Adsorption Behaviors of Cationic Methylene Blue and Anionic Reactive Blue 19 Dyes onto Nano-Carbon Adsorbent Carbonized from Small Precursors
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Preparation of N-CM
2.3. Characterization
2.4. Adsorption Studies
2.5. Statistical Analysis
3. Results
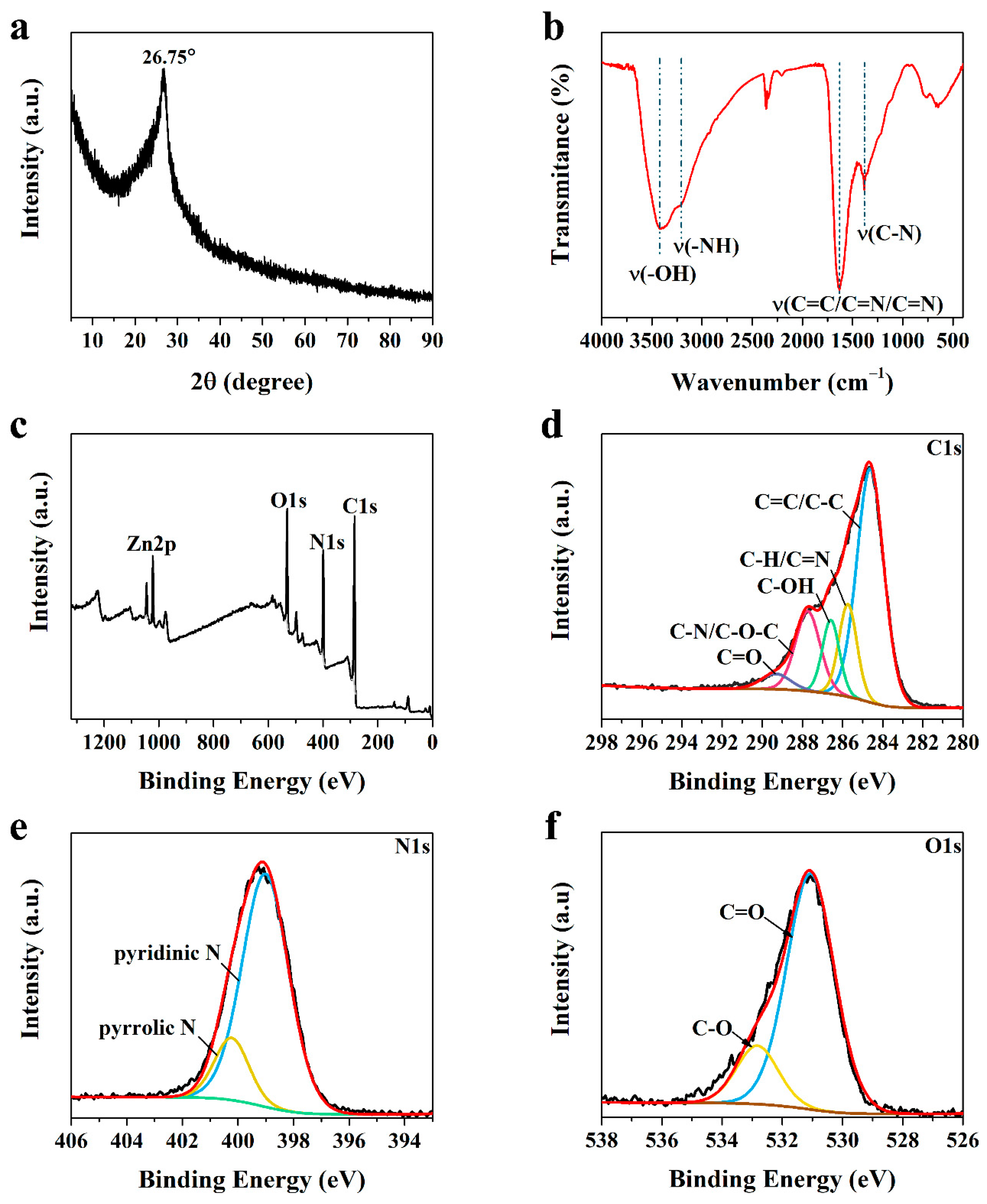
3.1. Structural Characterization
3.2. Adsorption Study
3.2.1. Adsorption Performance of N-CM toward MB and RB19
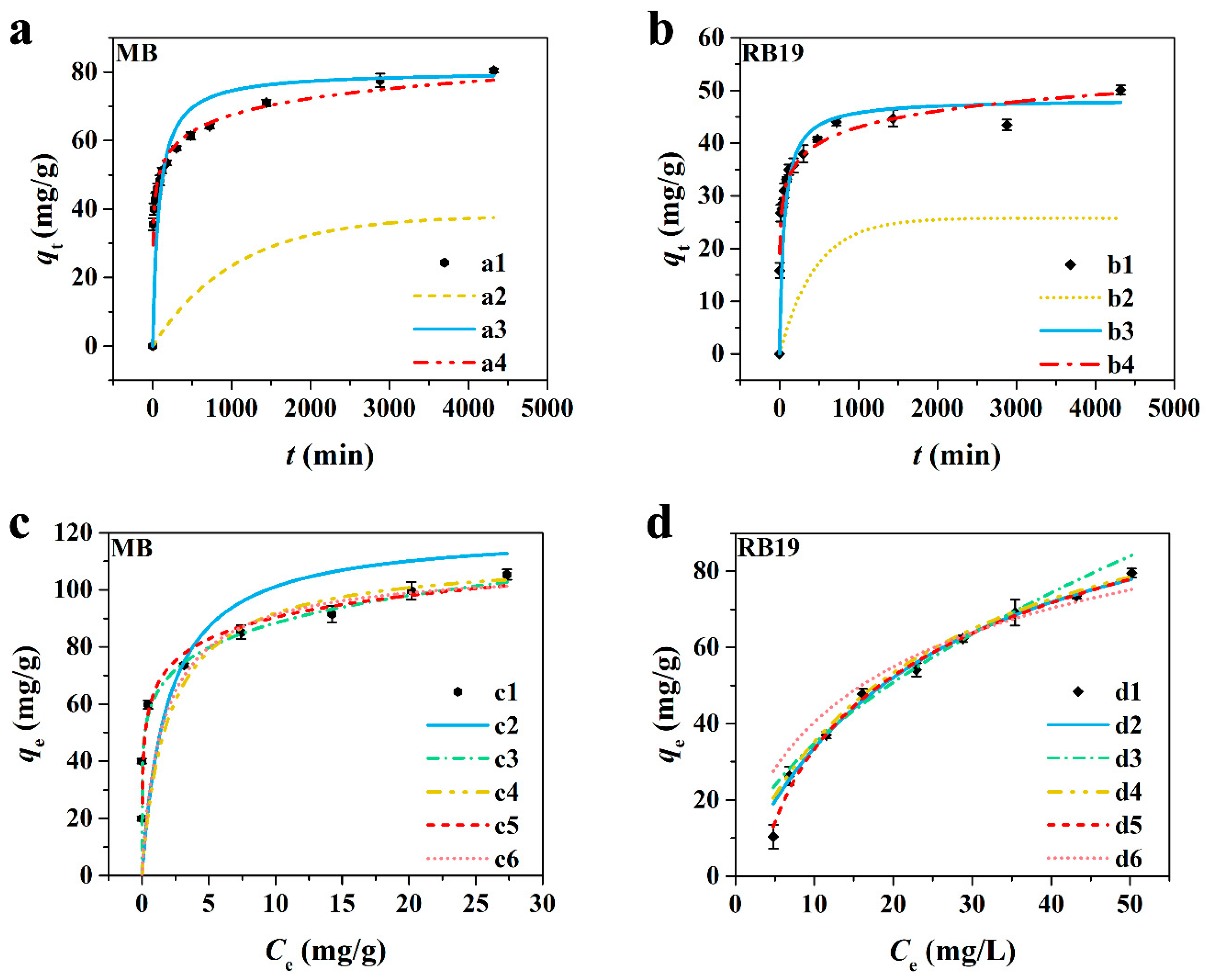
3.2.2. Adsorption Kinetics
3.2.3. Adsorption Isotherm
3.2.4. Adsorption Thermodynamics
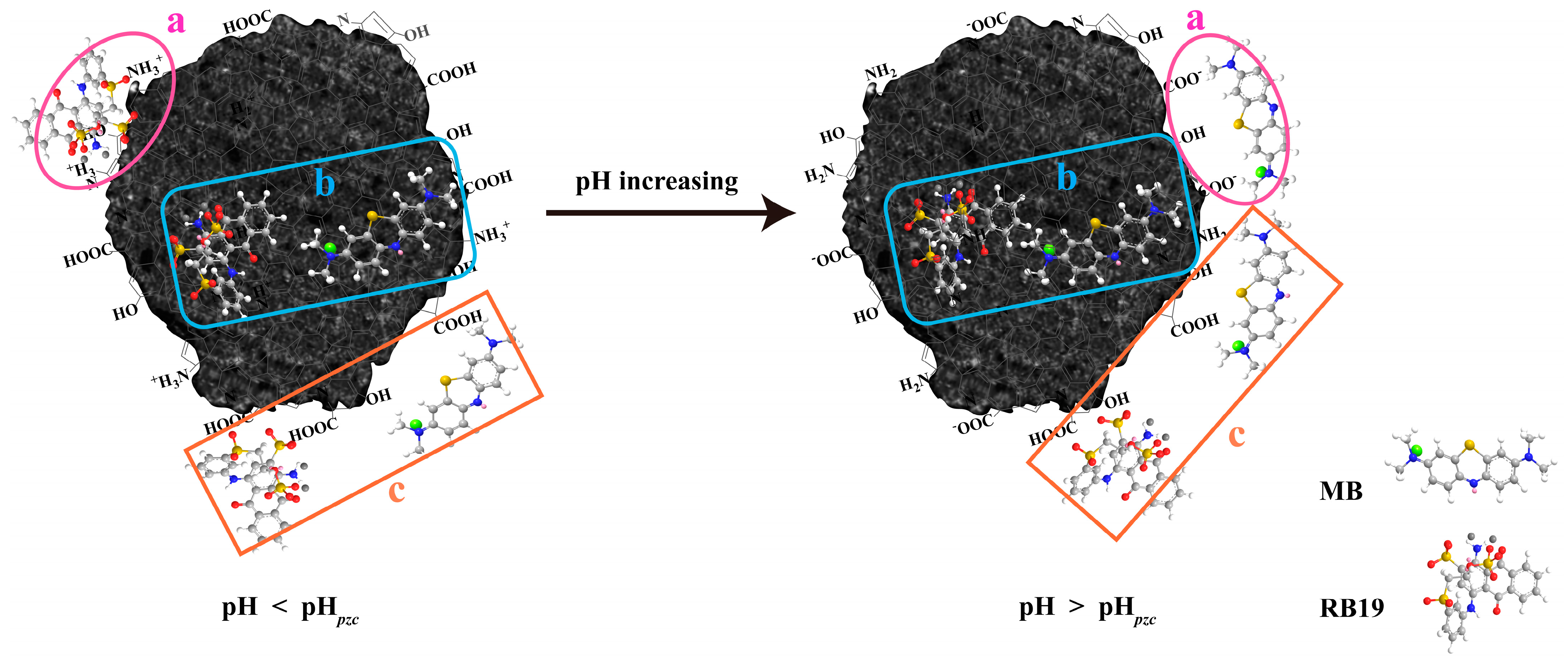
3.2.5. Possible Adsorption Mechanism
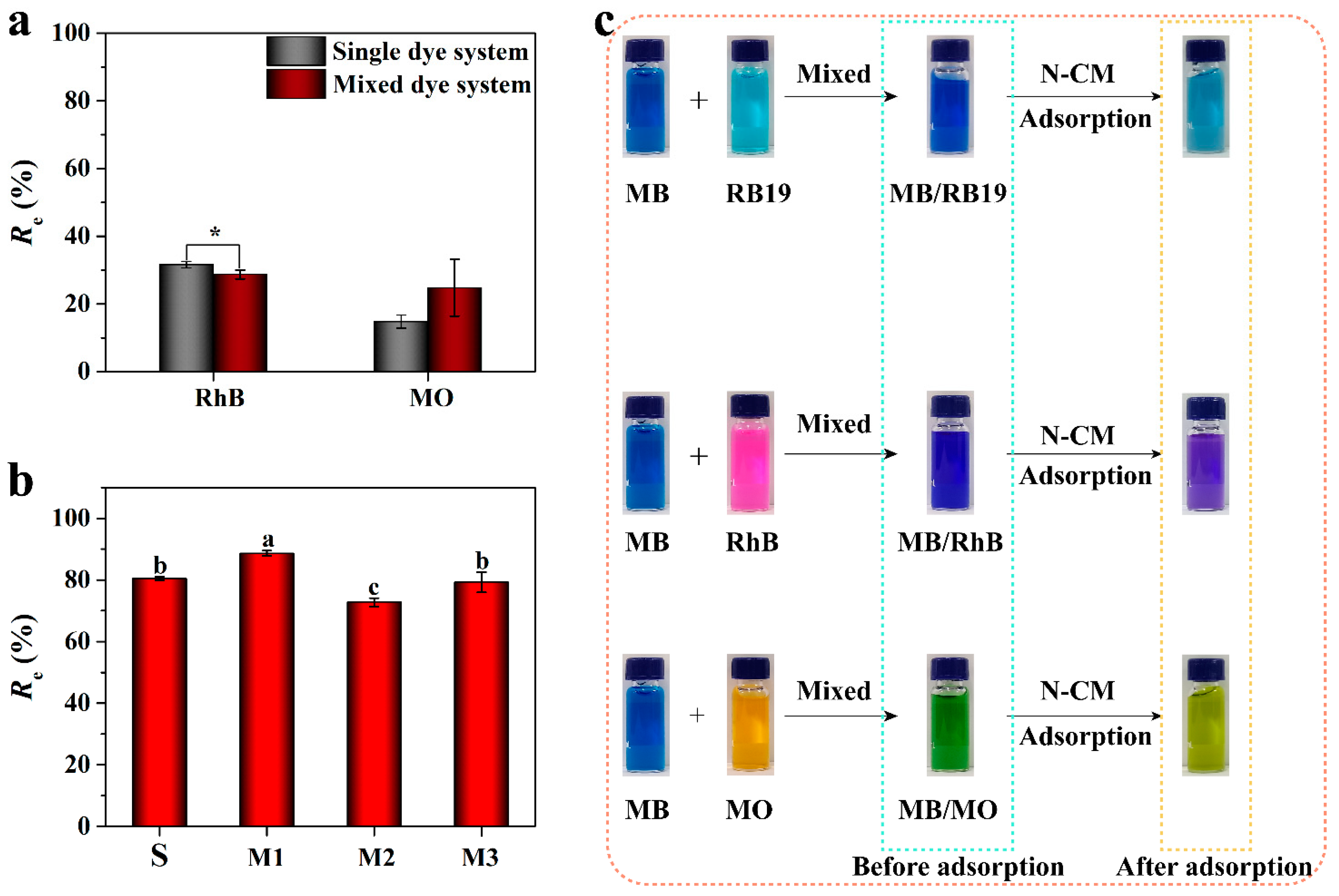
3.2.6. Selective Adsorption
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Dao, M.U.; Le, H.S.; Hoang, H.Y.; Tran, V.A.; Doan, V.D.; Le, T.T.N.; Sirotkin, A.; Le, V.T. Natural core-shell structure activated carbon beads derived from Litsea glutinosa seeds for removal of methylene blue: Facile preparation, characterization, and adsorption properties. Environ. Res. 2021, 198, 110481. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.; Hu, X.; Ding, H.; Li, Z.; Liang, R.; Sun, G. Villi-like poly(acrylic acid) based hydrogel adsorbent with fast and highly efficient methylene blue removing ability. J. Colloid Interface Sci. 2021, 594, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Zhao, E.; Kim, T.; Wang, J.; Hableel, G.; Reardon, P.J.T.; Ananthakrishna, S.J.; Wang, T.; Arconada-Alvarez, S.; Knowles, J.C.; et al. Organosilica nanoparticles with an intrinsic secondary amine: An efficient and reusable adsorbent for dyes. ACS Appl. Mater. Interfaces 2017, 9, 15566–15576. [Google Scholar] [CrossRef]
- Tang, X.; Ran, G.; Li, J.; Zhang, Z.; Xiang, C. Extremely efficient and rapidly adsorb methylene blue using porous adsorbent prepared from waste paper: Kinetics and equilibrium studies. J. Hazard. Mater. 2021, 402, 123579. [Google Scholar] [CrossRef] [PubMed]
- Siciliano, A.; Curcio, G.M.; Limonti, C.; Masi, S.; Greco, M. Methylene blue adsorption on thermo plasma expanded graphite in a multilayer column system. J. Environ. Manag. 2021, 296, 113365. [Google Scholar] [CrossRef] [PubMed]
- Chinoune, K.; Bentaleb, K.; Bouberka, Z.; Nadim, A.; Maschke, U. Adsorption of reactive dyes from aqueous solution by dirty bentonite. Appl. Clay Sci. 2016, 123, 64–75. [Google Scholar] [CrossRef]
