Atomistic Simulations of the Permeability and Dynamic Transportation Characteristics of Diamond Nanochannels
Abstract
:1. Introduction
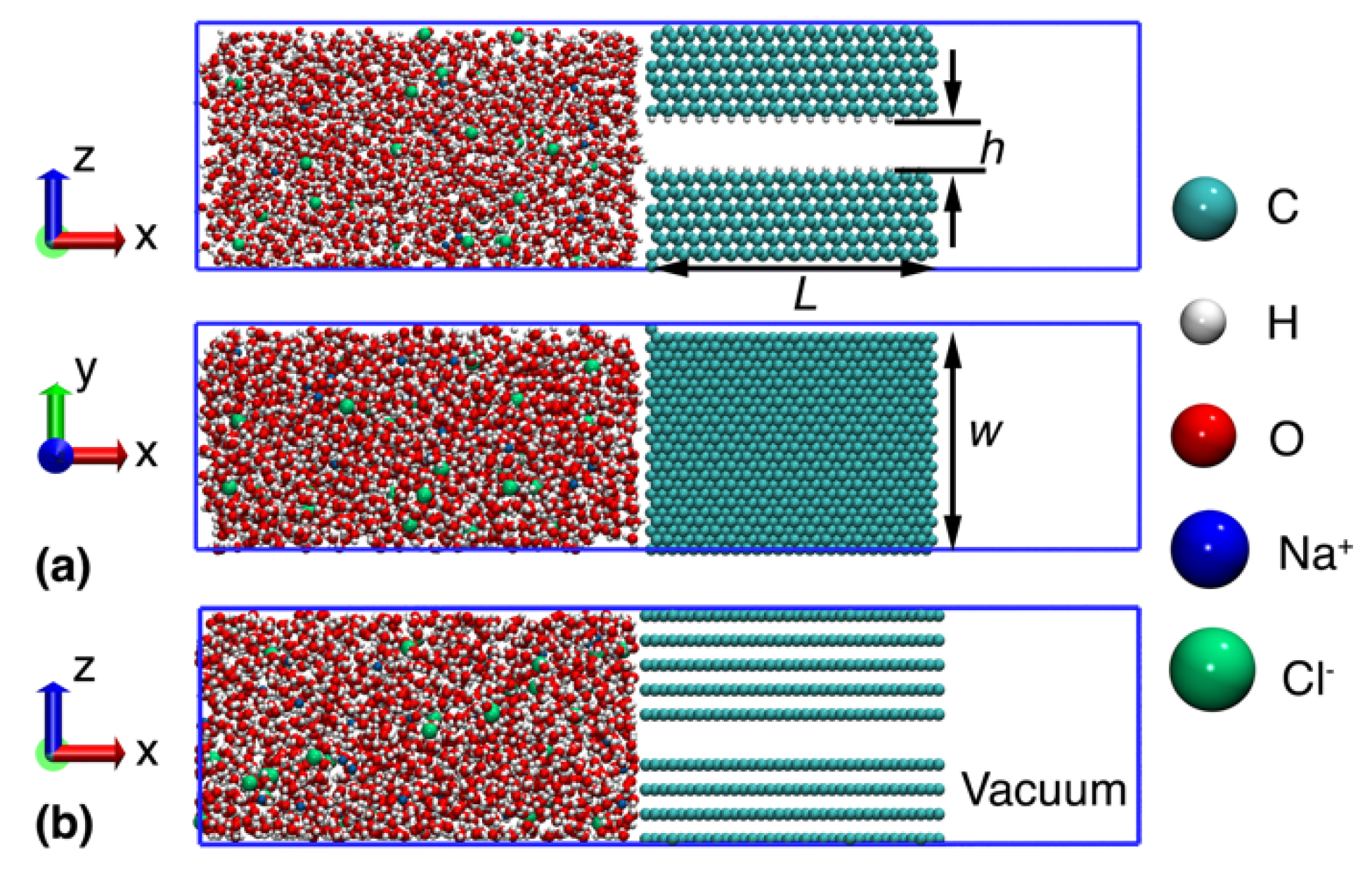
2. Methods
3. Results and Discussion
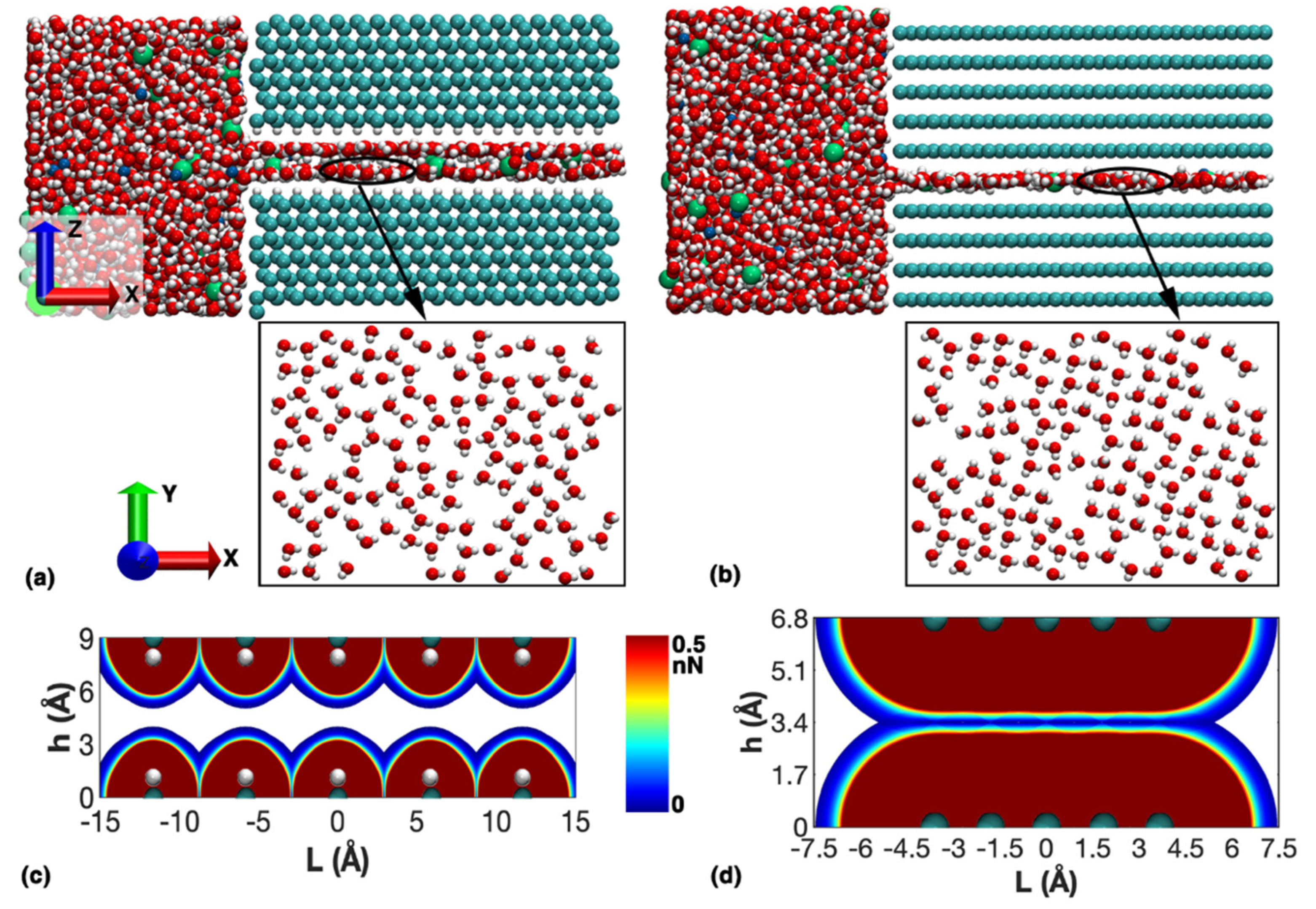
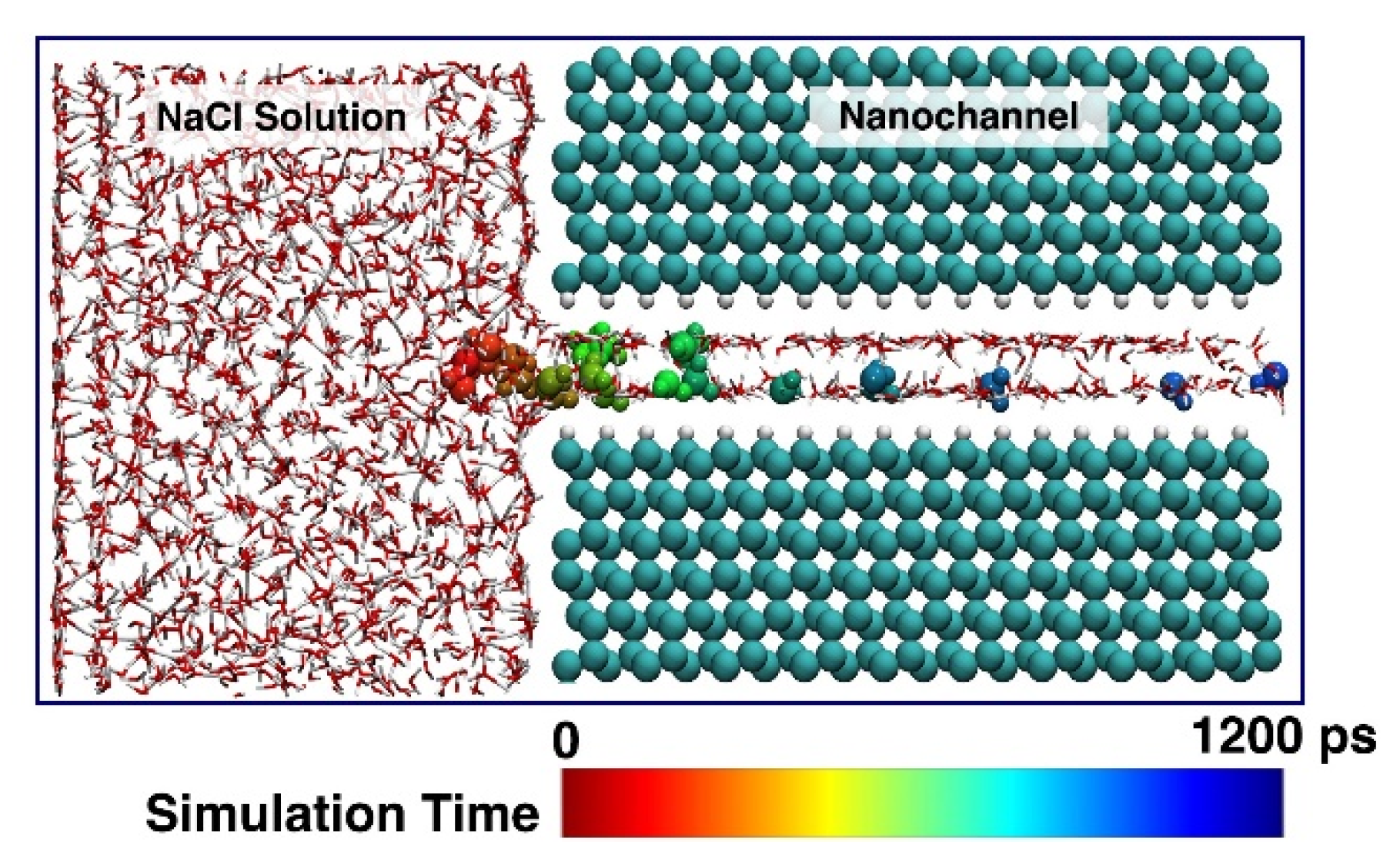
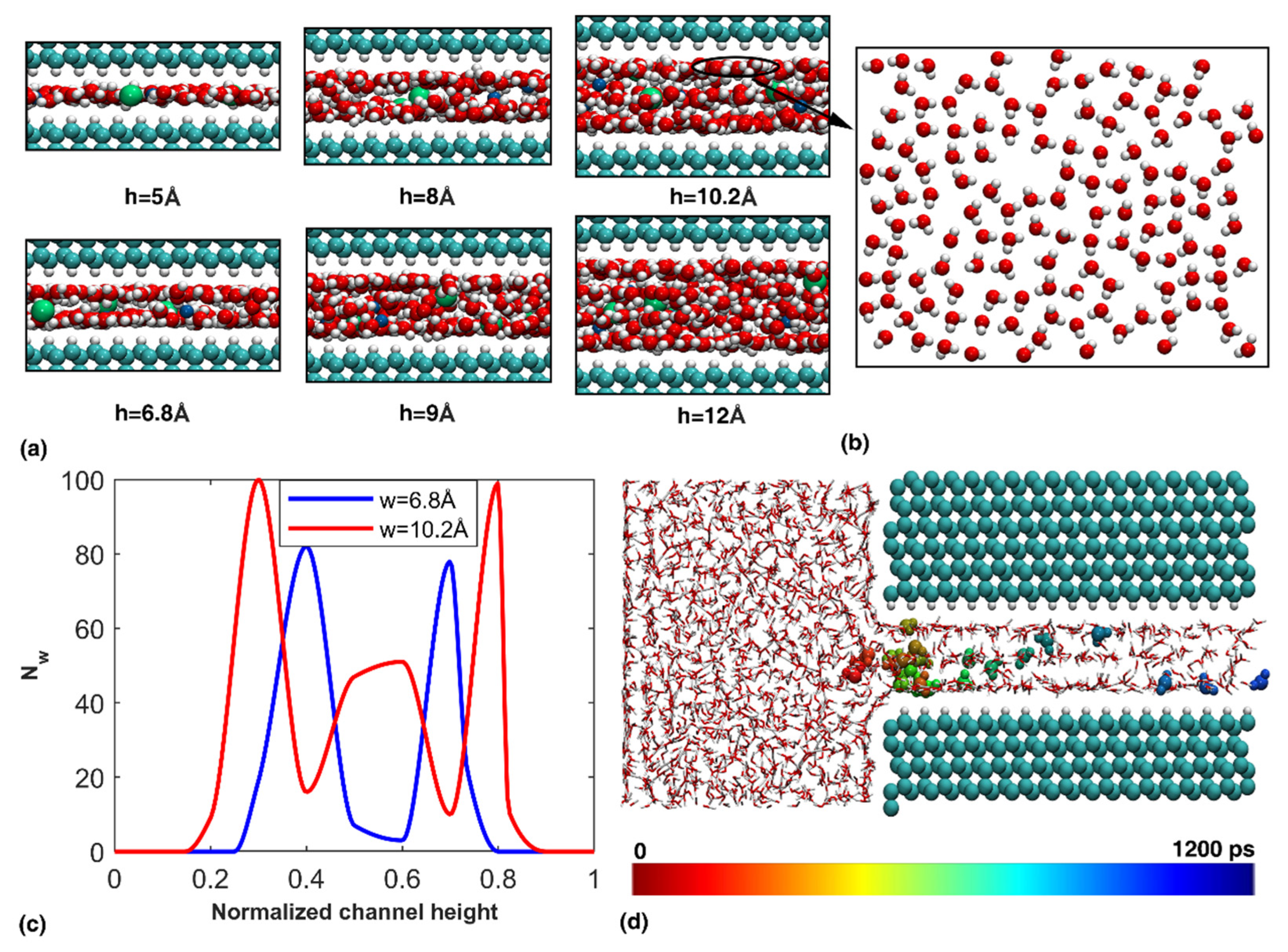
3.1. Transportation Characteristics in a Nanochannel
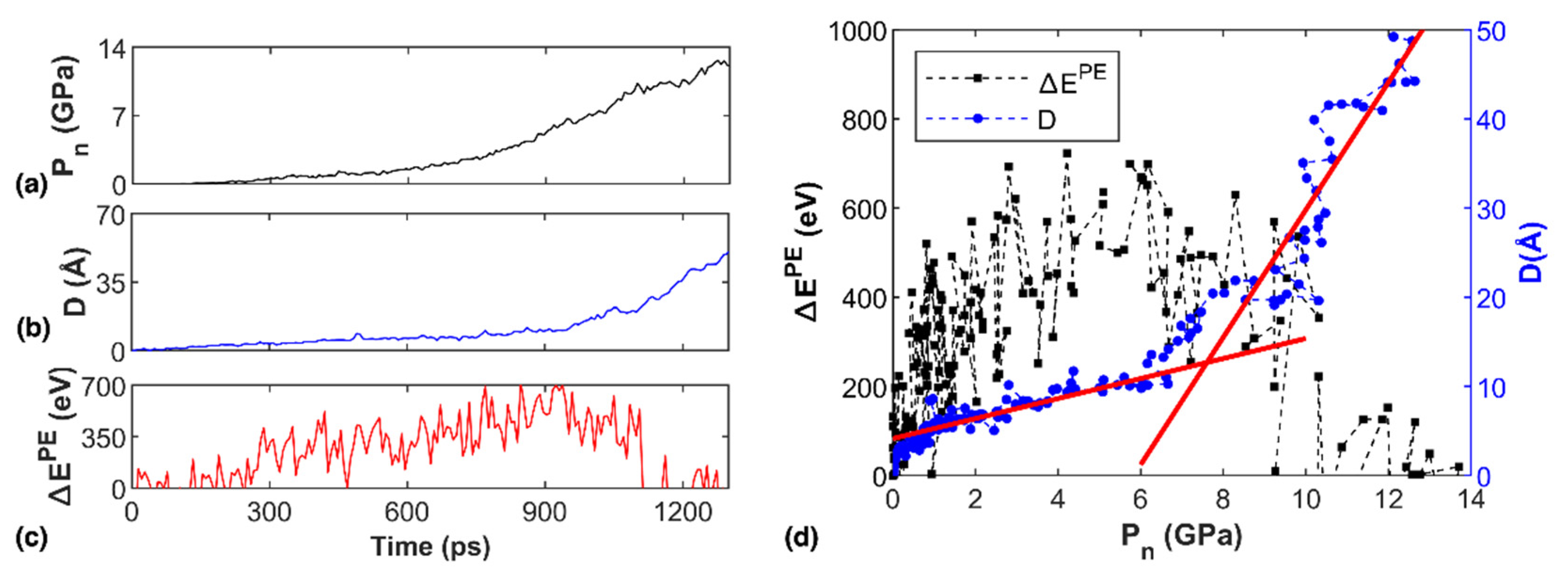
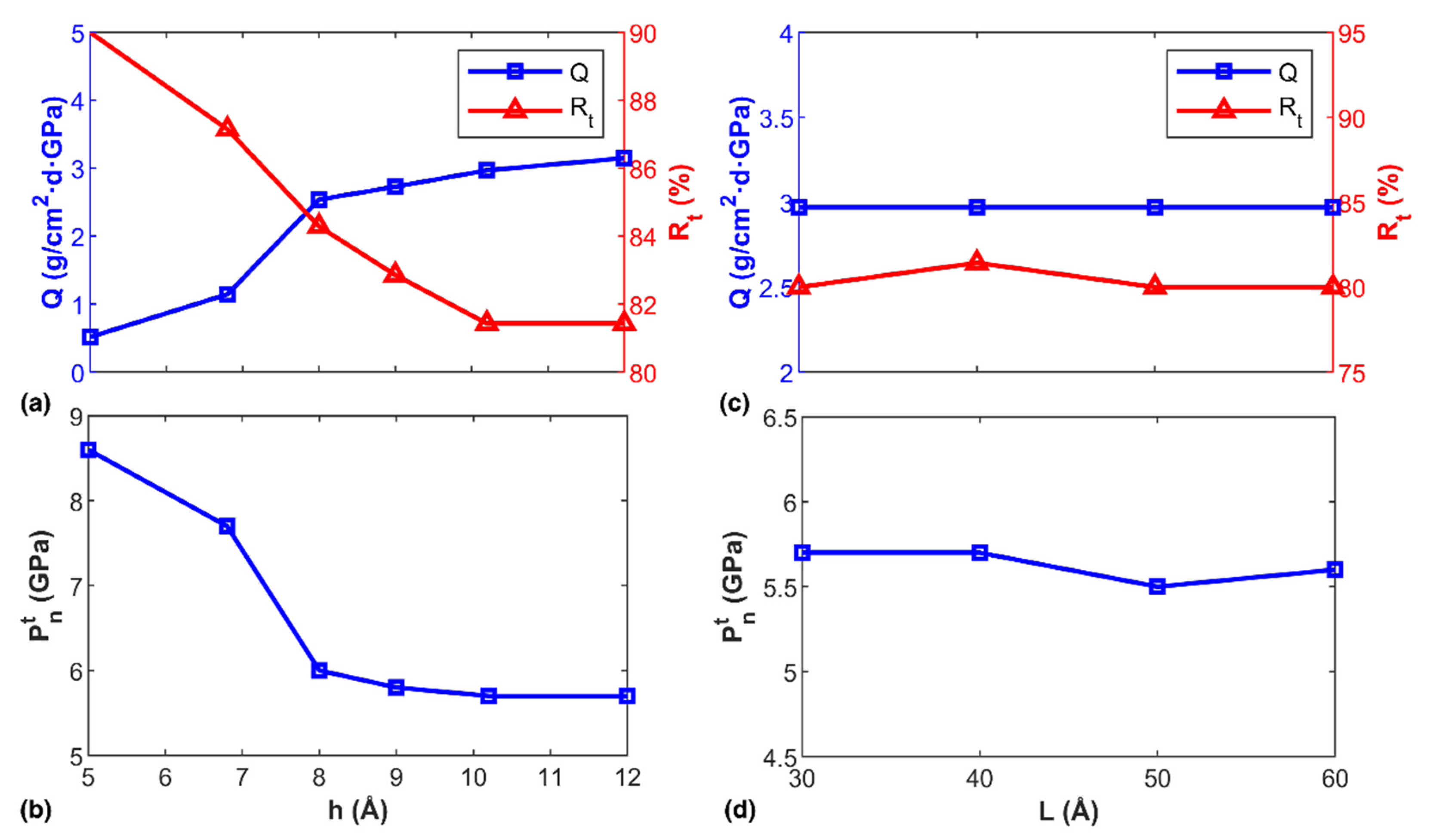
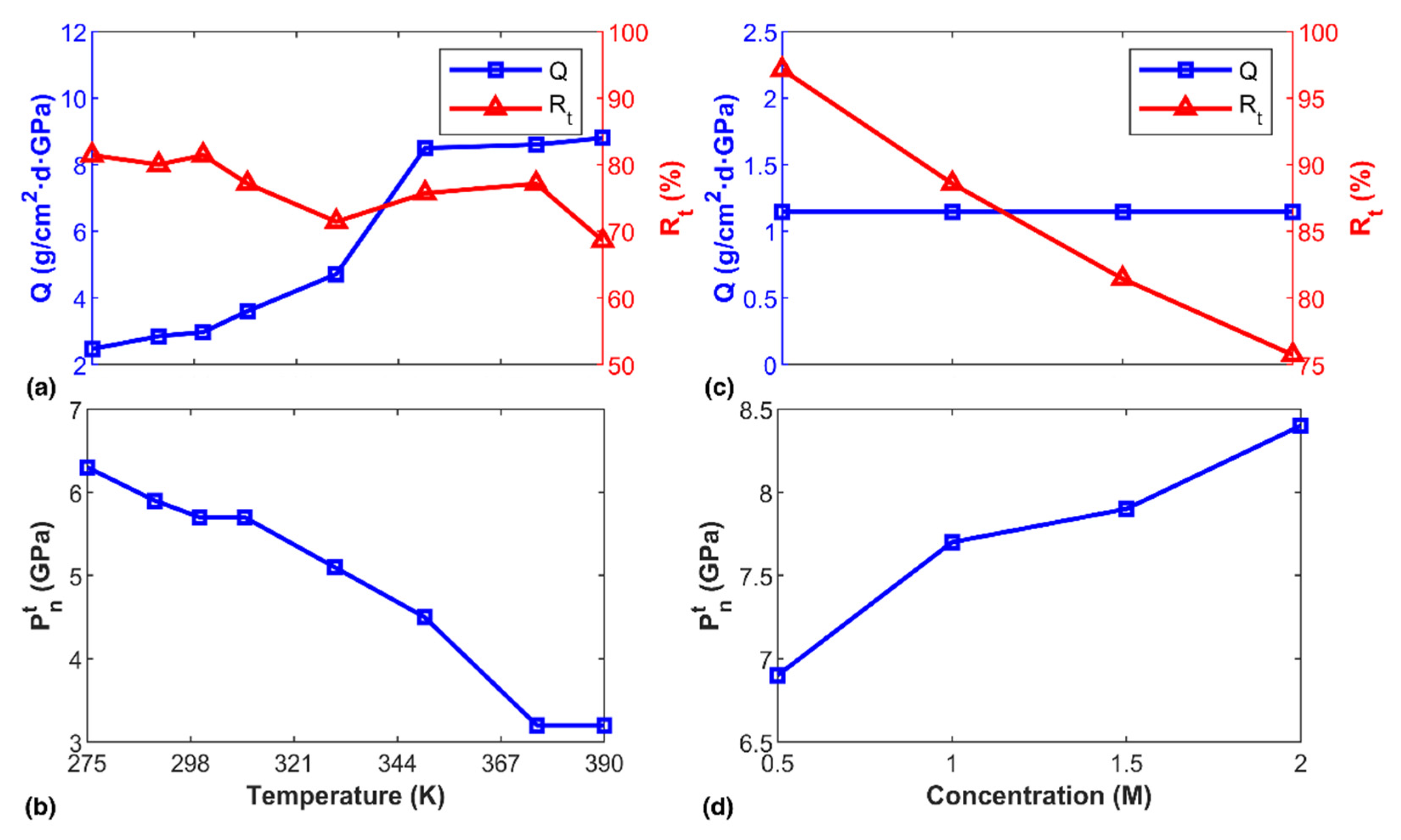
3.2. Factors Influencing the Transportation Behavior
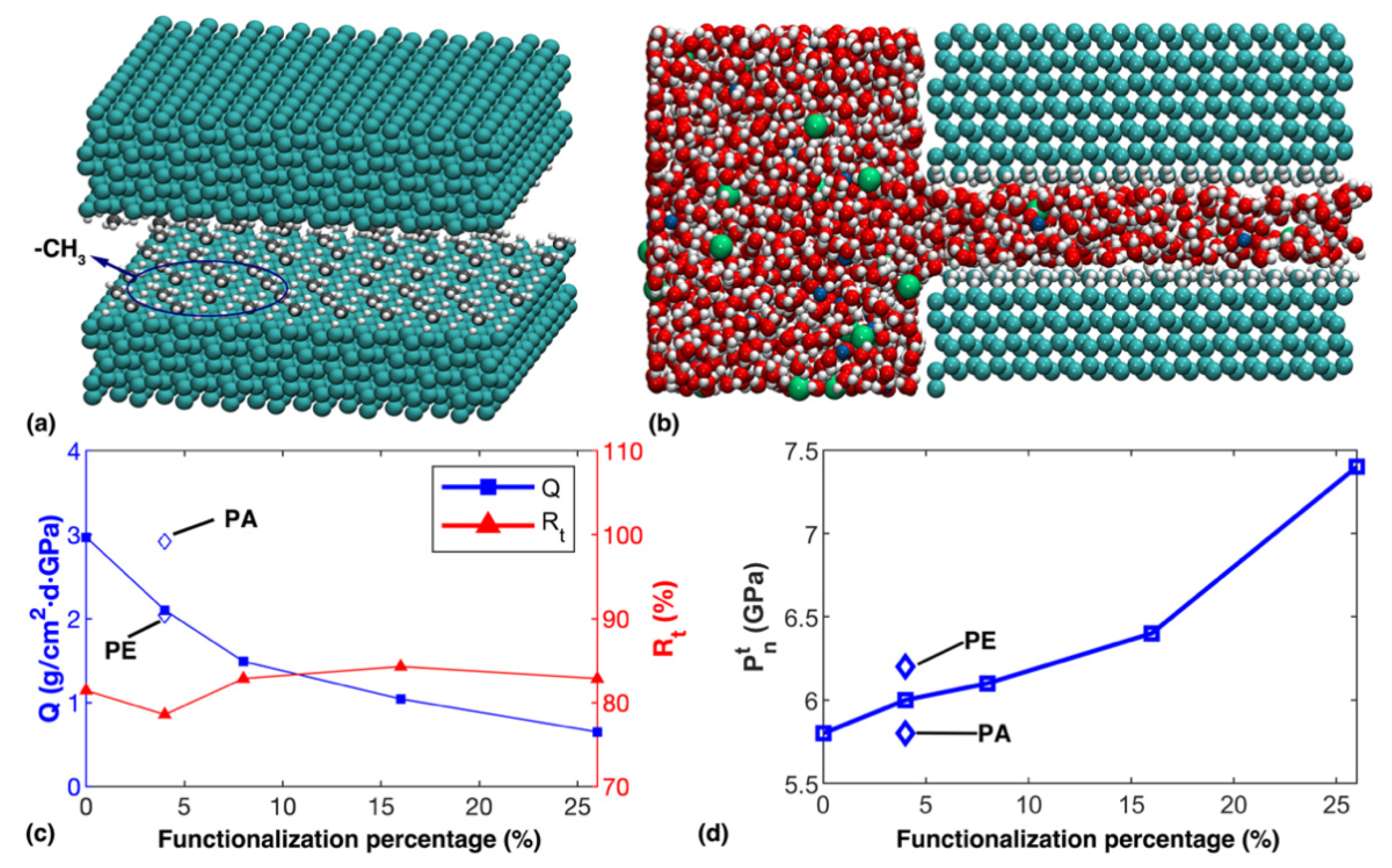
3.3. Functionalized Diamond Nanochannel
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gao, J.; Feng, Y.P.; Guo, W.; Jiang, L. Nanofluidics in two-dimensional layered materials: Inspirations from nature. Chem. Soc. Rev. 2017, 46, 5400–5424. [Google Scholar] [CrossRef] [PubMed]
- Guillet, J.F.; Flahaut, E.; Golzio, M. A hydrogel/carbon-nanotube needle-free device for electrostimulated skin drug delivery. Chem. Phys. Chem. 2017, 18, 2715–2723. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, X.H.; Xu, D.Q.; Liao, C.C.; Fang, Y.Q.; Guo, B.H. Development of a promising drug delivery for formononetin: Cyclodextrin-modified single-walled carbon nanotubes. J. Drug Deliv. Sci. Technol. 2018, 43, 461–468. [Google Scholar] [CrossRef]
- Park, H.B.; Kamcev, J.; Robeson, L.M.; Elimelech, M.; Freeman, B.D. Maximizing the right stuff: The trade-off between membrane permeability and selectivity. Science 2017, 356, 10. [Google Scholar] [CrossRef] [Green Version]
- Zhu, H.J.; Wang, Y.Y.; Fan, Y.Q.; Xu, J.B.; Yang, C. Structure and transport properties of water and hydrated ions in nano-confined channels. Adv. Theory Simul. 2019, 2, 18. [Google Scholar] [CrossRef]
- Werber, J.R.; Osuji, C.O.; Elimelech, M. Materials for next-generation desalination and water purification membranes. Nat. Rev. Mater. 2016, 1, 16. [Google Scholar] [CrossRef]
