Carbon Allotropes-Based Paints and Their Composite Coatings for Electromagnetic Shielding Applications
Abstract
:1. Introduction
2. Materials and Methods
- ⮚
- B1: 60% GAK-1, 20% PB and 20% PVB
- ⮚
- B2: 50% GAK-1, 16.7% PB, 16.7% Fe3O4, 16.7% PVB
- ⮚
- B3: 50% GAK-1, 16.7% PB, 16.7% Iron Ore, 16.7% PVB
- ⮚
- V: 25% natural graphite, 25%GNPs, 25% Fe3O4, 12.5% CB, 7.5% PEDOT:PSS, 5% PVB
Characterization Methods
3. Results and Discussion
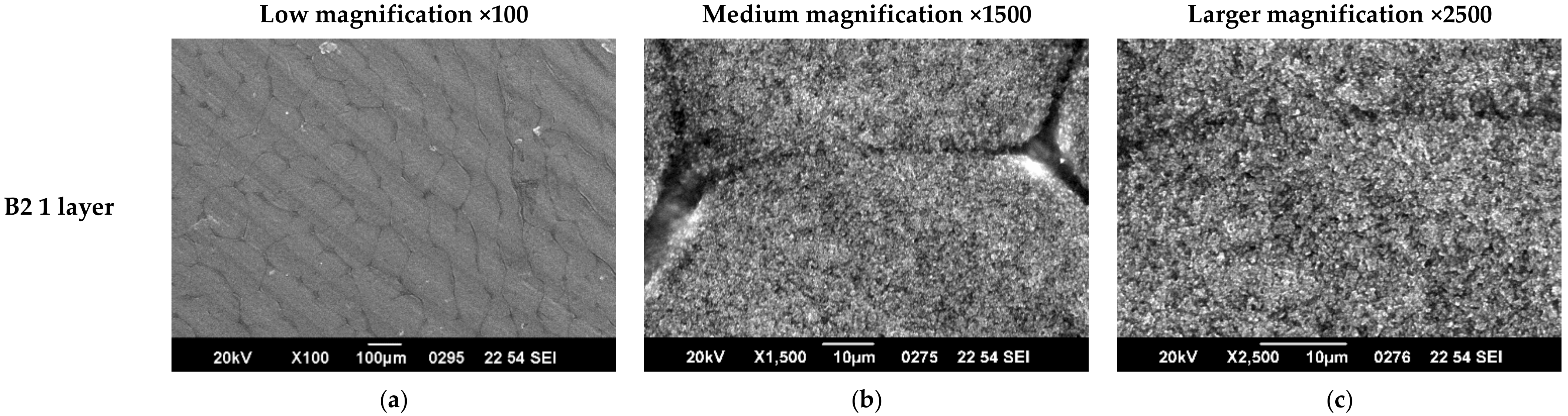
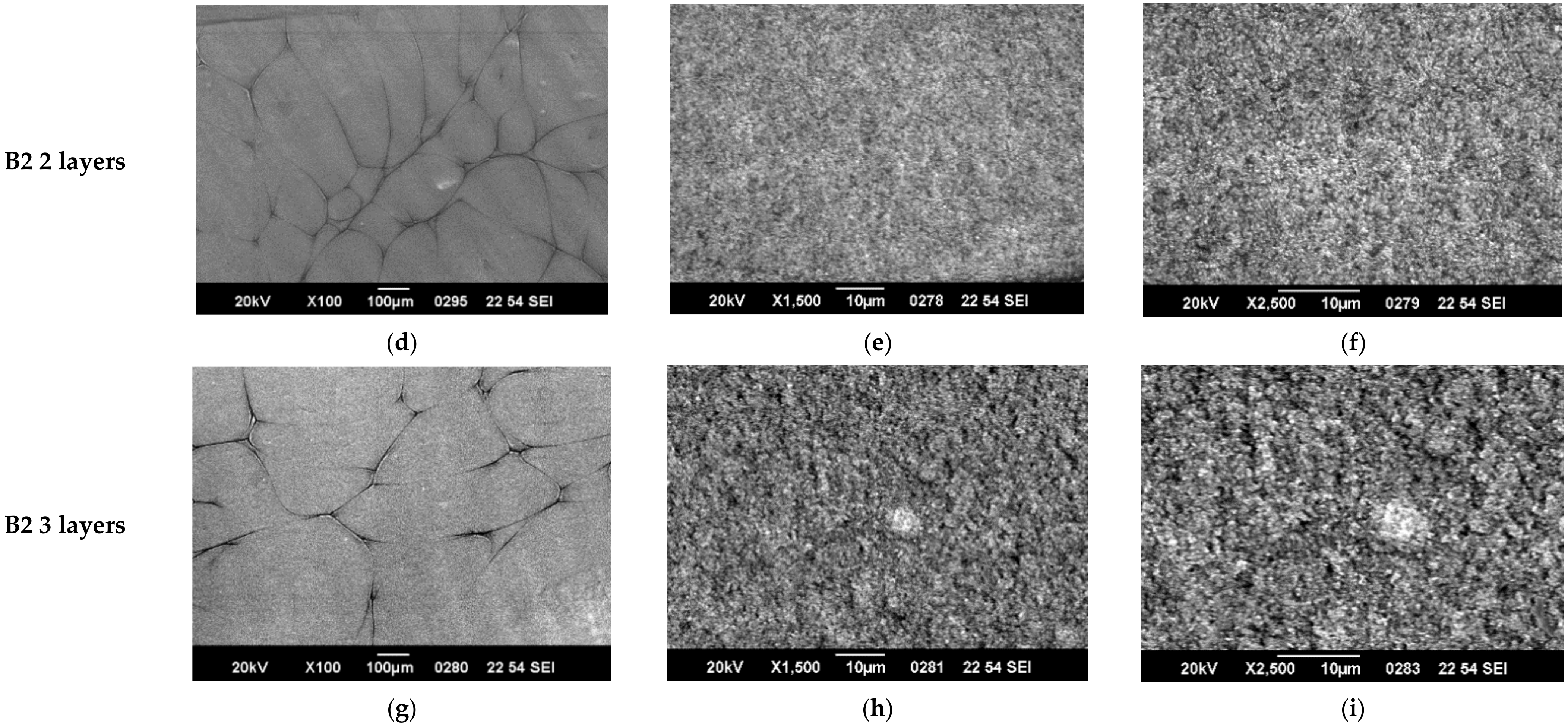
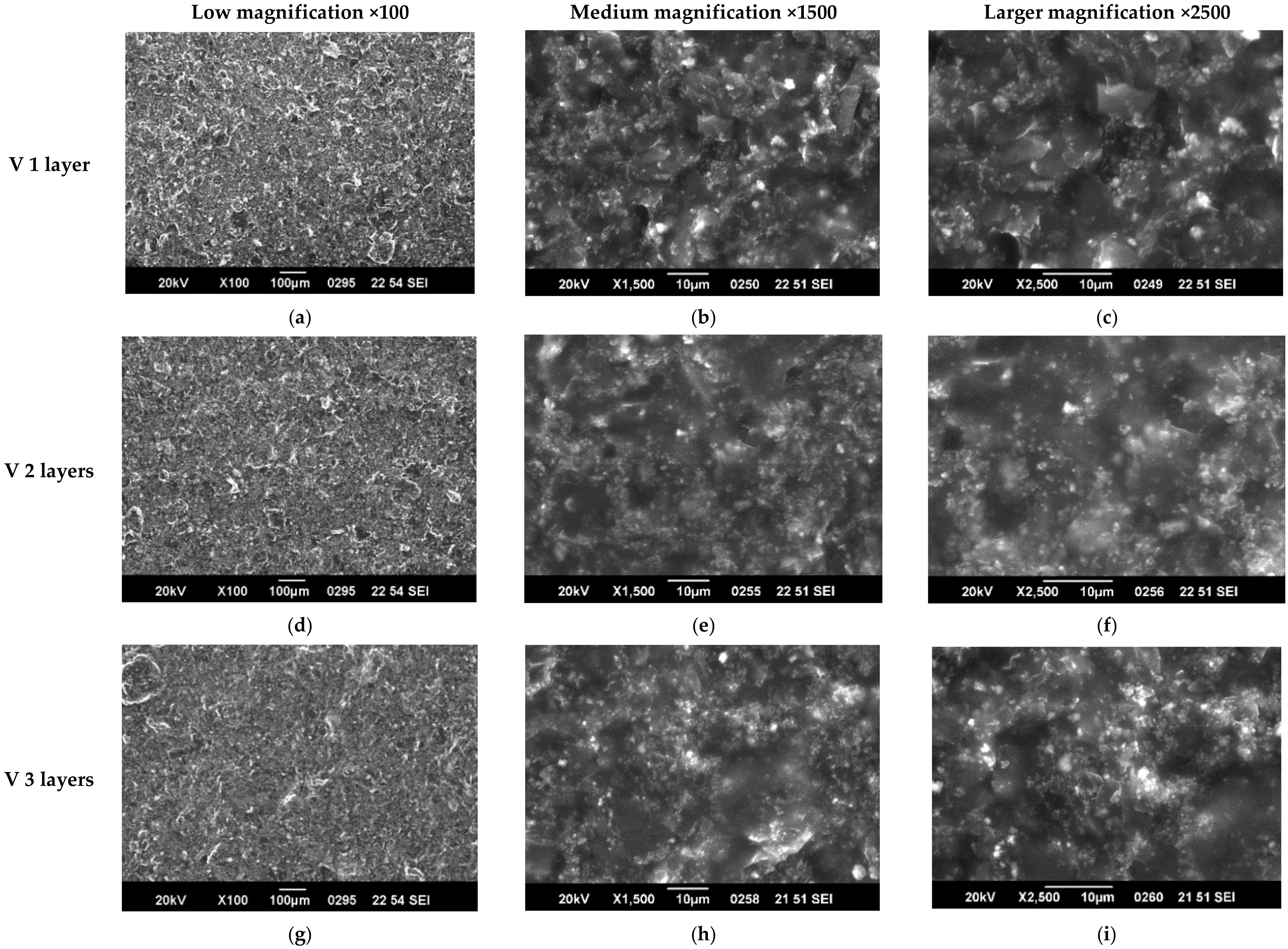
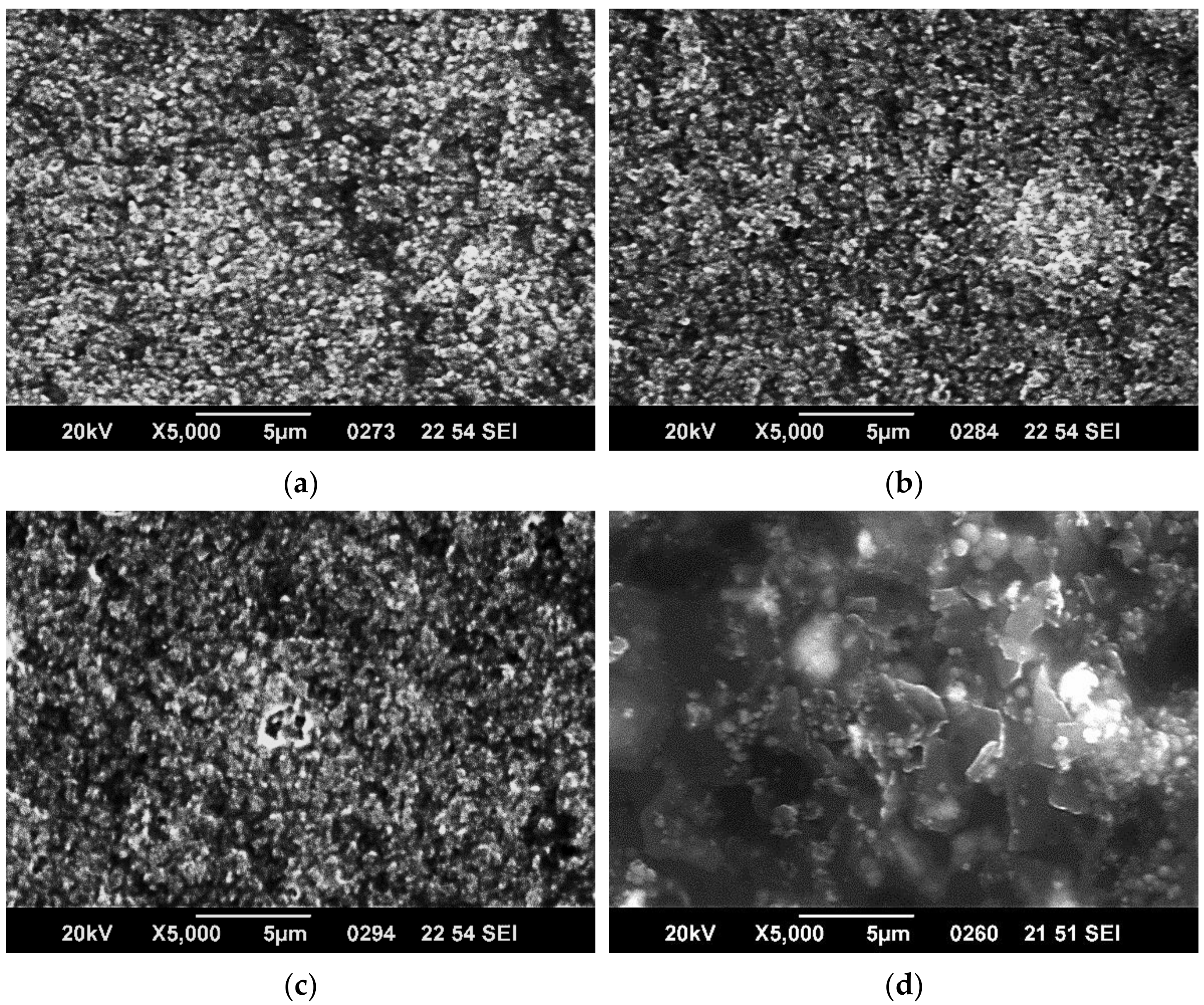
3.1. SEM Characterization
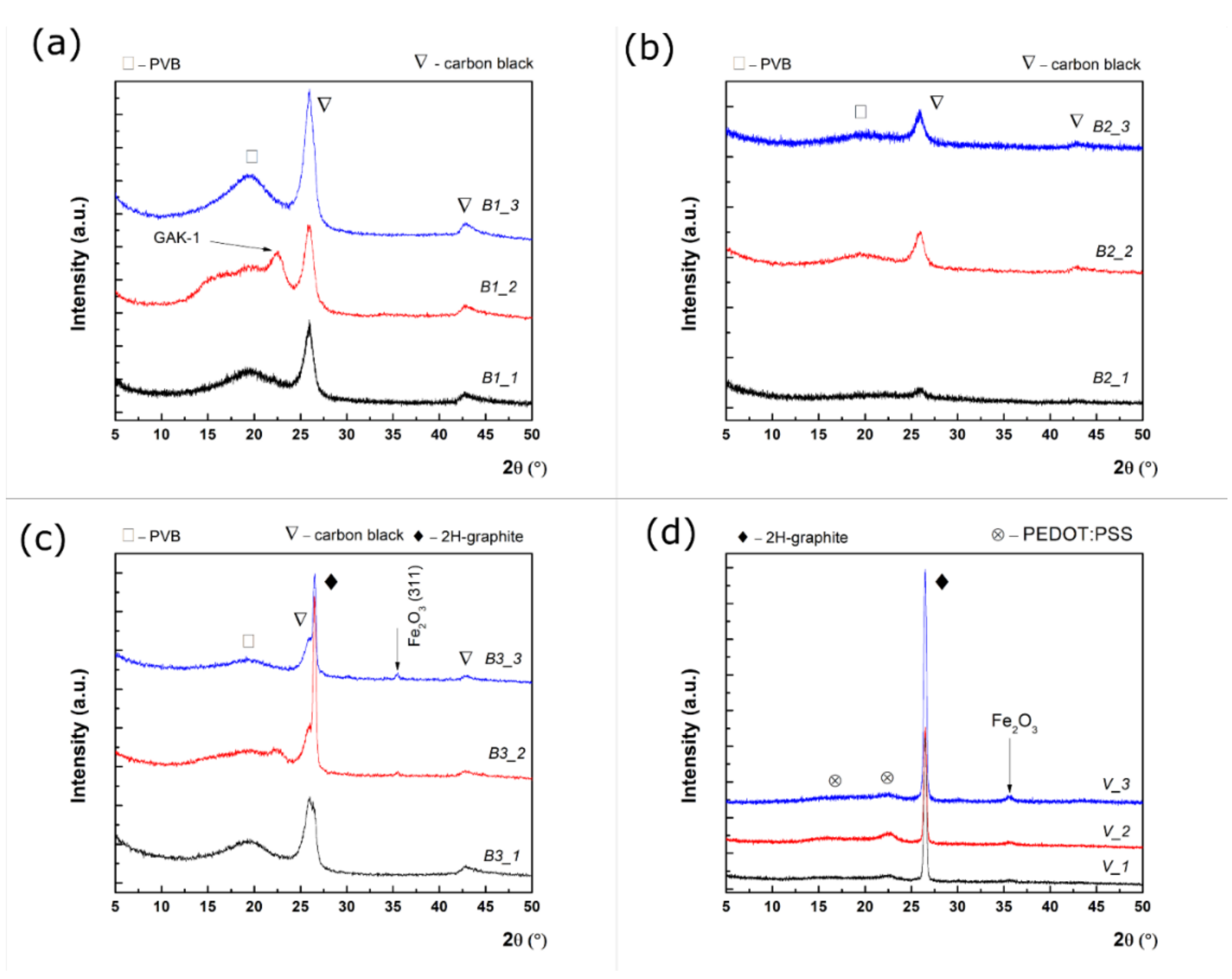
3.2. XRD Characterization
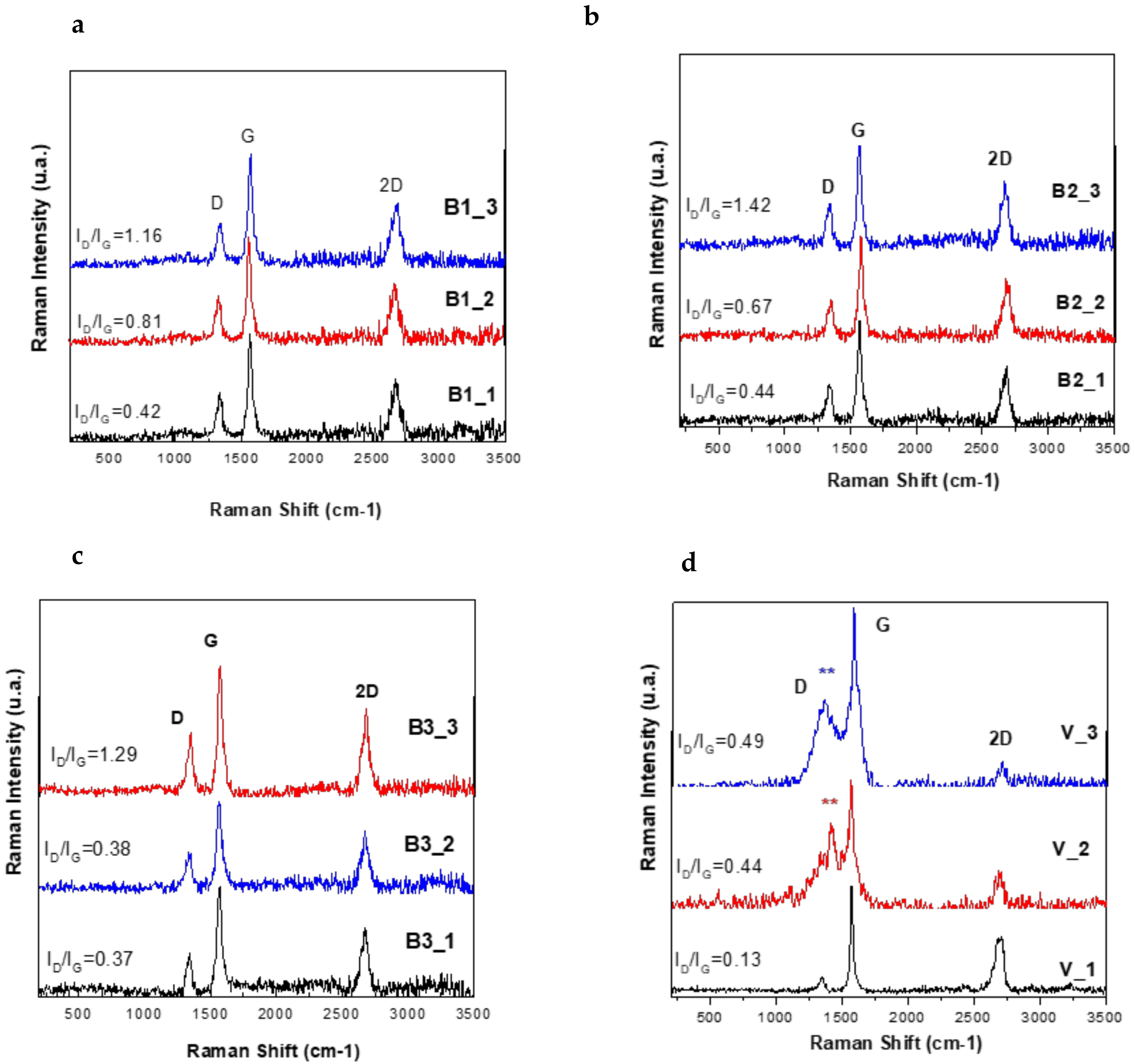
3.3. Raman Spectroscopy Characterisation
3.4. Resistance Measurements
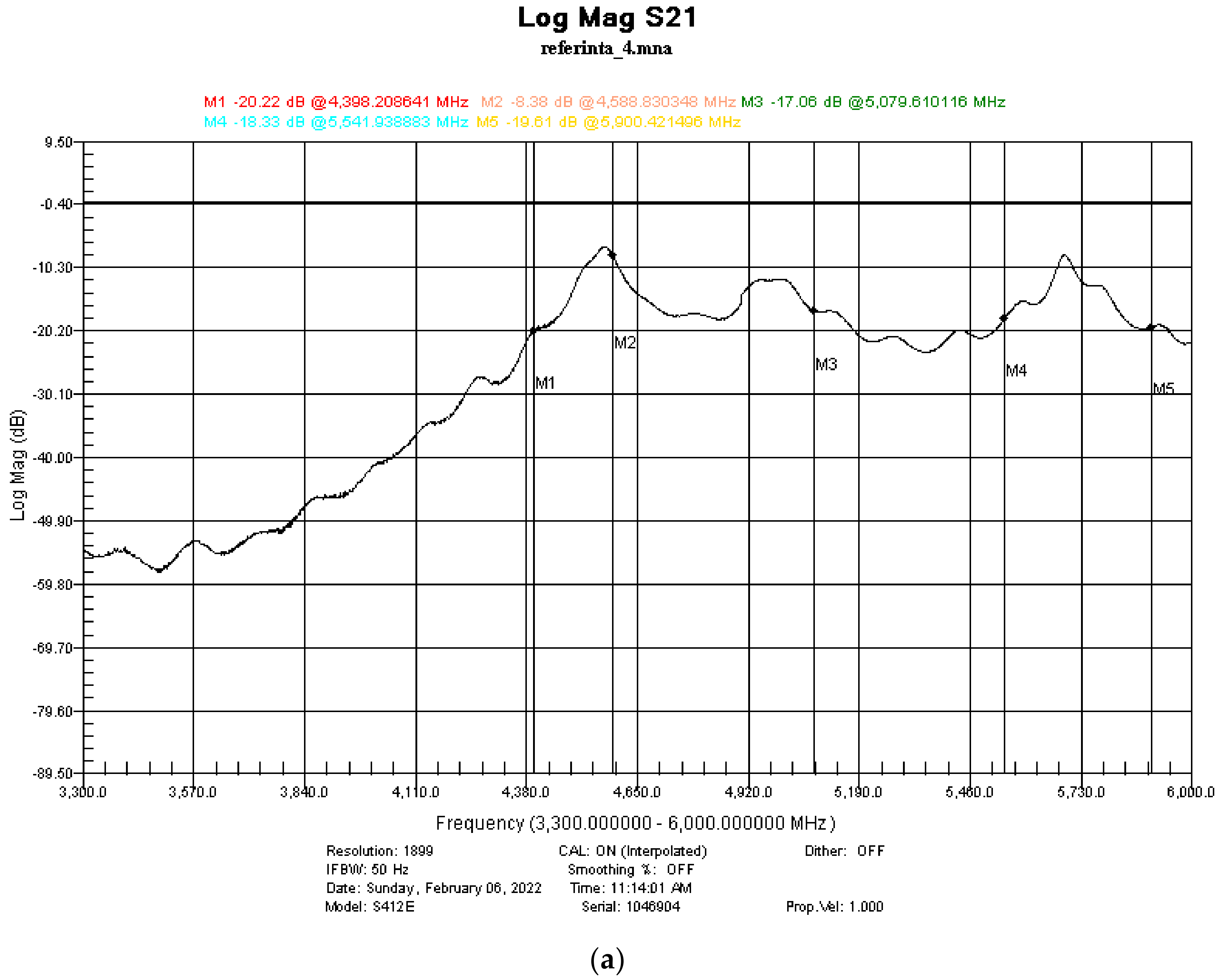
