Tunable Color-Variable Solar Absorber Based on Phase Change Material Sb2Se3
Abstract
:1. Introduction
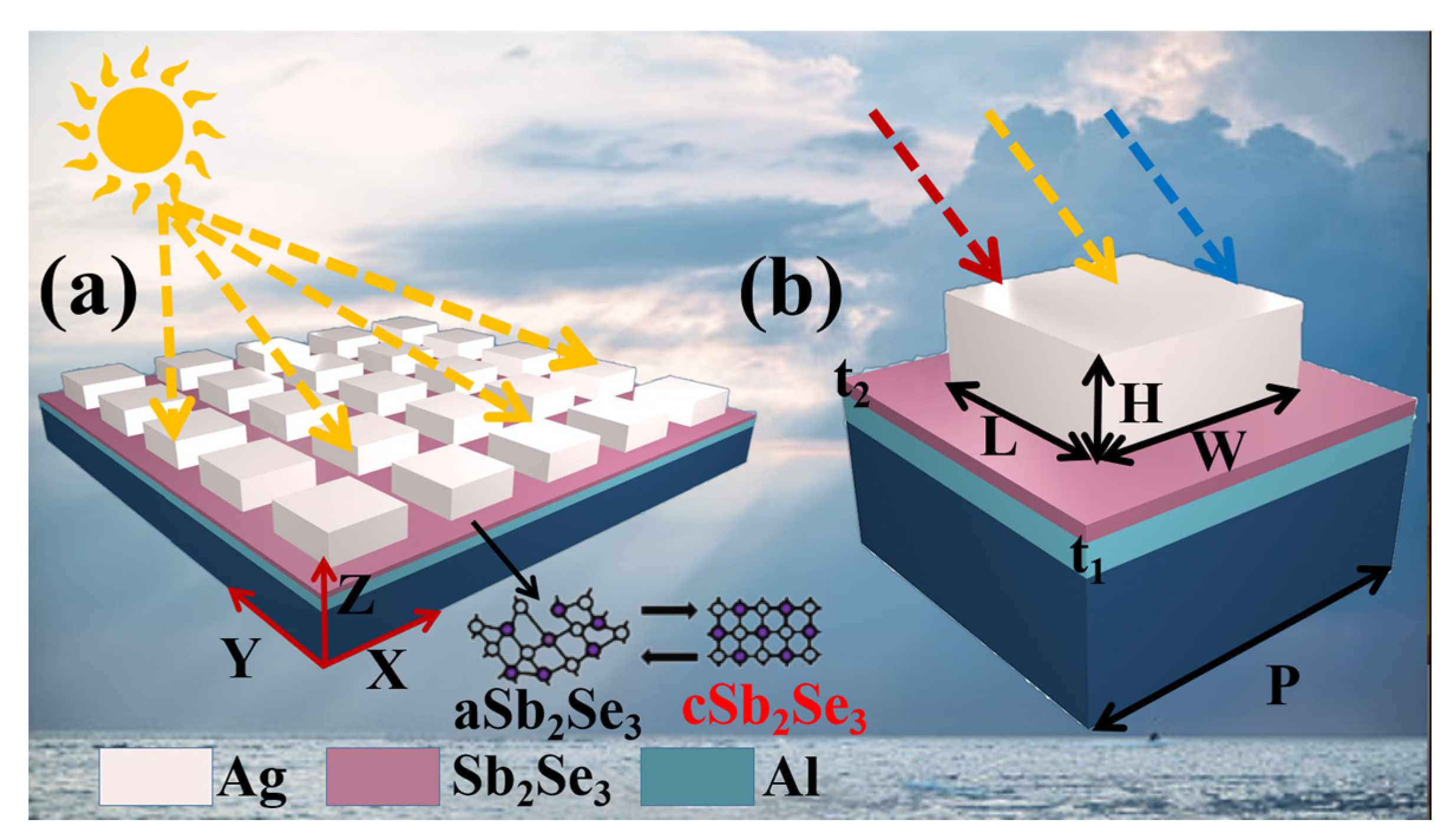
2. Material and Structure
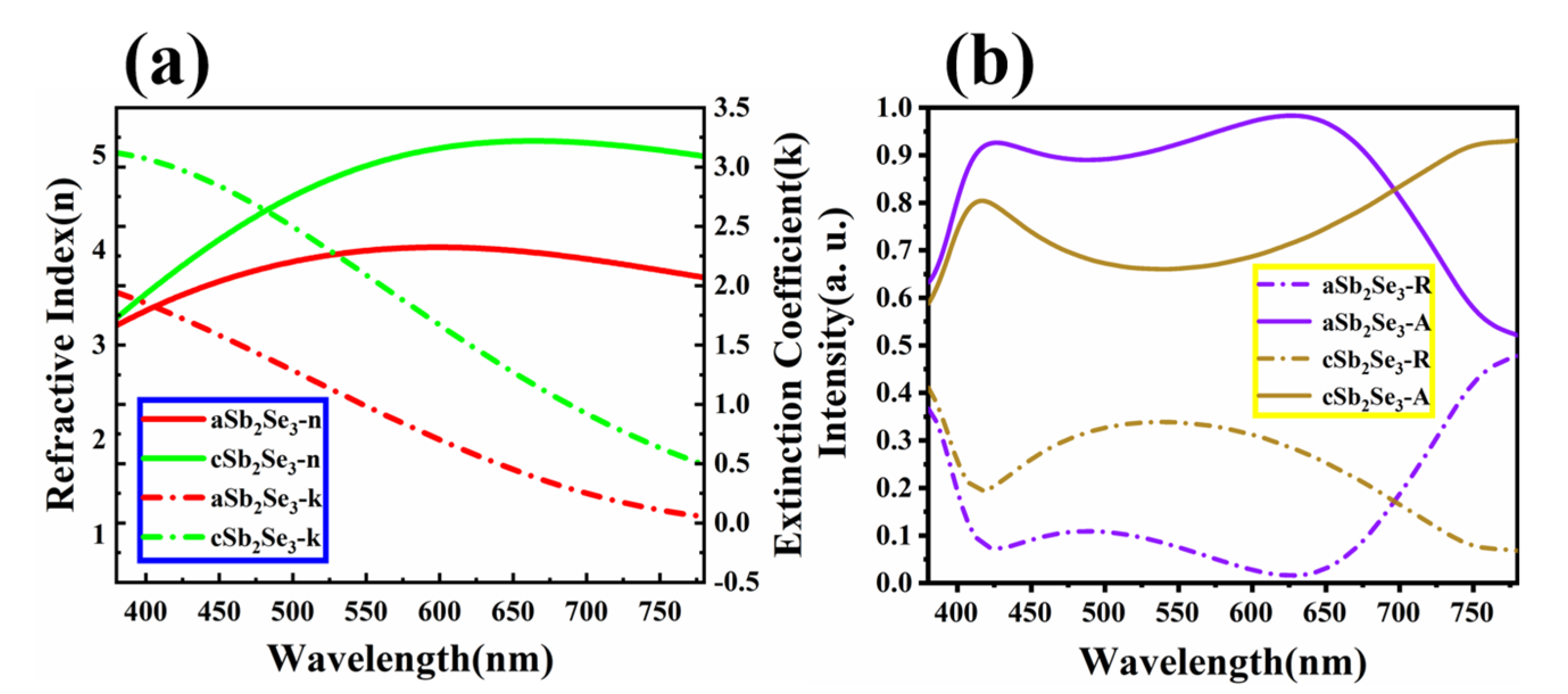
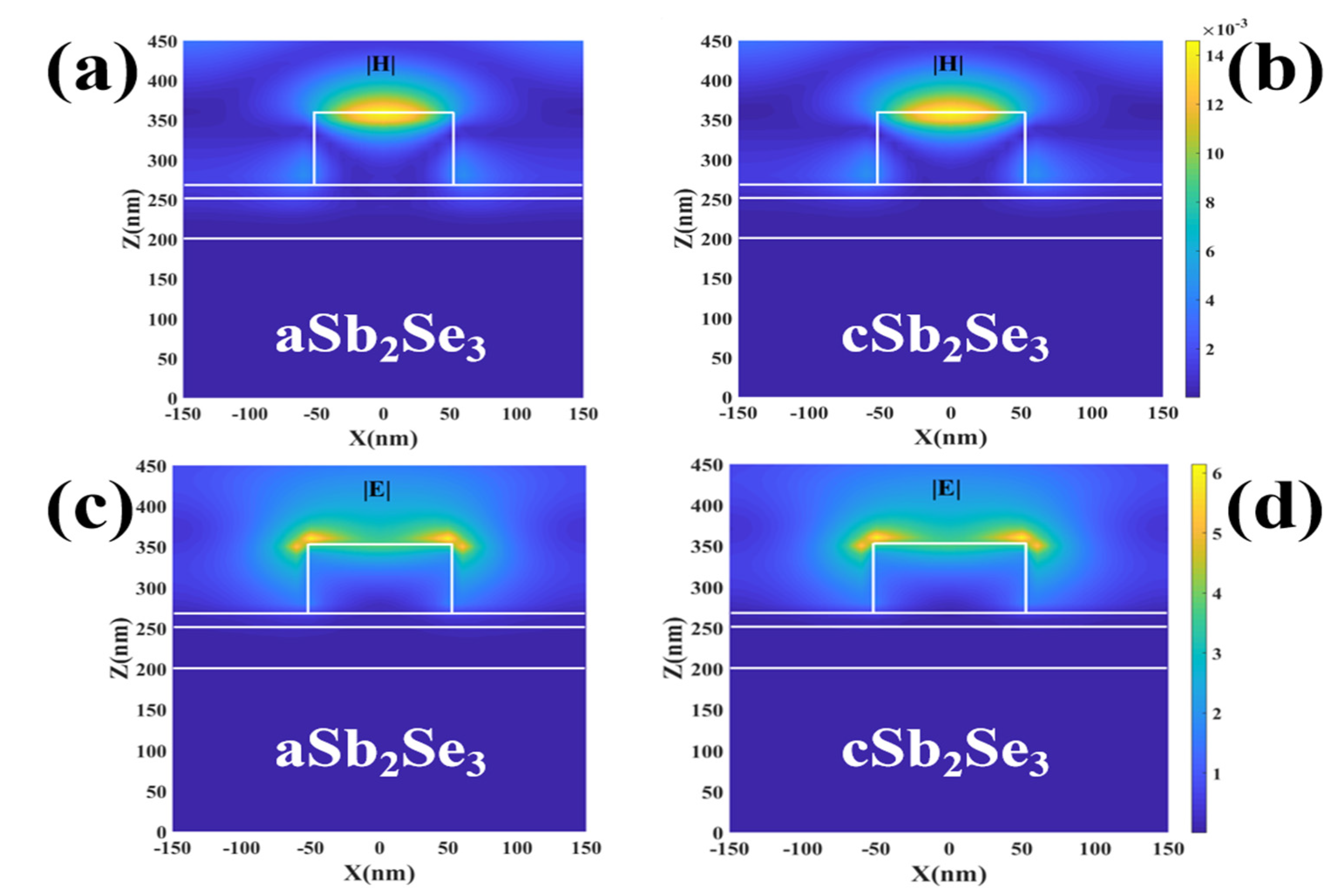
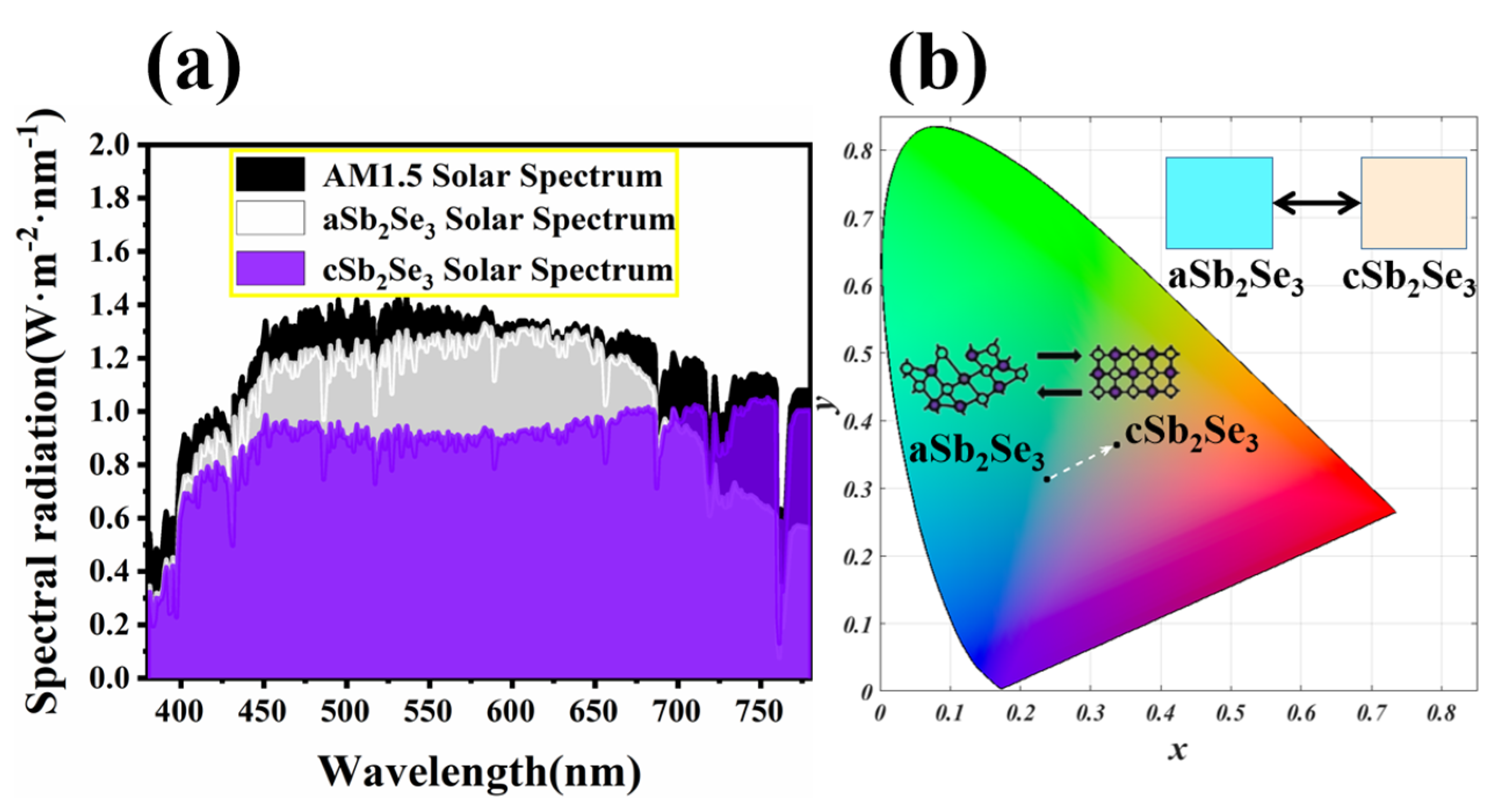
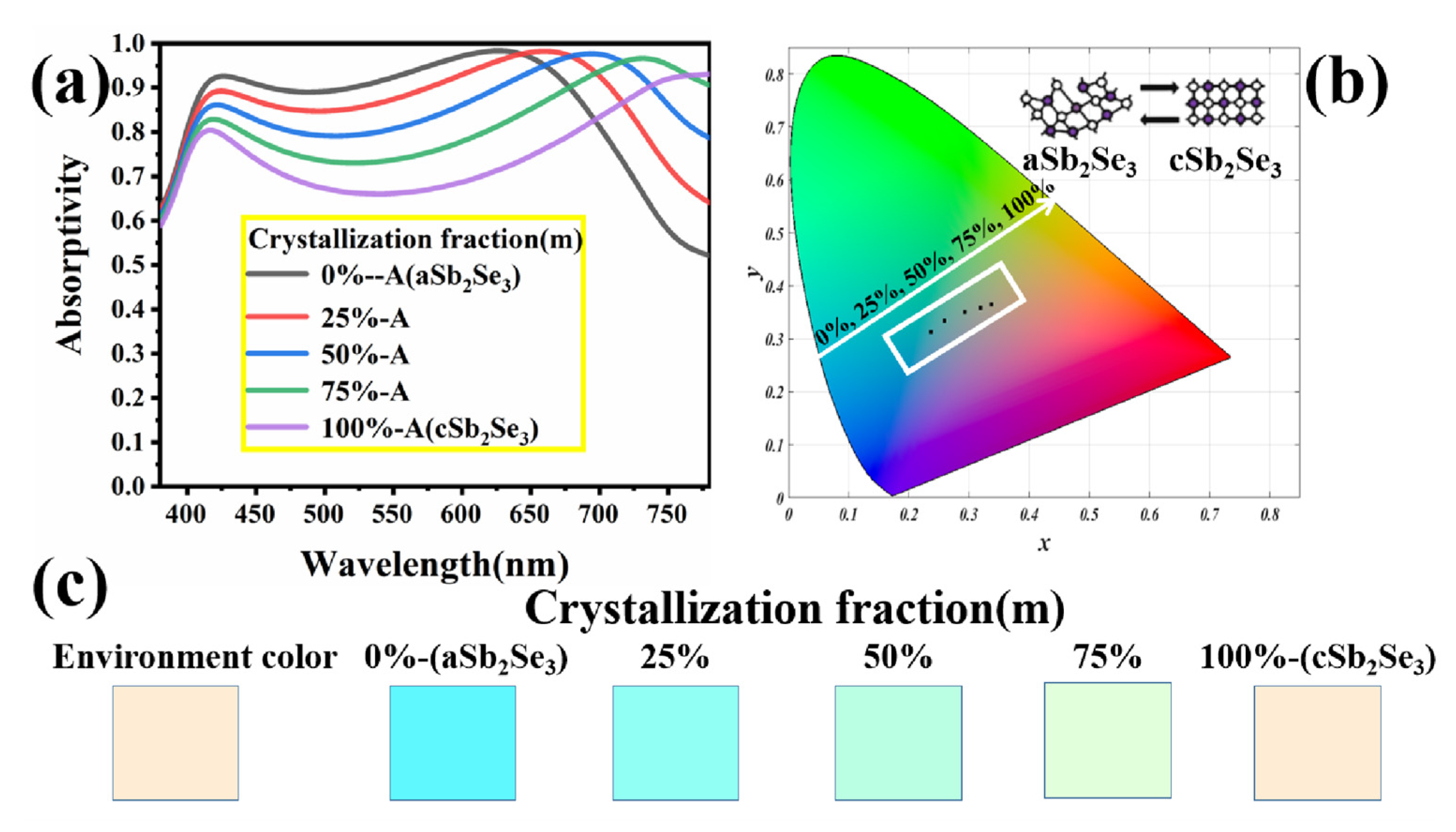
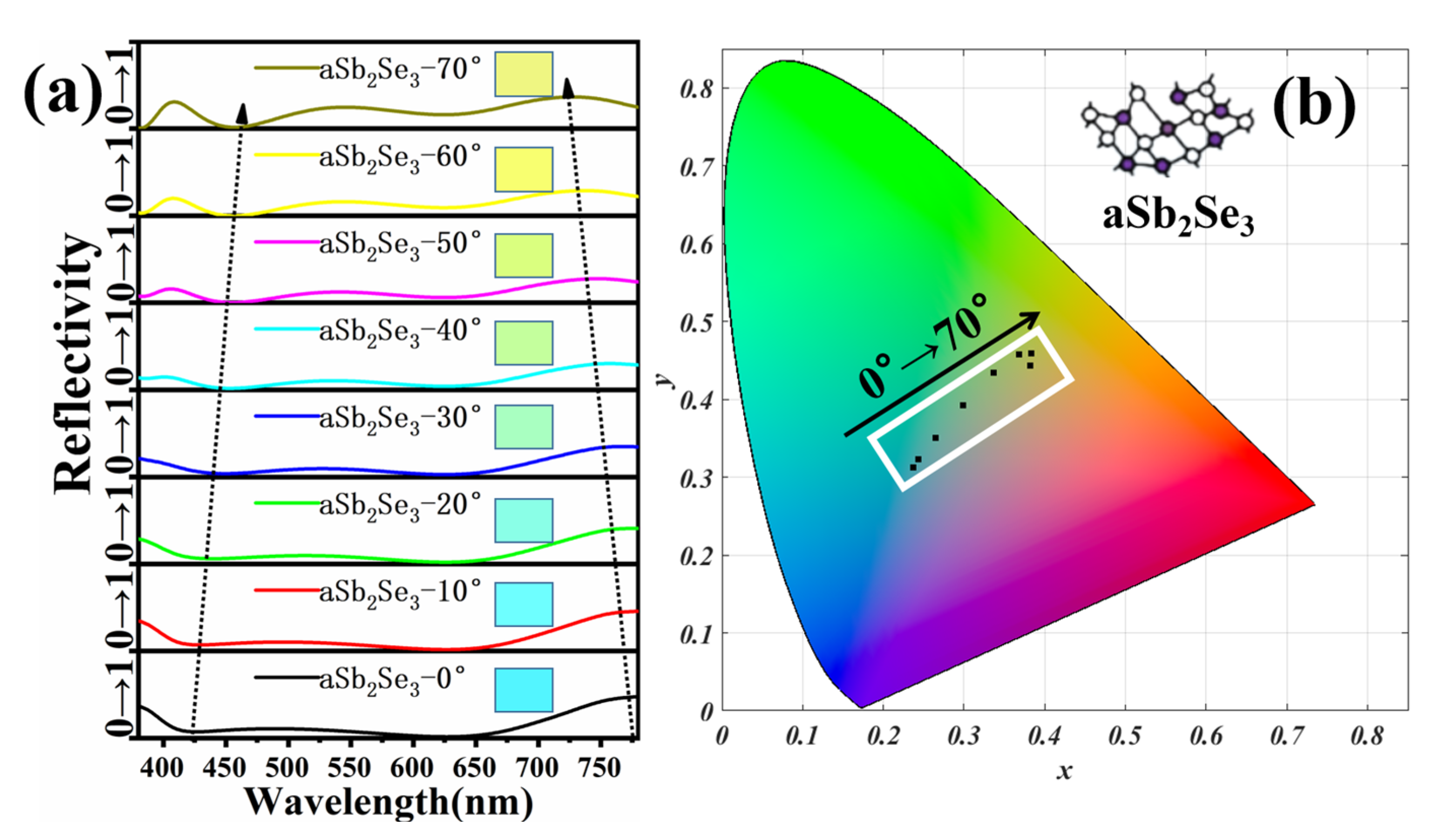
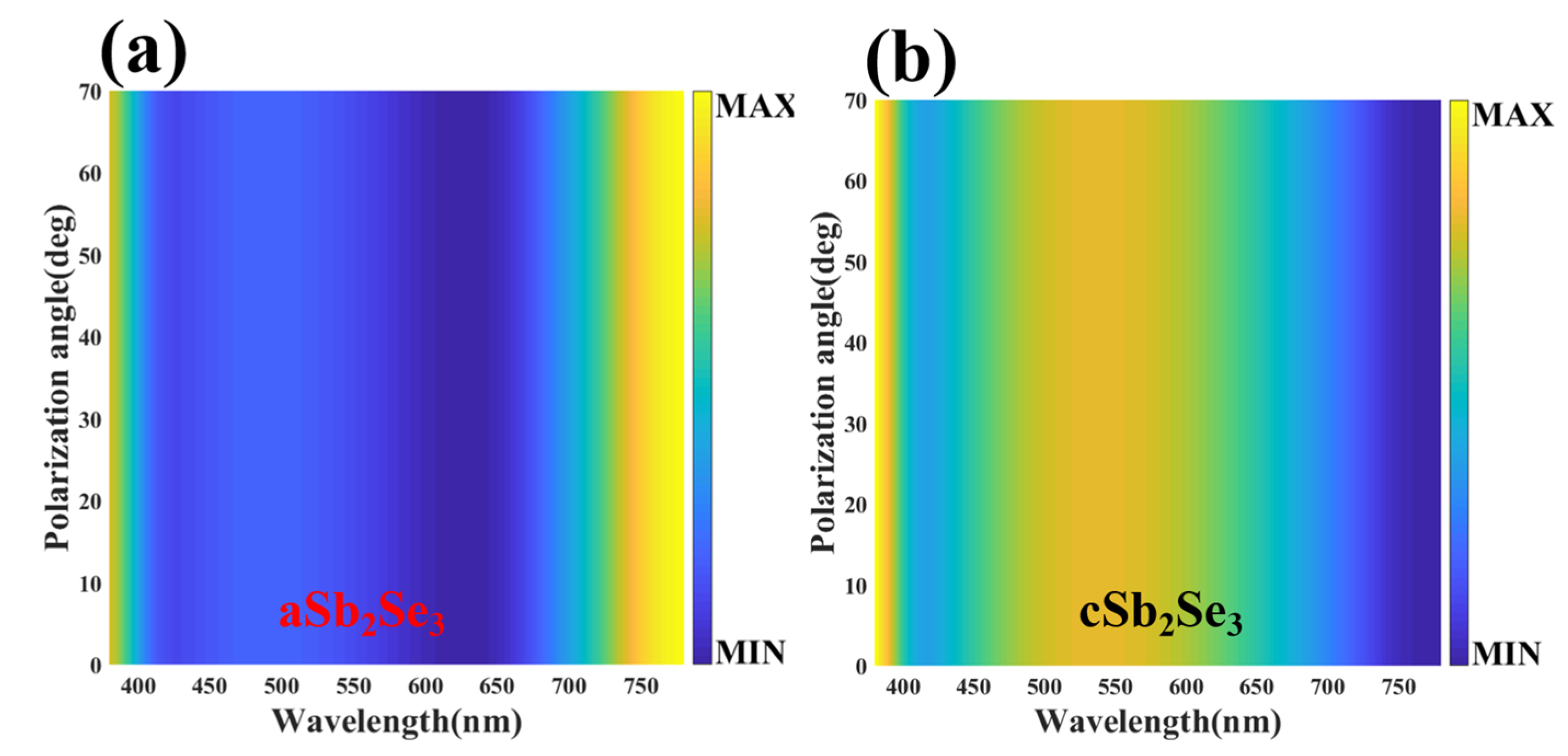
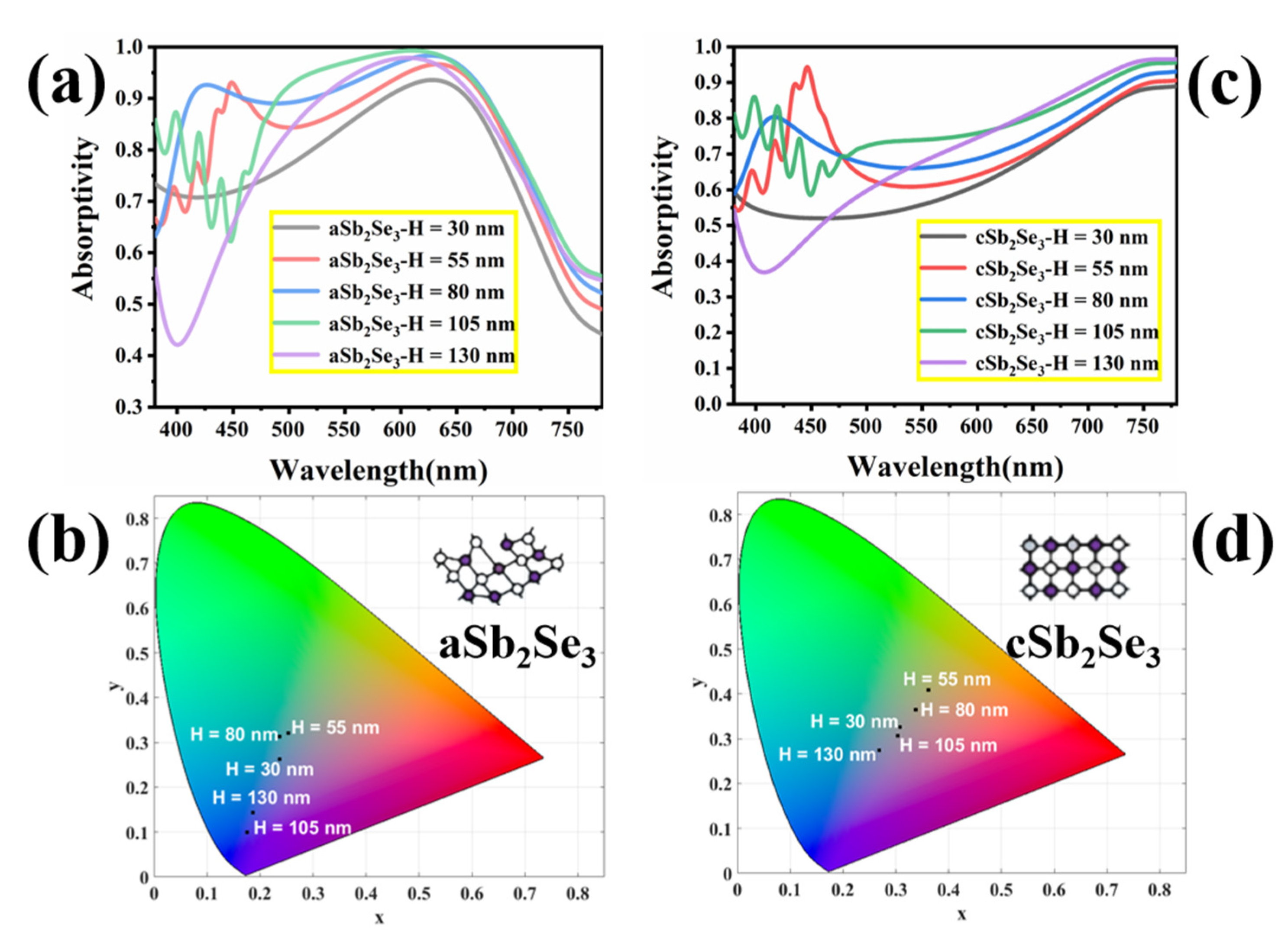
3. Results
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- An, Q.; Wang, J.; Gao, W.; Ma, X.; Hu, Z.; Gao, J.; Xu, C.; Hao, M.; Zhang, X.; Yang, C.; et al. Alloy-like ternary polymer solar cells with over 17.2% efficiency. Sci. Bull. 2020, 65, 538–545. [Google Scholar] [CrossRef] [Green Version]
- Atwater, H.A.; Polman, A. Plasmonics for improved photovoltaic devices. Nat. Mater. 2010, 9, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Raman, A.; Fan, S. Fundamental limit of nanophotonic light trapping in solar cells. Proc. Natl. Acad. Sci. USA 2010, 107, 17491–17496. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brongersma, M.L.; Cui, Y.; Fan, S. Light management for photovoltaics using high-index nanostructures. Nat. Mater. 2014, 13, 451–460. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Lai, Y. Near-perfect absorption by photonic crystals with a broadband and omnidirectional impedance-matching property. Opt. Express 2019, 27, 15800–15811. [Google Scholar] [CrossRef] [PubMed]