- Li, C.Y.; Meckler, S.M.; Smith, Z.P.; Bachman, J.E.; Maserati, L.; Long, J.R.; Helms, B.A. Engineered transport in microporous materials and membranes for clean energy technologies. Adv. Mater. 2018, 30, 33. [Google Scholar] [CrossRef] [Green Version]
- Ige, E.O.; Arun, R.K.; Singh, P.; Gope, M.; Saha, R.; Chanda, N.; Chakraborty, S. Water desalination using graphene oxide-embedded paper microfluidics. Microfluid. Nanofluid. 2019, 23, 8. [Google Scholar] [CrossRef]
- Han, X.; Wang, W.P.; Zuo, K.C.; Chen, L.; Yuan, L.; Liang, J.; Li, Q.L.; Ajayan, P.M.; Zhao, Y.; Lou, J. Bio-derived ultrathin membrane for solar driven water purification. Nano. Energy 2019, 60, 567–575. [Google Scholar] [CrossRef]
- Elimelech, M.; Phillip, W.A. The future of seawater desalination: Energy, technology, and the environment. Science 2011, 333, 712–717. [Google Scholar] [CrossRef]
- Yusuf, H.; Annuar, M.S.M.; Subramaniam, R.; Gumel, A.M. Amphiphilic biopolyester-carbon nanotube anode enhances electrochemical activities of microbial fuel cell. Chem. Eng. Technol. 2019, 42, 566–574. [Google Scholar] [CrossRef]
- Wu, W.G.; Niu, H.; Yang, D.Y.; Wang, S.B.; Wang, J.F.; Lin, J.; Hu, C.Y. Controlled layer-by-layer deposition of carbon nanotubes on electrodes for microbial fuel cells. Energies 2019, 12, 363. [Google Scholar] [CrossRef] [Green Version]
- Daiguji, H. Ion transport in nanofluidic channels. Chem. Soc. Rev. 2010, 39, 901–911. [Google Scholar] [CrossRef] [PubMed]
- Radha, B.; Esfandiar, A.; Wang, F.; Rooney, A.; Gopinadhan, K.; Keerthi, A.; Mishchenko, A.; Janardanan, A.; Blake, P.; Fumagalli, L. Molecular transport through capillaries made with atomic-scale precision. Nature 2016, 538, 222–225. [Google Scholar] [CrossRef] [Green Version]
- Ishii, Y.; Matubayasi, N.; Watanabe, G.; Kato, T.; Washizu, H. Molecular insights on confined water in the nanochannels of self-assembled ionic liquid crystal. Sci. Adv. 2021, 7, eabf0669. [Google Scholar] [CrossRef]
