The Biological Effects of Polystyrene Nanoplastics on Human Peripheral Blood Lymphocytes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Polystyrene Nanoplastic
2.3. Isolation and In-Vitro Culture of Lymphocytes
2.4. Cell Viability Assay
2.5. Hemolysis Assay
2.6. Chromosomal Aberration (CA) Assay and InVitro Cytokinesis—Block Micronucleus (CBMN) Assay
2.7. Statistical Analysis
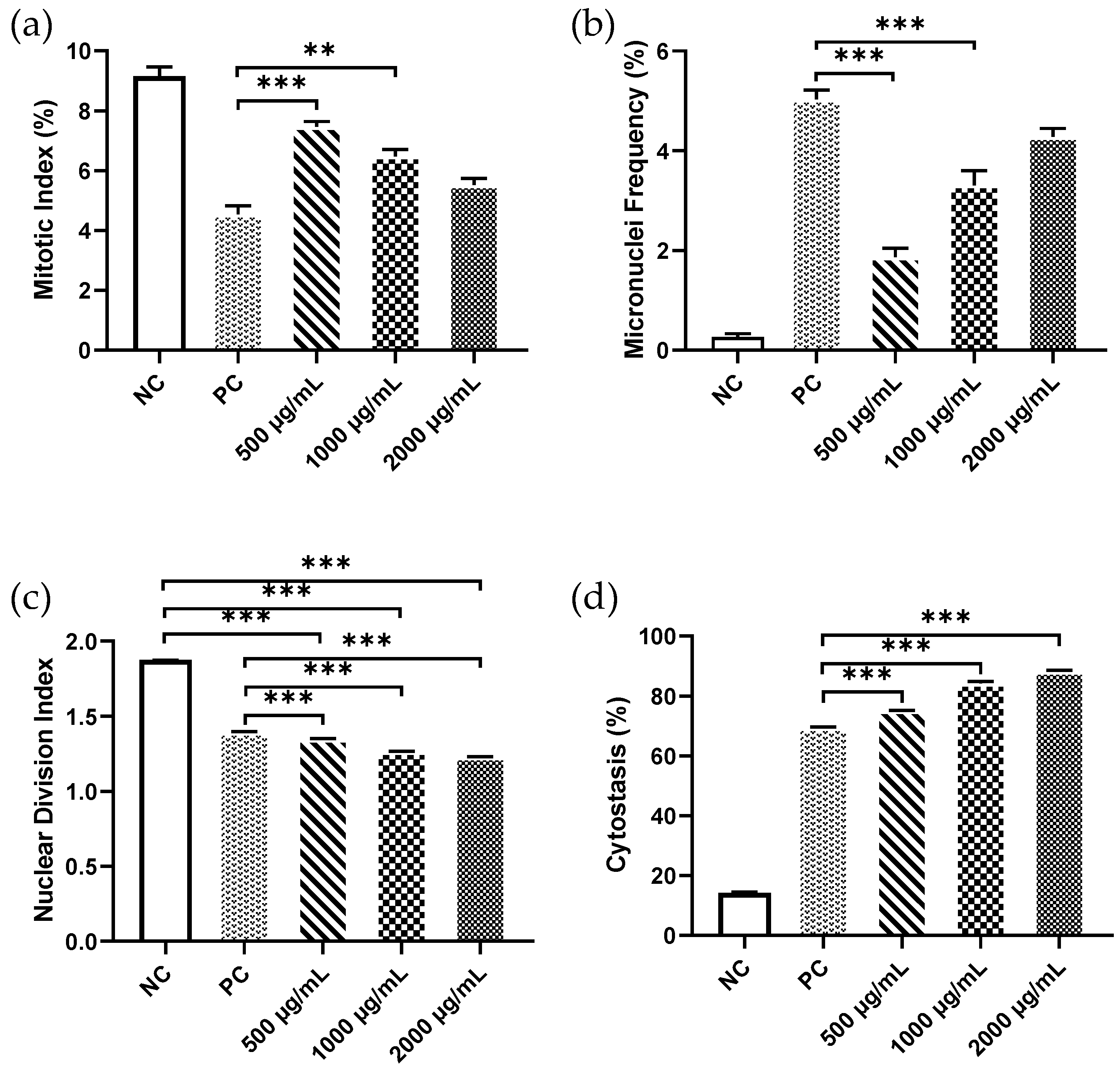
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Geyer, R.; Jambeck, J.R.; Law, K.L. Production, use, and fate of all plastics ever made. Sci. Adv. 2017, 3, e1700782. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Geyer, R. Production, use, and fate of synthetic polymers. In Plastic Waste and Recycling; Elsevier: Amsterdam, The Netherlands, 2020; Volume 1, pp. 13–32. ISBN 9780128178805. [Google Scholar]
- Rodrigues, M.O.; Abrantes, N.; Gonçalves, F.J.M.; Nogueira, H.; Marques, J.C.; Gonçalves, A.M.M. Impacts of plastic products used in daily life on the environment and human health: What is known? Environ. Toxicol. Pharmacol. 2019, 72, 103239. [Google Scholar] [CrossRef] [PubMed]
- Manzoor, J.; Sharma, M.; Sofi, I.R.; Dar, A.A. Plastic waste environmental and human health impacts. In Handbook of Research on Environmental and Human Health Impacts of Plastic Pollution; IGI Global: Hershey, PA, USA, 2020; pp. 29–37. ISBN 9781787859661. [Google Scholar]
- MacLeod, M.; Arp, H.P.H.; Tekman, M.B.; Jahnke, A. The global threat from plastic pollution. Science 2021, 373, 61–65. [Google Scholar] [CrossRef] [PubMed]
- Yee, M.S.; Hii, L.W.; Looi, C.K.; Lim, W.M.; Wong, S.F.; Kok, Y.Y.; Tan, B.K.; Wong, C.Y.; Leong, C.O. Impact of Microplastics and Nanoplastics on Human Health. Nanomaterials 2021, 11, 496. [Google Scholar] [CrossRef] [PubMed]
- Noventa, S.; Boyles, M.S.; Seifert, A.; Belluco, S.; Jiménez, A.S.; Johnston, H.J.; Tran, L.; Fernandes, T.F.; Mughini-Gras, L.; Orsini, M.J.M.; et al. Paradigms to assess the human health risks of nano-and microplastics. Microplastics Nanoplastics 2021, 1, 1–27. [Google Scholar] [CrossRef]
- Allen, S.; Allen, D.; Phoenix, V.R.; Le Roux, G.; Jiménez, P.D.; Simonneau, A.; Binet, S.; Galop, D. Atmospheric transport and deposition of microplastics in a remote mountain catchment. Nat. Geosci. 2019, 12, 339–344. [Google Scholar] [CrossRef]
- Kik, K.; Bukowska, B.; Sicińska, P. Polystyrene nanoparticles: Sources, occurrence in the environment, distribution in tissues, accumulation and toxicity to various organisms. Environ. Pollut. 2020, 262, 114297. [Google Scholar] [CrossRef]
