Luminescence Reduced Graphene Oxide Based Photothermal Purification of Seawater for Drinkable Purpose
Abstract
:1. Introduction
2. Experiment Section
2.1. Materials
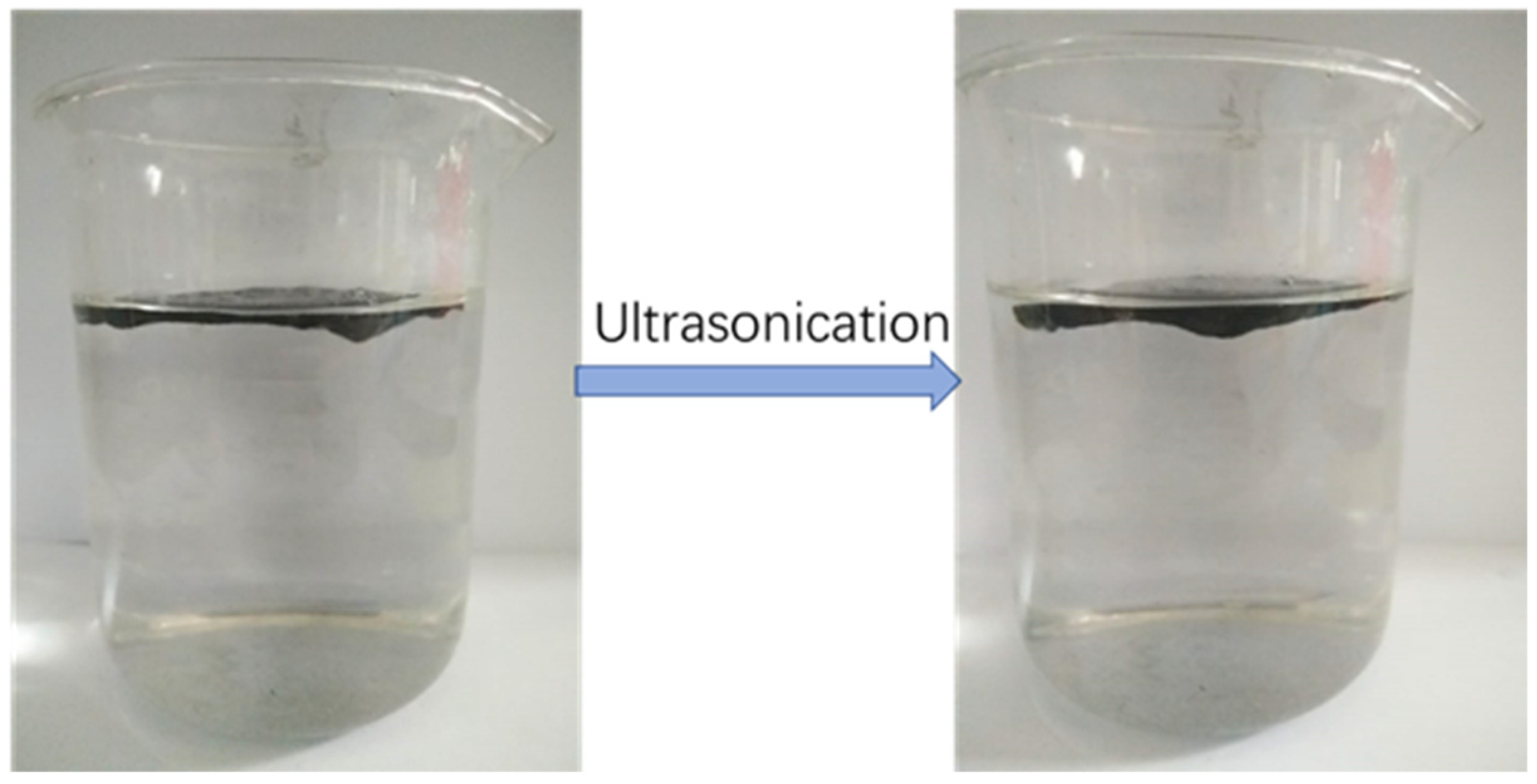
2.2. Preparation of Graphene Oxide (Go)