- Culp, T.E.; Khara, B.; Brickey, K.P.; Geitner, M.; Zimudzi, T.J.; Wilbur, J.D.; Jons, S.D.; Roy, A.; Paul, M.; Ganapathysubramanian, B.; et al. Nanoscale control of internal inhomogeneity enhances water transport in desalination membranes. Science 2021, 371, 72–75. [Google Scholar] [CrossRef]
- Seo, D.H.; Pineda, S.; Woo, Y.C.; Xie, M.; Murdock, A.T.; Ang, E.Y.M.; Jiao, Y.; Park, M.J.; Lim, S.I.; Lawn, M.; et al. Anti-fouling graphene-based membranes for effective water desalination. Nat. Commun. 2018, 9, 12. [Google Scholar] [CrossRef] [Green Version]
- Xie, Q.; Alibakhshi, M.A.; Jiao, S.P.; Xu, Z.P.; Hempel, M.; Kong, J.; Park, H.G.; Duan, C.H. Fast water transport in graphene nanofluidic channels. Nat. Nanotechnol. 2018, 13, 238–245. [Google Scholar] [CrossRef]
- Ma, Y.; Sun, M.; Duan, X.X.; van den Berg, A.; Eijkel, J.C.T.; Xie, Y.B. Dimension-reconfigurable bubble film nanochannel for wetting based sensing. Nat. Commun. 2020, 11, 11. [Google Scholar] [CrossRef] [Green Version]
- Guan, K.C.; Jia, Y.D.; Lin, Y.Q.; Wang, S.Y.; Matsuyama, H. Chemically converted graphene nanosheets for the construction of ion-exclusion nanochannel membranes. Nano Lett. 2021, 21, 3495–3502. [Google Scholar] [CrossRef]
- Liu, M.C.; Weston, P.J.; Hurt, R.H. Controlling nanochannel orientation and dimensions in graphene-based nanofluidic membranes. Nat. Commun. 2021, 12, 7. [Google Scholar] [CrossRef] [PubMed]
- Giri, A.K.; Teixeira, F.; Cordeiro, M. Salt separation from water using graphene oxide nanochannels: A molecular dynamics simulation study. Desalination 2019, 460, 1–14. [Google Scholar] [CrossRef]
- Kargar, M.; Lohrasebi, A. Water flow modeling through a graphene-based nanochannel: Theory and simulation. Phys. Chem. Chem. Phys. 2019, 21, 3304–3309. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Wang, W.S.; Zhang, Y.N.; Yan, Y.G.; Kral, P.; Zhang, J. Highly efficient water desalination in carbon nanocones. Carbon 2018, 129, 374–379. [Google Scholar] [CrossRef]
