Sensitive Detection of Industrial Pollutants Using Modified Electrochemical Platforms
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
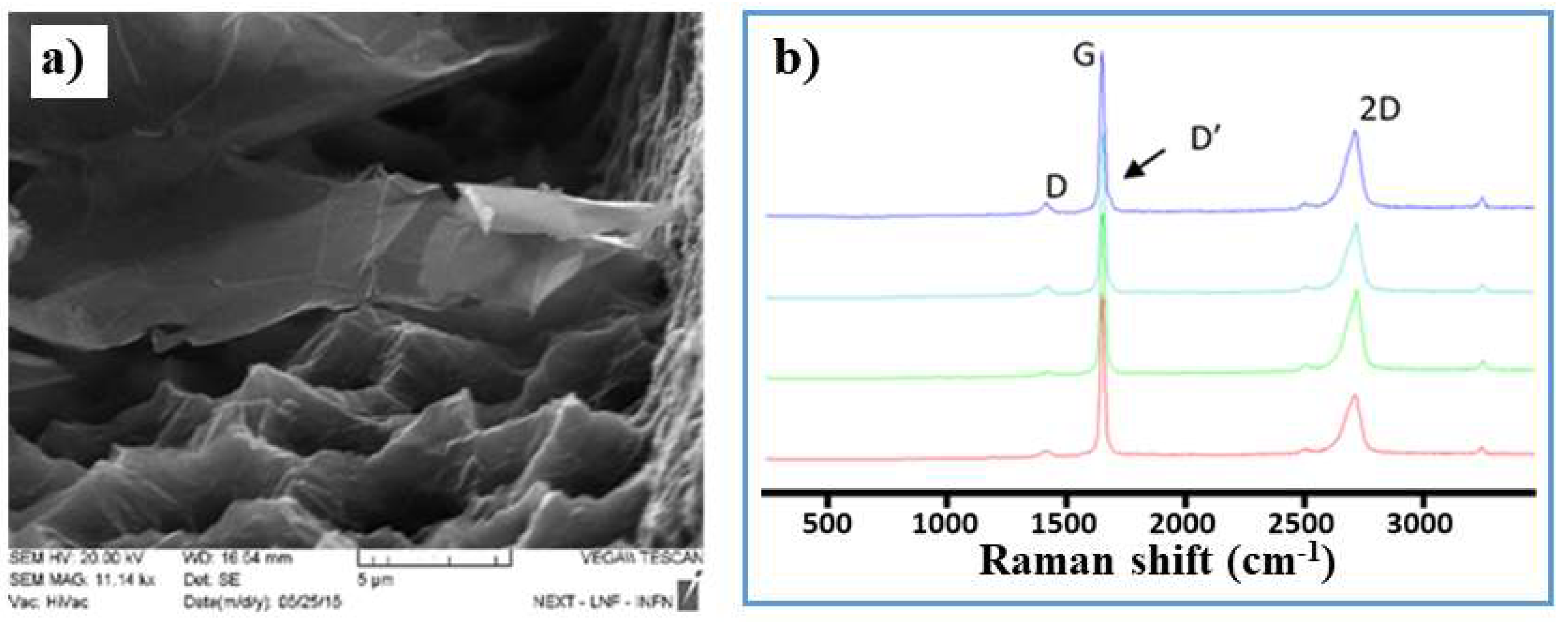
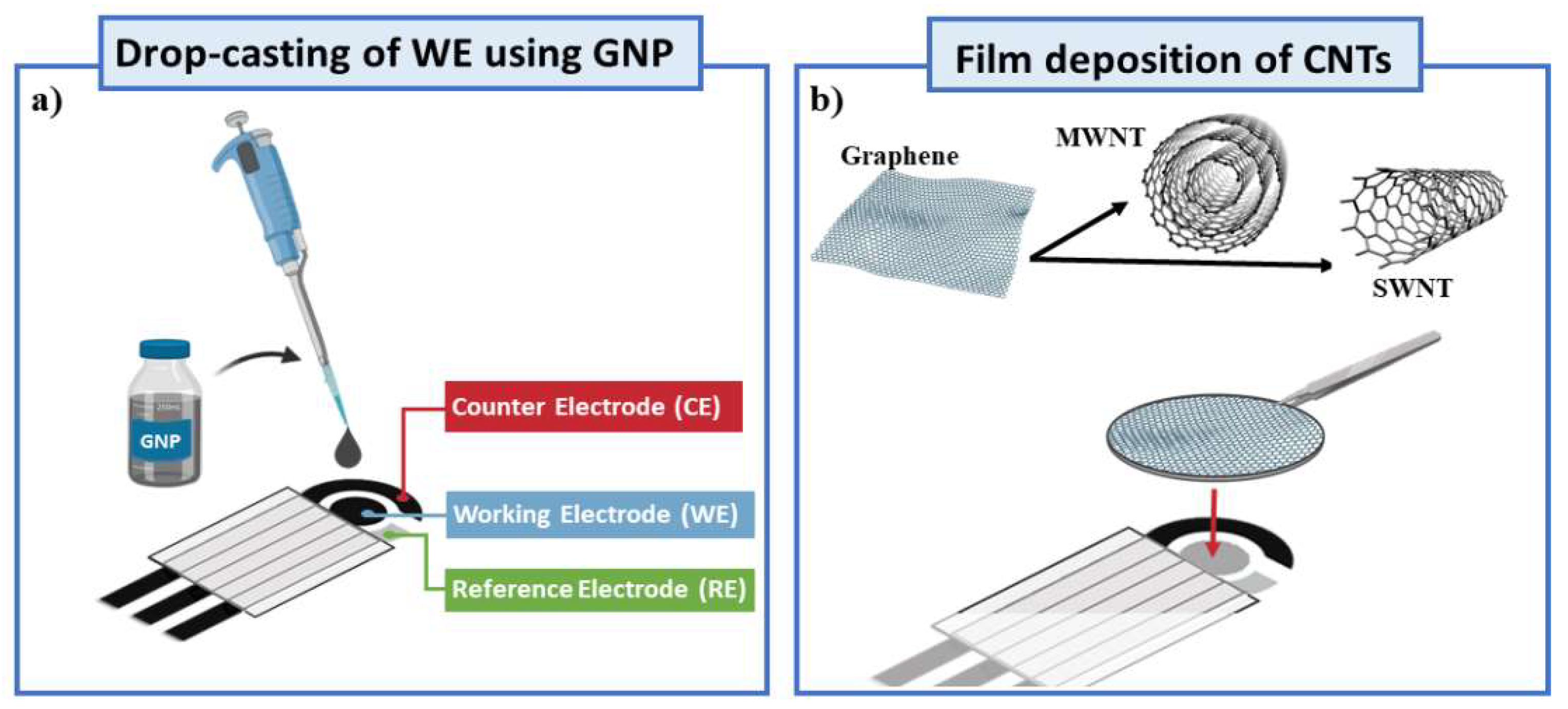
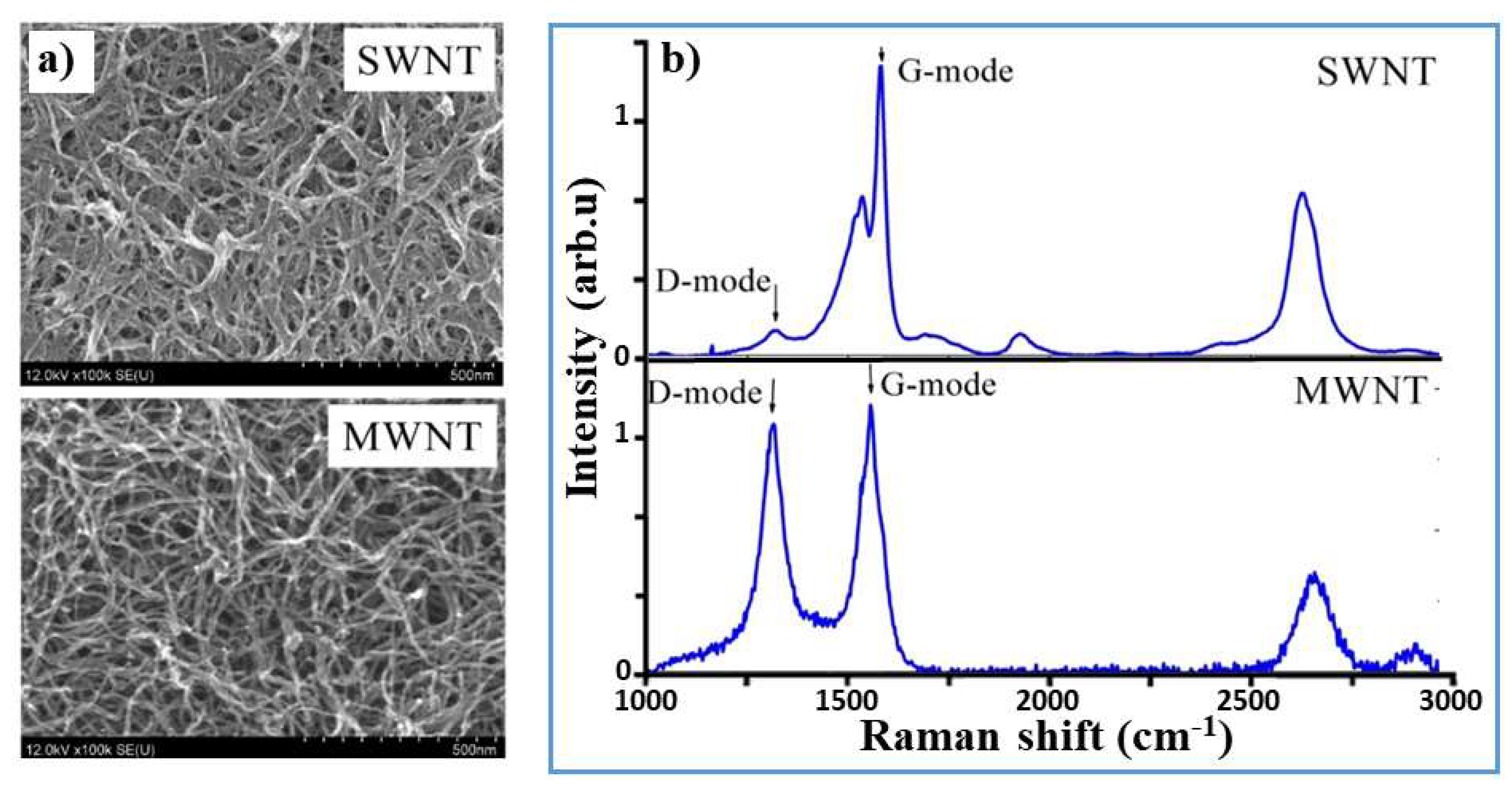
2.2. Fabrication of GNPs and CNTs and Integration into SPEs
2.3. Apparatus
2.4. Analytical Parameters Calculation
3. Results and Discussion
3.1. Electrochemical Performances of CNM-Modified Platforms
3.2. CNM-Based Sensor for the Determination of Hydroquinone and Benzoquinone in Buffer Solution
3.3. CNM-Based Sensor for the Determination of Hydroquinone and Benzoquinone in Water
3.4. Stability and Recovery of CNM-Based Sensors in HQ Determination
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vallero, D. Controlling Air Pollution from Sources. In Fundamentals of Air Pollution; Elsevier: Amsterdam, The Netherlands, 2014; pp. 881–925. ISBN 978-0-12-401733-7. [Google Scholar] [CrossRef]
- Jaeger, H.; Meneses, N.; Knorr, D. Food Technologies: Pulsed Electric Field Technology. In Encyclopedia of Food Safety; Elsevier: Amsterdam, The Netherlands, 2014; pp. 239–244. ISBN 978-0-12-378613-5. [Google Scholar] [CrossRef]
- Hou, L.; Zhang, X.; Wang, D.; Baccarelli, A. Environmental chemical exposures and human epigenetics. Int. J. Epidemiol. 2012, 41, 79–105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Enguita, F.J.; Leitão, A.L. Hydroquinone: Environmental Pollution, Toxicity, and Microbial Answers. BioMed Res. Int. 2013, 2013, 542168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johnson, E.S.; Langård, S.; Lin, Y.-S. A critique of benzene exposure in the general population. Sci. Total Environ. 2007, 374, 183–198. [Google Scholar] [CrossRef] [PubMed]
- Bahadar, H.; Mostafalou, S.; Abdollahi, M. Current understandings and perspectives on non-cancer health effects of benzene: A global concern. Toxicol. Appl. Pharmacol. 2014, 276, 83–94. [Google Scholar] [CrossRef] [Green Version]
- Yang, X.; Lu, Y.; He, F.; Hou, F.; Xing, C.; Xu, P.; Wang, Q.-F. Benzene metabolite hydroquinone promotes DNA homologous recombination repair via the NF-κB pathway. Carcinogenesis 2019, 40, 1021–1030. [Google Scholar] [CrossRef]
- Rappaport, S.M.; Kim, S.; Lan, Q.; Vermeulen, R.; Waidyanatha, S.; Zhang, L.; Li, G.; Yin, S.; Hayes, R.B.; Rothman, N.; et al. Evidence That Humans Metabolize Benzene via Two Pathways. Environ. Health Perspect. 2009, 117, 946–952. [Google Scholar] [CrossRef] [Green Version]