2.3. Preparation of rGO-Based Nonwoven Fabric Membrane (RGM)
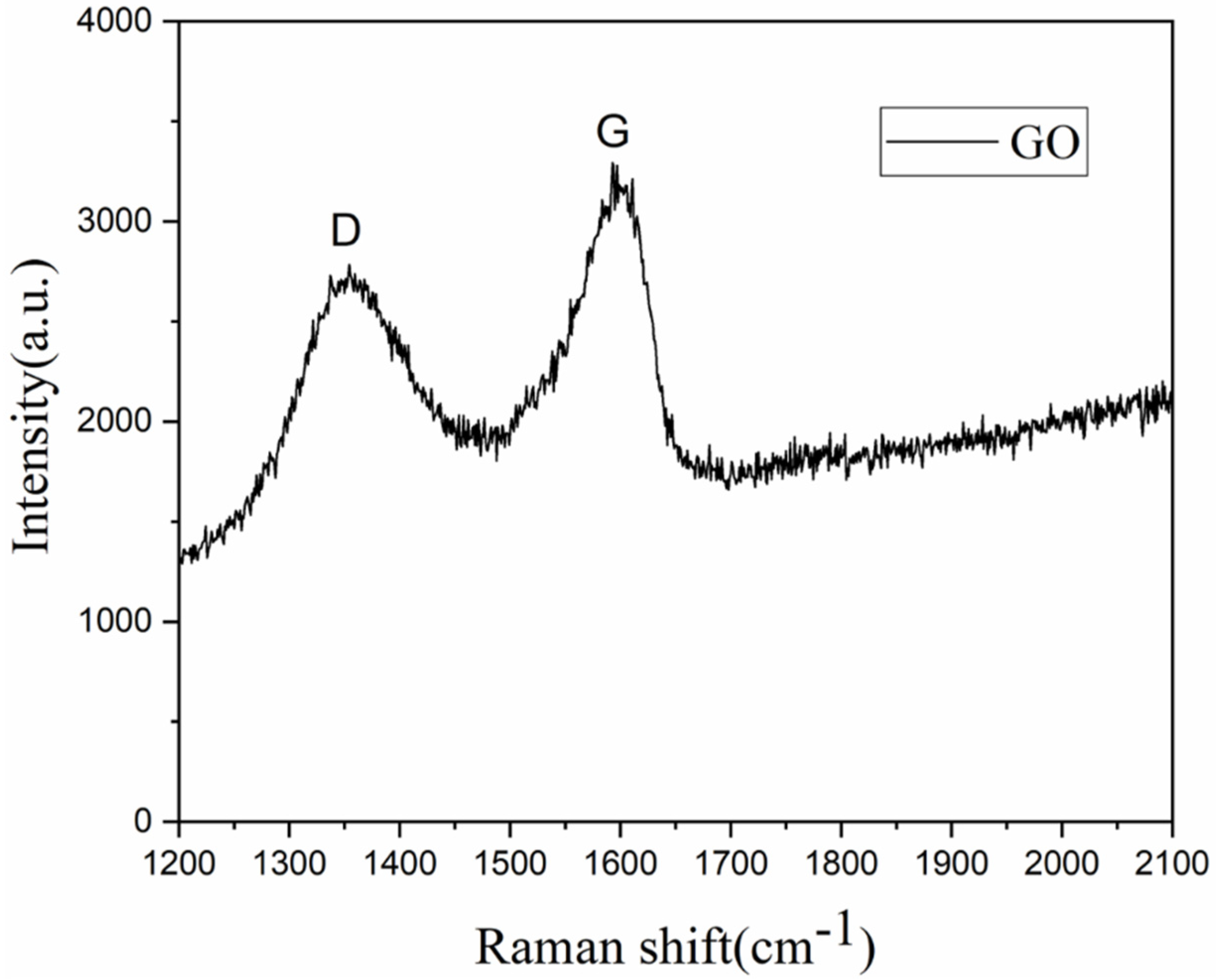