- Calabro, F. Modeling the effects of material chemistry on water flow enhancement in nanotube membranes. MRS Bull. 2017, 42, 289–293. [Google Scholar] [CrossRef]
- Wang, M.; Shen, W.H.; Ding, S.Y.; Wang, X.; Wang, Z.; Wang, Y.G.; Liu, F. A coupled effect of dehydration and electrostatic interactions on selective ion transport through charged nanochannels. Nanoscale 2018, 10, 18821–18828. [Google Scholar] [CrossRef]
- Esmaeilzadeh, H.; Su, J.W.; Charmchi, M.; Sun, H.W. Effect of hydrophobicity on the water flow in carbon nanotube-a molecular dynamics study. Theor. Appl. Mech. Lett. 2018, 8, 284–290. [Google Scholar] [CrossRef]
- Casanova, S.; Borg, M.K.; Chew, Y.M.J.; Mattia, D. Surface-controlled water flow in nanotube membranes. ACS Appl. Mater. Interfaces 2019, 11, 1689–1698. [Google Scholar] [CrossRef] [Green Version]
- Heiranian, M.; Aluru, N.R. Nanofluidic transport theory with enhancement factors approaching one. ACS Nano 2020, 14, 272–281. [Google Scholar] [CrossRef]
- Fang, C.; Lv, F.J.; Huang, D.C.; Su, J.Y. Coupling transport of water and ions through a carbon nanotube: A novel desalination phenomenon induced by tuning the pressure direction. Desalination 2020, 492, 8. [Google Scholar] [CrossRef]
- Otake, K.-i.; Otsubo, K.; Komatsu, T.; Dekura, S.; Taylor, J.M.; Ikeda, R.; Sugimoto, K.; Fujiwara, A.; Chou, C.-P.; Sakti, A.W. Confined water-mediated high proton conduction in hydrophobic channel of a synthetic nanotube. Nat. Commun. 2020, 11, 843. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Esfandiar, A.; Radha, B.; Wang, F.C.; Yang, Q.; Hu, S.; Garaj, S.; Nair, R.R.; Geim, A.K.; Gopinadhan, K. Size effect in ion transport through angstrom-scale slits. Science 2017, 358, 511–513. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Corry, B. Mechanisms of selective ion transport and salt rejection in carbon nanostructures. MRS Bull. 2017, 42, 306–310. [Google Scholar] [CrossRef]