- Marko, G.; Prajapati, A.; Shalev, G. Subwavelength nonimaging light concentrators for the harvesting of the solar radiation. Nano Energy 2019, 61, 275–283. [Google Scholar] [CrossRef]
- Chauhan, A.; Prajapati, A.; Calaza, C.; Fonseca, H.; Sousa, P.C.; Llobet, J.; Shalev, G. Near-Field Optical Excitations in Silicon Subwavelength Light Funnel Arrays for Broadband Absorption of the Solar Radiation. Sol. RRL 2021, 5, 2100721. [Google Scholar] [CrossRef]
- Xu, C.; Pu, K. Second near-infrared photothermal materials for combinational nanotheranostics. Chem. Soc. Rev. 2021, 50, 1111–1137. [Google Scholar] [CrossRef]
- Geisz, J.F.; France, R.M.; Schulte, K.L.; Steiner, M.A.; Norman, A.G.; Guthrey, H.L.; Young, M.R.; Song, T.; Moriarty, T. Six-junction III–V solar cells with 47.1% conversion efficiency under 143 Suns concentration. Nat. Energy 2020, 5, 326–335. [Google Scholar] [CrossRef]
- Ferrari, C.; Melino, F.; Pinelli, M.; Spina, P.R.; Venturini, M. Overview and status of thermophotovoltaic systems. Energy Procedia 2014, 45, 160–169. [Google Scholar] [CrossRef] [Green Version]
- Fan, D.; Burger, T.; McSherry, S.; Lee, B.; Lenert, A.; Forrest, S.R. Near-perfect photon utilization in an air-bridge thermophotovoltaic cell. Nature 2020, 586, 237–241. [Google Scholar] [CrossRef] [PubMed]