- Rahman, A.; Sarkar, A.; Yadav, O.P.; Achari, G.; Slobodnik, J. Potential human health risks due to environmental exposure to microplastics and knowledge gaps: A scoping review. Sci. Total Environ. 2021, 757, 143872. [Google Scholar] [CrossRef] [PubMed]
- Facciola, A.; Visalli, G.; Pruiti Ciarello, M.; Di Pietro, A. Newly Emerging Airborne Pollutants: Current Knowledge of Health Impact of Micro and Nanoplastics. Int. J. Environ. Res. Public Health 2021, 18, 2997. [Google Scholar] [CrossRef]
- Dong, C.D.; Chen, C.W.; Chen, Y.C.; Chen, H.H.; Lee, J.S.; Lin, C.H. Polystyrene microplastic particles: In vitro pulmonary toxicity assessment. J. Hazard. Mater. 2020, 385, 121575. [Google Scholar] [CrossRef]
- Gopinath, P.M.; Saranya, V.; Vijayakumar, S.; Mythili Meera, M.; Ruprekha, S.; Kunal, R.; Pranay, A.; Thomas, J.; Mukherjee, A.; Chandrasekaran, N. Assessment on interactive prospectives of nanoplastics with plasma proteins and the toxicological impacts of virgin, coronated and environmentally released-nanoplastics. Sci Rep. 2019, 9, 8860. [Google Scholar] [CrossRef] [PubMed]
- Guimaraes, A.T.B.; Estrela, F.N.; Pereira, P.S.; de Andrade Vieira, J.E.; de Lima Rodrigues, A.S.; Silva, F.G.; Malafaia, G. Toxicity of polystyrene nanoplastics in Ctenopharyngodon idella juveniles: A genotoxic, mutagenic and cytotoxic perspective. Sci. Total Environ. 2021, 752, 141937. [Google Scholar] [CrossRef] [PubMed]
- Lin, P.; Lu, Z.; Zhang, Y.; Liao, X.; He, L.; Guo, Y.; Zhou, C.; Qian, Z.-J.; Hong, P.; Liang, Y.-Q.J.E.S.N. Do polystyrene nanoplastics aggravate the toxicity of single contaminants (okadaic acid)? Using AGS cells as a biological model. Environ. Sci. Nano 2021, 8, 3186–3201. [Google Scholar] [CrossRef]
- Vecchiotti, G.; Colafarina, S.; Aloisi, M.; Zarivi, O.; Di Carlo, P.; Poma, A. Genotoxicity and oxidative stress induction by polystyrene nanoparticles in the colorectal cancer cell line HCT116. PLoS ONE 2021, 16, e0255120. [Google Scholar] [CrossRef]
- Poma, A.; Vecchiotti, G.; Colafarina, S.; Zarivi, O.; Aloisi, M.; Arrizza, L.; Chichiricco, G.; Di Carlo, P. In Vitro Genotoxicity of Polystyrene Nanoparticles on the Human Fibroblast Hs27 Cell Line. Nanomaterials 2019, 9, 1299. [Google Scholar] [CrossRef] [Green Version]
