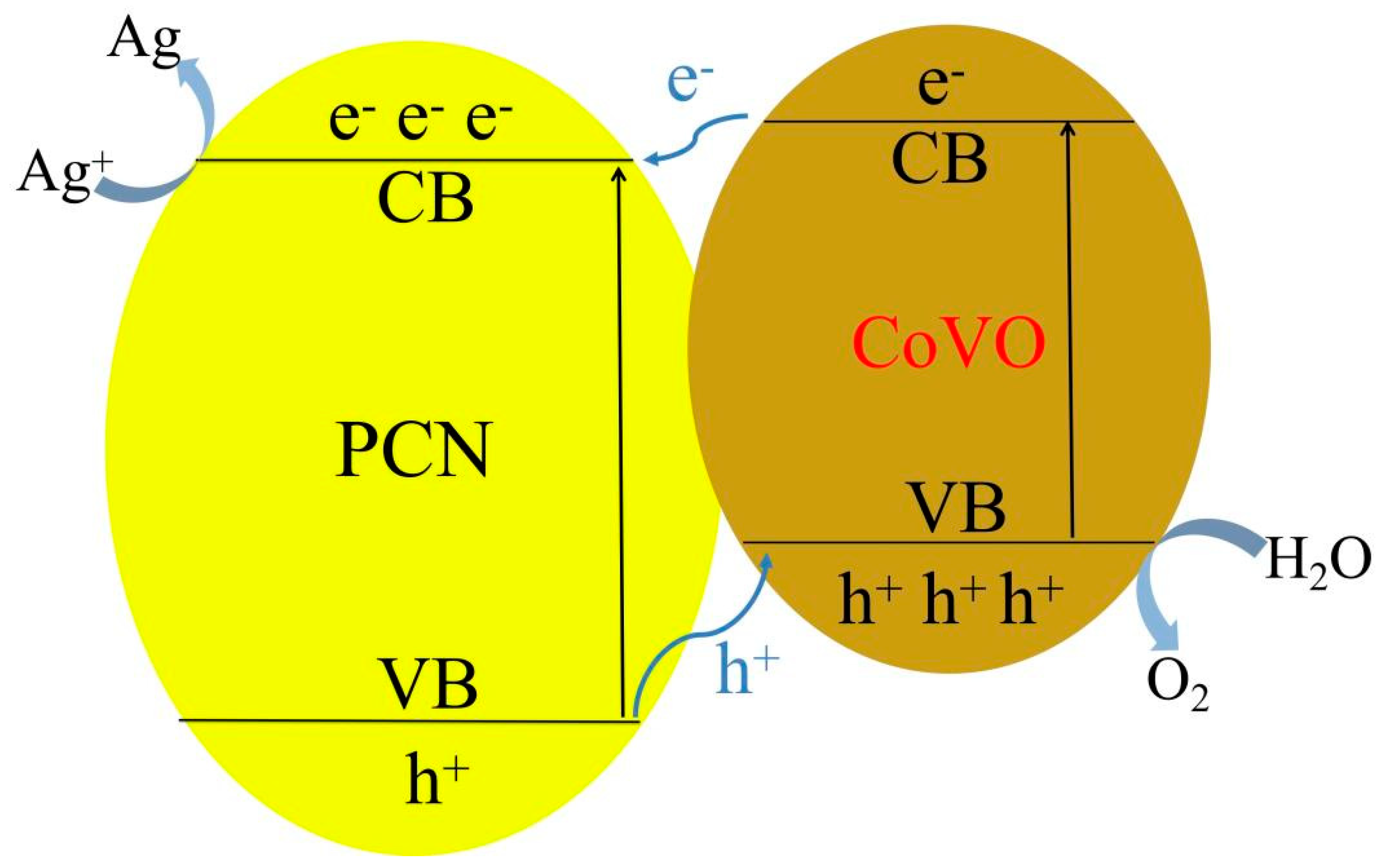
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Matsuoka, M.; Kitano, M.; Takeuchi, M.; Tsujimaru, K.; Anpo, M.; Thomas, J.M. Photocatalysis for new energy production: Recent advances in photocatalytic water splitting reactions for hydrogen production. Catal. Today 2007, 122, 51–61. [Google Scholar] [CrossRef]
- Kim, G.H.; Park, Y.S.; Yang, J.; Jang, M.J.; Jeong, J.; Lee, J.H.; Park, H.S.; Park, Y.H.; Choi, S.M.; Lee, J. Effects of annealing temperature on the oxygen evolution reaction activity of copper-cobalt oxide nanosheets. Nanomaterials 2021, 11, 657. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Guo, C.; Ning, J.; Zhong, Y.; Chen, D.; Hu, Y. Oxygen-vacancy-assisted construction of FeOOH/CdS heterostructure as an efficient bifunctional photocatalyst for CO2 conversion and water oxidation. Appl. Catal. B Environ. 2021, 293, 120203. [Google Scholar] [CrossRef]
- Fang, Y.; Hou, Y.; Fu, X.; Wang, X. Semiconducting polymers for oxygen evolution reaction under light illumination. Chem. Rev. 2022, 122, 4204–4256. [Google Scholar] [CrossRef]
- Ran, J.; Zhang, J.; Yu, J.; Jaroniec, M.; Qiao, S.Z. Earth-abundant cocatalysts for semiconductor-based photocatalytic water splitting. Chem Soc. Rev. 2014, 43, 7787–7812. [Google Scholar] [CrossRef]
- Wang, X.; Maeda, K.; Thomas, A.; Takanabe, K.; Xin, G.; Carlsson, J.M.; Domen, K.; Antonietti, M. A metal-free polymeric photocatalyst for hydrogen production from water under visible light. Nat. Mater. 2009, 8, 76–80. [Google Scholar] [CrossRef]
- Zhang, J.; Grzelczak, M.; Hou, Y.; Maeda, K.; Domen, K.; Fu, X.; Antonietti, M.; Wang, X. Photocatalytic oxidation of water by polymeric carbon nitride nanohybrids made of sustainable elements. Chem. Sci. 2012, 3, 443–446. [Google Scholar] [CrossRef]
- Zheng, D.; Yang, L.; Chen, W.; Fang, Y.; Wang, X. Coating polymeric carbon nitride on conductive carbon cloth to promote charge separation for photocatalytic water splitting. ChemSusChem 2021, 14, 3821–3824. [Google Scholar] [CrossRef]
- Li, Y.; Tsang, S.C.E. Recent progress and strategies for enhancing photocatalytic water splitting. Mater. Today Sustain. 2020, 9, 100032. [Google Scholar] [CrossRef]
