Design and Preparation of Avermectin Nanopesticide for Control and Prevention of Pine Wilt Disease
Abstract
1. Introduction
2. Materials and Methods
2.1. The Materials and Characterization
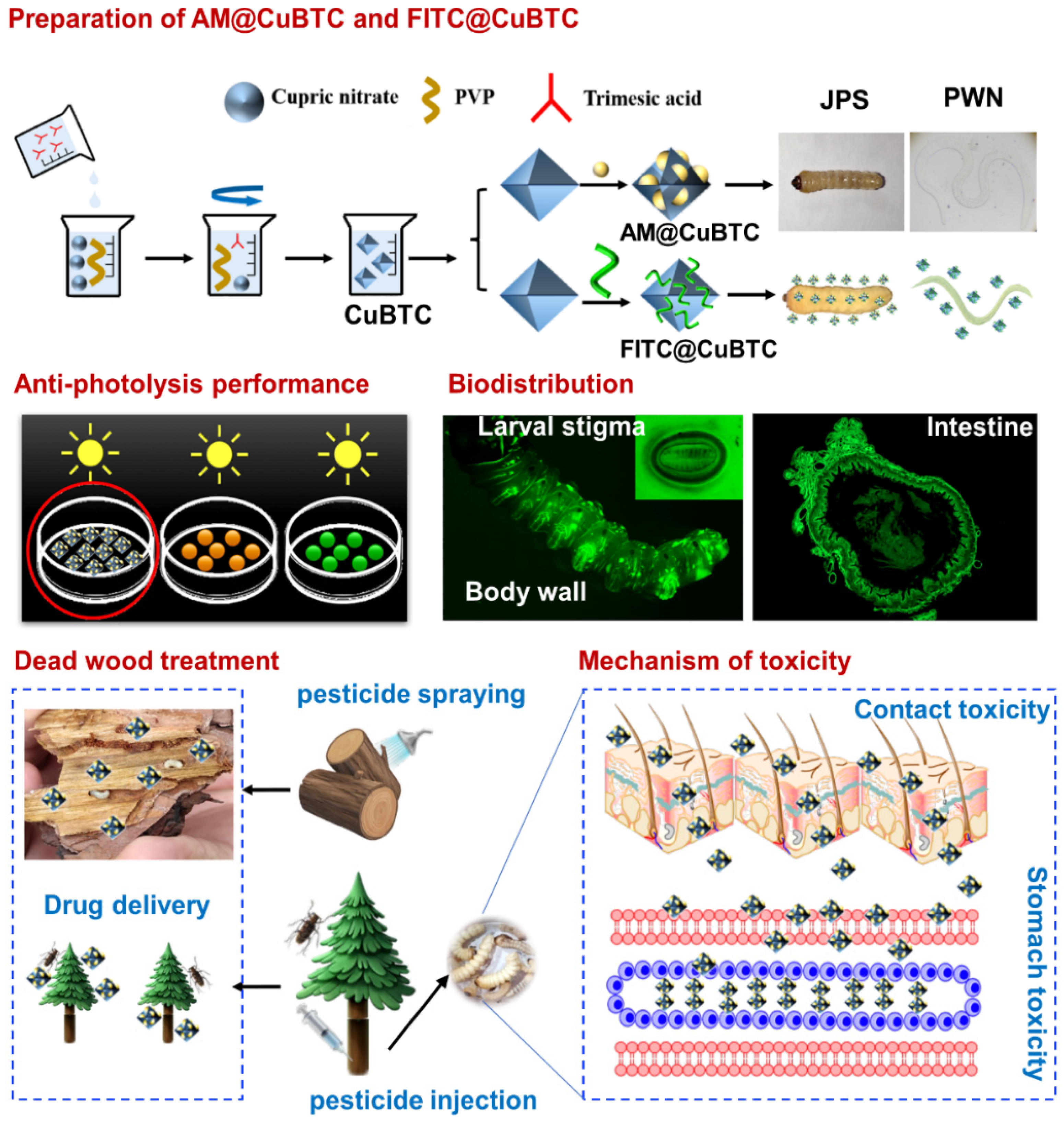
2.2. Preparation of AM@CuBTC
2.3. Preparation of FITC@CuBTC
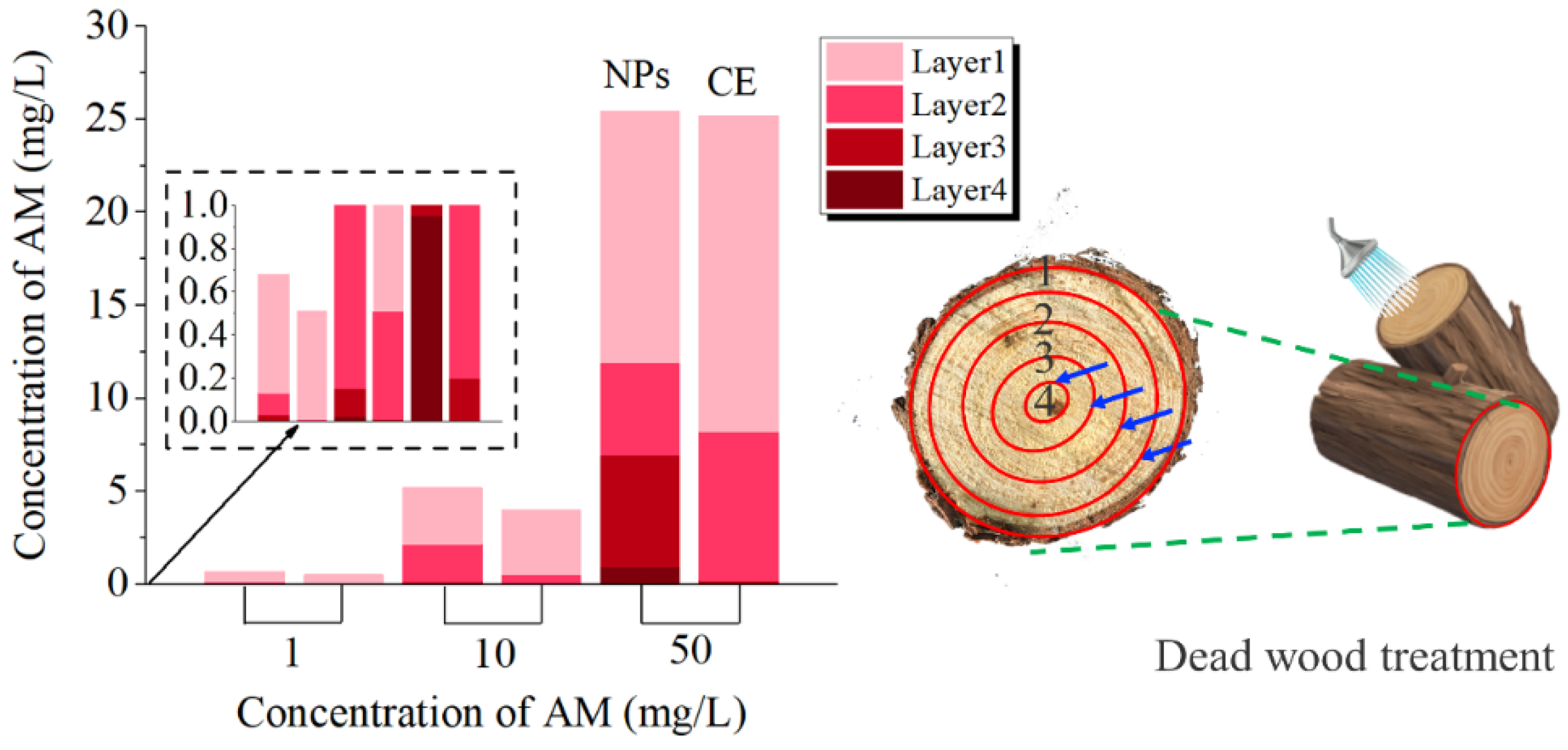
2.4. Pesticide Delivery
2.5. Pesticide Release
2.6. Photodegradability of AM@CuBTC
2.7. Biodistribution
2.8. Cellular Uptake
2.9. Cytotoxicity of AM@CuBTC
2.10. Mechanism of Toxicity
2.11. Transmission of AM@CuBTC
2.12. Statistical Analysis
3. Results and Discussion
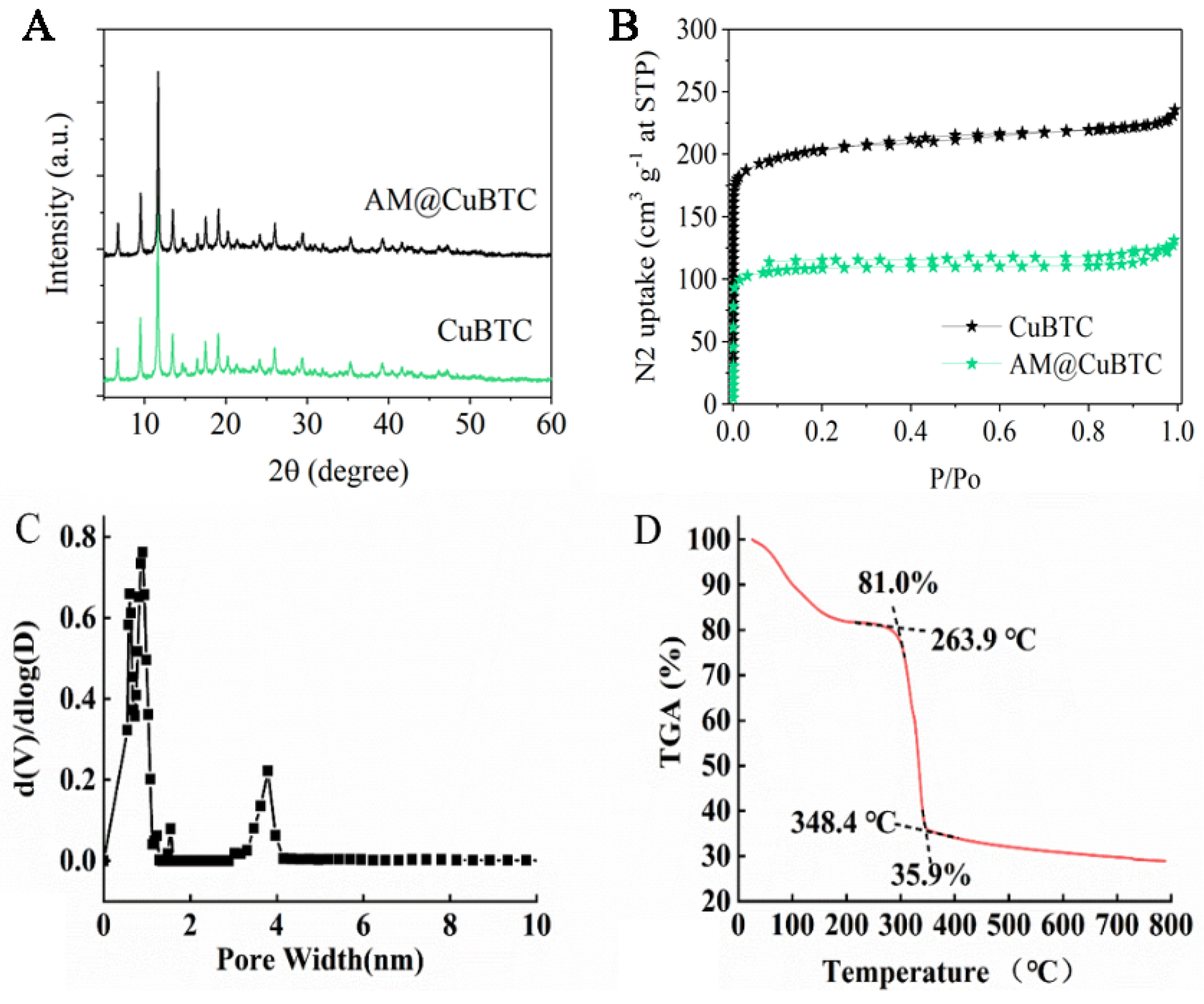
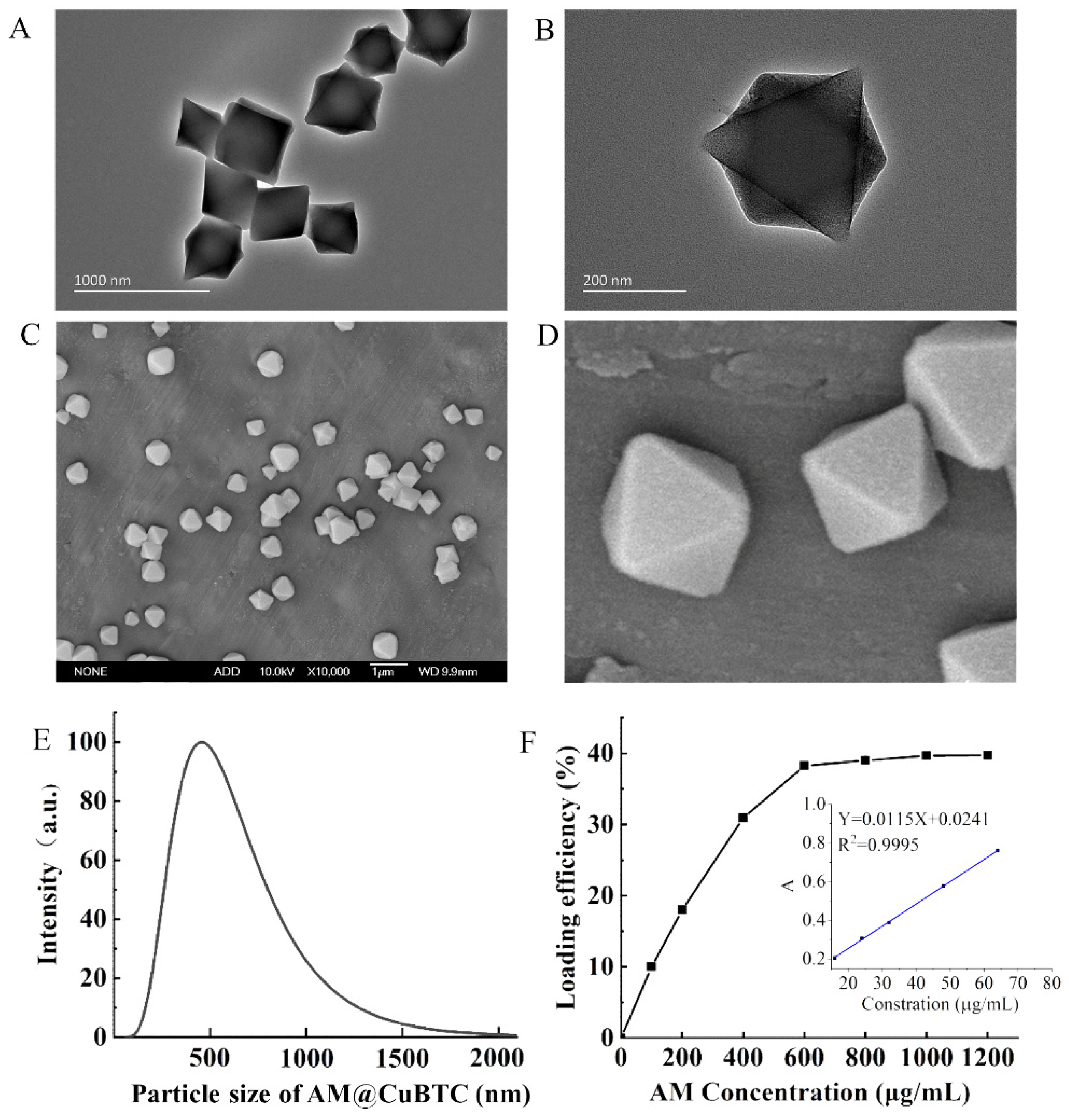
3.1. Characterization of AM@CuBTC
3.2. Pesticide Loading of AM@CuBTC
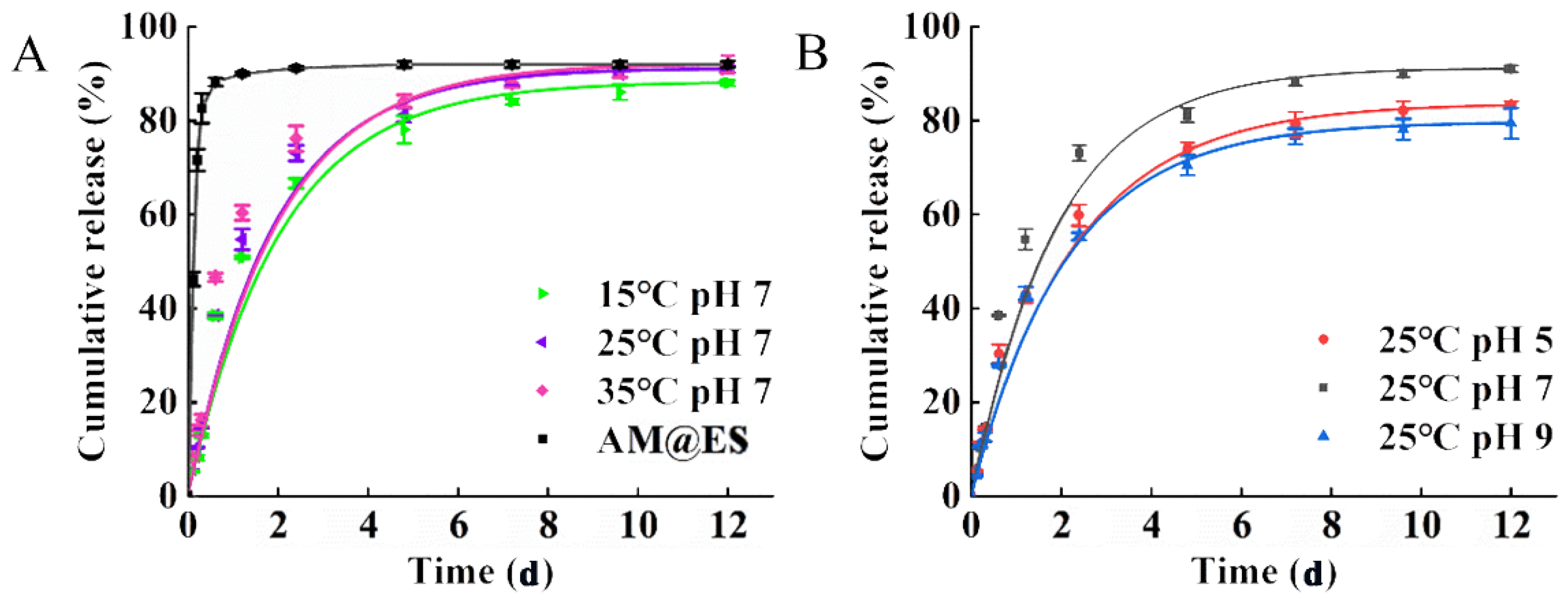
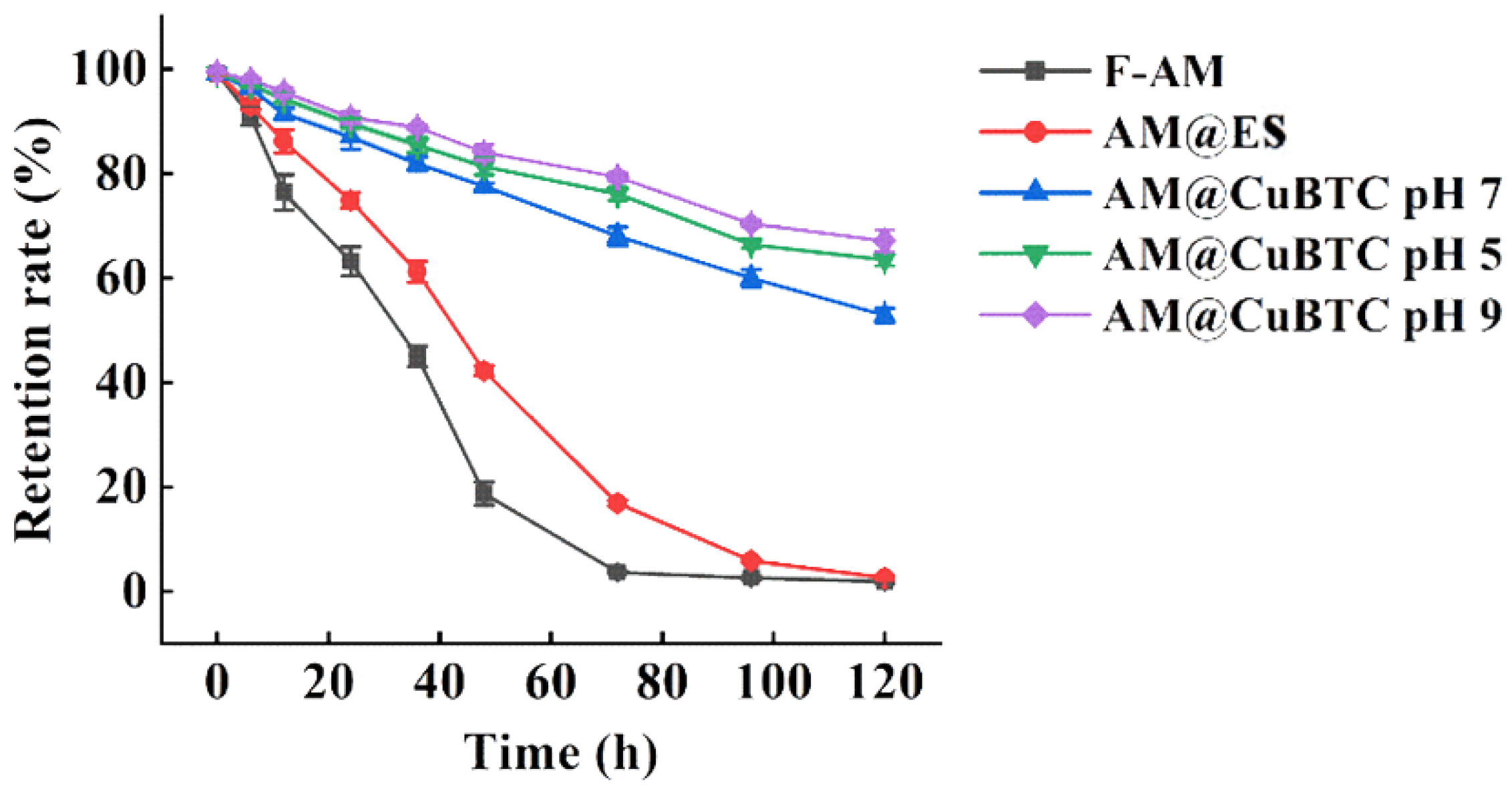
3.3. Assessment of Release Characteristics
3.4. Photodegradability of AM@CuBTC
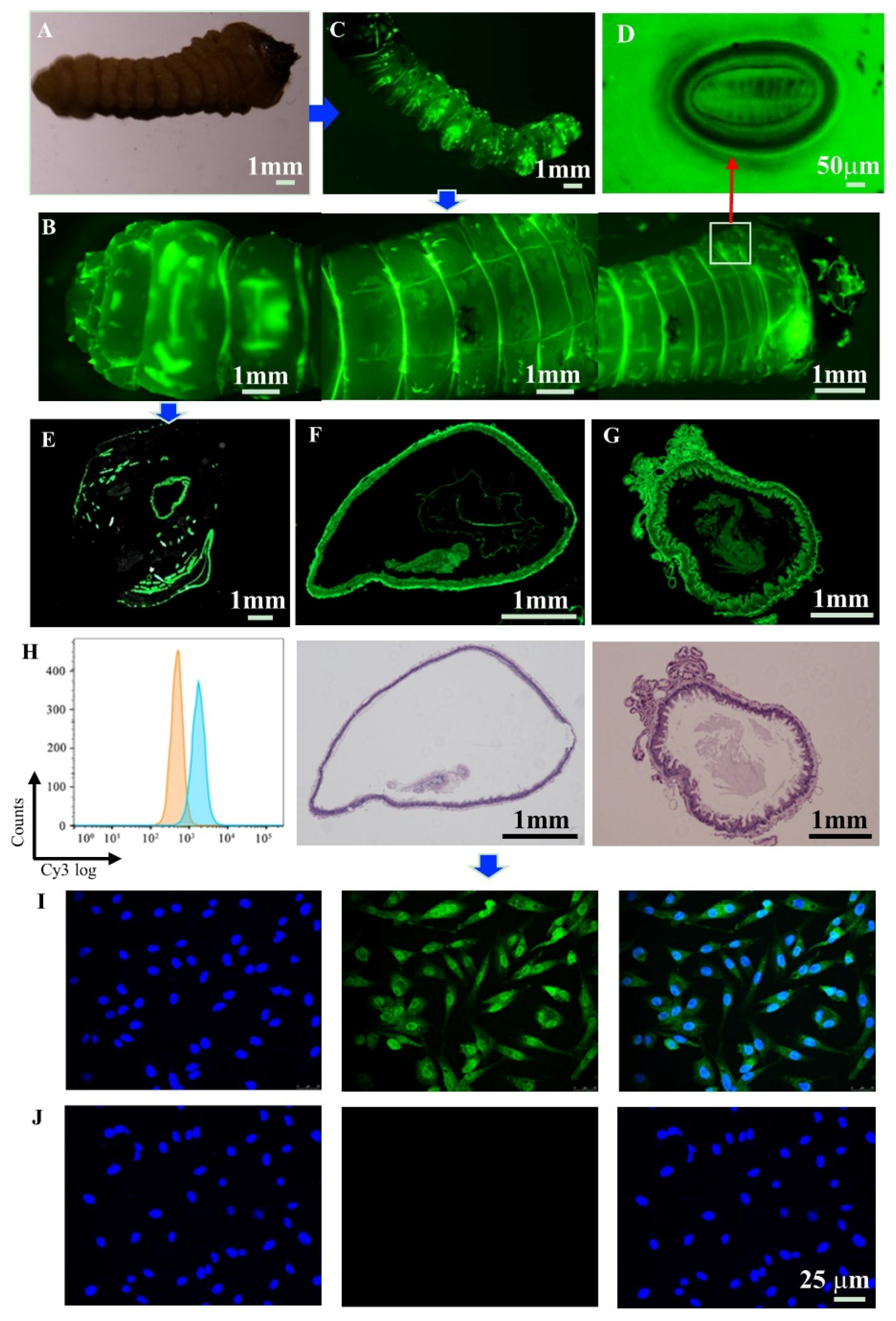
3.5. Biodistribution
3.6. Cellular Uptake and Detection of Adhesion
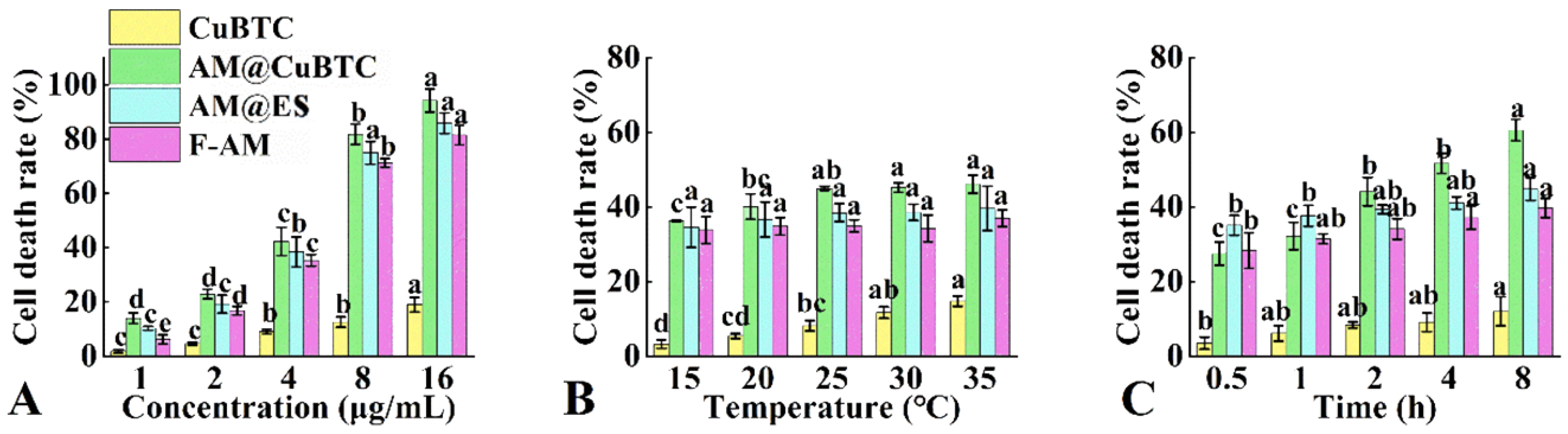
3.7. Cytotoxicity Assessment of AM@CuBTC
3.8. Mechanism of Toxicity
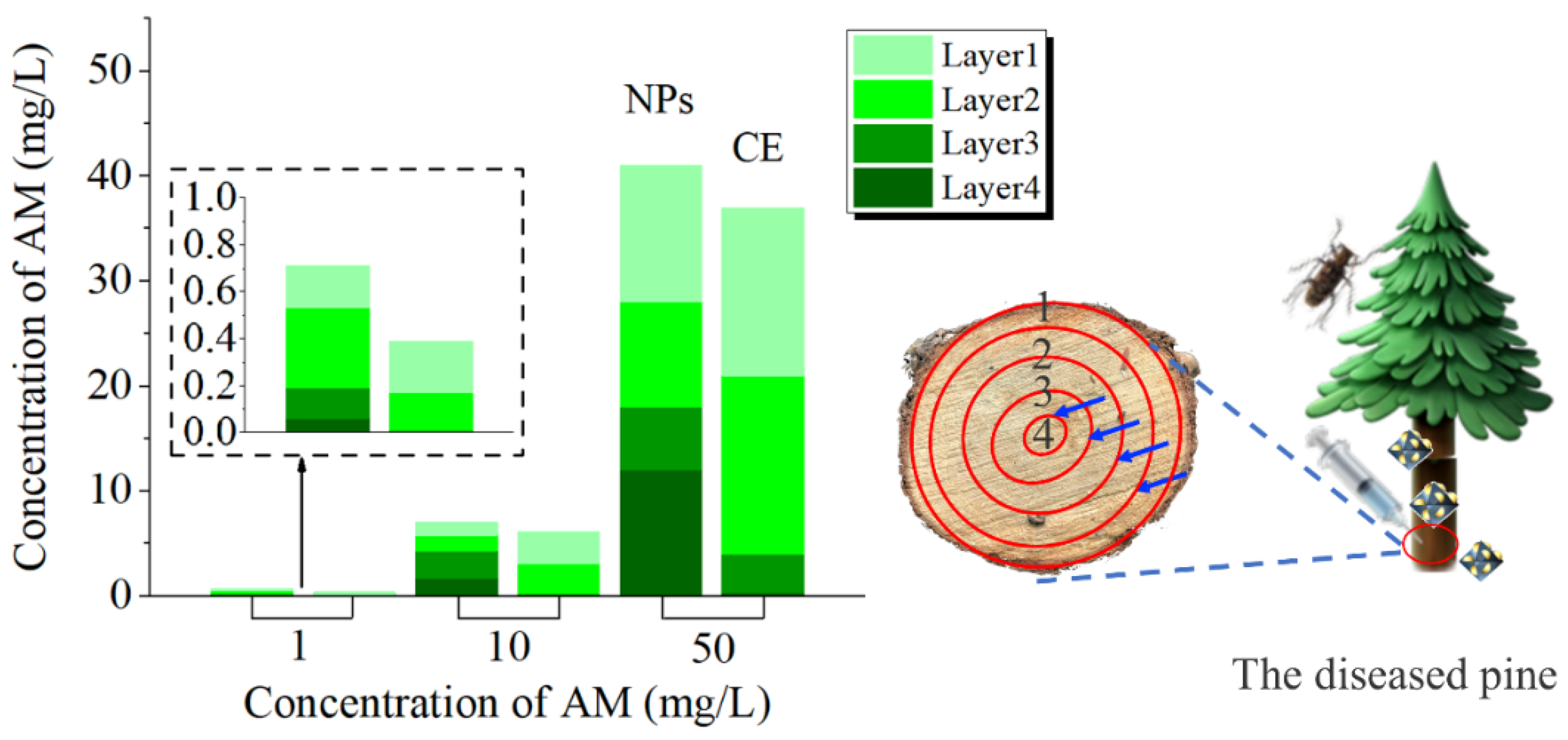
3.9. Transport Efficiency
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- da Silva, M.N.; Lima, M.R.M.; Vasconcelos, M.W. Susceptibility evaluation of Picea abies and Cupressus lusitanica to the pine wood nematode (Bursaphelenchus xylophilus). Plant Pathol. 2013, 62, 1398–1406. [Google Scholar] [CrossRef]
- Kim, N.; Jeon, H.W.; Mannaa, M.; Jeong, S.I.; Kim, J.; Lee, C.; Park, A.R.; Kim, J.C.; Seo, Y.S. Induction of resistance against pine wilt disease caused by Bursaphelenchus xylophilus using selected pine endophytic bacteria. Plant Pathol. 2019, 68, 434–444. [Google Scholar] [CrossRef]
- Wu, Y.X.; Wickham, J.D.; Zhao, L.L.; Sun, J.H. CO2 drives the pine wood nematode of its insect vector. Curr. Biol. 2019, 29, 619–620. [Google Scholar] [CrossRef] [PubMed]
- Futai, K. Pine wood nematode, Bursaphelenchus xylophilus. Ann. Rev. Phytopathol. 2013, 51, 61–83. [Google Scholar] [CrossRef] [PubMed]