- Ballesteros, S.; Domenech, J.; Barguilla, I.; Cortés, C.; Marcos, R.; Hernández, A.J.E.S.N. Genotoxic and immunomodulatory effects in human white blood cells after ex vivo exposure to polystyrene nanoplastics. Environ. Sci. Nano 2020, 7, 3431–3446. [Google Scholar] [CrossRef]
- Brunet, T.; King, N. The Origin of Animal Multicellularity and Cell Differentiation. Dev. Cell 2017, 43, 124–140. [Google Scholar] [CrossRef] [Green Version]
- Çobanoğlu, H.; Belivermiş, M.; Sıkdokur, E.; Kılıç, Ö.; Çayır, A.J.C. Genotoxic and cytotoxic effects of polyethylene microplastics on human peripheral blood lymphocytes. Chemosphere 2021, 272, 129805. [Google Scholar] [CrossRef]
- He, Y.; Li, J.; Chen, J.; Miao, X.; Li, G.; He, Q.; Xu, H.; Li, H.; Wei, Y. Cytotoxic effects of polystyrene nanoplastics with different surface functionalization on human HepG2 cells. Sci. Total. Environ. 2020, 723, 138180. [Google Scholar] [CrossRef]
- Shi, Q.; Tang, J.; Wang, L.; Liu, R.; Giesy, J.P. Combined cytotoxicity of polystyrene nanoplastics and phthalate esters on human lung epithelial A549 cells and its mechanism. Ecotoxicol. Environ. Saf. 2021, 213, 112041. [Google Scholar] [CrossRef]
- Jeong, C.B.; Won, E.J.; Kang, H.M.; Lee, M.C.; Hwang, D.S.; Hwang, U.K.; Zhou, B.; Souissi, S.; Lee, S.J.; Lee, J.S. Microplastic Size-Dependent Toxicity, Oxidative Stress Induction, and p-JNK and p-p38 Activation in the Monogonont Rotifer (Brachionus koreanus). Environ. Sci. Technol. 2016, 50, 8849–8857. [Google Scholar] [CrossRef]
- Hwang, J.; Choi, D.; Han, S.; Jung, S.Y.; Choi, J.; Hong, J. Potential toxicity of polystyrene microplastic particles. Sci. Rep. 2020, 10, 7391. [Google Scholar] [CrossRef] [PubMed]
- Yong, C.Q.Y.; Valiyaveetill, S.; Tang, B.L. Toxicity of Microplastics and Nanoplastics in Mammalian Systems. Int. J. Environ. Res. Public Health 2020, 17, 1509. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stock, V.; Bohmert, L.; Lisicki, E.; Block, R.; Cara-Carmona, J.; Pack, L.K.; Selb, R.; Lichtenstein, D.; Voss, L.; Henderson, C.J.; et al. Uptake and effects of orally ingested polystyrene microplastic particles in vitro and in vivo. Arch. Toxicol. 2019, 93, 1817–1833. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Huang, R.; Wang, X.; Tang, C.; Hu, C.; Wang, F. Cytotoxicity and Genotoxicity of Polystyrene Microplastics with Different Size and Surface Modification in A549 Human Lung Cells. Available online: https://papers.ssrn.com/sol3/papers.cfm?abstract_id=3935593 (accessed on 10 February 2022).