- Peng, C.; Arthur, D.; Liu, F.; Lee, J.; Xia, Q.; Lavin, M.F.; Ng, J.C. Genotoxicity of hydroquinone in A549 cells. Cell Biol. Toxicol. 2013, 29, 213–227. [Google Scholar] [CrossRef]
- Roza, L.; De Vogel, N.; Van Delft, J.H.M. Lack of clastogenic effects in cultured human lymphocytes treated with hydroquinone. Food Chem. Toxicol. 2003, 41, 1299–1305. [Google Scholar] [CrossRef]
- Link, T.A.; Haase, U.; Brandt, U.; von Jagow, G. What information do inhibitors provide about the structure of the hydroquinone oxidation site of ubihydroquinone: Cytochromec oxidoreductase? J. Bioenerg. Biomembr. 1993, 25, 221–232. [Google Scholar] [CrossRef]
- Bremer, P.T.; Hixon, M.S.; Janda, K.D. Benzoquinones as inhibitors of botulinum neurotoxin serotype A. Bioorganic Med. Chem. 2014, 22, 3971–3981. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Yu, Y.; Wang, Y.; Qian, J.; Zhi, J. The benzoquinone-mediated electrochemical microbial biosensor for water biotoxicity assay. Electrochim. Acta 2013, 97, 52–57. [Google Scholar] [CrossRef]
- Murthy, A.S.N.; Sharma, J. Electrocatalytic oxidation of ascorbic acid using benzoquinone as a mediator. Electroanalysis 1997, 9, 726–729. [Google Scholar] [CrossRef]
- Choi, H.; Kim, M.-C.; Park, Y.; Lee, S.; Ahn, W.; Hong, J.; Sohn, J.I.; Jang, A.-R.; Lee, Y.-W. Electrochemically active hydroquinone-based redox mediator for flexible energy storage system with improved charge storing ability. J. Colloid Interface Sci. 2021, 588, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Gao, Y.; Liu, H.; Du, J.; Xia, N. Electrochemical-chemical-chemical redox cycling triggered by thiocholine and hydroquinone with ferrocenecarboxylic acid as the redox mediator. Electrochim. Acta 2014, 139, 323–330. [Google Scholar] [CrossRef]
- Abdel-Lateff, A.; König, G.M.; Fisch, K.M.; Höller, U.; Jones, P.G.; Wright, A.D. New Antioxidant Hydroquinone Derivatives from the Algicolous Marine Fungus Acremonium sp. J. Nat. Prod. 2002, 65, 1605–1611. [Google Scholar] [CrossRef]
- Aknin, M.; Dayan, T.L.-A.; Rudi, A.; Kashman, Y.; Gaydou, E.M. Hydroquinone Antioxidants from the Indian Ocean Tunicate Aplidium savignyi. J. Agric. Food Chem. 1999, 47, 4175–4177. [Google Scholar] [CrossRef]
- Muralidharan, S.; Phani, K.L.N.; Pitchumani, S.; Ravichandran, S.; Iyer, S.V.K. Polyamino-Benzoquinone Polymers: A New Class of Corrosion Inhibitors for Mild Steel. J. Electrochem. Soc. 1995, 142, 1478–1483. [Google Scholar] [CrossRef]