- Mate, C.J.; Mishra, S. Synthesis of borax cross-linked Jhingan gum hydrogel for remediation of Remazol Brilliant Blue R (RBBR) dye from water: Adsorption isotherm, kinetic, thermodynamic and biodegradation studies. Int. J. Biol. Macromol. 2020, 151, 677–690. [Google Scholar] [CrossRef]
- Su, P.; Wan, Q.; Yang, Y.; Shu, J.; Zhao, H.; Meng, W.; Li, B.; Chen, M.; Liu, Z.; Liu, R. Hydroxylation of electrolytic manganese anode slime with EDTA-2Na and its adsorption of methylene blue. Sep. Purif. Technol. 2021, 278, 119526. [Google Scholar] [CrossRef]
- Dutta, S.; Gupta, B.; Srivastava, S.K.; Gupta, A.K. Recent advances on the removal of dyes from wastewater using various adsorbents: A critical review. Mater. Adv. 2021, 2, 4497–4531. [Google Scholar] [CrossRef]
- Yao, X.; Ji, L.; Guo, J.; Ge, S.; Lu, W.; Cai, L.; Wang, Y.; Song, W.; Zhang, H. Magnetic activated biochar nanocomposites derived from wakame and its application in methylene blue adsorption. Bioresour. Technol. 2020, 302, 122842. [Google Scholar] [CrossRef]
- Wang, B.; Yang, X.; Ma, L.; Zhai, L.; Xuan, J.; Liu, C.; Bai, Z. Ultra-high efficient pH induced selective removal of cationic and anionic dyes from complex coexisted solution by novel amphoteric biocomposite microspheres. Sep. Purif. Technol. 2020, 231, 115922. [Google Scholar] [CrossRef]
- Koupaie, E.H.; Moghaddam, M.R.A.; Hashemi, S.H. Post-treatment of anaerobically degraded azo dye Acid Red 18 using aerobic moving bed biofilm process: Enhanced removal of aromatic amines. J. Hazard. Mater. 2011, 195, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Shang, S.; Li, X.-y. Fabrication of sulfur-doped TiO2 nanotube array as a conductive interlayer of PbO2 anode for efficient electrochemical oxidation of organic pollutants. Sep. Purif. Technol. 2021, 258, 118035. [Google Scholar] [CrossRef]
- McYotto, F.; Wei, Q.; Macharia, D.K.; Huang, M.; Shen, C.; Chow, C.W.K. Effect of dye structure on color removal efficiency by coagulation. Chem. Eng. J. 2021, 405, 126674. [Google Scholar] [CrossRef]
- Hou, J.; Chen, Y.; Shi, W.; Bao, C.; Hu, X. Graphene oxide/methylene blue composite membrane for dyes separation: Formation mechanism and separation performance. Appl. Surf. Sci. 2020, 505, 144145. [Google Scholar] [CrossRef]