- Moon, Y.; Son, J.A.; Jung, C.S. Morphometric variation in Pine wood nematodes, Bursaphelenchus xylophilus and B. mucronatus, Isolated from multiple locations in South Korea. Plant Pathol. J. 2013, 29, 344–349. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sriwati, R.; Takemoto, S.; Futai, K. Seasonal changes in the nematode fauna in pine trees killed by the pine wood nematode, Bursaphelenchus xylophilus. Jpn. J. Nematol. 2007, 36, 87–100. [Google Scholar] [CrossRef][Green Version]
- Maehara, N.; Futai, K. Effect of fungal interactions on the numbers of the pine wood nematode, Bursaphelenchus xylophilus (Nematoda: Aphelenchoididae), carried by the Japanese pine sawyer, Monochamus alternatus (Coleoptera: Cerambycidae). Fundam. Appl. Nematol. 1997, 20, 611–617. [Google Scholar]
- Koichi, S.; Shin-ichiro, N.; Kunihiko, H. Abundance-dependent transmission of the pine wood nematode, Bursaphelenchus xylophilus (Nematoda: Aphelenchoididae), to the Japanese pine sawyer, Monochamus alternatus (Coleoptera: Cerambycidae), adult in its pupal chamber. J. Forest Res. 2011, 16, 82–86. [Google Scholar]
- Togashi, K.; Shigesada, N. Spread of the pinewood nematode vectored by the Japanese pine sawyer: Modeling and analytical approaches. Popul. Ecol. 2006, 48, 271–283. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, F.M.; Wang, L.C.; Zhou, L.F.; Song, J. Study of the departure of pine wood nematode, Bursaphelenchus xylophilus (Nematoda: Aphelenchoididae) from Monochamus alternatus (Coleoptera: Cerambycidae). J. Asia-Pac. Entomol. 2020, 23, 981–987. [Google Scholar] [CrossRef]
- Teale, S.A.; Wickham, J.D.; Zhang, F.P.; Su, J.; Chen, Y.; Xiao, W.; Hanks, L.M.; Millar, J.G. A Male-produced aggregation pheromone of Monochamus alternatus (Coleoptera: Cerambycidae), a major vector of Pine wood nematode. J. Econ. Entomol. 2011, 104, 1592–1598. [Google Scholar] [CrossRef] [PubMed]