- Rubio-Garcia, J.; Kucernak, A.; Parra-Puerto, A.; Liu, R.; Chakrabarti, B. Hydrogen/functionalized benzoquinone for a high-performance regenerative fuel cell as a potential large-scale energy storage platform. J. Mater. Chem. A 2020, 8, 3933–3941. [Google Scholar] [CrossRef]
- Xu, X.; Zhou, L.; Van Cleemput, O.; Wang, Z. Fate of urea-15N in a soil-wheat system as influenced by urease inhibitor hydroquinone and nitrification inhibitor dicyandiamide. Plant Soil 2000, 220, 261–270. [Google Scholar] [CrossRef]
- Gentry, S.T.; Fredericks, S.J.; Krchnavek, R. Controlled Particle Growth of Silver Sols through the Use of Hydroquinone as a Selective Reducing Agent. Langmuir 2009, 25, 2613–2621. [Google Scholar] [CrossRef]
- Cook, A.R.; Curtiss, L.A.; Miller, J.R. Fluorescence of the 1,4-Benzoquinone Radical Anion. J. Am. Chem. Soc. 1997, 119, 5729–5734. [Google Scholar] [CrossRef]
- Bang, S.-H.; Han, S.-J.; Kim, D.-H. Hydrolysis of arbutin to hydroquinone by human skin bacteria and its effect on antioxidant activity. J. Cosmet. Dermatol. 2008, 7, 189–193. [Google Scholar] [CrossRef] [PubMed]
- Uchimiya, M.; Stone, A.T. Reversible redox chemistry of quinones: Impact on biogeochemical cycles. Chemosphere 2009, 77, 451–458. [Google Scholar] [CrossRef] [PubMed]
- Macalady, D.L.; Tratnyek, P.G.; Grundl, T.J. Abiotic reduction reactions of anthropogenic organic chemicals in anaerobic systems: A critical review. J. Contam. Hydrol. 1986, 1, 1–28. [Google Scholar] [CrossRef]
- Bedner, M.; MacCrehan, W.A. Transformation of Acetaminophen by Chlorination Produces the Toxicants 1,4-Benzoquinone and N-Acetyl-p-benzoquinone Imine. Environ. Sci. Technol. 2006, 40, 516–522. [Google Scholar] [CrossRef]
- Flickinger, C.W. The benzenediols: Catechol, resorcinol and hydroquinone—A review of the industrial toxicology and current industrial exposure limits. Am. Ind. Hyg. Assoc. J. 1976, 37, 596–606. [Google Scholar] [CrossRef]
- Suresh, S.; Srivastava, V.C.; Mishra, I.M. Adsorption of catechol, resorcinol, hydroquinone, and their derivatives: A review. Int. J. Energy Environ. Eng. 2012, 3, 32. [Google Scholar] [CrossRef]
- Choukem, S.-P.; Efie, D.T.; Djiogue, S.; Kaze, F.F.; Mboue-Djieka, Y.; Boudjeko, T.; Dongo, E.; Gautier, J.-F.; Kengne, A.-P. Effects of hydroquinone-containing creams on capillary glycemia before and after serial hand washings in Africans. PLoS ONE 2018, 13, e0202271. [Google Scholar] [CrossRef]
- Nordlund, J.; Grimes, P.; Ortonne, J. The safety of hydroquinone: The safety of hydroquinone. J. Eur. Acad. Dermatol. Venereol. 2006, 20, 781–787. [Google Scholar] [CrossRef]