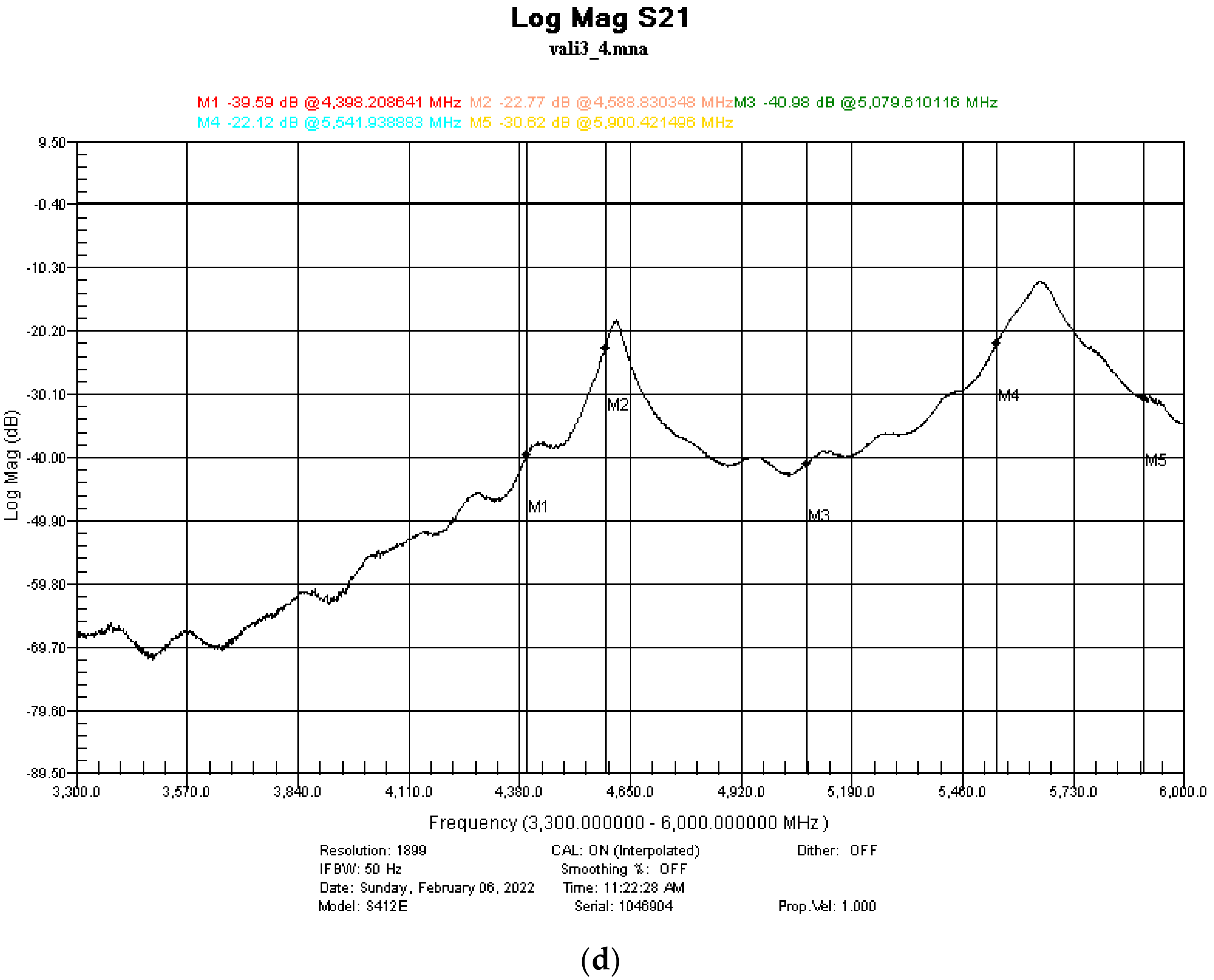
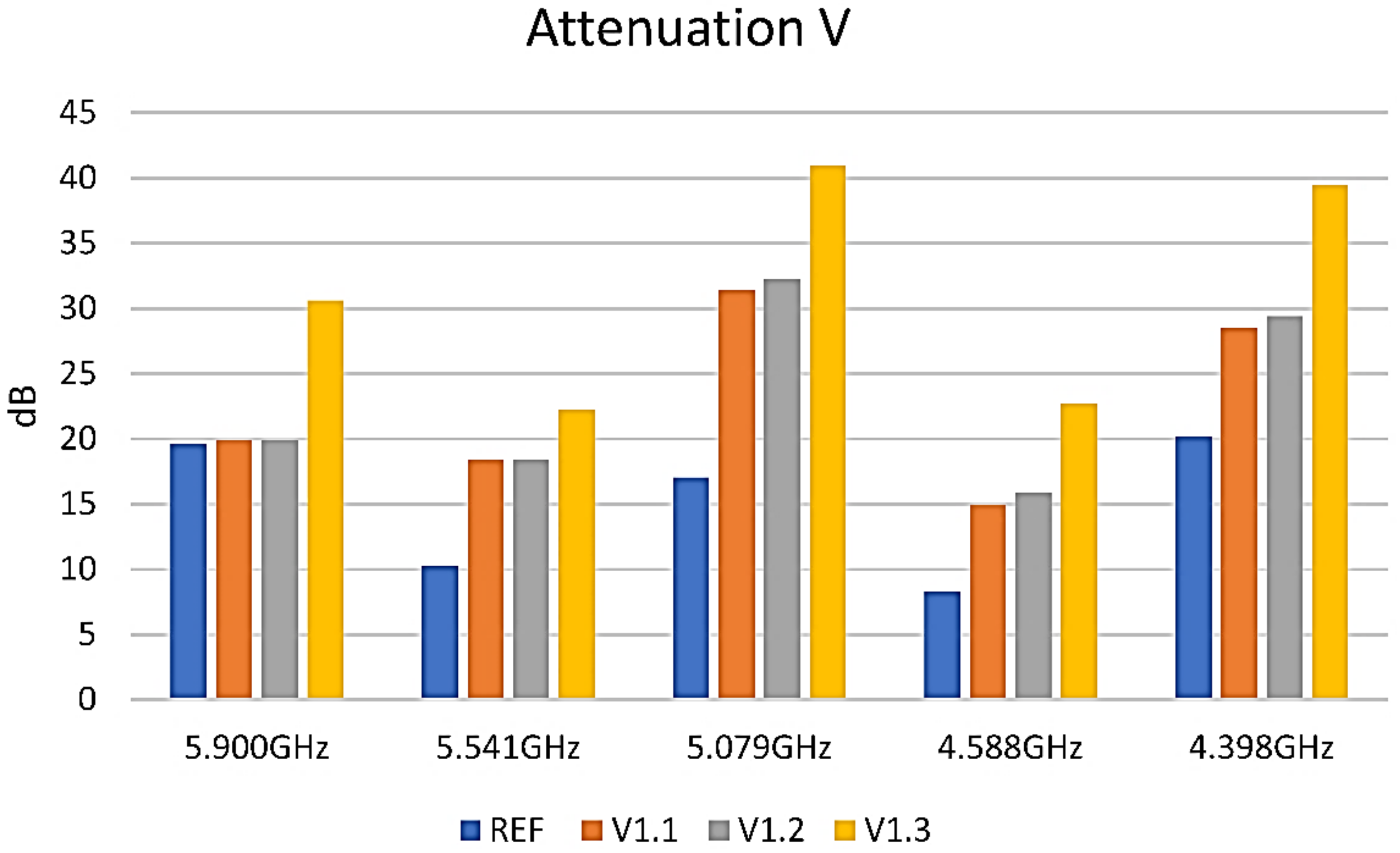
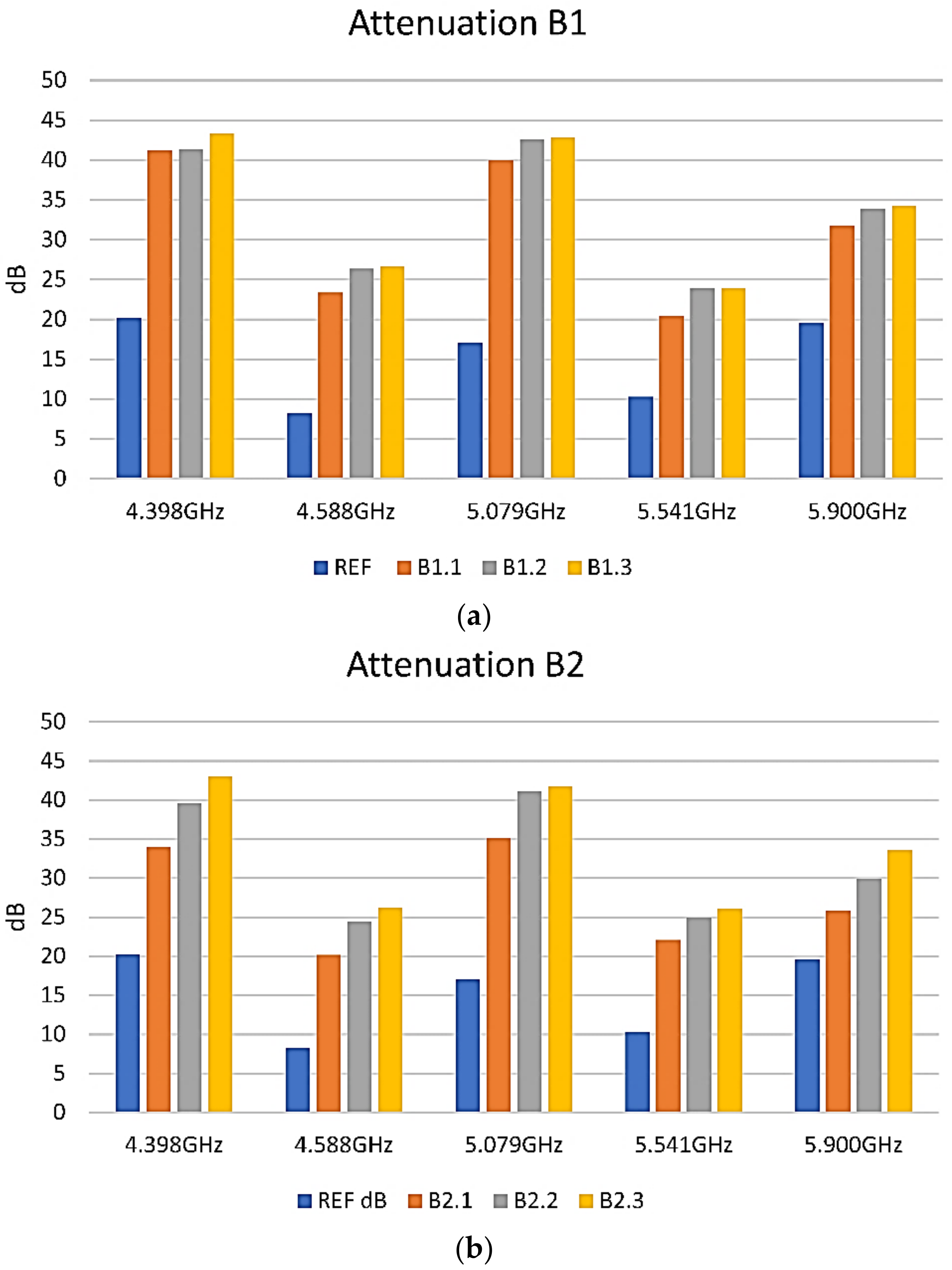
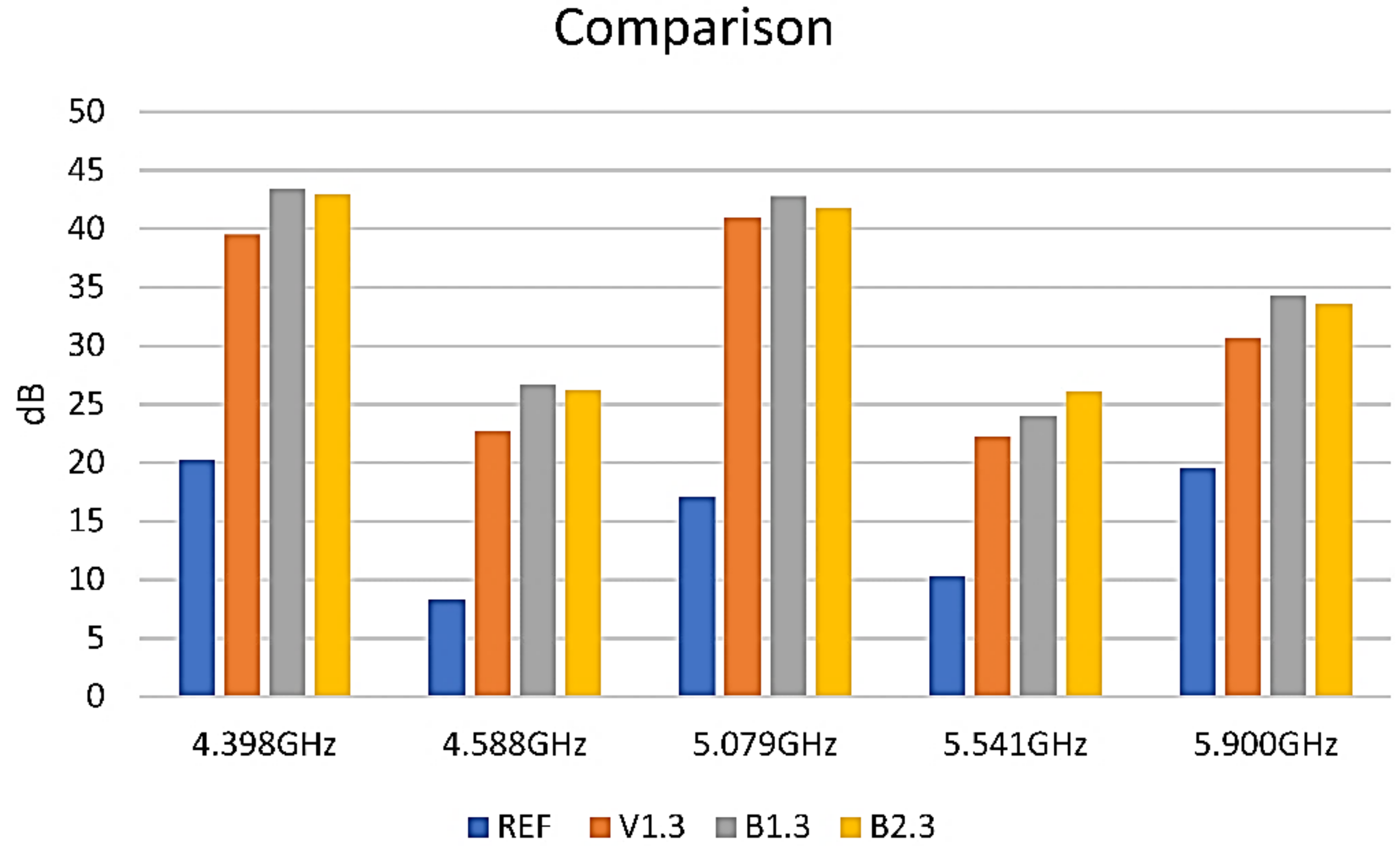
3.5. Shielding Properties
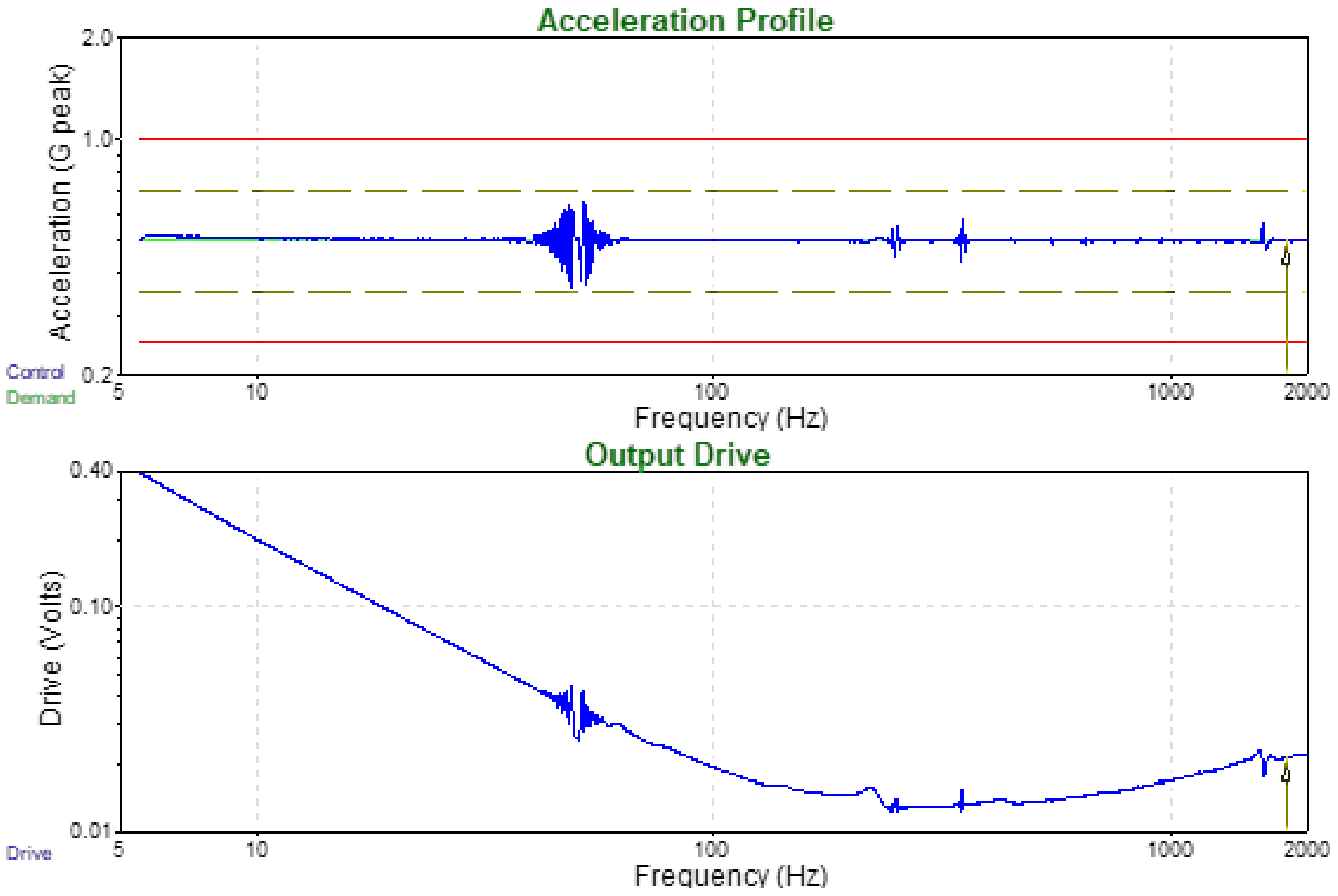
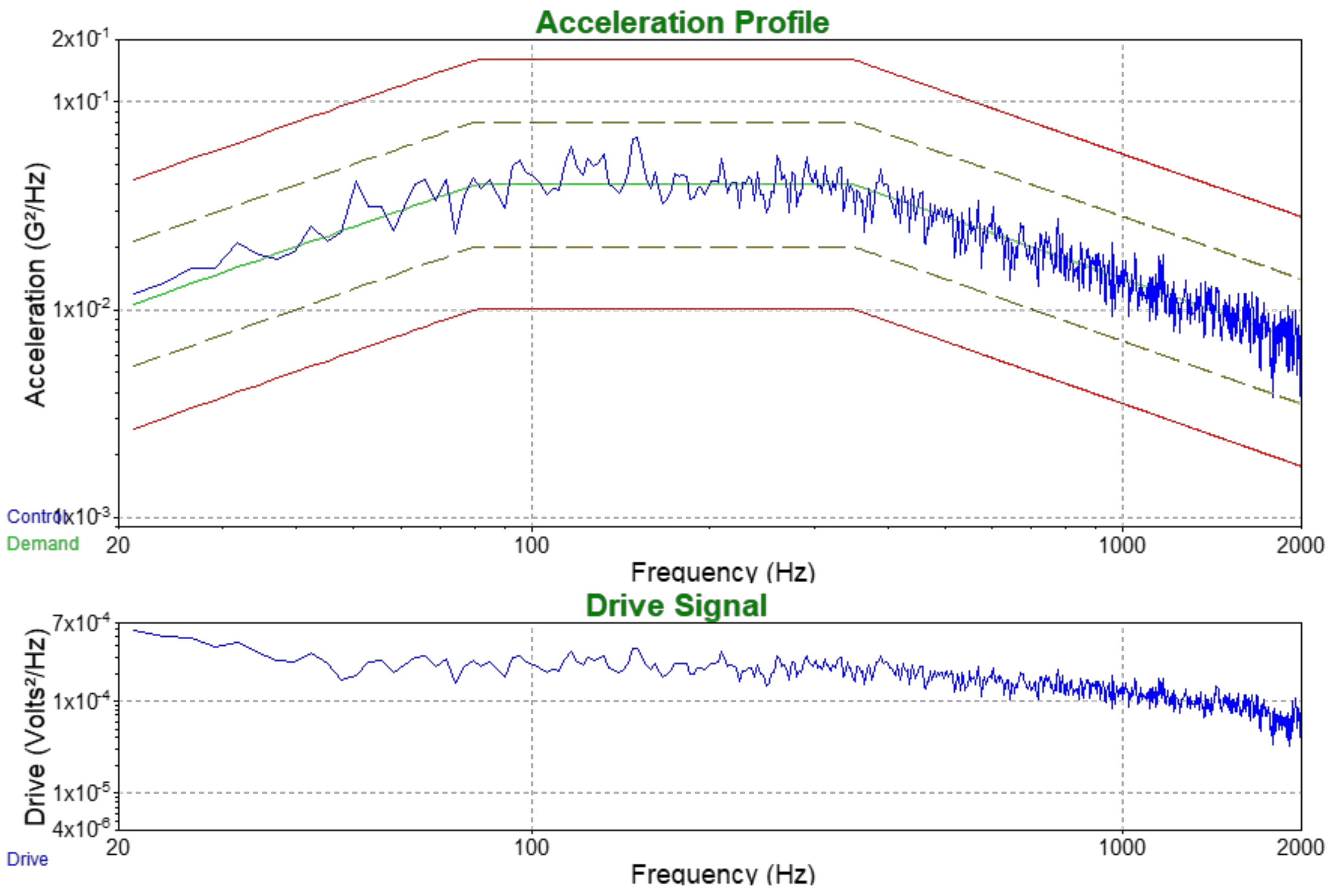
3.6. Environmental Testing for Potential Commercial Applications
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jaroszewsk, I.M.; Thomas, S.; Rane, A.V. Advanced Materials for Electromagnetic Shielding: Fundamentals, Properties, and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2018; p. 464. ISBN 978-1-119-12863. [Google Scholar]
- Suchea, M.; ITudose, I.V.; Pascariu, P.; Koudoumas, E. Chapter 12—Carbon-Based Nanocomposites for EMI Shielding: Recent Advances. In Materials for Potential EMI Shielding Applications; Joseph, K., Wilson, R., George, G., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 201–211. ISBN 9780128175903. [Google Scholar] [CrossRef]