- Peng, Y.; Wang, K.K.; Liu, T.; Xu, J.; Xu, B.G. Synthesis of one-dimensional Bi2O3-Bi2O2.33 heterojunctions with high interface quality for enhanced visible light photocatalysis in degradation of high-concentration phenol and MO dyes. Appl. Catal. B-Environ. 2017, 203, 946–954. [Google Scholar] [CrossRef]
- Mahmoodi, N.M.; Abdi, J.; Taghizadeh, M.; Taghizadeh, A.; Hayati, B.; Shekarchi, A.A.; Vossoughi, M. Activated carbon/metal-organic framework nanocomposite: Preparation and photocatalytic dye degradation mathematical modeling from wastewater by least squares support vector machine. J. Environ. Manag. 2019, 233, 660–672. [Google Scholar] [CrossRef]
- Tan, K.L.; Hameed, B.H. Insight into the adsorption kinetics models for the removal of contaminants from aqueous solutions. J. Taiwan Inst. Chem. Eng. 2017, 74, 25–48. [Google Scholar] [CrossRef]
- Mahmoodi, N.M.; Oveisi, M.; Taghizadeh, A.; Taghizadeh, M. Synthesis of pearl necklace-like ZIF-8@chitosan/PVA nanofiber with synergistic effect for recycling aqueous dye removal. Carbohydr. Polym. 2020, 227, 115364. [Google Scholar] [CrossRef]
- Shin, J.; Lee, Y.-G.; Lee, S.-H.; Kim, S.; Ochir, D.; Park, Y.; Kim, J.; Chon, K. Single and competitive adsorptions of micropollutants using pristine and alkali-modified biochars from spent coffee grounds. J. Hazard. Mater. 2020, 400, 123102. [Google Scholar] [CrossRef]
- Choudhary, M.; Kumar, R.; Neogi, S. Activated biochar derived from Opuntia ficus-indica for the efficient adsorption of malachite green dye, Cu2+ and Ni2+ from water. J. Hazard. Mater. 2020, 392, 122441. [Google Scholar] [CrossRef] [PubMed]
- Parra-Marfíl, A.; Ocampo-Pérez, R.; Collins-Martínez, V.H.; Flores-Vélez, L.M.; Gonzalez-Garcia, R.; Medellín-Castillo, N.A.; Labrada-Delgado, G.J. Synthesis and characterization of hydrochar from industrial Capsicum annuum seeds and its application for the adsorptive removal of methylene blue from water. Environ. Res. 2020, 184, 109334. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.D.; Lin, Y.C.; Ho, S.H.; Zhou, Y.; Ren, N.Q. Highly efficient adsorption of dyes by biochar derived from pigments-extracted macroalgae pyrolyzed at different temperature. Bioresour. Technol. 2018, 259, 104–110. [Google Scholar] [CrossRef]
