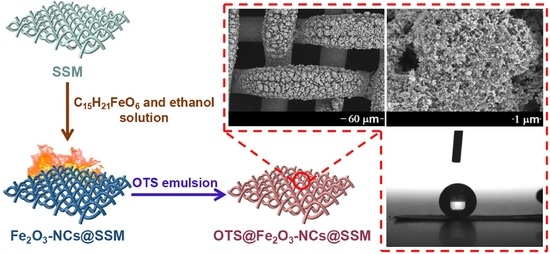
Facile Construction and Fabrication of a Superhydrophobic and Super Oleophilic Stainless Steel Mesh for Separation of Water and Oil
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
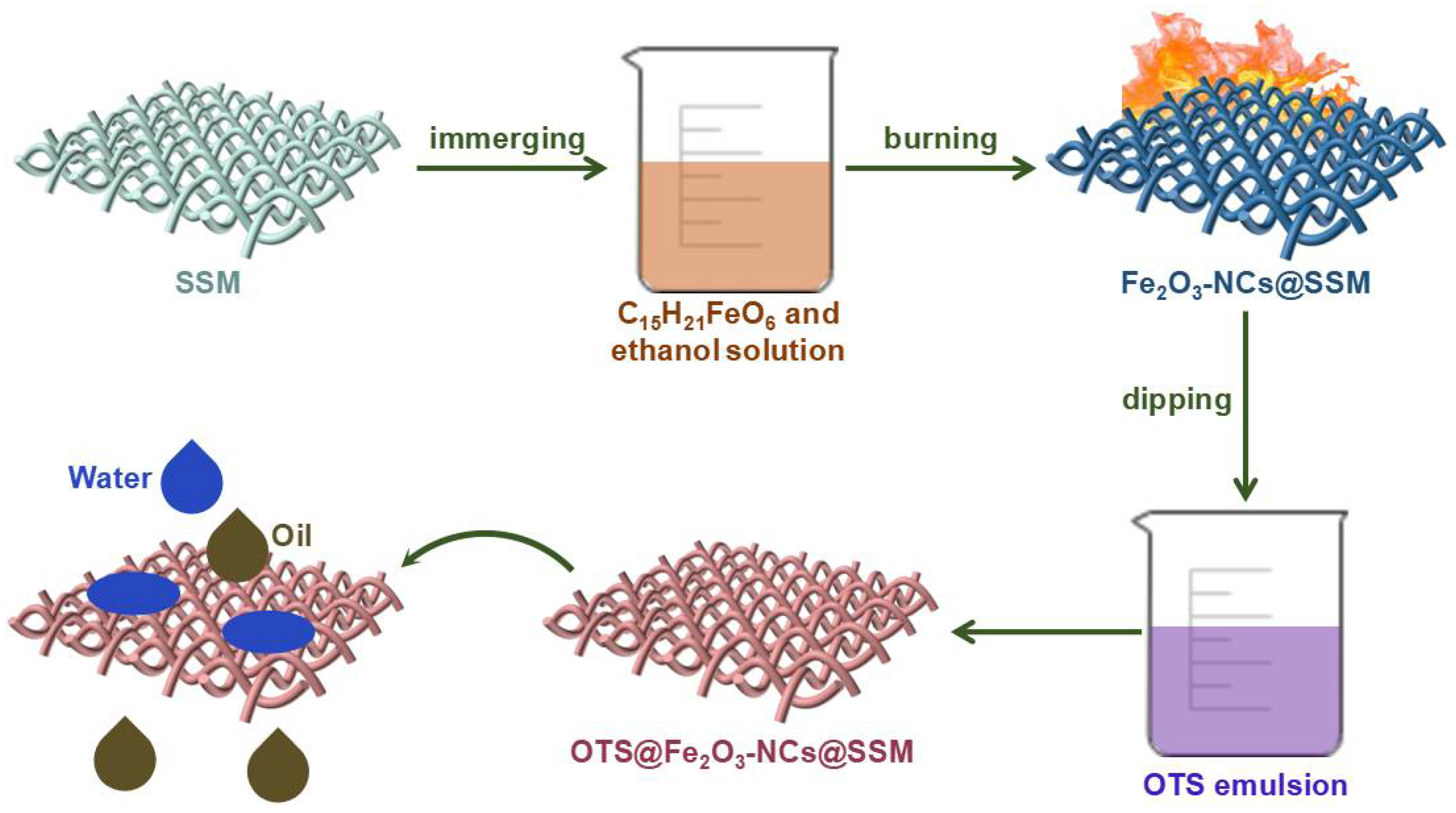
2.2. Preparation of OTS@Fe2O3-NCs@SSM
2.3. Physical Characterizations
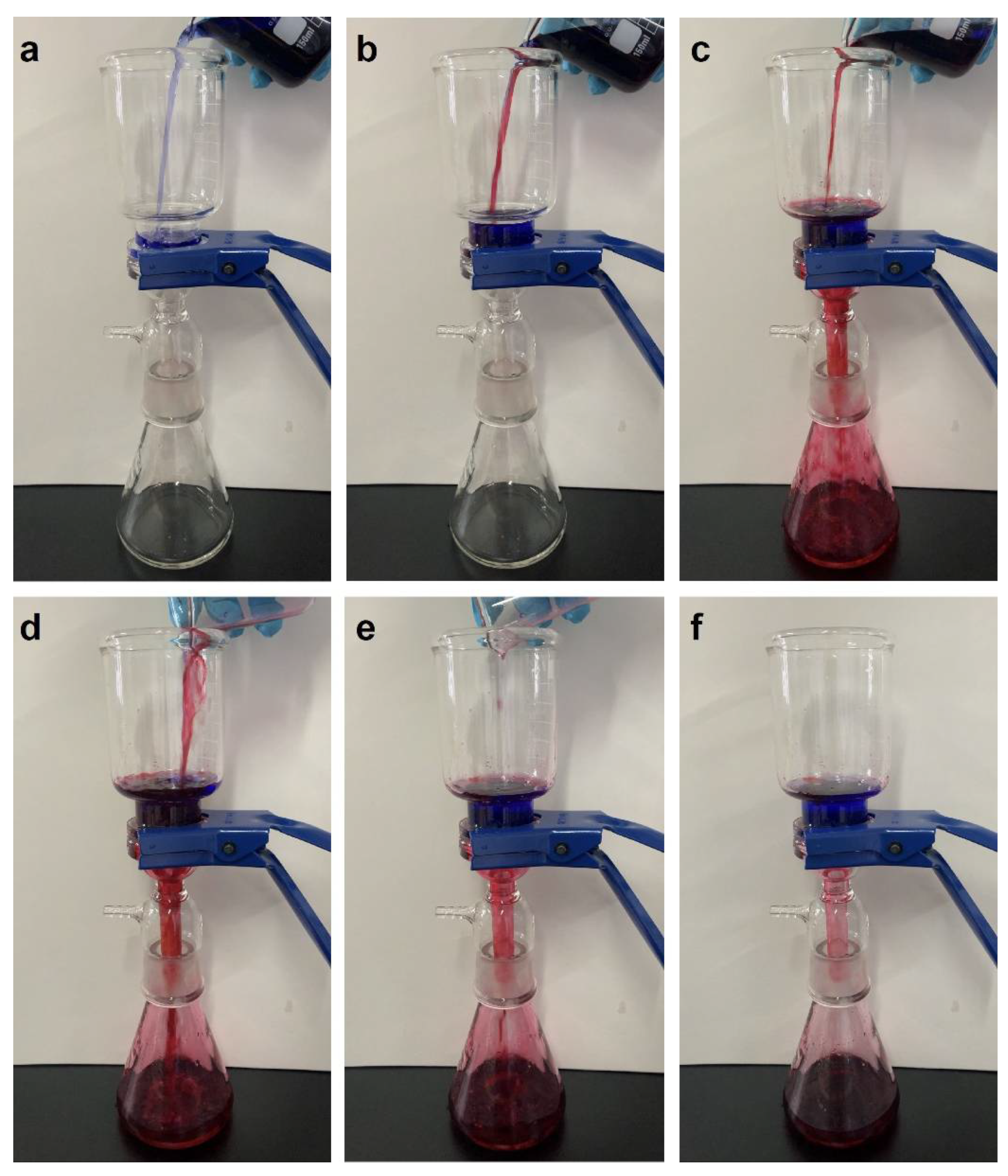
2.4. Oil–Water Separation and Recyclability Tests
3. Results and Discussion
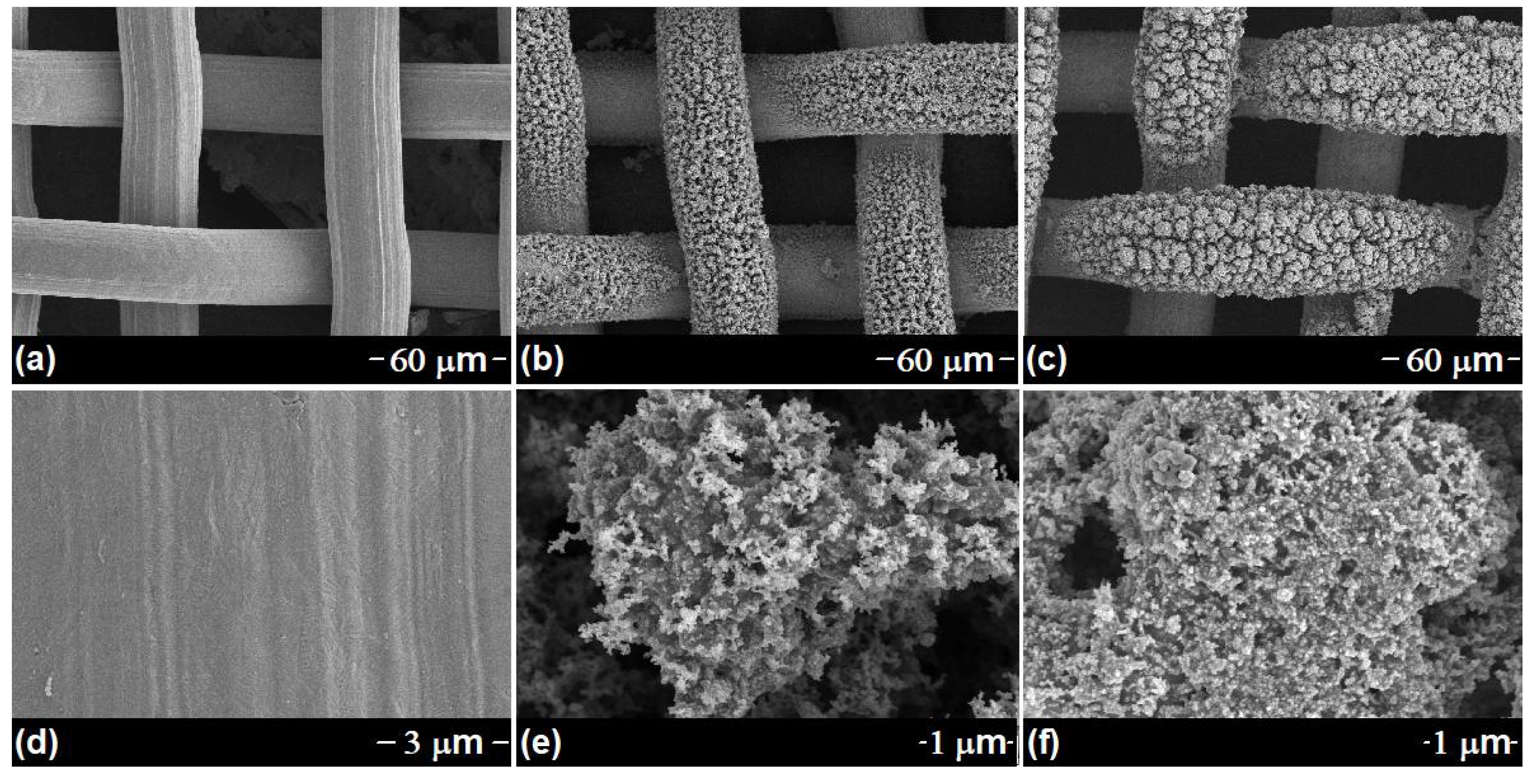
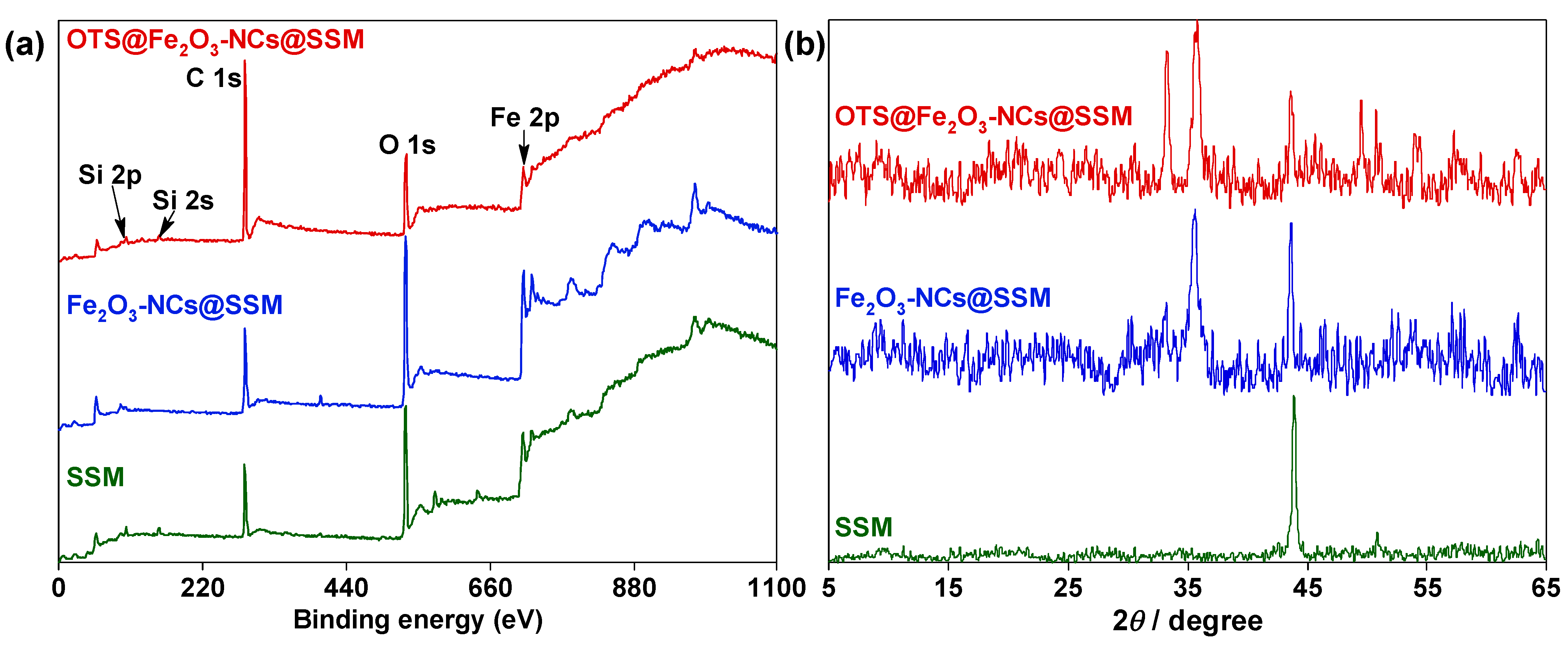
3.1. Characterization
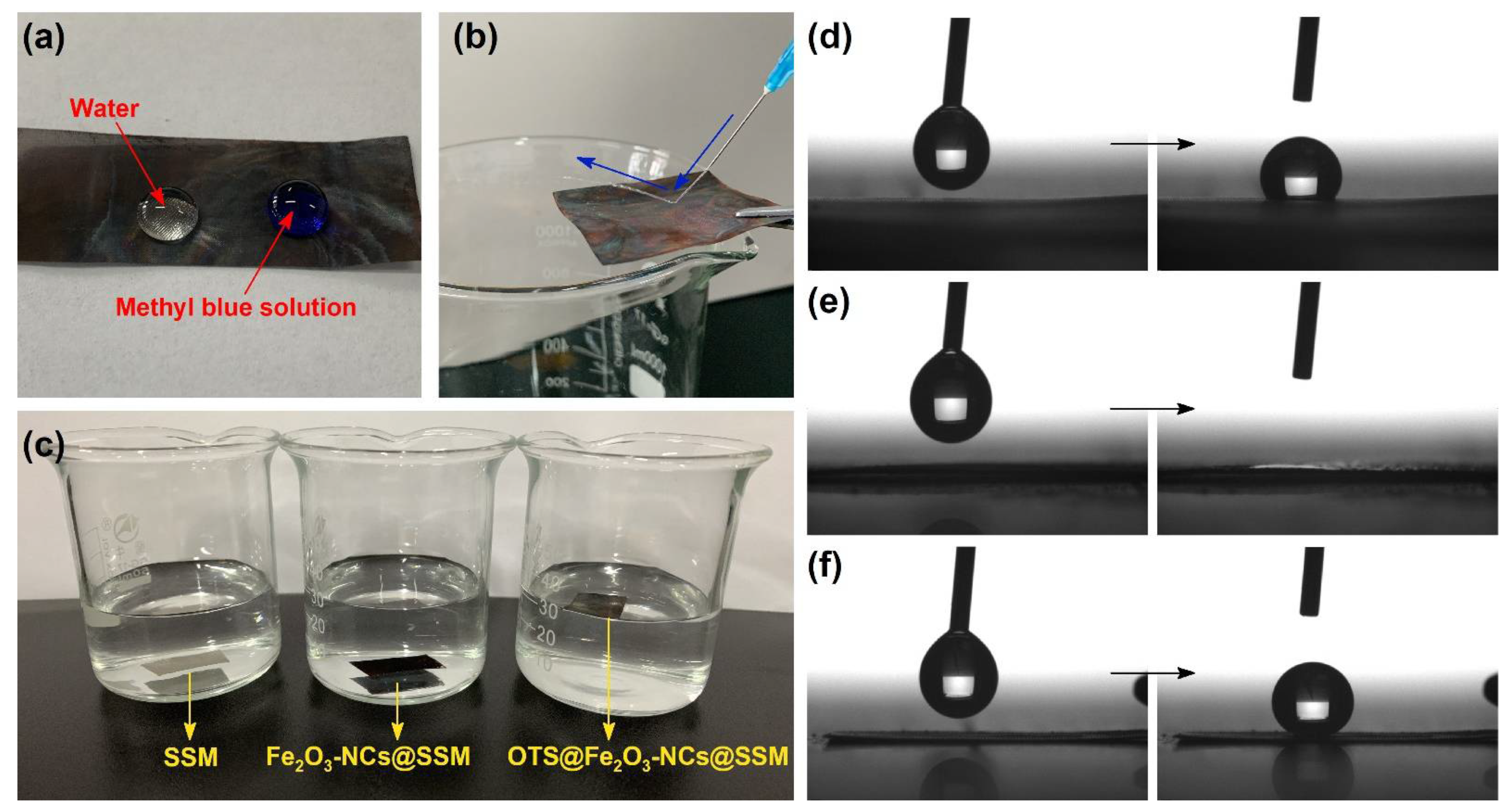
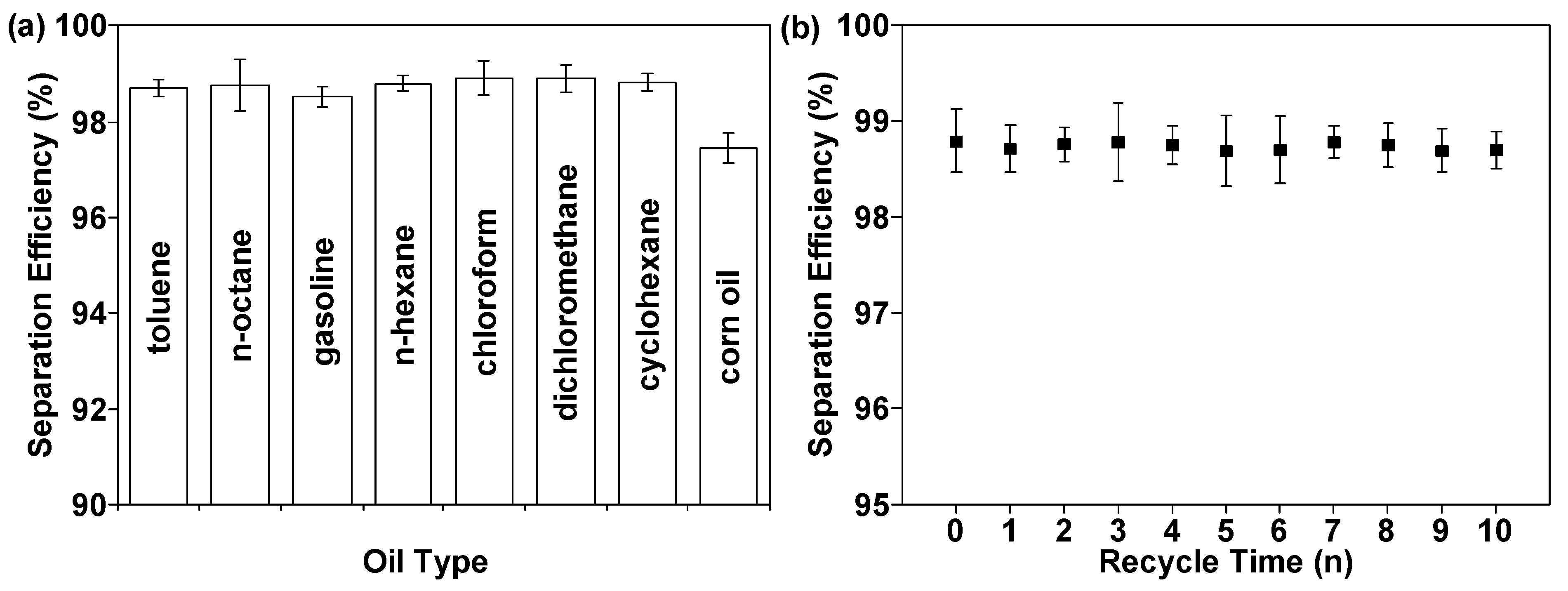
3.2. Superhydrophobic Performance
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sam, E.K.; Liu, J.; Lv, X. Surface engineering materials of superhydrophobic sponges for oil/water separation: A review. Ind. Eng. Chem. Res. 2021, 60, 2353–2364. [Google Scholar] [CrossRef]
- Luo, W.; Sun, D.; Chen, S.; Shanmugam, L.; Xiang, Y.; Yang, J. Robust microcapsules with durable superhydrophobicity and superoleophilicity for efficient oil-water separation. ACS Appl. Mater. Interfaces 2020, 12, 57547–57559. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Geng, B.; Chen, Y.; Wang, H. Review on the aerogel-type oil sorbents derived from nanocellulose. ACS Sustain. Chem. Eng. 2016, 5, 49–66. [Google Scholar] [CrossRef]
- Elhenawy, S.; Khraisheh, M.; AlMomani, F.; Hassan, M.K.; Al-Ghouti, M.A.; Selvaraj, R. Recent developments and advancements in graphene-based technologies for oil spill cleanup and oil-water separation processes. Nanomaterials 2021, 12, 87. [Google Scholar] [CrossRef]