- Singh, A.K.; Shishkin, A.; Koppel, T.; Gupta, N. Chapter 18—Porous materials for EMI shielding. In Materials for Potential EMI Shielding Applications; Joseph, K., Wilson, R., George, G., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 287–314. ISBN 9780128175903. [Google Scholar] [CrossRef]
- Wang, C.; Murugadoss, V.; Kong, J.; He, Z.; Ma, X.; Shao, Q.; Chen, Y.; Guo, L.; Liu, C.; Angaiah, S.; et al. Overview of carbon nanostructures and nanocomposites for electromagnetic wave shielding. Carbon 2018, 140, 696–733. [Google Scholar] [CrossRef]
- Gupta, S.; Tai, N.H. Carbon materials and their composites for electromagnetic interference shielding effectiveness in X-band. Carbon 2019, 152, 159–187. [Google Scholar] [CrossRef]
- Sankaran, S.; Deshmukh, K.; Basheer Ahamed, M.; Khadheer Pasha, S.K. Recent advances in electromagnetic interference shielding properties of metal and carbon filler reinforced flexible polymer composites: A review. Compos. Part A Appl. Sci. Manuf. 2018, 114, 49–71. [Google Scholar] [CrossRef]
- Kausar, K. Chapter 14—Hybrid polymeric nanocomposites with EMI shielding applications. In Materials for Potential EMI Shielding Applications; Joseph, K., Wilson, R., George, G., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 227–236. ISBN 9780128175903. [Google Scholar] [CrossRef]
- Pradhan, S.S.; Unnikrishnan, L.; Mohanty, S.; Nayak, S.K. Thermally Conducting Polymer Composites with EMI Shielding: A review. J. Electron. Mater. 2020, 49, 1749–1764. [Google Scholar] [CrossRef]
- Zubair, K.; Ashraf, A.; Gulzar, H.; Fayzan Shakir, M.; Nawab, Y.; Rehan, Z.A.; Rashid, I.A. Study of mechanical, electrical and EMI shielding properties of polymer-based nanocomposites incorporating polyaniline coated graphene nanoparticles. Nano Express. 