- Sahoo, M.; Babu, P.; Singh, C.P.; Krishnamurty, S.; Parida, K. Facile fabrication of nano silver phosphate on B-doped g-C3N4: An excellent p-n heterojunction photocatalyst towards water oxidation and Cr (VI) reduction. J. Alloys Compd. 2022, 898, 162853. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, Z.; Qiu, C.; Chen, S.; Ling, X.; Su, C. Photocatalytic water-splitting coupled with alkanol oxidation for selective N-alkylation reactions over carbon nitride. ChemSusChem 2021, 14, 582–589. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xu, W.; Zhang, Y.; Wu, Y.; Wang, Z.; Fu, L.; Bai, F.; Zhou, B.; Wang, T.; Cheng, L.; et al. In-situ annealed "M-scheme" MXene-based photocatalyst for enhanced photoelectric performance and highly selective CO2 photoreduction. Nano Energy 2021, 83, 106532. [Google Scholar]
- Ran, J.; Ma, T.Y.; Gao, G.; Du, X.-W.; Qiao, S.Z. Porous P-doped graphitic carbon nitride nanosheets for synergistically enhanced visible-light photocatalytic H2 production. Energy Environ. Sci. 2015, 8, 3708–3717. [Google Scholar] [CrossRef]
- Wu, B.; Zhang, L.; Jiang, B.; Li, Q.; Tian, C.; Xie, Y.; Li, W.; Fu, H. Ultrathin porous carbon nitride bundles with an adjustable energy band structure toward simultaneous solar photocatalytic water splitting and selective phenylcarbinol oxidation. Angew. Chem. Int. Ed. Engl. 2021, 60, 4815–4822. [Google Scholar] [CrossRef] [PubMed]
- Xia, J.; Mark, G.; Volokh, M.; Fang, Y.; Chen, H.; Wang, X.; Shalom, M. Supramolecular organization of melem for the synthesis of photoactive porous carbon nitride rods. Nanoscale 2021, 13, 19511–19517. [Google Scholar] [CrossRef] [PubMed]
- Zinin, P.V.; Acosta-Maeda, T.E.; Misra, A.K.; Sharma, S.K. Enhancement of the anti-stokes fluorescence of hollow spherical carbon nitride nanostructures by high intensity green laser. Nanomaterials 2021, 11, 2529. [Google Scholar] [CrossRef]
- Wang, Z.; Luo, Y.; Hisatomi, T.; Vequizo, J.J.M.; Suzuki, S.; Chen, S.; Nakabayashi, M.; Lin, L.; Pan, Z.; Kariya, N.; et al. Sequential cocatalyst decoration on BaTaO2N towards highly-active Z-scheme water splitting. Nat. Commun. 2021, 12, 1005–1013. [Google Scholar] [CrossRef]
- Low, J.; Yu, J.; Jaroniec, M.; Wageh, S.; Al-Ghamdi, A.A. Heterojunction photocatalysts. Adv. Mater. 2017, 29, 1601694. [Google Scholar] [CrossRef]
- Zuo, G.; Wang, Y.; Teo, W.L.; Xie, A.; Guo, Y.; Dai, Y.; Zhou, W.; Jana, D.; Xian, Q.; Dong, W.; et al. Enhanced photocatalytic water oxidation by hierarchical 2D-Bi2MoO6@2D-MXene Schottky junction nanohybrid. Chem. Eng. J. 2021, 403, 126328. [Google Scholar] [CrossRef]
- Zang, S.; Zhang, G.; Lan, Z.-A.; Zheng, D.; Wang, X. Enhancement of photocatalytic H2 evolution on pyrene-based polymer promoted by MoS2 and visible light. Appl. Catal. B Environ. 2019, 251, 102–111. [Google Scholar] [CrossRef]
- Denis, S.; Baudrin, E.; Orsini, F.; Ouvrard, G.; Touboul, M.; Tarascon, J.M. Synthesis and electrochemical properties of numerous classes of vanadates. J. Power Sources 1999, 81–82, 79–84. [Google Scholar] [CrossRef]