- Suh, D.Y.; Jung, J.K.; Lee, S.K.; Seo, S.T. Effect of aerial spraying of thiacloprid on pine sawyer beetles (Monochamus alternatus) and honey bees (Apis mellifera) in pine forests. Entomol. Res. 2020, 51, 83–89. [Google Scholar] [CrossRef]
- Zhang, H.F.; Wei, Z.Y.; Wei, C.J.; Huang, H.X.; Cai, J.B.; Zhi, Z.Y. Experiment against Monochamus alternatus with thiacloprid 1% micro-capsule granular in the forest. For. Pest Dis. 2010, 29, 25–37. (In Chinese) [Google Scholar]
- Toshihiro, Y.; Shin-ichiro, I. Chemical defense responses of Wilt-resistant pine Species, Pinus strobus and P. taeda, against Bursaphelenchus xylophilus infection. Jpn. J. Phytopathol. 1993, 59, 666–672. [Google Scholar]
- Keiko, K.; Toshihiro, Y.; Kazuhiko, M.; Hirotada, T. Effects of cavitation on the development of pine wilt disease caused by Bursaphelenchus xylophilus. Ann. Phytopath. Soc. Jpn. 1988, 54, 606–615. [Google Scholar]
- Hirao, T.; Fukatsu, E.; Watanabe, A. Characterization of resistance to pine wood nematode infection in Pinus thunbergii using suppression subtractive hybridization. BMC Plant Biol. 2012, 13, 12. [Google Scholar]
- Liu, J.; Zheng, X.P.; Gu, Z.J.; Chen, C.Y.; Zhao, Y.L. Bismuth sulfide nanorods as a precision nanomedicine for in vivo multimodal imaging-guided photothermal therapy of tumor. ACS Nano 2016, 12, 486–487. [Google Scholar]
- Gopinath, A.; Miyazono, E.; Faraon, A.; Rothemund, P.W.K. Engineering and mapping nanocavity emission via precision placement of DNA origami. Nature 2016, 535, 401–405. [Google Scholar] [CrossRef]
- Zhang, H.F.; Wang, D.; Butler, R.; Campbell, N.L.; Long, J.; Tan, B.; Duncalf, D.J.; Foster, A.J.; Hopkinson, A.; Taylor, D.; et al. Formation and enhanced biocidal activity of water-dispersable organic nanoparticles. Nat. Nanotechnol. 2018, 3, 506–511. [Google Scholar] [CrossRef]
- Jia, X.; Sheng, W.B.; Li, W.; Tong, Y.B.; Liu, Z.Y.; Zhou, F. Adhesive polydopamine coated Avermectin microcapsules for prolonging foliar pesticide retention. ACS Appl. Mater. Interfaces 2014, 6, 19552–19558. [Google Scholar] [CrossRef]