- Lucchesi, C.; Cakiroglu, D.; Perez, J.P.; Taliercio, T.; Tournié, E.; Chapuis, P.O.; Vaillon, R. Near-field thermophotovoltaic conversion with high electrical power density and cell efficiency above 14%. Nano Lett. 2021, 21, 4524–4529. [Google Scholar] [CrossRef] [PubMed]
- Omair, Z.; Scranton, G.; Pazos-Outón, L.M.; Xiao, T.P.; Steiner, M.A.; Ganapati, V.; Peterson, P.F.; Holzrichter, J.; Atwater, H.; Yablonovitch, E. Ultraefficient thermophotovoltaic power conversion by band-edge spectral filtering. Proc. Natl. Acad. Sci. USA 2019, 116, 15356–15361. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lam, P.L.; Shiu, A. Efficiency and productivity of China’s thermal power generation. Rev. Ind. Organ. 2004, 24, 73–93. [Google Scholar] [CrossRef]
- Gupta, M.K.; Kaushik, S.C.; Ranjan, K.R.; Panwar, N.L.; Reddy, V.S.; Tyagi, S.K. Thermodynamic performance evaluation of solar and other thermal power generation systems: A review. Renew. Sustain. Energy Rev. 2015, 50, 567–582. [Google Scholar] [CrossRef]
- Sharma, C.; Sharma, A.K.; Mullick, S.C.; Kandpal, T.C. Assessment of solar thermal power generation potential in India. Renew. Sustain. Energy Rev. 2015, 42, 902–912. [Google Scholar] [CrossRef]