- Heiland, M.; Seliverstov, A.; Schwarz, B.; Anjass, M.H.; Streb, C. Homogeneous and heterogeneous anion sensing by a molecular cobalt-vanadium oxide. Chem. Eur. J. 2017, 23, 2201–2205. [Google Scholar] [CrossRef] [PubMed]
- Yao, G.; Zhang, N.; Zhang, Y.; Zhou, T. Nanostructured transition metal vanadates as electrodes for pseudo-supercapacitors: A review. J. Nanoparticle Res. 2021, 23, 57. [Google Scholar] [CrossRef]
- Wu, Y.; Sun, Y.; Tong, Y.; Liu, X.; Zheng, J.; Han, D.; Li, H.; Niu, L. Recent advances in potassium-ion hybrid capacitors: Electrode materials, storage mechanisms and performance evaluation. Energy Storage Mater. 2021, 41, 108–132. [Google Scholar] [CrossRef]
- Andrukaitis, E.; Cooper, J.P.; Smit, J.H. Lithium intercalation in the divalent metal vanadates MeV2O6 (Me=Cu, Co, Ni, Mn or Zn). J. Power Sources 1995, 54, 465–469. [Google Scholar] [CrossRef]
- Laruelle, S.; Poizot, P.; Baudrin, E.; Briois, V.; Touboul, M.; Tarascon, J.-M. X-ray absorption study of cobalt vanadates during cycling usable as negative electrode in lithium battery. J. Power Sources 2001, 97–98, 251–253. [Google Scholar] [CrossRef]
- Liang, H.; Zhang, Y.; Hao, S.; Cao, L.; Li, Y.; Li, Q.; Chen, D.; Wang, X.; Guo, X.; Li, H. Fast potassium storage in porous CoV2O6 nanosphere@graphene oxide towards high-performance potassium-ion capacitors. Energy Storage Mater. 2021, 40, 250–258. [Google Scholar] [CrossRef]
- Lv, X.; Huang, W.; Shi, Q.; Tang, L.; Tang, J. Synthesis of CoV2O6/CNTs composites via ultrasound as electrode materials for supercapacitors. J. Mater. Sci. Mater. Electron. 2020, 31, 2388–2397. [Google Scholar] [CrossRef]
- Wang, Y.; Chai, H.; Dong, H.; Xu, J.; Jia, D.; Zhou, W. Superior cycle stability performance of quasi-cuboidal CoV2O6 microstructures as electrode material for supercapacitors. ACS Appl Mater. Interfaces 2016, 8, 27291–27297. [Google Scholar] [CrossRef]
- Xing, M.; Kong, L.; Liu, M.; Liu, L.; Kang, L.; Luo, Y. Cobalt vanadate as highly active, stable, noble metal-free oxygen evolution electrocatalyst. J. Mater. Chem. A 2014, 2, 18435–18443. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, Y.; Du, X.; Han, R.; Ding, Y. Hexagonal assembly of Co3V2O8 nanoparticles acting as an efficient catalyst for visible light-driven water oxidation. J. Mater. Chem. A 2014, 2, 19308–19314. [Google Scholar] [CrossRef]
- Ghiyasiyan-Arani, M.; Masjedi-Arani, M.; Salavati-Niasari, M. Size controllable synthesis of cobalt vanadate nanostructures with enhanced photocatalytic activity for the degradation of organic dyes. J. Mol. Catal. A Chem. 2016, 425, 31–42. [Google Scholar] [CrossRef] [Green Version]