2021, 2, 010038. [Google Scholar] [CrossRef]
- Ramanujam BT, S.; Sabu, A.; Pranesh, M.; Thiruvengadathan, R. Carbon Nanostructures Fundamentals to Applications. In Chapter 15 Conducting Polymer Nanocomposites with Carbon Nanostructures as Advanced EMI Shielding Materials: Recent Advancements and Challenges; Thiruvengadathan, R., Roy, S.C., Sundriyal, P., Bhattacharya, S., Eds.; AIP Publishing: Long Island, NY, USA, 2021. [Google Scholar] [CrossRef]
- Ram, R.; Rahaman, M.; Khastgir, D. Carbon-Containing Polymer Composites; Rahaman, M., Khastgir, D., Aldalbahi, A., Eds.; Springer Series on Polymer and Composite Materials; Springer: Singapore, 2019. [Google Scholar] [CrossRef]
- Abbasi, H.; Antunes, M.; Velasco, J.I. Recent advances in carbon-based polymer nanocomposites for electromagnetic interference shielding. Prog. Mater. Sci. 2019, 103, 319–373. [Google Scholar] [CrossRef]
- Tudose, I.V.; Mouratis, K.; Ionescu, O.N.; Romanitan, C.; Pachiu, C.; Popescu, M.; Khomenko, V.; Butenko, O.; Chernysh, O.; Kenanakis, G.; et al. Novel Water-Based Paints for Composite Materials Used in Electromagnetic Shielding Applications. Nanomaterials 2022, 12, 487. [Google Scholar] [CrossRef] [PubMed]
- Ru, J.; Fan, Y.; Zhou, W.; Zhou, Z.; Wang, T.; Liu, R.; Yang, J.; Lu, X.; Wang, J.; Ji, C.; et al. Electrically Conductive and Mechanically Strong Graphene/Mullite Ceramic Composites for High-Performance Electromagnetic Interference Shielding. ACS Appl. Mater. Interfaces 2018, 10, 39245–39256. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Pang, L.; Li, Y.; Luo, H.; Duan, G.; Mei, C.; Xu, W.; Zhou, W.; Liu, K.; Jiang, S. Ultra-thin and highly flexible cellulose nanofiber/silver nanowire conductive paper for effective electromagnetic interference shielding. Compos. Part A Appl. Sci. Manuf. 2020, 135, 105960. [Google Scholar] [CrossRef]
- Khomenko, V.; Butenko, O.; Chernysh, O.; Barsukov, V.; Suchea, M.P.; Koudoumas, E. Electromagnetic Shielding of Composite Films Based on Graphite, Graphitized Carbon Black and Iron-Oxide. Coatings 2022, 12, 665. [Google Scholar] [CrossRef]
- Waremra, R.S.; Betaubun, P. Analysis of Electrical Properties Using the four point Probe Method. E3S Web Conf. 2018, 73, 13019. [Google Scholar] [CrossRef] [Green Version]
- Choma, J. Scattering Parameters: Concept, Theory, and Applications. 2021. Available online: https://www.ieee.li/pdf/essay/scattering_parameters_concept_theory_applications.pdf (accessed on 1 April 2022).