- Li, Y.H.; Yu, Y.Z.; Qian, J.H.; Wu, H.A.; Wang, F.C. Anomalous ion transport through angstrom-scale pores: Effect of hydration shell exchange on ion mobility. Appl. Surf. Sci. 2021, 560, 7. [Google Scholar] [CrossRef]
- Demingos, P.G.; Pagnussatti, R.A.; Muniz, A.R. Strain-tunable carbon nanothread-derived membranes for water desalination. J. Phys. Chem. B 2021, 125, 7311–7319. [Google Scholar] [CrossRef]
- Wang, Y.H.; He, Z.J.; Gupta, K.M.; Shi, Q.; Lu, R.F. Molecular dynamics study on water desalination through functionalized nanoporous graphene. Carbon 2017, 116, 120–127. [Google Scholar] [CrossRef]
- Yu, T.F.; Xu, Z.J.; Liu, S.Y.; Liu, H.; Yang, X.N. Enhanced hydrophilicity and water-permeating of functionalized graphene-oxide nanopores: Molecular dynamics simulations. J. Membr. Sci. 2018, 550, 510–517. [Google Scholar] [CrossRef]
- Li, X.Q.; Zhu, B.; Zhu, J. Graphene oxide based materials for desalination. Carbon 2019, 146, 320–328. [Google Scholar] [CrossRef]
- Vishnu, P.K.; Sathian, S.P. The effect of temperature on water desalination through two-dimensional nanopores. J. Chem. Phys. 2020, 152, 9. [Google Scholar]
- Li, Y.Y.; Yue, X.Y.; Huang, G.; Wang, M.; Zhang, Q.W.; Wang, C.C.; Yi, H.B.; Wang, S.Y. Li+ selectivity of carboxylate graphene nanopores inspired by electric field and nanoconfinement. Small 2021, 17, 9. [Google Scholar] [CrossRef]
- Kang, J.; Choi, Y.; Kim, J.H.; Choi, E.; Choi, S.E.; Kwon, O.; Kim, D.W. Functionalized nanoporous graphene membrane with ultrafast and stable nanofiltration. J. Membr. Sci. 2021, 618, 8. [Google Scholar] [CrossRef]