- Chariou, P.L.; Steinmetz, N.F. Delivery of pesticides to plant parasitic nematodes using tobacco mild green mosaic virus as a nanocarrier. ACS Nano 2017, 11, 4719–4730. [Google Scholar] [CrossRef] [PubMed]
- Li, B.X.; Wang, W.C.; Zhang, X.P.; Zhang, D.X.; Ren, Y.P.; Gao, Y.; Mu, W.; Liu, F. Using coordination assembly as the microencapsulation strategy to promote the efficacy and environmental safety of pyraclostrobin. Adv. Funct. Mater. 2017, 27, 1701841. [Google Scholar] [CrossRef]
- Liang, Y.; Fan, C.; Dong, H.Q.; Zhang, W.B.; Tang, G.; Yang, J.L.; Jiang, N.; Cao, Y.S. Preparation of MSNs-Chitosan@Prochloraz Nanoparticles for Reducing Toxicity and Improving Release Properties of Prochloraz. ACS Sustain. Chem. Eng. 2018, 6, 10211–10220. [Google Scholar] [CrossRef]
- Liang, Y.; Guo, M.C.; Fan, C.; Dong, H.Q.; Ding, G.L.; Zhang, W.B.; Tang, G.; Yang, J.L.; Kong, D.D.; Cao, Y.S. Development of novel urease-responsive pendimethalin microcapsules using silica-IPTS-PEI As controlled release carrier materials. ACS Sustain. Chem. Eng. 2017, 5, 4802–4810. [Google Scholar] [CrossRef]
- Jagadeesh, R.V.; Murugesan, K.; Alshammari, A.S.; Neumann, H.; Pohl, M.M.; Radnik, J.R.; Beller, M. MOF-derived cobalt nanoparticles catalyze a general synthesis of amines. Science 2017, 358, 326–332. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Gao, Y.; Liu, Y.K.; Huang, X.P.; Zhang, D.X.; Cao, H.C.; Jing, T.F.; Liu, F.; Li, B.X. Self-Assembled Degradable Nanogels Provide Foliar Affinity and Pinning for Pesticide Delivery by Flexibility and Adhesiveness Adjustment. ACS Nano 2021, 15, 14598–14609. [Google Scholar] [CrossRef]
- Grillo, R.; Abhilash, P.C.; Fraceto, L.F. Nanotechnology Applied to Bio-Encapsulation of Pesticides. J. Nanosci. Nanotechnol. 2016, 16, 1231–1244. [Google Scholar] [CrossRef]
- Rai, M.; Ingle, A. Role of nanotechnology in agriculture with special reference to management of insect pests. Appl. Microbiol. Biotechnol. 2012, 94, 287–293. [Google Scholar] [CrossRef]
- Hurtley, S.; Yeston, J. A MOF sets the stage to make amines. Science 2017, 358, 312–318. [Google Scholar] [CrossRef]