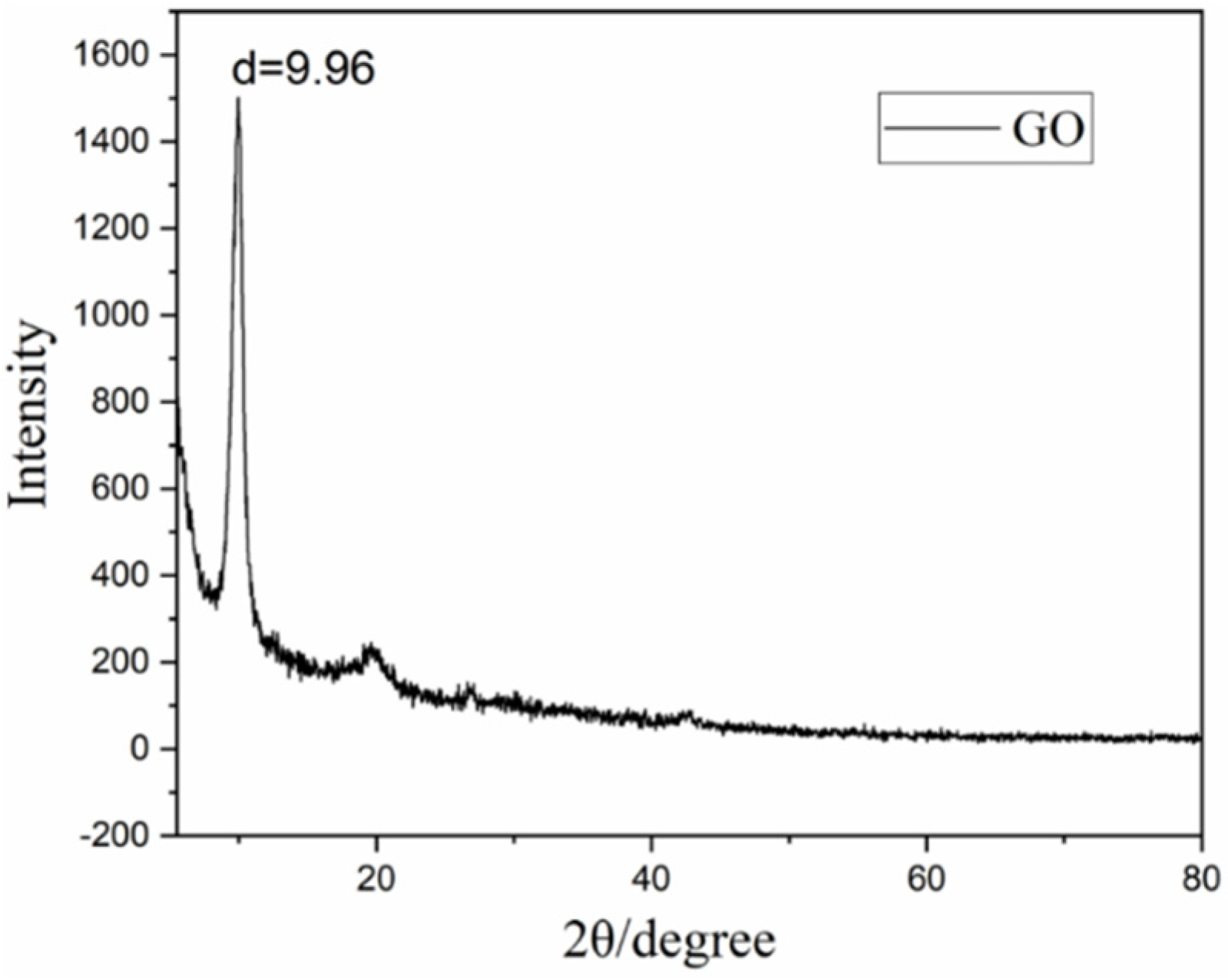
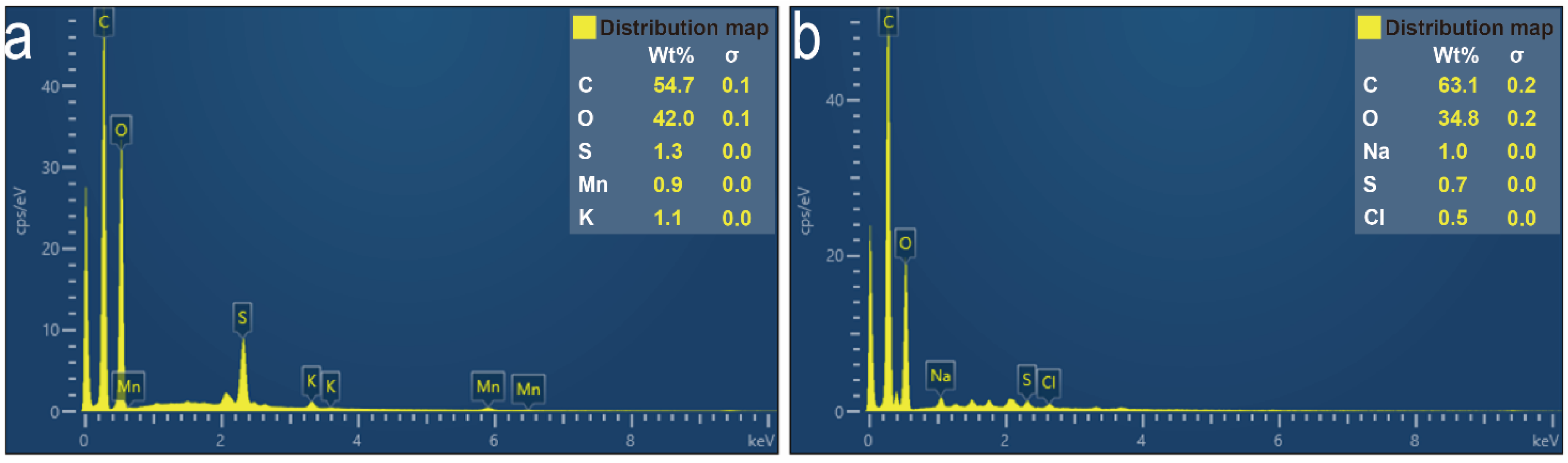
2.4. Characterization