- Pavliuk, M.V.; Mijangos, E.; Makhankova, V.G.; Kokozay, V.N.; Pullen, S.; Liu, J.; Zhu, J.; Styring, S.; Thapper, A. Homogeneous cobalt/vanadium complexes as precursors for functionalized mixed oxides in visible-light-driven water oxidation. ChemSusChem 2016, 9, 2957–2966. [Google Scholar] [CrossRef] [PubMed]
- Shen, F.; Wang, Y.; Tang, Y.; Li, S.; Wang, Y.; Dong, L.; Li, Y.; Xu, Y.; Lan, Y. CoV2O6–V2O5 coupled with porous N-doped reduced graphene oxide composite as a highly efficient electrocatalyst for oxygen evolution. ACS Energy Lett. 2017, 2, 1327–1333. [Google Scholar] [CrossRef]
- Mondal, A.; Ganguli, S.; Inta, H.R.; Mahalingam, V. Influence of vanadate structure on electrochemical surface reconstruction and OER performance of CoV2O6 and Co3V2O8. ACS Appl. Energy Mater. 2021, 4, 5381–5387. [Google Scholar] [CrossRef]
- Zang, S.; Zhang, G.; Yang, P.; Zheng, D.; Wang, X. Polymeric donor-acceptor heterostructures for enhanced photocatalytic H2 evolution without using Pt cocatalysts. Chem. Eur. J. 2019, 25, 6102–6107. [Google Scholar] [CrossRef]
- Zhang, L.; Gonçalves, A.; Jaroniec, M. Identification of preferentially exposed crystal facets by X-ray diffraction. RSC Adv. 2020, 10, 5585–5589. [Google Scholar] [CrossRef] [Green Version]
- Perez-Molina, A.; Pastrana-Martinez, L.M.; Perez-Poyatos, L.T.; Morales-Torres, S.; Maldonado-Hodar, F.J. One-pot thermal synthesis of g-C3N4/ZnO composites for the degradation of 5-fluoruracil cytostatic drug under UV-LED irradiation. Nanomaterials 2022, 12, 340. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, H.; Luo, W.; Sun, T.; Xu, Y. Ultrathin crystalline covalent-triazine-framework nanosheets with electron donor groups for synergistically enhanced photocatalytic water splitting. Angew. Chem. Int. Ed. 2021, 60, 25381–25390. [Google Scholar] [CrossRef]
- Sohail, M.; Altalhi, T.; Al-Sehemi, A.G.; Taha, T.A.M.; El-Nasser, K.S.; Al-Ghamdi, A.A.; Boukhari, M.; Palamanit, A.; Hayat, A.; Amin, M.A.; et al. Nanostructure engineering via intramolecular construction of carbon nitride as efficient photocatalyst for CO2 reduction. Nanomaterials 2021, 11, 3245. [Google Scholar] [CrossRef]
- Guo, F.; Hu, B.; Yang, C.; Zhang, J.; Hou, Y.; Wang, X. On-Surface polymerization of in-plane highly ordered carbon nitride nanosheets toward photocatalytic mineralization of mercaptan gas. Adv. Mater. 2021, 33, e2101466. [Google Scholar] [CrossRef] [PubMed]