- Yu, Y.Z.; Fan, J.C.; Xia, J.; Zhu, Y.B.; Wu, H.A.; Wang, F.C. Dehydration impeding ionic conductance through two-dimensional angstrom-scale slits. Nanoscale 2019, 11, 8449–8457. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.Z.; Fan, J.C.; Esfandiar, A.; Zhu, Y.B.; Wu, H.A.; Wang, F.C. Charge asymmetry effect in ion transport through angstrom-scale channels. J. Phys. Chem. C 2019, 123, 1462–1469. [Google Scholar] [CrossRef]
- Wang, Y.L.; Wang, C.L.; Zhang, Y.Q.; Huo, F.; He, H.Y.; Zhang, S.J. Molecular insights into the regulatable interfacial property and flow behavior of confined ionic liquids in graphene nanochannels. Small 2019, 15, 10. [Google Scholar] [CrossRef] [Green Version]
- Chen, Z.; Yang, J.W.; Ma, C.P.; Zhou, K.; Jiao, S.P. Continuous water filling in a graphene nanochannel: A molecular dynamics study. J. Phys. Chem. B 2021, 125, 9824–9833. [Google Scholar] [CrossRef]
- Shi, Q.; Gao, H.Q.; Zhang, Y.D.; Meng, Z.S.; Rao, D.W.; Su, J.Y.; Liu, Y.Z.; Wang, Y.H.; Lu, R.F. Bilayer graphene with ripples for reverse osmosis desalination. Carbon 2018, 136, 21–27. [Google Scholar] [CrossRef]
- Yang, L.; Guo, Y. Dynamics of water confined in a graphene nanochannel: Dependence of friction on graphene chirality. Nanotechnology 2020, 31, 235702. [Google Scholar] [CrossRef]
- Yang, L.; Guo, Y.J.; Diao, D.F. Structure and dynamics of water confined in a graphene nanochannel under gigapascal high pressure: Dependence of friction on pressure and confinement. Phys. Chem. Chem. Phys. 2017, 19, 14048–14054. [Google Scholar] [CrossRef]
- Xiong, W.; Liu, J.Z.; Ma, M.; Xu, Z.P.; Sheridan, J.; Zheng, Q.S. Strain engineering water transport in graphene nanochannels. Phys. Rev. E 2011, 84, 7. [Google Scholar] [CrossRef] [Green Version]
- Jiao, S.P.; Liu, M.C. Snap-through in graphene nanochannels: With application to fluidic control. ACS Appl. Mater. Interfaces 2021, 13, 1158–1168. [Google Scholar] [CrossRef]
- Sorokin, P.B.; Yakobson, B.I. Two-dimensional diamond-diamane: Current state and further prospects. Nano Lett. 2021, 21, 5475–5484. [Google Scholar] [CrossRef] [PubMed]