- Hoslett, J.; Ghazal, H.; Mohamad, N.; Jouhara, H. Removal of methylene blue from aqueous solutions by biochar prepared from the pyrolysis of mixed municipal discarded material. Sci. Total Environ. 2020, 714, 136832. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Peng, N.; Sun, J.; Lu, G.; Chen, M.; Deng, F.; Dou, R.; Nie, L.; Zhong, Y. Synthesis of silica-composited biochars from alkali-fused fly ash and agricultural wastes for enhanced adsorption of methylene blue. Sci. Total Environ. 2020, 729, 139055. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Zhang, Z.; Xu, G.; Li, G. Pyrolysis of penicillin fermentation residue and sludge to produce biochar: Antibiotic resistance genes destruction and biochar application in the adsorption of penicillin in water. J. Hazard. Mater. 2021, 413, 125385. [Google Scholar] [CrossRef] [PubMed]
- Ahlawat, W.; Kataria, N.; Dilbaghi, N.; Hassan, A.A.; Kumar, S.; Kim, K.-H. Carbonaceous nanomaterials as effective and efficient platforms for removal of dyes from aqueous systems. Environ. Res. 2020, 181, 108904. [Google Scholar] [CrossRef]
- Dai, C.; Zhang, M.; Guo, X.; Ma, X. Mesoporous composite Ni-C-N/SA for selective adsorption of methylene blue from water. Chem. Eng. J. 2021, 407, 127181. [Google Scholar] [CrossRef]
- Sharma, A.; Das, J. Small molecules derived carbon dots: Synthesis and applications in sensing, catalysis, imaging, and biomedicine. J. Nanobiotechnol. 2019, 17, 92. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.C.; Zhao, L.; Mao, L.J.; Han, B.H. One-step solvothermal carbonization to microporous carbon materials derived from cyclodextrins. J. Mater. Chem. A 2013, 1, 9456. [Google Scholar] [CrossRef]
- Tong, Z.; Tong, Y.; Wang, D.; Shi, Y.C. Whole maize flour and isolated maize starch for production of citric acid by Aspergillus niger: A review. Starch-Stärke 2021, 73, 2000014. [Google Scholar] [CrossRef]
- Zhu, S.; Zhao, X.; Song, Y.; Lu, S.; Yang, B. Beyond bottom-up carbon nanodots: Citric-acid derived organic molecules. Nano Today 2016, 11, 128–132. [Google Scholar] [CrossRef]
- Ranote, S.; Kumar, D.; Kumari, S.; Kumar, R.; Chauhan, G.S.; Joshi, V. Green synthesis of Moringa oleifera gum-based bifunctional polyurethane foam braced with ash for rapid and efficient dye removal. Chem. Eng. J. 2019, 361, 1586–1596. [Google Scholar] [CrossRef]