- Greathouse, J.A.; Allendorf, M.D. The interaction of water with MOF-5 simulated by molecular dynamics. J. Am. Chem. Soc. 2006, 128, 10678–10679. [Google Scholar] [CrossRef]
- Gould, S.L.; Tranchemontagne, D.; Yaghi, O.M.; Garcia-Garibay, M.A. Amphidynamic character of crystalline MOF-5: Rotational dynamics of terephthalate phenylenes in a free-volume, sterically unhindered environment. J. Am. Chem. Soc. 2008, 130, 3246–3247. [Google Scholar] [CrossRef] [PubMed]
- Yao, C.; Lykourinou, V.; Vetromile, C.; Hoang, T.; Ming, L.J.; Larsen, R.W.; Ma, S.Q. How can proteins enter the interior of a MOF? Investigation of cytochrome c translocation into a MOF consisting of mesoporous cages with microporous windows. J. Am. Chem. Soc. 2012, 134, 13188–13191. [Google Scholar]
- Pan, T.; Shen, Y.; Wu, P.; Gu, Z.D.; Zheng, B.; Wu, J.S.; Li, S.; Fu, Y.; Zhang, W.N.; Huo, F.W. Thermal Shrinkage Behavior of Metal–Organic Frameworks. Adv. Funct. Mater. 2020, 30, 2001389. [Google Scholar] [CrossRef]
- Kang, W.; Zhang, Y.; Fan, L.; Dai, F.; Wang, R.; Sun, D. Metal-Organic Framework Derived Porous Hollow Co3O4/N-C Polyhedron Composite with Excellent Energy Storage Capability. ACS Appl. Mater. Interfaces 2017, 9, 10602–10609. [Google Scholar] [CrossRef] [PubMed]
- Rosi, N.L.; Eddaoudi, M.; Kim, J.; O’Keeffe, M.; Yaghi, O.M. Advances in the chemistry of metal–organic frameworks. Crystengcomm 2002, 4, 401–404. [Google Scholar] [CrossRef]
- Su, C.Y.; Liu, S.S.; Cao, S.H.; Yin, S.Y.; Zhou, C.G.; Gao, S.K.; Jia, C.Y.; Ji, Y.C.; Liu, Y.X. Self-assembled bovine serum albumin nanoparticles as pesticide delivery vectors for controlling trunk-boring pests. J. Nanobiotechnol. 2020, 18, 165. [Google Scholar] [CrossRef]
- Su, C.Y.; Ji, Y.C.; Liu, S.S.; Gao, S.K.; Cao, S.H.; Xu, X.Y.; Zhou, C.G.; Liu, Y.X. Fluorescence-labeled abamectin nanopesticide for comprehensive control of pinewood nematode and Monochamus alternatus Hope. ACS Sustain. Chem. Eng. 2020, 8, 16555–16564. [Google Scholar] [CrossRef]