- Yu, Z.L.; Li, G.C.; Fechler, N.; Yang, N.; Ma, Z.Y.; Wang, X.; Antonietti, M.; Yu, S.H. Polymerization under hypersaline conditions: A robust route to phenolic polymer-derived carbon aerogels. Angew. Chem. Int. Ed. 2016, 55, 14623–14627. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Tong, Z.; Ngai, T.; Wang, C. Nitrogen-rich and fire-resistant carbon aerogels for the removal of oil contaminants from water. ACS Appl. Mater. Interfaces 2014, 6, 6351–6360. [Google Scholar] [CrossRef]
- Bi, H.; Yin, Z.; Cao, X.; Xie, X.; Tan, C.; Huang, X.; Chen, B.; Chen, F.; Yang, Q.; Bu, X.; et al. Carbon fiber aerogel made from raw cotton: A novel, efficient and recyclable sorbent for oils and organic solvents. Adv. Mater. 2013, 25, 5916–5921. [Google Scholar] [CrossRef]
- Bi, H.; Huang, X.; Wu, X.; Cao, X.; Tan, C.; Yin, Z.; Lu, X.; Sun, L.; Zhang, H. Carbon microbelt aerogel prepared by waste paper: An efficient and recyclable sorbent for oils and organic solvents. Small 2014, 10, 3544–3550. [Google Scholar] [CrossRef]
- Zhang, M.; Xiao, C.; Zhang, C.; Qi, J.; Wang, C.; Sun, X.; Wang, L.; Xu, Q.; Li, J. Large-scale synthesis of biomass@MOF-derived porous carbon/cobalt nanofiber for environmental remediation by advanced oxidation processes. ACS EST Eng. 2020, 1, 249–260. [Google Scholar] [CrossRef]
- Wen, H.; Hsu, Y.I.; Uyama, H. Superhydrophobic PDMS-pCA@CNWF composite with UV-resistant and self-cleaning properties for oil/water separation. Materials 2022, 15, 376. [Google Scholar] [CrossRef]
- Tudu, B.K.; Sinhamahapatra, A.; Kumar, A. Surface modification of cotton fabric using TiO2 nanoparticles for self-cleaning, oil-water separation, antistain, anti-water absorption, and antibacterial properties. ACS Omega 2020, 5, 7850–7860. [Google Scholar] [CrossRef] [Green Version]
- Du, J.; Liu, L.; Hu, Z.; Yu, Y.; Zhang, Y.; Hou, S.; Chen, A. Raw-cotton-derived N-doped carbon fiber aerogel as an efficient electrode for electrochemical capacitors. ACS Sustain. Chem. Eng. 2018, 6, 4008–4015. [Google Scholar] [CrossRef]