- Martins, L.G.P.; Matos, M.J.S.; Paschoal, A.R.; Freire, P.T.C.; Andrade, N.F.; Aguiar, A.L.; Kong, J.; Neves, B.R.A.; de Oliveira, A.B.; Mazzoni, M.S.C.; et al. Raman evidence for pressure-induced formation of diamondene. Nat. Commun. 2017, 8, 96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bakharev, P.V.; Huang, M.; Saxena, M.; Lee, S.W.; Joo, S.H.; Park, S.O.; Dong, J.; Camacho-Mojica, D.C.; Jin, S.; Kwon, Y.; et al. Chemically induced transformation of chemical vapour deposition grown bilayer graphene into fluorinated single-layer diamond. Nat. Nanotechnol. 2019, 15, 59–66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, Y.C.; Shao, J.L.; Zheng, Z.Q.; Zhan, H.F. Mechanical properties of a single-layer diamane under tension and bending. J. Phys. Chem. C 2021, 125, 915–922. [Google Scholar] [CrossRef]
- Martinez, L.; Andrade, R.; Birgin, E.G.; Martinez, J.M. Packmol: A package for building initial configurations for molecular dynamics simulations. J. Comput. Chem. 2009, 30, 2157–2164. [Google Scholar] [CrossRef]
- Gao, H.Q.; Wang, J.; Zhang, X.R.; Hu, M.A.; Xu, Q.H.; Xie, Y.N.; Liu, Y.Z.; Lu, R.F. Confined lamellar channels structured by multilayer graphene for high-efficiency desalination. Desalination 2022, 530, 7. [Google Scholar] [CrossRef]
- Berendsen, H.J.C.; Grigera, J.R.; Straatsma, T.P. The missing term in effective pair potentials. J. Phys. Chem. 1987, 91, 6269–6271. [Google Scholar] [CrossRef]
- Golzari, A.; Pishkenari, H.N. Vibrational analysis of fullerene hydrides using AIREBO potential. Sci. Iran. 2020, 27, 1933–1944. [Google Scholar] [CrossRef] [Green Version]
- Plimpton, S. Fast parallel algorithms for short-range molecular dynamics. J. Comput. Phys. 1995, 117, 1–19. [Google Scholar] [CrossRef] [Green Version]
- Prinos, J.; Panayiotou, C. Glass-transition temperature in hydrogen-bonded polymer mixtures. Polymer 1995, 36, 1223–1227. [Google Scholar] [CrossRef]