- Nuruzzaman, M.; Rahman, M.M.; Liu, Y.; Naidu, R. Nanoencapsulation, Nano-guard for pesticides: A new window for safe application. J. Agric. Food Chem. 2016, 64, 1447–1483. [Google Scholar] [CrossRef]
- Zhang, S.G.; Yang, Y.C.; Tong, Z.H.; Gao, B.; Gao, N.; Shen, T.L.; Wan, Y.S.; Yu, Z.; Liu, L.; Ma, X.X.; et al. Self-assembly of hydrophobic and self-healing bio-nanocomposite-coated controlled release fertilizer. ACS Appl. Mater. Interfaces 2020, 12, 27598–27606. [Google Scholar] [CrossRef]
- Liu, D.L.; Li, Y.; Sun, R.; Xu, J.Y.; Chen, Y.; Sun, C.Y. Colorimetric detection of organophosphorus pesticides based on the broad-spectrum aptamer. J. Nanosci. Nanotechnol. 2020, 20, 2114–2121. [Google Scholar] [CrossRef]
- Widawsky, D.; Rozelle, B.; Jin, S.Q.; Huang, J.K. Pesticide productivity, host-plant resistance and productivity in China. Agric. Ecom. 1998, 19, 203–217. [Google Scholar]
- Albersschoenberg, G.; Arison, B.H.; Chabala, J.C.; Douglas, A.W.; Eskola, P.; Fisher, M.H.; Lusi, A.; Mrozik, H.; Smith, J.L.; Tolman, R.L. Avermectins, Structure determination. J. Am. Chem. Soc. 1981, 103, 4216–4221. [Google Scholar] [CrossRef]
- Raliya, R.; Saharan, V.; Dimkpa, C.; Biswas, P. Nanofertilizer for precision and sustainable agriculture: Current state and future perspectives. J. Agric. Food Chem. 2017, 66, 6487–6503. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.Z.; Chen, J.F.; Fan, L.; Liu, A.Q.; Wen, L.X. Study of UV-shielding properties of novel porous hollow silica nanoparticle carriers for Avermectin. Pest Manag. Sci. 2010, 63, 241–246. [Google Scholar] [CrossRef]
- Webb, L.; Beaumont, D.J.; Nager, R.G.; Mccracken, D.I. Field-scale dispersal of Aphodius dung beetles (Coleoptera: Scarabaeidae) in response to Avermectin treatments on pastured cattle. Bull. Entomol. Res. 2010, 100, 175–183. [Google Scholar] [CrossRef]
- Barbosa, P.; Peters, T.M. Some effects of overcrowding on the respiration of larval Aedes aegypti. Entomol. Exp. Appl. 2011, 16, 146–156. [Google Scholar] [CrossRef]