- Zhang, G.D.; Wu, Z.H.; Xia, Q.Q.; Qu, Y.X.; Pan, H.T.; Hu, W.J.; Zhao, L.; Cao, K.; Chen, E.Y.; Yuan, Z.; et al. Ultrafast flame-induced pyrolysis of poly(dimethylsiloxane) foam materials toward exceptional superhydrophobic surfaces and reliable mechanical robustness. ACS Appl. Mater. Interfaces 2021, 13, 23161–23172. [Google Scholar] [CrossRef]
- Wu, F.; Pickett, K.; Panchal, A.; Liu, M.; Lvov, Y. Superhydrophobic polyurethane foam coated with polysiloxane-modified clay nanotubes for efficient and recyclable oil absorption. ACS Appl. Mater. Interfaces 2019, 11, 25445–25456. [Google Scholar] [CrossRef]
- Ju, G.; Zhou, L.; Jiao, C.; Shen, J.; Luan, Y.; Zhao, X. One-step fabrication of a functionally integrated device based on polydimethylsiloxane-coated SiO2 NPs for efficient and continuous oil absorption. Materials 2021, 14, 5998. [Google Scholar] [CrossRef]
- Wang, J.; Fan, Z.; Liu, Q.; Tong, Q.; Wang, B. Fabrication of PDMS@Fe3O4/MS composite materials and its application for oil-water separation. Materials 2021, 15, 115. [Google Scholar] [CrossRef]
- Panickar, R.; Sobhan, C.B.; Chakravorti, S. Highly efficient amorphous carbon sphere-based superhydrophobic and superoleophilic sponges for oil/water separation. Langmuir 2021, 37, 12501–12511. [Google Scholar] [CrossRef]
- Liu, D.; Wang, S.; Wu, T.; Li, Y. A robust superhydrophobic polyurethane sponge loaded with multi-walled carbon nanotubes for efficient and selective oil-water separation. Nanomaterials 2021, 11, 3344. [Google Scholar] [CrossRef]
- Han, S.; Song, Q.; Feng, X.; Wang, J.; Zhang, X.; Zhang, Y. Flame-retardant silanized boron nitride nanosheet-infused superhydrophobic sponges for oil/water separation. ACS Appl. Nano Mater. 2021, 4, 11809–11819. [Google Scholar] [CrossRef]