- Carson, P.A.; Mumford, C.J. Hazardous Chemicals Handbook, 2nd ed.; Butterworth-Heinemann: Oxford, UK; Boston, MA, USA, 2002. [Google Scholar]
- Zuo, Y.-T.; Hu, Y.; Lu, W.-W.; Cao, J.-J.; Wang, F.; Han, X.; Lu, W.-Q.; Liu, A.-L. Toxicity of 2,6-dichloro-1,4-benzoquinone and five regulated drinking water disinfection by-products for the Caenorhabditis elegans nematode. J. Hazard. Mater. 2017, 321, 456–463. [Google Scholar] [CrossRef]
- Moore, M.; Thor, H.; Moore, G.; Nelson, S.; Moldéus, P.; Orrenius, S. The toxicity of acetaminophen and N-acetyl-p-benzoquinone imine in isolated hepatocytes is associated with thiol depletion and increased cytosolic Ca2+. J. Biol. Chem. 1985, 260, 13035–13040. [Google Scholar] [CrossRef]
- Chan, K.; Jensen, N.; O’Brien, P.J. Structure–activity relationships for thiol reactivity and rat or human hepatocyte toxicity induced by substituted p-benzoquinone compounds. J. Appl. Toxicol. 2008, 28, 608–620. [Google Scholar] [CrossRef] [PubMed]
- Moldovan, Z.; Popa, D.E.; David, I.G.; Buleandra, M.; Badea, I.A. A Derivative Spectrometric Method for Hydroquinone Determination in the Presence of Kojic Acid, Glycolic Acid, and Ascorbic Acid. J. Spectrosc. 2017, 2017, 6929520. [Google Scholar] [CrossRef]
- Yuan, Y.; Li, C.; Zhu, J.; Liu, S.; Liu, Z.; Yang, J.; Qiao, M.; Shi, Y.; Duan, R.; Hu, X. Determination of hydroquinone based on the formation of Turnbull’s blue nanoparticles using resonance Rayleigh scattering. Anal. Methods 2015, 7, 9347–9353. [Google Scholar] [CrossRef]
- Gimeno, P.; Maggio, A.-F.; Bancilhon, M.; Lassu, N.; Gornes, H.; Brenier, C.; Lempereur, L. HPLC–UV Method for the Identification and Screening of Hydroquinone, Ethers of Hydroquinone and Corticosteroids Possibly Used as Skin-Whitening Agents in Illicit Cosmetic Products. J. Chromatogr. Sci. 2015, 54, 343–352. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uddin, S.; Rauf, A.; Kazi, T.G.; Afridi, H.I.; Lutfullah, G. Highly sensitive spectrometric method for determination of hydroquinone in skin lightening creams: Application in cosmetics: Highly sensitive spectrometric method for determination of hydroquinone in skin. Int. J. Cosmet. Sci. 2011, 33, 132–137. [Google Scholar] [CrossRef] [PubMed]
- Wang, J. Analytical Electrochemistry; Wiley-VCH: Hoboken, NJ, USA, 2006; Available online: http://www.123library.org/book_details/?id=48289 (accessed on 15 February 2022).