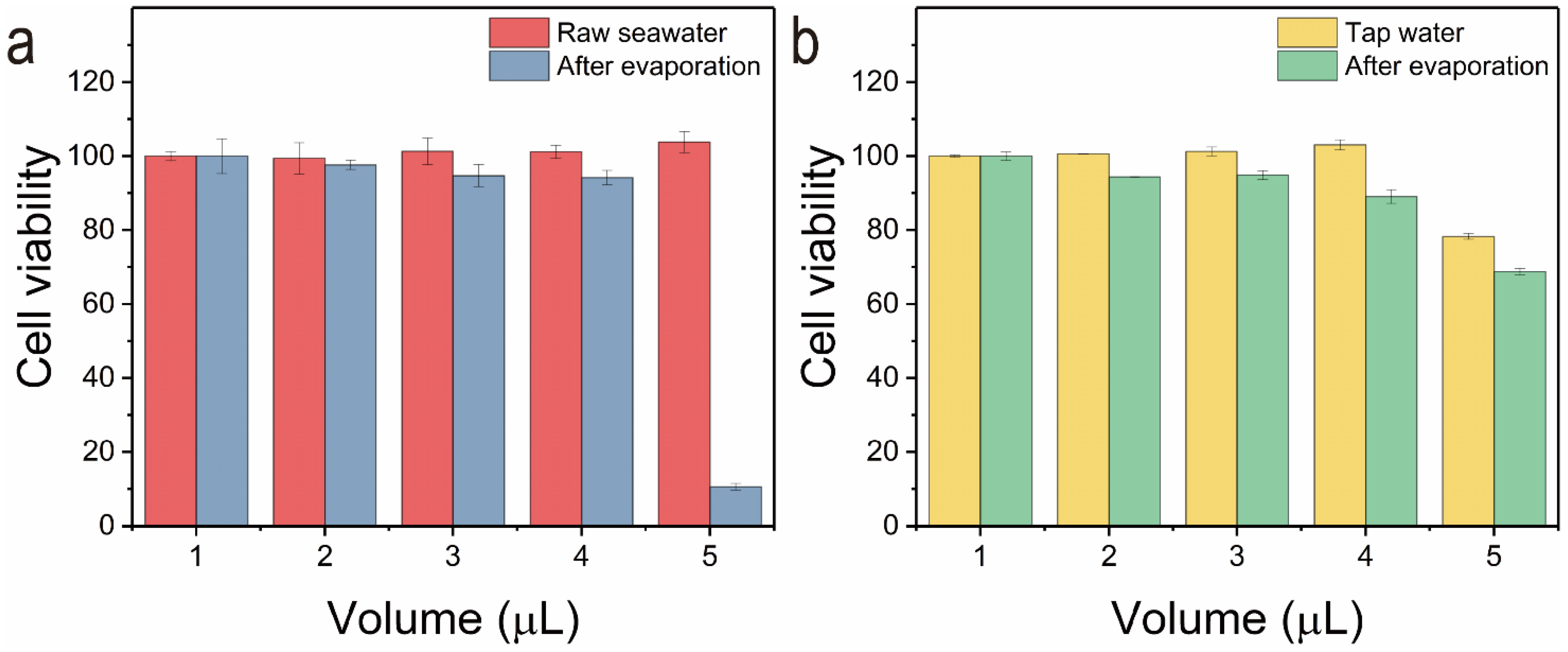
2.5. Cell Toxicity Studies by MTT (3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium Bromide) Assay