- Zhou, B.; Bashir, B.H.; Liu, Y.; Zhang, B. Facile construction and fabrication of a superhydrophobic copper mesh for ultraefficient oil/water separation. Ind. Eng. Chem. Res. 2021, 60, 8139–8146. [Google Scholar] [CrossRef]
- Liang, T.; Wang, B.; Fan, Z.; Liu, Q. Fabrication of superhydrophobic SA-CeO2@Cu mesh and its application in oil-water separation. ACS Omega 2021, 6, 25323–25328. [Google Scholar] [CrossRef]
- Kong, L.H.; Chen, X.H.; Yu, L.G.; Wu, Z.S.; Zhang, P.Y. Superhydrophobic cuprous oxide nanostructures on phosphor-copper meshes and their oil-water separation and oil spill cleanup. ACS Appl. Mater. Interfaces 2015, 7, 2616–2625. [Google Scholar] [CrossRef]
- Yin, X.; He, Y.; Li, H.; Ma, X.; Zhou, L.; He, T.; Li, S. One-step in-situ fabrication of carbon nanotube/stainless steel mesh membrane with excellent anti-fouling properties for effective gravity-driven filtration of oil-in-water emulsions. J. Colloid Interface Sci. 2021, 592, 87–94. [Google Scholar] [CrossRef]
- Wang, J.; Xu, J.; Chen, G.; Lian, Z.; Yu, H. Reversible wettability between underwater superoleophobicity and superhydrophobicity of stainless steel mesh for efficient oil-water separation. ACS Omega 2021, 6, 77–84. [Google Scholar] [CrossRef]
- Song, J.; Liu, N.; Li, J.; Cao, Y.; Cao, H. Facile fabrication of highly hydrophobic onion-like candle soot-coated mesh for durable oil/water separation. Nanomaterials 2022, 12, 761. [Google Scholar] [CrossRef]
- Avasthi, P.; Kumar, A.; Balakrishnan, V. Aligned CNT forests on stainless steel mesh for flexible supercapacitor electrode with high capacitance and power density. ACS Appl. Nano Mater. 2019, 2, 1484–1495. [Google Scholar] [CrossRef]