- OECD. Guidance Document on Revisions to OECD Genetic Toxicology Test Guidelines; OECD Workgroup of National Coordinators for Test 42 Guidelines (WNT); OECD: Paris, France, 2015. [Google Scholar]
- Panda, S.K.; Ravindran, B. Isolation of human PBMCs. Bio-Protocol 2013, 3, e323. [Google Scholar] [CrossRef]
- Lefort, C.T.; Kim, M. Human T lymphocyte isolation, culture and analysis of migration in vitro. J. Vis. Exp. JoVE 2010, 40, e2017. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Strober, W. Trypan Blue Exclusion Test of Cell Viability. Curr. Protoc. Immunol. 2015, 111, A3 B 1–A3 B 3. [Google Scholar] [CrossRef]
- Evans, B.C.; Nelson, C.E.; Yu, S.S.; Beavers, K.R.; Kim, A.J.; Li, H.; Nelson, H.M.; Giorgio, T.D.; Duvall, C.L. Ex vivo red blood cell hemolysis assay for the evaluation of pH-responsive endosomolytic agents for cytosolic delivery of biomacromolecular drugs. J. Vis. Exp. JoVE 2013, 73, e50166. [Google Scholar] [CrossRef] [Green Version]
- Fenech, M. Cytokinesis-block micronucleus cytome assay. Nat. Protoc. 2007, 2, 1084–1104. [Google Scholar] [CrossRef] [Green Version]
- Samarth, R.M.; Khan, T.; Srivas, S.; Mishra, P.K.; Tiwari, R.R. Evaluation of cyclophosphamide-induced genotoxicity and cytotoxicity in cultured human lymphocytes. J. Radiat. Cancer Res. 2018, 9, 28. [Google Scholar] [CrossRef]
- Lorge, E.; Hayashi, M.; Albertini, S.; Kirkland, D. Comparison of different methods for an accurate assessment of cytotoxicity in the in vitro micronucleus test. I. Theoretical aspects. Mutat. Res. 2008, 655, 1–3. [Google Scholar] [CrossRef] [PubMed]
- McCarroll, N.; Keshava, N.; Cimino, M.; Chu, M.; Dearfield, K.; Keshava, C.; Kligerman, A.; Owen, R.; Protzel, A.; Putzrath, R.; et al. An evaluation of the mode of action framework for mutagenic carcinogens case study: Cyclophosphamide. Environ. Mol. Mutagenesis 2008, 49, 117–131. [Google Scholar] [CrossRef] [PubMed]
- Barshtein, G.; Livshits, L.; Shvartsman, L.D.; Shlomai, N.O.; Yedgar, S.; Arbell, D. Polystyrene Nanoparticles Activate Erythrocyte Aggregation and Adhesion to Endothelial Cells. Cell Biochem. Biophys. 2016, 74, 19–27. [Google Scholar] [CrossRef]
- Xu, M.; Halimu, G.; Zhang, Q.; Song, Y.; Fu, X.; Li, Y.; Li, Y.; Zhang, H. Internalization and toxicity: A preliminary study of effects of nanoplastic particles on human lung epithelial cell. Sci. Total. Environ. 2019, 694, 133794. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Wu, M.; Tian, D.; Qiu, L.; Li, T. Effects of polystyrene microbeads on cytotoxicity and transcriptomic profiles in human Caco-2 cells. Environ. Toxicol. 2020, 35, 495–506. [Google Scholar] [CrossRef]
- Jiang, B.; Kauffman, A.E.; Li, L.; McFee, W.; Cai, B.; Weinstein, J.; Lead, J.R.; Chatterjee, S.; Scott, G.I.; Xiao, S. Health impacts of environmental contamination of micro- and nanoplastics: A review. Environ. Health Prev. Med. 2020, 25, 29. [Google Scholar] [CrossRef] [PubMed]
- Llorca, M.; Farré, M. Current Insights into Potential Effects of Micro-Nanoplastics on Human Health by in-vitro Tests. Front. Toxicol. 2021, 3, 752140. [Google Scholar] [CrossRef]