3. Results and Discussion
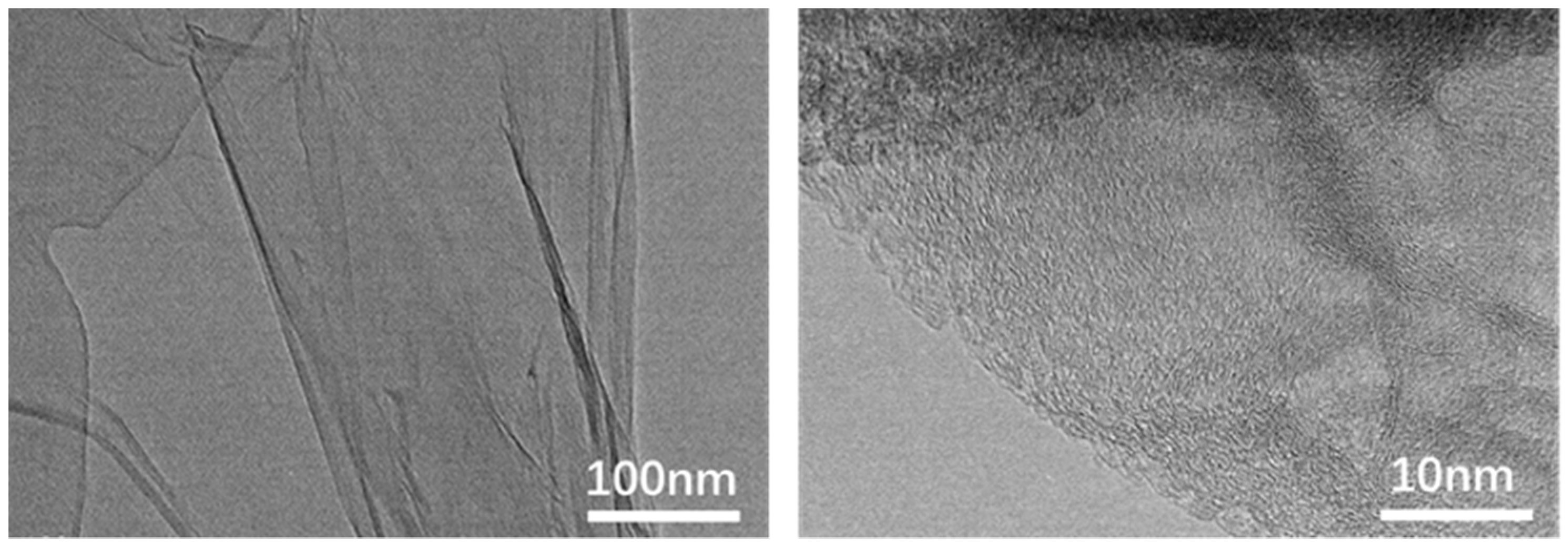
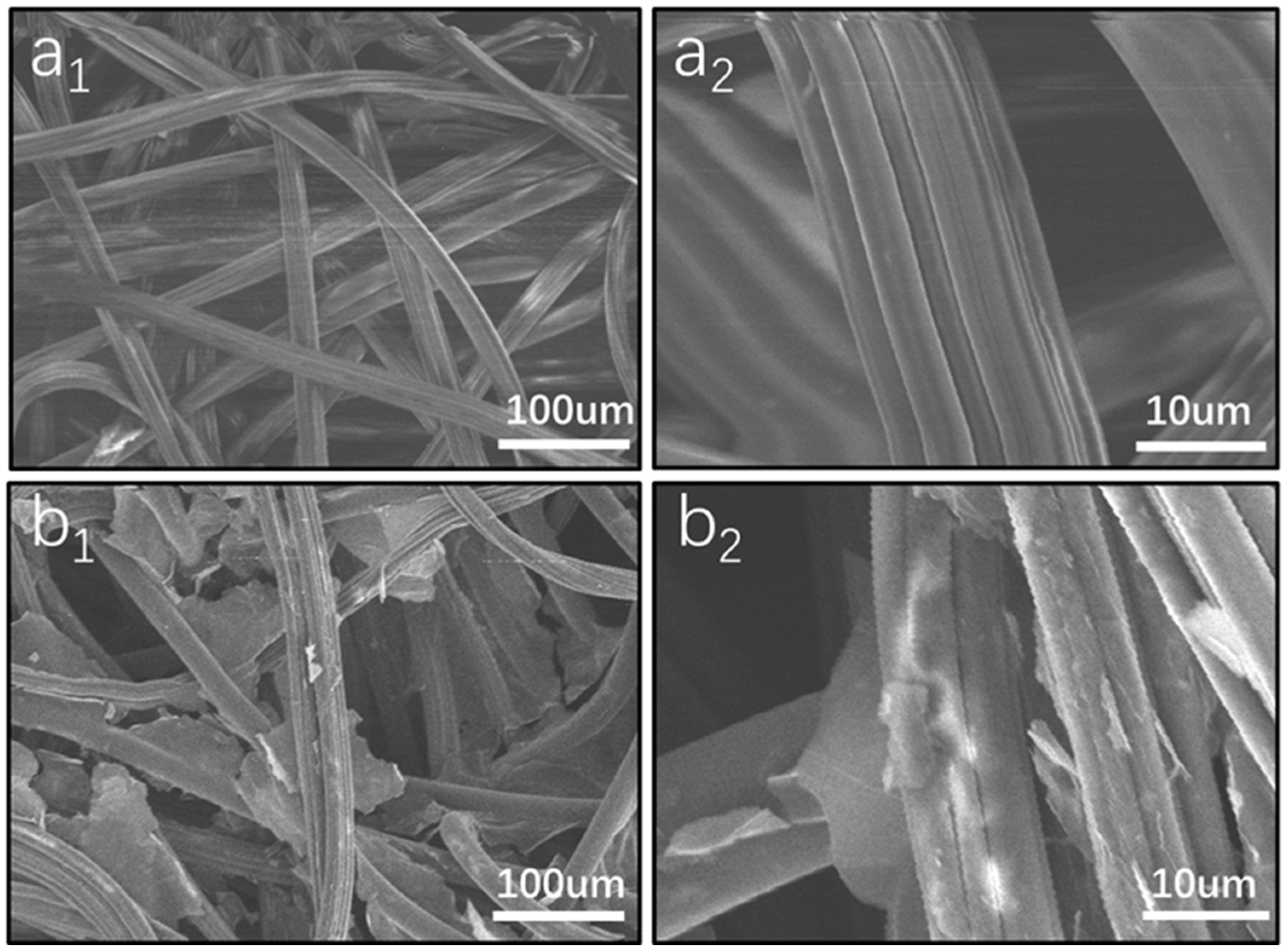