- Wang, Z.; Kong, W.; Si, L.; Niu, J.; Liu, Y.; Yin, L.; Tian, Z. Robust and thermally stable butterfly-like Co(OH)2/hexadecyltrimethoxysilane superhydrophobic mesh filters prepared by electrodeposition for highly efficient oil/water separation. Ind. Eng. Chem. Res. 2019, 58, 9576–9584. [Google Scholar] [CrossRef]
- Raturi, P.; Yadav, K.; Singh, J.P. ZnO-nanowires-coated smart surface mesh with reversible wettability for efficient on-demand oil/water separation. ACS Appl. Mater. Interfaces 2017, 9, 6007–6013. [Google Scholar] [CrossRef]
- Li, Y.; Liu, X.; Zhang, B. Durability and stability of superhydrophobic stainless steel mesh supported pure-silica zeolite beta coatings. Ind. Eng. Chem. Res. 2019, 58, 8044–8049. [Google Scholar] [CrossRef]
- Yue, X.; Fu, D.; Zhang, T.; Yang, D.; Qiu, F. Superhydrophobic stainless-steel mesh with excellent electrothermal properties for efficient separation of highly viscous water-in-crude oil emulsions. Ind. Eng. Chem. Res. 2020, 59, 17918–17926. [Google Scholar] [CrossRef]
- Feng, L.; Zhang, Z.; Mai, Z.; Ma, Y.; Liu, B.; Jiang, L.; Zhu, D. A super-hydrophobic and super-oleophilic coating mesh film for the separation of oil and water. Angew. Chem. Int. Ed. 2004, 43, 2012–2014. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Li, K.; Zhang, H.; Liu, J.; Wu, S.; Fan, Q.; Xue, H. Highly efficient and robust oil/water separation materials based on wire mesh coated by reduced graphene oxide. Langmuir 2017, 33, 9590–9597. [Google Scholar] [CrossRef] [PubMed]
- Fu, C.; Gu, L.; Zeng, Z.; Xue, Q. One-step transformation of metal meshes to robust superhydrophobic and superoleophilic meshes for highly efficient oil spill cleanup and oil/water separation. ACS Appl. Mater. Interfaces 2020, 12, 1850–1857. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Li, X.; Liu, J.; Zeng, M.J.; Feng, X.; Jia, X.; Yu, Z.Z. Coating of wood with Fe2O3-decorated carbon nanotubes by one-step combustion for efficient solar steam generation. ACS Appl. Mater. Interfaces 2021, 13, 22845–22854. [Google Scholar] [CrossRef]