- Kalogeras, I.M.; Brostow, W. Glass transition temperatures in binary polymer blends. J. Polym. Sci. Pt. B-Polym. Phys. 2009, 47, 80–95. [Google Scholar] [CrossRef]
- Chen, M.; Jabeen, F.; Rasulev, B.; Ossowski, M.; Boudjouk, P. A computational structure-property relationship study of glass transition temperatures for a diverse set of polymers. J. Polym. Sci. Pt. B-Polym. Phys. 2018, 56, 877–885. [Google Scholar] [CrossRef]
- Halim, S.I.A.; Chan, C.H.; Kammer, H.W. About glass transition in polymer-salt mixtures. Polym. Test. 2019, 79, 5. [Google Scholar]
- Cohen-Tanugi, D.; Grossman, J.C. Water desalination across nanoporous graphene. Nano Lett. 2012, 12, 3602–3608. [Google Scholar] [CrossRef]
- Gao, H.Q.; Xu, Q.H.; Wang, J.; Ning, C.; Liu, Y.Z.; Xie, Y.N.; Lu, R.F. Beyond the pore size limitation of a nanoporous graphene monolayer membrane for water desalination assisted by an external electric field. J. Phys. Chem. Lett. 2022, 13, 258–266. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, B.; Dong, B.; Shi, T.; Zhan, H.; Zhang, Y. Atomistic Simulations of the Permeability and Dynamic Transportation Characteristics of Diamond Nanochannels. Nanomaterials 2022, 12, 1785. https://doi.org/10.3390/nano12111785
Li B, Dong B, Shi T, Zhan H, Zhang Y. Atomistic Simulations of the Permeability and Dynamic Transportation Characteristics of Diamond Nanochannels. Nanomaterials. 2022; 12(11):1785. https://doi.org/10.3390/nano12111785
Chicago/Turabian StyleLi, Bingqing, Bin Dong, Tianxiang Shi, Haifei Zhan, and Yongqiang Zhang. 2022. "Atomistic Simulations of the Permeability and Dynamic Transportation Characteristics of Diamond Nanochannels" Nanomaterials 12, no. 11: 1785. https://doi.org/10.3390/nano12111785
APA StyleLi, B., Dong, B., Shi, T., Zhan, H., & Zhang, Y. (2022). Atomistic Simulations of the Permeability and Dynamic Transportation Characteristics of Diamond Nanochannels. Nanomaterials, 12(11), 1785. https://doi.org/10.3390/nano12111785