- Li, X.; Zhu, H.; Liu, C.; Yuan, P.; Lin, Z.; Yang, J.; Yue, Y.; Bai, Z.; Wang, T.; Bao, X. Synthesis, modification, and application of hollow mesoporous carbon submicrospheres for adsorptive desulfurization. Ind. Eng. Chem. Res. 2018, 57, 15020–15030. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, C.; Huang, D.; Zeng, G.; Huang, J.; Lai, C.; Zhou, C.; Wang, W.; Guo, H.; Xue, W.; et al. Boron nitride quantum dots decorated ultrathin porous g-C3N4: Intensified exciton dissociation and charge transfer for promoting visible-light-driven molecular oxygen activation. Appl. Catal. B-Environ. 2019, 245, 87–99. [Google Scholar] [CrossRef]
- Mahmoodi, N.M.; Saffar-Dastgerdi, M.H. Clean Laccase immobilized nanobiocatalysts (graphene oxide-zeolite nanocomposites): From production to detailed biocatalytic degradation of organic pollutant. Appl. Catal. B-Environ. 2020, 268, 118443. [Google Scholar] [CrossRef]
- Wu, F.-C.; Tseng, R.-L.; Juang, R.-S. Kinetic modeling of liquid-phase adsorption of reactive dyes and metal ions on chitosan. Water Res. 2001, 35, 613–618. [Google Scholar] [CrossRef]
- Mclintock, S.I. The Elovich Equation in Chemisorption Kinetics. Nature 1967, 216, 1204–1205. [Google Scholar] [CrossRef]
- Mahmoodi, N.M.; Oveisi, M.; Taghizadeh, A.; Taghizadeh, M. Novel magnetic amine functionalized carbon nanotube/metal-organic framework nanocomposites: From green ultrasound-assisted synthesis to detailed selective pollutant removal modelling from binary systems. J. Hazard. Mater. 2019, 368, 746–759. [Google Scholar] [CrossRef]
- Aljeboree, A.M.; Alshirifi, A.N.; Alkaim, A.F. Kinetics and equilibrium study for the adsorption of textile dyes on coconut shell activated carbon. Arab. J. Chem. 2017, 10, S3381–S3393. [Google Scholar] [CrossRef] [Green Version]
- Fu, Q.; Zhang, L.; Zhang, H.; Chen, X.; Li, M.; Gong, M. Ice- and MOF-templated porous carbonaceous monoliths for adsorptive removal of dyes in water with easy recycling. Environ. Res. 2020, 186, 109608. [Google Scholar] [CrossRef] [PubMed]
- Doğan, M.; Alkan, M.; Demirbaş, Ö.; Özdemir, Y.; Özmetin, C. Adsorption kinetics of maxilon blue GRL onto sepiolite from aqueous solutions. Chem. Eng. J. 2006, 124, 89–101. [Google Scholar] [CrossRef]
- Islam, M.A.; Sabar, S.; Benhouria, A.; Khanday, W.A.; Asif, M.; Hameed, B.H. Nanoporous activated carbon prepared from karanj (Pongamia pinnata) fruit hulls for methylene blue adsorption. J. Taiwan Inst. Chem. Eng. 2017, 74, 96–104. [Google Scholar] [CrossRef]