- Bae, S.H.; Karthikeyan, K.; Lee, Y.S.; Oh, I.K. Microwave self-assembly of 3D graphene-carbon nanotube-nickel nanostructure for high capacity anode material in lithium ion battery. Carbon 2013, 64, 527–536. [Google Scholar] [CrossRef]
- Pham-Huu, C.; Vieira, R.; Louis, B.; Carvalho, A.; Amadou, J.; Dintzer, T.; Ledoux, M.J. About the octopus-like growth mechanism of carbon nanofibers over graphite supported nickel catalyst. J. Catal. 2006, 240, 194–202. [Google Scholar] [CrossRef]
- Zhao, M.; Cao, K.; Liu, M.; Zhang, J.; Chen, R.; Zhang, Q.; Xia, Z. Dual-shelled RbLi(Li3SiO4)2:Eu(2+) @Al2O3@ODTMS phosphor as a stable green emitter for high-power LED backlights. Angew. Chem. Int. Ed. 2020, 59, 12938–12943. [Google Scholar] [CrossRef]
- Zhang, L.; Zhou, A.G.; Sun, B.R.; Chen, K.S.; Yu, H.Z. Functional and versatile superhydrophobic coatings via stoichiometric silanization. Nat. Commun. 2021, 12, 982. [Google Scholar] [CrossRef]
- Han, S.; Yang, J.; Li, X.; Li, W.; Zhang, X.; Koratkar, N.; Yu, Z.Z. Flame synthesis of superhydrophilic carbon nanotubes/Ni foam decorated with Fe2O3 nanoparticles for water purification via solar steam generation. ACS Appl. Mater. Interfaces 2020, 12, 13229–13238. [Google Scholar] [CrossRef]
- Friis, J.E.; Brons, K.; Salmi, Z.; Shimizu, K.; Subbiahdoss, G.; Holm, A.H.; Santos, O.; Pedersen, S.U.; Meyer, R.L.; Daasbjerg, K.; et al. Hydrophilic polymer brush layers on stainless steel using multilayered ATRP initiator layer. ACS Appl. Mater. Interfaces 2016, 8, 30616–30627. [Google Scholar] [CrossRef]