- Hernandez, L.M.; Xu, E.G.; Larsson, H.C.; Tahara, R.; Maisuria, V.B.; Tufenkji, N. Plastic teabags release billions of microparticles and nanoparticles into tea. Environ. Sci. Technol. 2019, 53, 12300–12310. [Google Scholar] [CrossRef]
- Hernandez, L.M.; Yousefi, N.; Tufenkji, N. Are there nanoplastics in your personal care products? Environ. Sci. Technol. Lett. 2017, 4, 280–285. [Google Scholar] [CrossRef] [Green Version]
- Molenaar, R.; Chatterjee, S.; Kamphuis, B.; Segers-Nolten, I.M.; Claessens, M.M.; Blum, C. Nanoplastic sizes and numbers: Quantification by single particle tracking. Environ. Sci. Nano 2021, 8, 723–730. [Google Scholar] [CrossRef]
- Li, D.; Shi, Y.; Yang, L.; Xiao, L.; Kehoe, D.K.; Gun’ko, Y.K.; Boland, J.J.; Wang, J.J. Microplastic release from the degradation of polypropylene feeding bottles during infant formula preparation. Nat. Food 2020, 1, 746–754. [Google Scholar] [CrossRef]
- Domenech, J.; de Britto, M.; Velazquez, A.; Pastor, S.; Hernandez, A.; Marcos, R.; Cortes, C. Long-Term Effects of Polystyrene Nanoplastics in Human Intestinal Caco-2 Cells. Biomolecules 2021, 11, 1442. [Google Scholar] [CrossRef] [PubMed]

| Concentrations (μg/mL) | NPB (%) | NBUD (%) | Chromosomal Breaks (%) | Dicentric Chromosomes (%) | Fragments (%) | Rings (%) |
|---|---|---|---|---|---|---|
| NC | - | - | 2.12 ± 1.02 | 0.00 ± 0.00 | 2.16 ± 0.12 | 0.00 ± 0.00 |
| 500 | 2.4 ± 0.19 | 2.6 ± 0.18 | 3.88 ± 1.20 | 1.54 ± 0.46 | 3.46 ± 0.84 | 0.62 ± 0.46 |
| 1000 | 2.0 ± 0.27 | 3.4 ± 0.19 | 5.24 ± 1.34 | 2.64 ± 0.62 | 4.82 ± 1.96 | 1.28 ± 0.86 |
| 2000 | 3.6 ± 0.25 | 5.6 ± 0.29 | 6.48 ± 1.22 | 2.88 ± 0.68 | 6.46 ± 1.86 | 1.44 ± 0.88 |
| PC | 5.0 ± 0.22 | 7.2 ± 0.43 | 7.10 ± 1.42 | 3.42 ± 0.80 | 9.24 ± 2.12 | 1.62 ± 0.86 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sarma, D.K.; Dubey, R.; Samarth, R.M.; Shubham, S.; Chowdhury, P.; Kumawat, M.; Verma, V.; Tiwari, R.R.; Kumar, M. The Biological Effects of Polystyrene Nanoplastics on Human Peripheral Blood Lymphocytes. Nanomaterials 2022, 12, 1632. https://doi.org/10.3390/nano12101632
Sarma DK, Dubey R, Samarth RM, Shubham S, Chowdhury P, Kumawat M, Verma V, Tiwari RR, Kumar M. The Biological Effects of Polystyrene Nanoplastics on Human Peripheral Blood Lymphocytes. Nanomaterials. 2022; 12(10):1632. https://doi.org/10.3390/nano12101632
Chicago/Turabian StyleSarma, Devojit Kumar, Ruchi Dubey, Ravindra M. Samarth, Swasti Shubham, Pritom Chowdhury, Manoj Kumawat, Vinod Verma, Rajnarayan R. Tiwari, and Manoj Kumar. 2022. "The Biological Effects of Polystyrene Nanoplastics on Human Peripheral Blood Lymphocytes" Nanomaterials 12, no. 10: 1632. https://doi.org/10.3390/nano12101632
APA StyleSarma, D. K., Dubey, R., Samarth, R. M., Shubham, S., Chowdhury, P., Kumawat, M., Verma, V., Tiwari, R. R., & Kumar, M. (2022). The Biological Effects of Polystyrene Nanoplastics on Human Peripheral Blood Lymphocytes. Nanomaterials, 12(10), 1632. https://doi.org/10.3390/nano12101632