- He, F.; Meng, A.; Cheng, B.; Ho, W.; Yu, J. Enhanced photocatalytic H2-production activity of WO3/TiO2 step-scheme heterojunction by graphene modification. Chin. J. Catal. 2020, 41, 9–20. [Google Scholar] [CrossRef]
- Kirlikovali, K.O.; Chen, Z.; Islamoglu, T.; Hupp, J.T.; Farha, O.K. Zirconium-based metal–organic frameworks for the catalytic hydrolysis of organophosphorus nerve agents. ACS Appl. Mater. Interfaces 2020, 12, 14702–14720. [Google Scholar] [CrossRef]
- Ren, X.; Lv, H.; Yang, S.; Wang, Y.; Li, J.; Wei, R.; Xu, D.; Liu, B. Promoting effect of heterostructured NiO/Ni on Pt nanocatalysts toward catalytic hydrolysis of ammonia borane. J. Phys. Chem. Lett. 2019, 10, 7374–7382. [Google Scholar] [CrossRef]
- Momeni, M.R.; Cramer, C.J. Dual role of water in heterogeneous catalytic hydrolysis of sarin by zirconium-based metal–organic frameworks. ACS Appl. Mater. Interaces 2018, 10, 18435–18439. [Google Scholar] [CrossRef]
- Lei, L.; Li, S.; Huang, H.; Tao, K.; Xu, P. Ultra-broadband absorber from visible to near-infrared using plasmonic metamaterial. Opt. Express 2018, 26, 5686–5693. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Xu, K.D.; Feng, N.; Guo, R.; Lin, H.; Zhu, J. Anisotropic infrared plasmonic broadband absorber based on graphene-black phosphorus multilayers. Opt. Express 2019, 27, 3101–3112. [Google Scholar] [CrossRef] [PubMed]