- Hacet, N.; Amur-Elpek, B.; Kirgiz, T. A Study on the Odonate Larvae of Turkish Thrace: With Larval Identification Keys to the Considered Taxa. J. Entomol. Res. Soc. 2009, 12, 57–74. [Google Scholar]
- Van Rensburg, G.J.; Wepener, V.; Horn, S.; Richard, G. Oxidative stress in the freshwater shrimp Caridina africana following exposure to atrazine. Bull. Environ. Contam. Toxicol. 2022. [Google Scholar] [CrossRef]
- Stanley, J.; Sah, K.; Jain, S.K.; Bhatt, J.C.; Sushil, S.N. Evaluation of pesticide toxicity at their field recommended doses to honeybees, Apis cerana and A. mellifera through laboratory, semi-field and field studies. Chemosphere 2015, 119, 668–674. [Google Scholar] [CrossRef]
- Kuotsu, K.; Das, A.; Verma, B.C. Nanotechnology and Agriculture; Lap Lambert Academic Publishing GmbH & Co. KG.: Saarbrücken, Germany, 2012; pp. 2–10. [Google Scholar]
- Seaman, D. Trends in the formulation of pesticides—An overview. Pest Manag. Sci. 2010, 29, 437–449. [Google Scholar] [CrossRef]
- Karmakar, R.; Hazra, D.K. Recent advances in pesticide formulations for eco-friendly and sustainable vegetable pest management: A review. Arch. Agric. Environ. Sci. 2017, 2, 232–237. [Google Scholar]
- Zhao, X.; Cui, H.; Wang, Y.; Sun, C.; Cui, B.; Zeng, Z. Development strategies and prospects of nano-based smart pesticide formulation. J. Agric. Food Chem. 2018, 66, 6504–6512. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Bai, B.; Wang, H.; Suo, Y. A Near-Infrared and Temperature-Responsive Pesticide Release Platform through Core–Shell Polydopamine@PNIPAm Nanocomposites. ACS Appl. Mater. Interfaces 2017, 9, 6424–6432. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.P.; Jing, T.F.; Zhang, D.X.; Luo, J.; Li, B.X.; Liu, F. Assessment of ethylene glycol diacetate as an alternative carrier for use in agrochemical emulsifiable concentrate formulation. Ecotoxicol. Environ. Saf. 2018, 163, 349–355. [Google Scholar] [CrossRef] [PubMed]