- ISO 16750-1. Road Vehicles—Environmental Conditions and Testing for Electrical and Electronic Equipment—Part 1. General. Available online: https://www.iso.org/standard/39008.html (accessed on 1 April 2022).
- IEC 60068-2, 6. Environmental Testing—Part 2–6: Testing, Test Fc: Vibration (Sinusoidal). Available online: https://www.saiglobal.com/PDFTemp/Previews/OSH/iec/iec60000/60000/iec60068-2-6%7Bed7.0%7Db.pdf (accessed on 1 April 2022).
- IEC 60068-2, 14. Basic Environmental Testing Procedures—Part 2–14: Tests—Test Nb: Change of Temperature. Available online: https://www.saiglobal.com/PDFTemp/Previews/OSH/iec/iec60000/60000/iec60068-2-6%7Bed7.0%7Db.pdf (accessed on 1 April 2022).
- IEC 60068-2, 64. Environmental Testing—Part 2–64: Test Methods—Test Fh—Vibration, Broad-Band Random (Digital Control) and Guidance. Available online: https://www.saiglobal.com/PDFTemp/Previews/OSH/iec/iec60000/60000/iec60068-2-6%7Bed7.0%7Db.pdf (accessed on 1 April 2022).
- IEC 60068-2, 80. Environmental Testing—Part 2–80: Tests—Test Fi: Vibration—Mixed Mode Testing. Available online: https://www.saiglobal.com/PDFTemp/Previews/OSH/iec/iec60000/60000/iec60068-2-6%7Bed7.0%7Db.pdf (accessed on 1 April 2022).
- Bragg, W.L. The Diffraction of Short Electromagnetic Waves by a Crystal. Proc. Camb. Philos. Soc. 1913, 17, 43–57. [Google Scholar]
- Zhang, Y.; Ding, Y.; Li, Y.; Gao, J.; Yang, J. Synthesis and characterization of polyvinyl butyral–Al(NO3)3 composite sol used for alumina based fibers. J. Sol Gel Sci. Technol. 2009, 49, 385–390. [Google Scholar] [CrossRef]
- Wen, Y.; He, K.; Zhu, Y.; Han, F.; Xu, Y.; Matsuda, I.; Ishii, Y.; Cumings, J.; Wang, C. Expanded graphite as superior anode for sodium-ion batteries. Nat. Commun. 2014, 5, 4033. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patterson, A.L. The Diffraction of X-rays by Small Crystalline Particles. Phys. Rev. 1939, 56, 972. [Google Scholar] [CrossRef]
- Arthisree, D.L.; Sumathi, R.R.; Joshi, G. Effect of graphene quantum dots on photoluminescence property of polyvinyl butyral nanocomposite. Polym. Adv. Technol. 2019, 30, 790–798. [Google Scholar] [CrossRef]
- Bokobza, L.; Bruneel, J.-L.; Couzi, M. Raman Spectra of Carbon-Based Materials (from Graphite to Carbon Black) and of Some Silicone Composites. C 2015, 1, 77–94. [Google Scholar] [CrossRef] [Green Version]
- Hatel, R.; Majdoub, S.E.; Bakour, A.; Khenfouch, M.; Baitoul, M. Graphene oxide/Fe3O4 nanorods composite: Structural and Raman investigation. J. Phys. 2018, 1081, 012006. [Google Scholar] [CrossRef] [Green Version]
- Dresselhaus, M.S.; Jorio, A.; Saito, R. Characterizing Graphene, Graphite, and Carbon Nanotubes by Raman Spectroscopy. Annu. Rev. Condens. Matter Phys. 2010, 1, 89–108. [Google Scholar] [CrossRef]






| Paint | Resistance (ohm) ± 0.01 |
|---|---|
| B1.1 | 322.10 |
| B1.2 | 142.90 |
| B1.3 | 60.12 |
| B2.1 | 217.30 |
| B2.2 | 156.80 |
| B2.3 | 66.09 |
| B3.1 | 215.10 |
| B3.2 | 158.60 |
| B3.3 | 69.01 |
| V1.1 | 336.37 |
| V1.2 | 148.70 |
| V1.3 | 70.80 |
| Frequency | Amplitude | Durate |
|---|---|---|
| 5–2000 Hz | 0.5 (g) | 2 (Oct/min) (up) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tudose, I.V.; Mouratis, K.; Ionescu, O.N.; Romanitan, C.; Pachiu, C.; Pricop, E.; Khomenko, V.H.; Butenko, O.; Chernysh, O.; Barsukov, V.Z.; et al. Carbon Allotropes-Based Paints and Their Composite Coatings for Electromagnetic Shielding Applications. Nanomaterials 2022, 12, 1839. https://doi.org/10.3390/nano12111839
Tudose IV, Mouratis K, Ionescu ON, Romanitan C, Pachiu C, Pricop E, Khomenko VH, Butenko O, Chernysh O, Barsukov VZ, et al. Carbon Allotropes-Based Paints and Their Composite Coatings for Electromagnetic Shielding Applications. Nanomaterials. 2022; 12(11):1839. https://doi.org/10.3390/nano12111839
Chicago/Turabian StyleTudose, Ioan Valentin, Kyriakos Mouratis, Octavian Narcis Ionescu, Cosmin Romanitan, Cristina Pachiu, Emil Pricop, Volodymyr H. Khomenko, Oksana Butenko, Oksana Chernysh, Viacheslav Z. Barsukov, and et al. 2022. "Carbon Allotropes-Based Paints and Their Composite Coatings for Electromagnetic Shielding Applications" Nanomaterials 12, no. 11: 1839. https://doi.org/10.3390/nano12111839
APA StyleTudose, I. V., Mouratis, K., Ionescu, O. N., Romanitan, C., Pachiu, C., Pricop, E., Khomenko, V. H., Butenko, O., Chernysh, O., Barsukov, V. Z., Suchea, M. P., & Koudoumas, E. (2022). Carbon Allotropes-Based Paints and Their Composite Coatings for Electromagnetic Shielding Applications. Nanomaterials, 12(11), 1839. https://doi.org/10.3390/nano12111839