- Kinoshita, S.; Yoshioka, S.; Miyazaki, J. Physics of structural colors. Rep. Prog. Phys. 2008, 71, 076401. [Google Scholar] [CrossRef] [Green Version]
- Hosseini, P.; Wright, C.D.; Bhaskaran, H. An optoelectronic framework enabled by low-dimensional phase-change films. Nature 2014, 511, 206–211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gerislioglu, B.; Bakan, G.; Ahuja, R.; Adam, J.; Mishra, Y.K.; Ahmadivand, A. The role of Ge2Sb2Te5 in enhancing the performance of functional plasmonic devices. Mater. Today Phys. 2020, 12, 100178. [Google Scholar] [CrossRef]
- Mocanu, F.C.; Konstantinou, K.; Lee, T.H.; Bernstein, N.; Deringer, V.L.; Csányi, G.; Elliott, S.R. Modeling the phase-change memory material, Ge2Sb2Te5, with a machine-learned interatomic potential. J. Phys. Chem. B 2018, 122, 8998–9006. [Google Scholar] [CrossRef] [Green Version]
- Gholipour, B.; Karvounis, A.; Yin, J.; Soci, C.; MacDonald, K.F.; Zheludev, N.I. Phase-change-driven dielectric-plasmonic transitions in chalcogenide metasurfaces. NPG Asia Mater. 2018, 10, 533–539. [Google Scholar] [CrossRef] [Green Version]
- Ni, Z.; Mou, S.; Zhou, T.; Cheng, Z. Broader color gamut of color-modulating optical coating display based on indium tin oxide and phase change materials. Appl. Opt. 2018, 57, 3385–3389. [Google Scholar] [CrossRef]