- Foo, K.Y.; Hameed, B.H. Insights into the modeling of adsorption isotherm systems. Chem. Eng. J. 2010, 156, 2–10. [Google Scholar] [CrossRef]
- Kumar, K.V.; Porkodi, K.; Rocha, F. Isotherms and thermodynamics by linear and non-linear regression analysis for the sorption of methylene blue onto activated carbon: Comparison of various error functions. J. Hazard. Mater. 2008, 151, 794–804. [Google Scholar] [CrossRef]
- Kumar, P.S.; Ramalingam, S.; Senthamarai, C.; Niranjanaa, M.; Vijayalakshmi, P.; Sivanesan, S. Adsorption of dye from aqueous solution by cashew nut shell: Studies on equilibrium isotherm, kinetics and thermodynamics of interactions. Desalination 2010, 261, 52–60. [Google Scholar] [CrossRef]
- Tang, L.; Yu, J.; Pang, Y.; Zeng, G.; Deng, Y.; Wang, J.; Ren, X.; Ye, S.; Peng, B.; Feng, H. Sustainable efficient adsorbent: Alkali-acid modified magnetic biochar derived from sewage sludge for aqueous organic contaminant removal. Chem. Eng. J. 2018, 336, 160–169. [Google Scholar] [CrossRef]
- Eltaweil, A.S.; Elgarhy, G.S.; El-Subruiti, G.M.; Omer, A.M. Carboxymethyl cellulose/carboxylated graphene oxide composite microbeads for efficient adsorption of cationic methylene blue dye. Int. J. Biol. Macromol. 2020, 154, 307–318. [Google Scholar] [CrossRef]
- Chang, S.; Zhang, Q.; Lu, Y.; Wu, S.; Wang, W. High-efficiency and selective adsorption of organic pollutants by magnetic CoFe2O4/graphene oxide adsorbents: Experimental and molecular dynamics simulation study. Sep. Purif. Technol. 2020, 238, 116400. [Google Scholar] [CrossRef]
- Dai, H.; Chen, Y.; Ma, L.; Zhang, Y.; Cui, B. Direct regeneration of hydrogels based on lemon peel and its isolated microcrystalline cellulose: Characterization and application for methylene blue adsorption. Int. J. Biol. Macromol. 2021, 191, 129–138. [Google Scholar] [CrossRef]
- Mohammed, N.; Lian, H.; Islam, M.S.; Strong, M.; Shi, Z.; Berry, R.M.; Yu, H.-Y.; Tam, K.C. Selective adsorption and separation of organic dyes using functionalized cellulose nanocrystals. Chem. Eng. J. 2021, 417, 129237. [Google Scholar] [CrossRef]
- You, X.; Wang, R.; Zhu, Y.; Sui, W.; Cheng, D. Comparison of adsorption properties of a cellulose-rich modified rice husk for the removal of methylene blue and aluminum(III) from their aqueous solution. Ind. Crop. Prod. 2021, 170, 113687. [Google Scholar] [CrossRef]