- Borremans, M.; De Beer, J.; Goeyens, L. Experimental and statistical validation of HPLC analysis of hydroquinone and its 4-methoxyphenol, 4-ethoxyphenol and 4-benzyloxyphenol ethers in cosmetic products. Chromatographia 1999, 50, 346–352. [Google Scholar] [CrossRef]
- Raymundo-Pereira, P.A.; Gomes, N.O.; Machado, S.A.S.; Oliveira, O.N., Jr. Simultaneous, ultrasensitive detection of hydroquinone, paracetamol and estradiol for quality control of tap water with a simple electrochemical method. J. Electroanal. Chem. 2019, 848, 113319. [Google Scholar] [CrossRef]
- García, P.L.; Santoro, M.I.R.M.; Kedor-Hackman, E.R.M.; Singh, A.K. Development and validation of a HPLC and a UV derivative spectrophotometric methods for determination of hydroquinone in gel and cream preparations. J. Pharm. Biomed. Anal. 2005, 39, 764–768. [Google Scholar] [CrossRef]
- Lin, C.-H.; Sheu, J.-Y.; Wu, H.-L.; Huang, Y.-L. Determination of hydroquinone in cosmetic emulsion using microdialysis sampling coupled with high-performance liquid chromatography. J. Pharm. Biomed. Anal. 2005, 38, 414–419. [Google Scholar] [CrossRef]
- Galimany-Rovira, F.; Pérez-Lozano, P.; Suñé-Negre, J.M.; García-Montoya, E.; Miñarro, M.; Ticó, J.R. Development and validation of a new RP-HPLC method for the simultaneous determination of hydroquinone, kojic acid, octinoxate, avobenzone, BHA and BHT in skin-whitening cream. Anal. Methods 2016, 8, 1170–1180. [Google Scholar] [CrossRef]
- Bielicka-Daszkiewicz, K.; Hadzicka, M.; Voelkel, A. Optimization of SPE/GC/HPLC Analytical Procedure for Determination of Phenol, Quinones, and Carboxylic Acids in Water Samples. ISRN Chromatogr. 2012, 2012, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Kaur, S.; Kaur, T.; Kaur, G.; Verma, S. Development and Validation of UV-Spectrophotometric Method for Estimation of Hydroquinone in Bulk, Marketed Cream and Preapared NLC Formulation. Int. J. Appl. Pharm. 2017, 9, 102. [Google Scholar] [CrossRef] [Green Version]
- Elferjani, H.S.; Ahmida, N.H.S.; Ahmida, A. Determination of Hydroquinone in Some Pharmaceutical and Cosmetic Preparations by Spectrophotometric Method. Int. J. Sci. Res. 2017, 6, 2219–2224. [Google Scholar] [CrossRef]
- Iskander, M.L.; Medien, H.A.A.; Khalil, L.H. Spectrophotometric Determination of Some Quinones. Anal. Lett. 1995, 28, 1513–1523. [Google Scholar] [CrossRef]
- Cancelliere, R.; Di Tinno, A.; Di Lellis, A.M.; Tedeschi, Y.; Bellucci, S.; Carbone, K.; Signori, E.; Contini, G.; Micheli, L. An inverse-designed electrochemical platform for analytical applications. Electrochem. Commun. 2020, 121, 106862. [Google Scholar] [CrossRef]