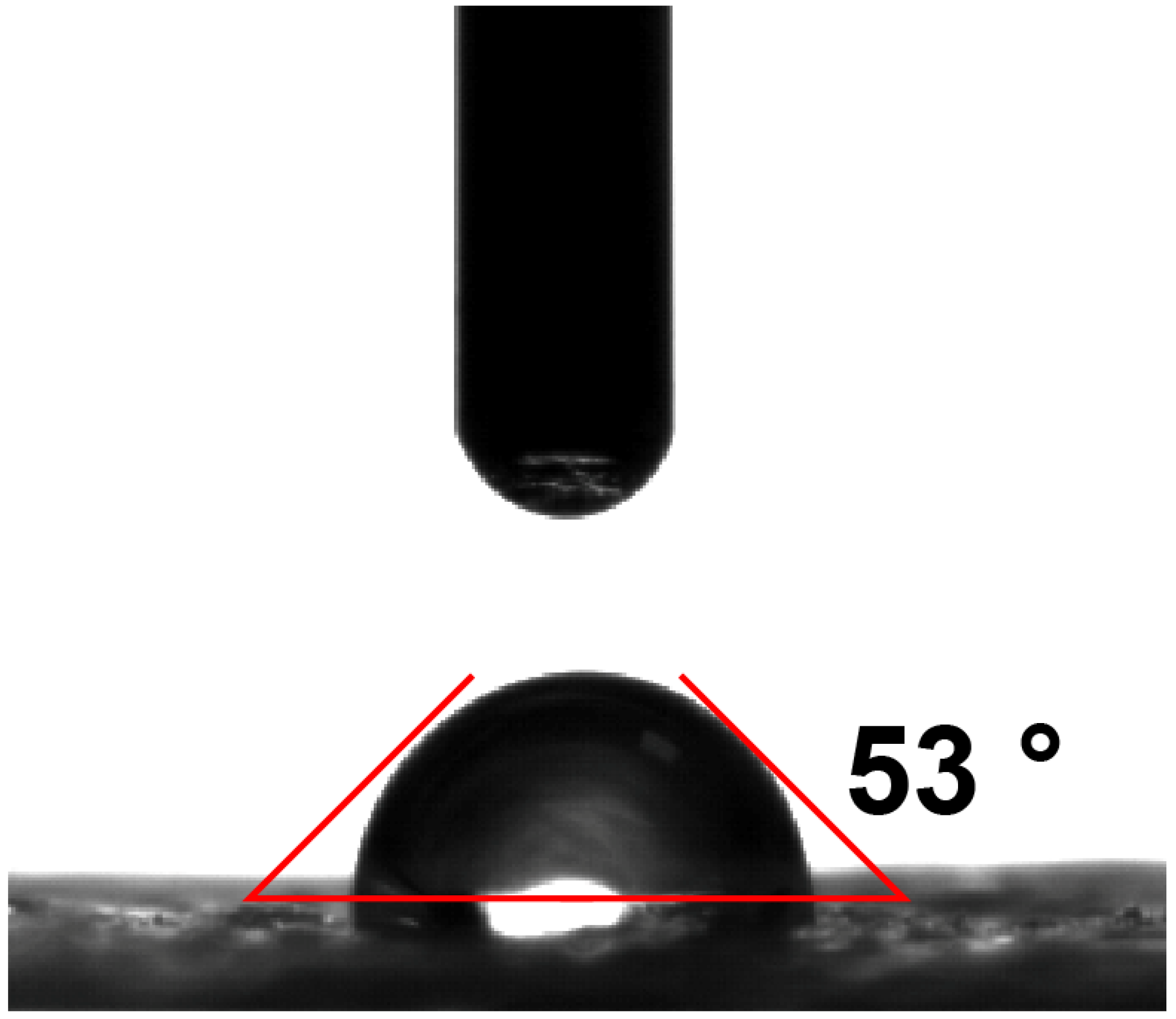
3.1. Preparation of the Graphene Oxide-Loaded Hydrophilic Nonwoven