- Xie, J.; Lin, R.; Liang, Z.; Zhao, Z.; Yang, C.; Cui, F. Effect of cations on the enhanced adsorption of cationic dye in Fe3O4-loaded biochar and mechanism. J. Environ. Chem. Eng. 2021, 9, 105744. [Google Scholar] [CrossRef]
- Liu, Z.; Chen, G.; Hu, F.; Li, X. Synthesis of mesoporous magnetic MnFe2O4@CS-SiO2 microsphere and its adsorption performance of Zn2+ and MB studies. J. Environ. Manag. 2020, 263, 110377. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Xiang, M.; Liu, H.; Yang, C.; Deng, S. Novel two-dimensional magnetic titanium carbide for methylene blue removal over a wide ph range: Insight into removal performance and mechanism. ACS Appl. Mater. Interfaces 2019, 11, 24027–24036. [Google Scholar] [CrossRef] [PubMed]
- Raj, A.; Yadav, A.; Rawat, A.P.; Singh, A.K.; Kumar, S.; Pandey, A.K.; Sirohi, R.; Pandey, A. Kinetic and thermodynamic investigations of sewage sludge biochar in removal of Remazol Brilliant Blue R dye from aqueous solution and evaluation of residual dyes cytotoxicity. Environ. Technol. Inno. 2021, 23, 101556. [Google Scholar] [CrossRef]
- Bedin, K.C.; de Azevedo, S.P.; Leandro, P.K.T.; Cazetta, A.L.; Almeida, V.C. Bone char prepared by CO2 atmosphere: Preparation optimization and adsorption studies of Remazol Brilliant Blue R. J. Clean. Prod. 2017, 161, 288–298. [Google Scholar] [CrossRef]
- Silva, T.L.; Ronix, A.; Pezoti, O.; Souza, L.S.; Leandro, P.K.T.; Bedin, K.C.; Beltrame, K.K.; Cazetta, A.L.; Almeida, V.C. Mesoporous activated carbon from industrial laundry sewage sludge: Adsorption studies of reactive dye Remazol Brilliant Blue R. Chem. Eng. J. 2016, 303, 467–476. [Google Scholar] [CrossRef]
- Karatay, S.E.; Aksu, Z.; Özeren, İ.; Dönmez, G. Potentiality of newly isolated Aspergillus tubingensis in biosorption of textile dyes: Equilibrium and kinetic modeling. Biomass Conv. Bioref. 2021, 155, 1–8. [Google Scholar] [CrossRef]



| Kinetic Models | Parameters | Adsorbates | |
|---|---|---|---|
| MB | RB19 | ||
| qe,exp | 80.490 | 45.250 | |
| Pseudo-first order | qe,cal | 33.369 | 20.437 |
| k1 | 0.00110 | 0.00181 | |
| R2 | 0.717 | 0.816 | |
| Pseudo-second order | qe,cal | 81.900 | 48.900 |
| k2 | 0.000112 | 0.000329 | |
| R2 | 0.998 | 0.995 | |
| Elovich | α | 60.529 | 73.183 |
| β | 0.132 | 0.223 | |
| R2 | 0.983 | 0.959 | |