- Takai, K.; Soejima, T.; Suzuki, T.; Kawazu, K. Emamectin benzoate as a candidate for a trunk-injection agent against the pine wood nematode, Bursaphelenchus xylophilus. Pest Manag. Sci. 2015, 56, 937–941. [Google Scholar] [CrossRef]
- Blaikie, S.J.; Leonardi, J.; Chacko, E.K.; Muller, W.J.; Scott, N.S. Effect of cincturing and chemical treatments on growth, flowering and yield of mango (Mangifera indica L.) cv. Kensington Pride. Aust. J. Exp. Agric. 1999, 39, 761–770. [Google Scholar] [CrossRef]
- Kosaka, H.; Aikawa, T.; Ogura, N.; Tabata, K.; Kiyohara, T. Pine Wilt Disease Caused by the Pine Wood Nematode: The Induced Resistance of Pine Trees by the Avirulent Isolates of Nematode. Eur. J. Plant Pathol. 2001, 107, 667–675. [Google Scholar] [CrossRef]
- Kim, B.N.; Kim, H.K.; Ahn, J.Y.; Kim, S.; Min, J. A short review of the pinewood nematode, Bursaphelenchus xylophilus. Toxicol. Environ. Health Sci. 2020, 12, 297–304. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Zhang, Y.; Xin, X.; Xu, X.; Wang, G.; Gao, S.; Qiao, L.; Yin, S.; Liu, H.; Jia, C.; et al. Design and Preparation of Avermectin Nanopesticide for Control and Prevention of Pine Wilt Disease. Nanomaterials 2022, 12, 1863. https://doi.org/10.3390/nano12111863
Liu Y, Zhang Y, Xin X, Xu X, Wang G, Gao S, Qiao L, Yin S, Liu H, Jia C, et al. Design and Preparation of Avermectin Nanopesticide for Control and Prevention of Pine Wilt Disease. Nanomaterials. 2022; 12(11):1863. https://doi.org/10.3390/nano12111863
Chicago/Turabian StyleLiu, Yanxue, Yiwu Zhang, Xin Xin, Xueying Xu, Gehui Wang, Shangkun Gao, Luqin Qiao, Shuyan Yin, Huixiang Liu, Chunyan Jia, and et al. 2022. "Design and Preparation of Avermectin Nanopesticide for Control and Prevention of Pine Wilt Disease" Nanomaterials 12, no. 11: 1863. https://doi.org/10.3390/nano12111863
APA StyleLiu, Y., Zhang, Y., Xin, X., Xu, X., Wang, G., Gao, S., Qiao, L., Yin, S., Liu, H., Jia, C., Shen, W., Xu, L., Ji, Y., & Zhou, C. (2022). Design and Preparation of Avermectin Nanopesticide for Control and Prevention of Pine Wilt Disease. Nanomaterials, 12(11), 1863. https://doi.org/10.3390/nano12111863