- Delaney, M.; Zeimpekis, I.; Lawson, D.; Hewak, D.W.; Muskens, O.L. A new family of ultralow loss reversible phase-change materials for photonic integrated circuits: Sb2S3 and Sb2Se3. Adv. Func. Mater. 2020, 30, 2002447. [Google Scholar] [CrossRef]
- Delaney, M.; Zeimpekis, I.; Du, H.; Yan, X.; Banakar, M.; Thomson, D.J.; Hewak, D.W.; Muskens, O.L. Nonvolatile programmable silicon photonics using an ultralow-loss Sb2Se3 phase change material. Sci. Adv. 2021, 7, eabg3500. [Google Scholar] [CrossRef]
- Yim, W.M.; Fitzke, E.V.; Rosi, F.D. Thermoelectric properties of Bi2Te3-Sb2Te3-Sb2Se3 pseudo-ternary alloys in the temperature range 77 to 300 K. J. Mater. Sci. 1966, 1, 52–65. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, L.; Chen, S.; Qin, S.; Liu, X.; Chen, J.; Xue, D.; Luo, M.; Cao, Y.; Cheng, Y.; et al. Thin-film Sb2Se3 photovoltaics with oriented one-dimensional ribbons and benign grain boundaries. Nat. Photonics 2015, 9, 409–415. [Google Scholar] [CrossRef]
- Liu, X.; Chen, J.; Luo, M.; Leng, M.; Xia, Z.; Zhou, Y.; Qin, S.; Xue, D.; Lv, L.; Huang, H.; et al. Thermal evaporation and characterization of Sb2Se3 thin film for substrate Sb2Se3/CdS solar cells. ACS. Appl. Mater. Interfaces 2014, 6, 10687–10695. [Google Scholar] [CrossRef] [PubMed]
- Mavlonov, A.; Razykov, T.; Raziq, F.; Gan, J.; Chantana, J.; Kawano, Y.; Nishimura, T.; Wei, H.; Zakutayev, A.; Minemoto, T.; et al. A review of Sb2Se3 photovoltaic absorber materials and thin-film solar cells. Sol. Energy 2020, 201, 227–246. [Google Scholar] [CrossRef]