| Intra-particle diffusion | kint1 | 1.496 | 1.372 |
| C1 | 34.003 | 21.027 | |
| R2 | 0.984 | 0.979 | |
| kint2 | 0.454 | 0.191 | |
| C2 | 51.256 | 35.555 | |
| R2 | 0.980 | 0.769 | |
| Diffusion-chemisorption | kDC | 10.564 | 9.536 |
| qe,cal | 89.445 | 51.813 | |
| R2 | 0.994 | 0.993 | |
| Isothermal Models | Parameters | Adsorbates | |
|---|---|---|---|
| MB | RB19 | ||
| Langmuir | qm,cal | 120.773 | 116.009 |
| kL | 0.515 | 0.0407 | |
| R2 | 0.990 | 0.997 | |
| Freundlich | kF | 57.489 | 9.816 |
| 1/nF | 0.200 | 0.549 | |
| R2 | 0.944 | 0.979 | |
| Langmuir-Freundlich | qm,cal | 112.5 | 119.92 |
| kL-F | 0.480 | 0.041 | |
| nL-F | 0.950 | 0.960 | |
| R2 | 0.975 | 0.972 | |
| Temkin | BT | 10.987 | 27.760 |
| kT | 374.398 | 0.331 | |
| R2 | 0.928 | 0.996 | |
| Hill | qH | 109.350 | 123.950 |
| kH | 1.610 | 10.870 | |
| nH | 0.910 | 0.720 | |
| R2 | 0.900 | 0.904 | |
| Adsorbate | ΔHΘ (kJ/mol) | ΔSΘ (kJ mol−1 K−1) | ΔGΘ (kJ/mol) | ||||
|---|---|---|---|---|---|---|---|
| 288 K | 298 K | 308 K | 318 K | 328 K | |||
| MB | 31.169 | 0.122 | −3.561 | −5.217 | −8.162 | −8.034 | −8.542 |
| RB19 | 48.755 | 0.169 | −1.045 | −1.630 | −2.780 | −3.106 | −7.862 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, C.; Shi, Q.; Feng, J.; Yao, J.; Huang, H.; Xie, X. Adsorption Behaviors of Cationic Methylene Blue and Anionic Reactive Blue 19 Dyes onto Nano-Carbon Adsorbent Carbonized from Small Precursors. Nanomaterials 2022, 12, 1814. https://doi.org/10.3390/nano12111814
Liang C, Shi Q, Feng J, Yao J, Huang H, Xie X. Adsorption Behaviors of Cationic Methylene Blue and Anionic Reactive Blue 19 Dyes onto Nano-Carbon Adsorbent Carbonized from Small Precursors. Nanomaterials. 2022; 12(11):1814. https://doi.org/10.3390/nano12111814
Chicago/Turabian StyleLiang, Caizhen, Qingshan Shi, Jin Feng, Junwei Yao, Hui Huang, and Xiaobao Xie. 2022. "Adsorption Behaviors of Cationic Methylene Blue and Anionic Reactive Blue 19 Dyes onto Nano-Carbon Adsorbent Carbonized from Small Precursors" Nanomaterials 12, no. 11: 1814. https://doi.org/10.3390/nano12111814
APA StyleLiang, C., Shi, Q., Feng, J., Yao, J., Huang, H., & Xie, X. (2022). Adsorption Behaviors of Cationic Methylene Blue and Anionic Reactive Blue 19 Dyes onto Nano-Carbon Adsorbent Carbonized from Small Precursors. Nanomaterials, 12(11), 1814. https://doi.org/10.3390/nano12111814