- Di Tinno, A.; Cancelliere, R.; Micheli, L. Determination of Folic Acid Using Biosensors—A Short Review of Recent Progress. Sensors 2021, 21, 3360. [Google Scholar] [CrossRef]
- Renedo, O.D.; Alonso-Lomillo, M.A.; Martínez, M.J.A. Recent developments in the field of screen-printed electrodes and their related applications. Talanta 2007, 73, 202–219. [Google Scholar] [CrossRef]
- Cotchim, S.; Promsuwan, K.; Dueramae, M.; Duerama, S.; Dueraning, A.; Thavarungkul, P.; Kanatharana, P.; Limbut, W. Development and Application of an Electrochemical Sensor for Hydroquinone in Pharmaceutical Products. J. Electrochem. Soc. 2020, 167, 155528. [Google Scholar] [CrossRef]
- Sawczuk, R.B.S.; Pinheiro, H.A.; Santos, J.R.N.; Alves, I.C.B.; Viegas, H.D.C.; Lacerda, C.A.; Sousa, J.K.C.; Marques, E.P.; Marques, A.L.B. A sensitive electrochemical nanosensor based on iron oxide nanoparticles and multiwalled carbon nanotubes for simultaneous determination of benzoquinone and catechol in groundwater. Int. J. Environ. Anal. Chem. 2021, 101, 1–18. [Google Scholar] [CrossRef]
- Guin, P.S.; Das, S.; Mandal, P.C. Electrochemical Reduction of Quinones in Different Media: A Review. Int. J. Electrochem. 2011, 2011, 816202. [Google Scholar] [CrossRef] [Green Version]
- Cancelliere, R.; Di Tinno, A.; Cataldo, A.; Bellucci, S.; Micheli, L. Powerful Electron-Transfer Screen-Printed Platforms as Biosensing Tools: The Case of Uric Acid Biosensor. Biosensors 2021, 12, 2. [Google Scholar] [CrossRef]
- Ramaseshan, R.; Sundarrajan, S.; Jose, R.; Ramakrishna, S. Nanostructured ceramics by electrospinning. J. Appl. Phys. 2007, 102, 111101. [Google Scholar] [CrossRef]
- Dabrowska, A.; Bellucci, S.; Cataldo, A.; Micciulla, F.; Huczko, A. Nanocomposites of epoxy resin with graphene nanoplates and exfoliated graphite: Synthesis and electrical properties: Nanocomposites of epoxy resin with GNP and exfoliated graphite. Phys. Status Solidi B 2014, 251, 2599–2602. [Google Scholar] [CrossRef]
- Maffucci, A.; Micciulla, F.; Cataldo, A.; Miano, G.; Bellucci, S. Bottom-up realization and electrical characterization of a graphene-based device. Nanotechnology 2016, 27, 095204. [Google Scholar] [CrossRef]
- Gomez, F.J.V.; Martín, A.; Silva, M.F.; Escarpa, A. Screen-printed electrodes modified with carbon nanotubes or graphene for simultaneous determination of melatonin and serotonin. Microchim. Acta 2015, 182, 1925–1931. [Google Scholar] [CrossRef]