- Huang, M.; Xu, P.; Han, D.; Tang, J.; Chen, S. Complicated and Unconventional Defect Properties of the Quasi-One-Dimensional Photovoltaic Semiconductor Sb2Se3. ACS Appl. Mater. Interfaces 2019, 11, 15564–15572. [Google Scholar] [CrossRef]
- Liang, G.X.; Luo, Y.D.; Chen, S.; Tang, R.; Zheng, Z.H.; Li, X.J.; Fan, P. Sputtered and selenized Sb2Se3 thin-film solar cells with open-circuit voltage exceeding 500 mV. Nano Energy 2020, 73, 104806. [Google Scholar] [CrossRef]
- Fang, Y.; Yu, X.Y.; Lou, X.W. Formation of Polypyrrole-Coated Sb2Se3 Microclips with Enhanced Sodium-Storage Properties. Angew. Chem. 2018, 130, 10007–10011. [Google Scholar] [CrossRef]
- Hobson, T.D.; Phillips, L.J.; Hutter, O.S.; Shiel, H.; Swallow, J.E.; Savory, C.N.; Major, J.D. Isotype heterojunction solar cells using n-type Sb2Se3 thin films. Chem. Mater. 2020, 32, 2621–2630. [Google Scholar] [CrossRef] [Green Version]
- Palik, E.D. Handbook of Optical Constants of Solids; Elsevier: Amsterdam, The Netherlands, 1985. [Google Scholar]
- Dong, W.; Liu, H.; Behera, J.K.; Lu, L.; Ng, R.J.; Sreekanth, K.V.; Zhou, X.; Yang, J.K.W.; Simpson, R.E. Wide bandgap phase change material tuned visible photonics. Adv. Func. Mater. 2019, 29, 1806181. [Google Scholar] [CrossRef] [Green Version]