- Qin, H.; Cao, H.; Zhao, Y.; Jin, G.; Cheng, M.; Wang, J.; Jiang, Y.; An, Z.; Zhang, X.; Liu, X. Antimicrobial and osteogenic properties of silver-ion-implanted stainless steel. ACS Appl. Mater. Interfaces 2015, 7, 10785–10794. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, K.T.; Yao, W.G.; Zhang, C.C.; Han, Z.W.; Ren, L.Q. A facile electrodeposition process for the fabrication of superhydrophobic and superoleophilic copper mesh for efficient oil–water separation. Ind. Eng. Chem. Res. 2016, 55, 2704–2712. [Google Scholar] [CrossRef]
- Cao, H.; Liu, Y. Facile design of a stable and inorganic underwater superoleophobic copper mesh modified by self-assembly sodium silicate and aluminum oxide for oil/water separation with high flux. J. Colloid Interface Sci. 2021, 598, 483–491. [Google Scholar] [CrossRef]
- Gunatilake, U.B.; Bandara, J. Fabrication of highly hydrophilic filter using natural and hydrothermally treated mica nanoparticles for efficient waste oil-water separation. J. Environ. Manag. 2017, 191, 96–104. [Google Scholar] [CrossRef] [Green Version]
- Gunatilake, U.B.; Bandara, J. Efficient removal of oil from oil contaminated water by superhydrophilic and underwater superoleophobic nano/micro structured TiO2 nanofibers coated mesh. Chemosphere 2017, 171, 134–141. [Google Scholar] [CrossRef] [Green Version]
- Ahlawat, S.; Singh, A.; Mukhopadhyay, P.K.; Singh, R.; Bindra, K.S. Nanosecond laser induced glass particle deposition over steel mesh for long-term superhydrophilicity and gravity driven oil water separation. Mater. Chem. Phys. 2021, 263, 124343. [Google Scholar] [CrossRef]


| Matrix | Modified Materials | Method | Separation Efficiency (%) | Ref. |
|---|---|---|---|---|
| wire mesh | Graphene oxide | O2 plasma and thermal annealing | >98 | [32] |
| Cu mesh | 1-dodecanethiol | Immersion and modification | >96 | [22] |
| Cu Mesh | Lauric acid | Electrodeposition and modification | >93 | [40] |