- Cancelliere, R.; Carbone, K.; Pagano, M.; Cacciotti, I.; Micheli, L. Biochar from Brewers’ Spent Grain: A Green and Low-Cost Smart Material to Modify Screen-Printed Electrodes. Biosensors 2019, 9, 139. [Google Scholar] [CrossRef]
- Paton, K.R.; Varrla, E.; Backes, C.; Smith, R.J.; Khan, U.; O’Neill, A.; Boland, C.S.; Lotya, M.; Istrate, O.M.; King, P.; et al. Scalable production of large quantities of defect-free few-layer graphene by shear exfoliation in liquids. Nat. Mater. 2014, 13, 624–630. [Google Scholar] [CrossRef]
- Cancelliere, R.; Zurlo, F.; Micheli, L.; Melino, S. Vegetable waste scaffolds for 3D-stem cell proliferating systems and low cost biosensors. Talanta 2021, 223, 121671. [Google Scholar] [CrossRef]
- Eng, A.Y.S.; Chua, C.K.; Pumera, M. Intrinsic electrochemical performance and precise control of surface porosity of graphene-modified electrodes using the drop-casting technique. Electrochem. Commun. 2015, 59, 86–90. [Google Scholar] [CrossRef]
- Cancelliere, R.; Albano, D.; Brugnoli, B.; Buonasera, K.; Leo, G.; Margonelli, A.; Rea, G. Electrochemical and morphological layer-by-layer characterization of electrode interfaces during a label-free impedimetric immunosensor build-up: The case of ochratoxin A. Appl. Surf. Sci. 2021, 567, 150791. [Google Scholar] [CrossRef]
- Wang, J. Analytical Electrochemistry: Wang/Analytical Electrochemistry, 3rd ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2006. [Google Scholar] [CrossRef]
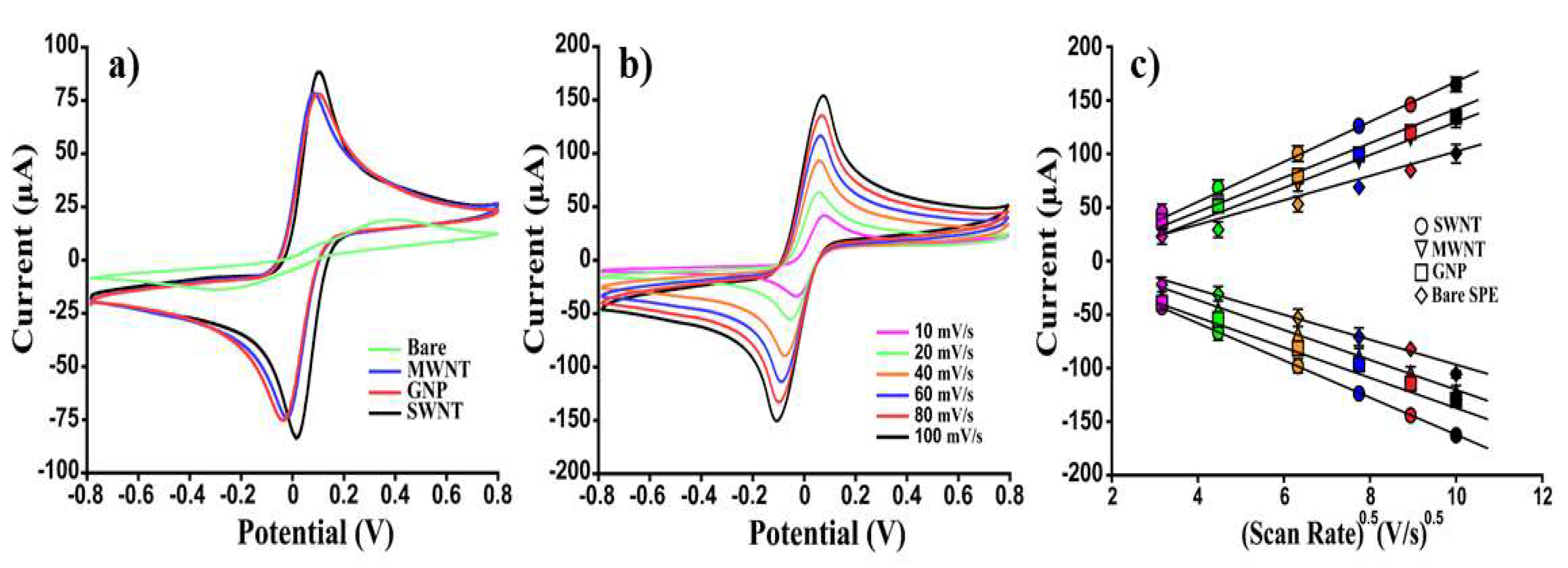

| [Fe(CN)6] 4−/3− (10 mM) | Bare Electrode | SWNT | MWNT | GNP |
|---|---|---|---|---|
| |Iap| [µA] | 17 ± 2 | 325 ± 5 | 274 ± 4 | 186 ± 17 |
| |Icp| [µA] | 22 ± 1 | 321 ± 4 | 269 ± 5 | 181 ± 15 |
| |Ipa|/|Ipc| | 0.80 | 1.01 | 1.02 | 1.02 |
| |k0 | [cm/s] | 1.9 × 10−5 | 5.6 × 10−3 | 2.1 × 10−3 | 1.4 × 10−3 |
| A [cm2] | 0.13 | 0.58 | 0.43 | 0.33 |
| ΔE [mV] | 197 | 85 | 110 | 124 |
| D0 [cm2/s] | 1.7 × 10−7 | 4.6 × 10−6 | 3.3 × 10−6 | 3.5 × 10−6 |
| LOD [μM] | 34.8 | 1.5 | 5.4 | 9.4 |
| Sensitivity [mA/M cm2] | 12 | 8.3 | 4.5 | 6.5 |
| Reproducibility | 10 | 5 | 3 | 5 |
| % Increase |Ipa| | / | 258 | 294 | 276 |
| % Increase |Ipc| | / | 480 | 580 | 312 |
| |Iap| [µA] | 17 ± 2 | 325 ± 5 | 274 ± 4 | 186 ± 17 |
| Bare Electrode | SWNT | MWNT | GNP | ||
|---|---|---|---|---|---|
| HydroQuinone (HQ) | |||||
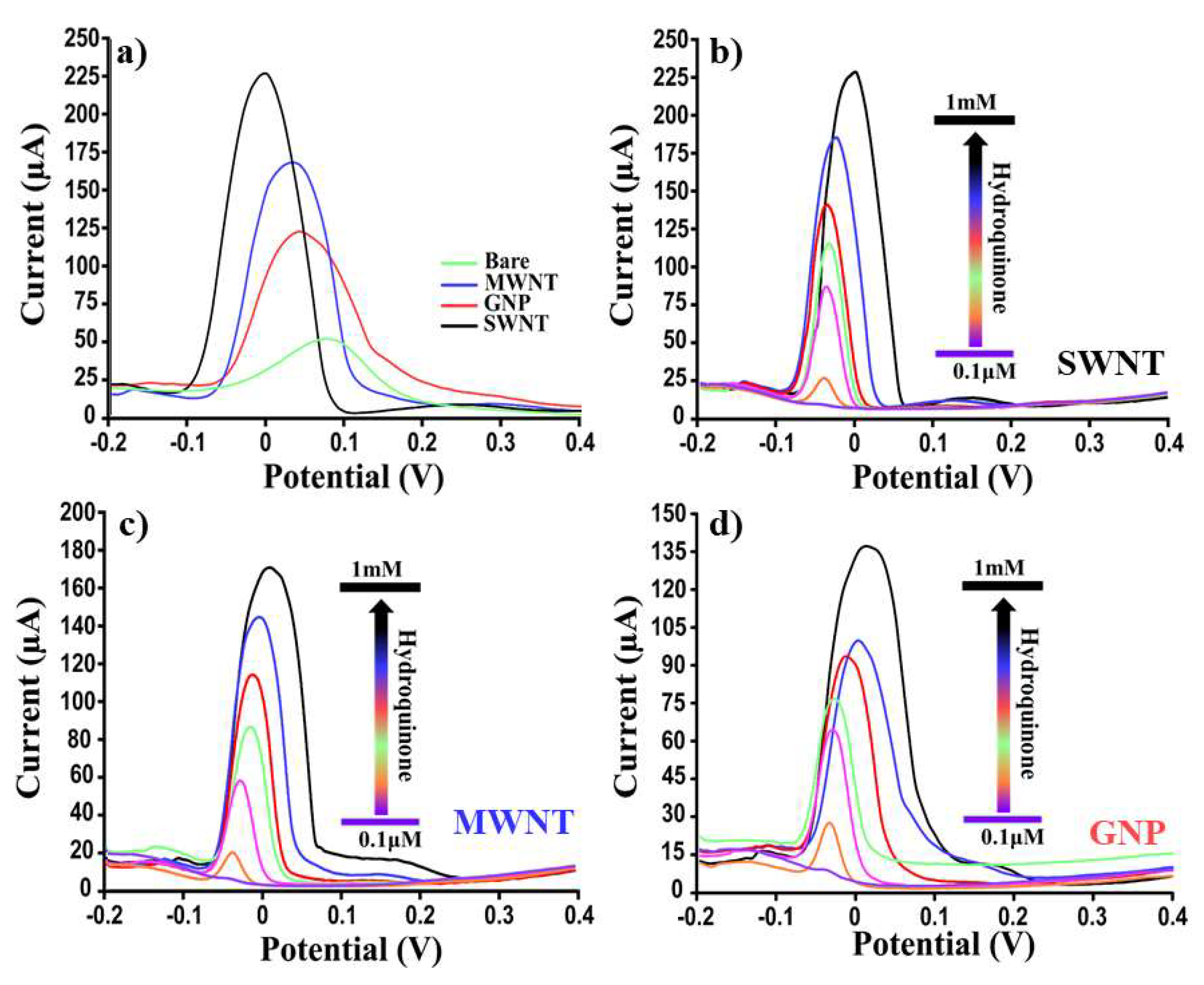
| CV | LOD (μM) | 90.3 | 8.5 | 11.6 | 15.8 |
| Reproducibility (RSD%) | 15 | 4 | 6 | 7 | |