- Aspnes, D.E. Local-field effects and effective-medium theory: A microscopic perspective. Am. J. Phys. 1982, 50, 704–709. [Google Scholar] [CrossRef]
- Voshchinnikov, N.V.; Videen, G.; Henning, T. Effective medium theories for irregular fluffy structures: Aggregation of small particles. Appl. Opt. 2007, 46, 4065–4072. [Google Scholar] [CrossRef] [PubMed]
- Kang, Q.; Li, D.; Guo, K.; Gao, J.; Guo, Z. Tunable thermal camouflage based on GST plasmonic metamaterial. Nanomaterials 2021, 11, 260. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Luo, M.; Jiang, X.; Luo, S.; Yang, J. Tunable Color-Variable Solar Absorber Based on Phase Change Material Sb2Se3. Nanomaterials 2022, 12, 1903. https://doi.org/10.3390/nano12111903
Li X, Luo M, Jiang X, Luo S, Yang J. Tunable Color-Variable Solar Absorber Based on Phase Change Material Sb2Se3. Nanomaterials. 2022; 12(11):1903. https://doi.org/10.3390/nano12111903
Chicago/Turabian StyleLi, Xin, Mingyu Luo, Xinpeng Jiang, Shishang Luo, and Junbo Yang. 2022. "Tunable Color-Variable Solar Absorber Based on Phase Change Material Sb2Se3" Nanomaterials 12, no. 11: 1903. https://doi.org/10.3390/nano12111903
APA StyleLi, X., Luo, M., Jiang, X., Luo, S., & Yang, J. (2022). Tunable Color-Variable Solar Absorber Based on Phase Change Material Sb2Se3. Nanomaterials, 12(11), 1903. https://doi.org/10.3390/nano12111903