| Cu mesh | Na2SiO3 + Al2O3 | Self-assemble | >95 | [41] |
| Cu mesh | Stearic acid | Co-precipitation and modification | >99 | [21] |
| SSM | Phytic acid and vinyltriethoxysilane | Immersion | >90 | [33] |
| SSM | Natural flake mica | Hydrothermal synthesis and electrodeposition | >90 | [42] |
| SSM | — | Laser ablation | >96 | [24] |
| SSM | Hexadecyltrimethoxysilane | Pulse electrodeposition and modification | >99 | [27] |
| SSM | TiO2 nanofibers | Hydrothermal synthesis and spray deposition | >90 | [43] |
| SSM | Glass particles | Laser texturing | >96 | [44] |
| SSM | Fe2O3-NCs and OTS | Flame synthesis and modification | >97 | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, Y.; Ke, Z.; Shen, C.; Wei, Q.; Sun, R.; Yang, W.; Yin, Z. Facile Construction and Fabrication of a Superhydrophobic and Super Oleophilic Stainless Steel Mesh for Separation of Water and Oil. Nanomaterials 2022, 12, 1661. https://doi.org/10.3390/nano12101661
Sun Y, Ke Z, Shen C, Wei Q, Sun R, Yang W, Yin Z. Facile Construction and Fabrication of a Superhydrophobic and Super Oleophilic Stainless Steel Mesh for Separation of Water and Oil. Nanomaterials. 2022; 12(10):1661. https://doi.org/10.3390/nano12101661
Chicago/Turabian StyleSun, Yinyu, Zhongcheng Ke, Caiyun Shen, Qing Wei, Ruikang Sun, Wei Yang, and Zihan Yin. 2022. "Facile Construction and Fabrication of a Superhydrophobic and Super Oleophilic Stainless Steel Mesh for Separation of Water and Oil" Nanomaterials 12, no. 10: 1661. https://doi.org/10.3390/nano12101661
APA StyleSun, Y., Ke, Z., Shen, C., Wei, Q., Sun, R., Yang, W., & Yin, Z. (2022). Facile Construction and Fabrication of a Superhydrophobic and Super Oleophilic Stainless Steel Mesh for Separation of Water and Oil. Nanomaterials, 12(10), 1661. https://doi.org/10.3390/nano12101661