| SWV | LOD (μM) | 12.3 | 0.04 | 0.07 | 0.3 |
| Reproducibility (RSD%) | 17 | 5 | 8 | 8 | |
| BenzoQuinone (BQ) | |||||
| CV | LOD (μM) | 95.7 | 9.3 | 12.3 | 17.5 |
| Reproducibility (RSD%) | 15 | 5 | 5 | 8 | |
| SWV | LOD (μM) | 17.3 | 0.05 | 0.08 | 1.4 |
| Reproducibility (RSD%) | 18 | 6 | 7 | 9 | |
| Bare Electrode | SWNT | MWNT | GNP | ||
|---|---|---|---|---|---|
| HydroQuinone (HQ) | |||||
| CV | LOD (μM) | 334.5 | 80.3 | 13.7 | 166.6 |
| Reproducibility (RSD%) | 17 | 8 | 9 | 9 | |
| SWV | LOD (μM) | 132.3 | 2.2 | 1.7 | 5.3 |
| Reproducibility (RSD%) | 17 | 10 | 10 | 10 | |
| BenzoQuinone (BQ) | |||||
| CV | LOD (μM) | 275.5 | 1.4 | 102.5 | 108.5 |
| Reproducibility (RSD%) | 14 | 10 | 10 | 10 | |
| SWV | LOD (μM) | 121.2 | 1.7 | 2.9 | 4.3 |
| Reproducibility (RSD%) | 18 | 11 | 12 | 11 | |
| Spiked HQ Concentration CS (μM) | Recovered HQ Concentration (CT–C0) (μM) | Recovery % (n = 6) | RSD% (n = 6) | |
|---|---|---|---|---|
| SWNT | 100 | 85 | 85.0 | 10 |
| 200 | 177 | 88.5 | 9 | |
| 400 | 374 | 93.5 | 7 | |
| 800 | 743 | 92.8 | 6 | |
| MWNT | 100 | 35 | 5.0 | 20 |
| 200 | 129 | 64.5 | 17 | |
| 400 | 310 | 77.5 | 14 | |
| 800 | 633 | 79.1 | 12 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Tinno, A.; Cancelliere, R.; Mantegazza, P.; Cataldo, A.; Paddubskaya, A.; Ferrigno, L.; Kuzhir, P.; Maksimenko, S.; Shuba, M.; Maffucci, A.; et al. Sensitive Detection of Industrial Pollutants Using Modified Electrochemical Platforms. Nanomaterials 2022, 12, 1779. https://doi.org/10.3390/nano12101779
Di Tinno A, Cancelliere R, Mantegazza P, Cataldo A, Paddubskaya A, Ferrigno L, Kuzhir P, Maksimenko S, Shuba M, Maffucci A, et al. Sensitive Detection of Industrial Pollutants Using Modified Electrochemical Platforms. Nanomaterials. 2022; 12(10):1779. https://doi.org/10.3390/nano12101779
Chicago/Turabian StyleDi Tinno, Alessio, Rocco Cancelliere, Pietro Mantegazza, Antonino Cataldo, Alesia Paddubskaya, Luigi Ferrigno, Polina Kuzhir, Sergey Maksimenko, Mikhail Shuba, Antonio Maffucci, and et al. 2022. "Sensitive Detection of Industrial Pollutants Using Modified Electrochemical Platforms" Nanomaterials 12, no. 10: 1779. https://doi.org/10.3390/nano12101779
APA StyleDi Tinno, A., Cancelliere, R., Mantegazza, P., Cataldo, A., Paddubskaya, A., Ferrigno, L., Kuzhir, P., Maksimenko, S., Shuba, M., Maffucci, A., Bellucci, S., & Micheli, L. (2022). Sensitive Detection of Industrial Pollutants Using Modified Electrochemical Platforms. Nanomaterials, 12(10), 1779. https://doi.org/10.3390/nano12101779