- Ming, H.; Zhang, P.; Yang, Y.; Zou, Y.; Yang, C.; Hou, Y.; Ding, K.; Zhang, J.; Wang, X. Tailored poly-heptazine units in carbon nitride for activating peroxymonosulfate to degrade organic contaminants with visible light. Appl. Catal. B Environ. 2022, 311, 121341. [Google Scholar] [CrossRef]
- Kong, H.J.; Won, D.H.; Kim, J.; Woo, S.I. Sulfur-Doped g-C3N4/BiVO4 composite photocatalyst for water oxidation under visible light. Chem. Mater. 2016, 28, 1318–1324. [Google Scholar] [CrossRef]
- Sprick, R.S.; Chen, Z.; Cowan, A.J.; Bai, Y.; Aitchison, C.M.; Fang, Y.; Zwijnenburg, M.A.; Cooper, A.I.; Wang, X. Water oxidation with cobalt-loaded linear conjugated polymer photocatalysts. Angew. Chem. Int. Ed. Engl. 2020, 59, 18695–18700. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Zang, S.; Wang, X. Layered Co(OH)2 deposited polymeric carbon nitrides for photocatalytic water oxidation. ACS Catal. 2015, 5, 941–947. [Google Scholar] [CrossRef]
- Zhang, G.; Liu, M.; Heil, T.; Zafeiratos, S.; Savateev, A.; Antonietti, M.; Wang, X. Electron deficient monomers that optimize nucleation and enhance the photocatalytic redox activity of carbon nitrides. Angew. Chem. Int. Ed. Engl. 2019, 58, 14950–14954. [Google Scholar] [CrossRef] [Green Version]
- Lin, F.; Jing, X.; Wang, Y.; Zang, S. Polarization-induced carrier separation in conjugated polyimide for boosted visible light driven H2O2 production. Appl. Surf. Sci. 2022, 594, 153478. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zang, S.; Cai, X.; Chen, M.; Teng, D.; Jing, F.; Leng, Z.; Zhou, Y.; Lin, F. Tunable Carrier Transfer of Polymeric Carbon Nitride with Charge-Conducting CoV2O6∙2H2O for Photocatalytic O2 Evolution. Nanomaterials 2022, 12, 1931. https://doi.org/10.3390/nano12111931
Zang S, Cai X, Chen M, Teng D, Jing F, Leng Z, Zhou Y, Lin F. Tunable Carrier Transfer of Polymeric Carbon Nitride with Charge-Conducting CoV2O6∙2H2O for Photocatalytic O2 Evolution. Nanomaterials. 2022; 12(11):1931. https://doi.org/10.3390/nano12111931
Chicago/Turabian StyleZang, Shaohong, Xiaorong Cai, Mengshan Chen, Dehong Teng, Fei Jing, Zhe Leng, Yingtang Zhou, and Feng Lin. 2022. "Tunable Carrier Transfer of Polymeric Carbon Nitride with Charge-Conducting CoV2O6∙2H2O for Photocatalytic O2 Evolution" Nanomaterials 12, no. 11: 1931. https://doi.org/10.3390/nano12111931
APA StyleZang, S., Cai, X., Chen, M., Teng, D., Jing, F., Leng, Z., Zhou, Y., & Lin, F. (2022). Tunable Carrier Transfer of Polymeric Carbon Nitride with Charge-Conducting CoV2O6∙2H2O for Photocatalytic O2 Evolution. Nanomaterials, 12(11), 1931. https://doi.org/10.3390/nano12111931