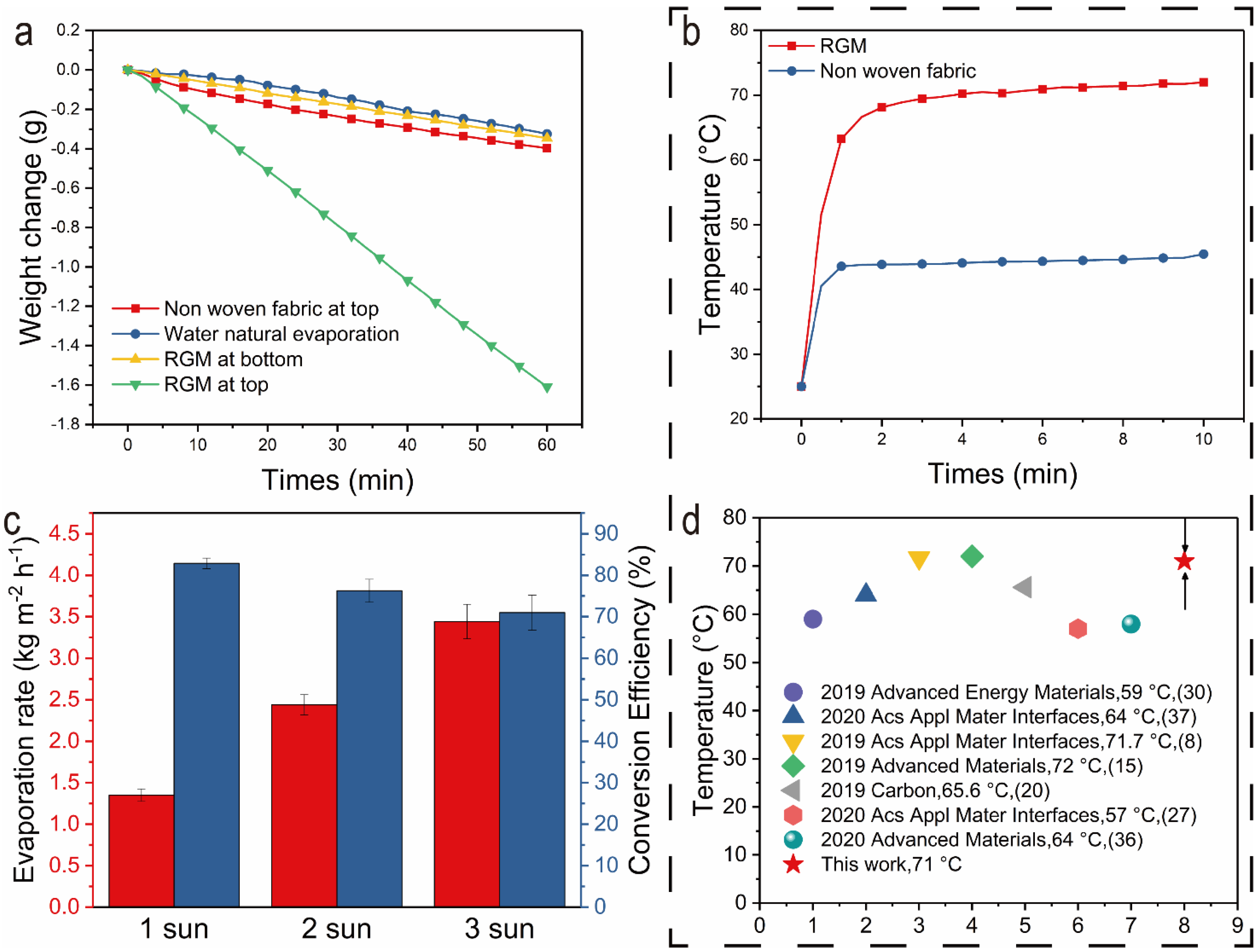
3.2. Light-Induced Evaporation Enhancement via the rGO-Loaded Hydrophilic Nonwoven
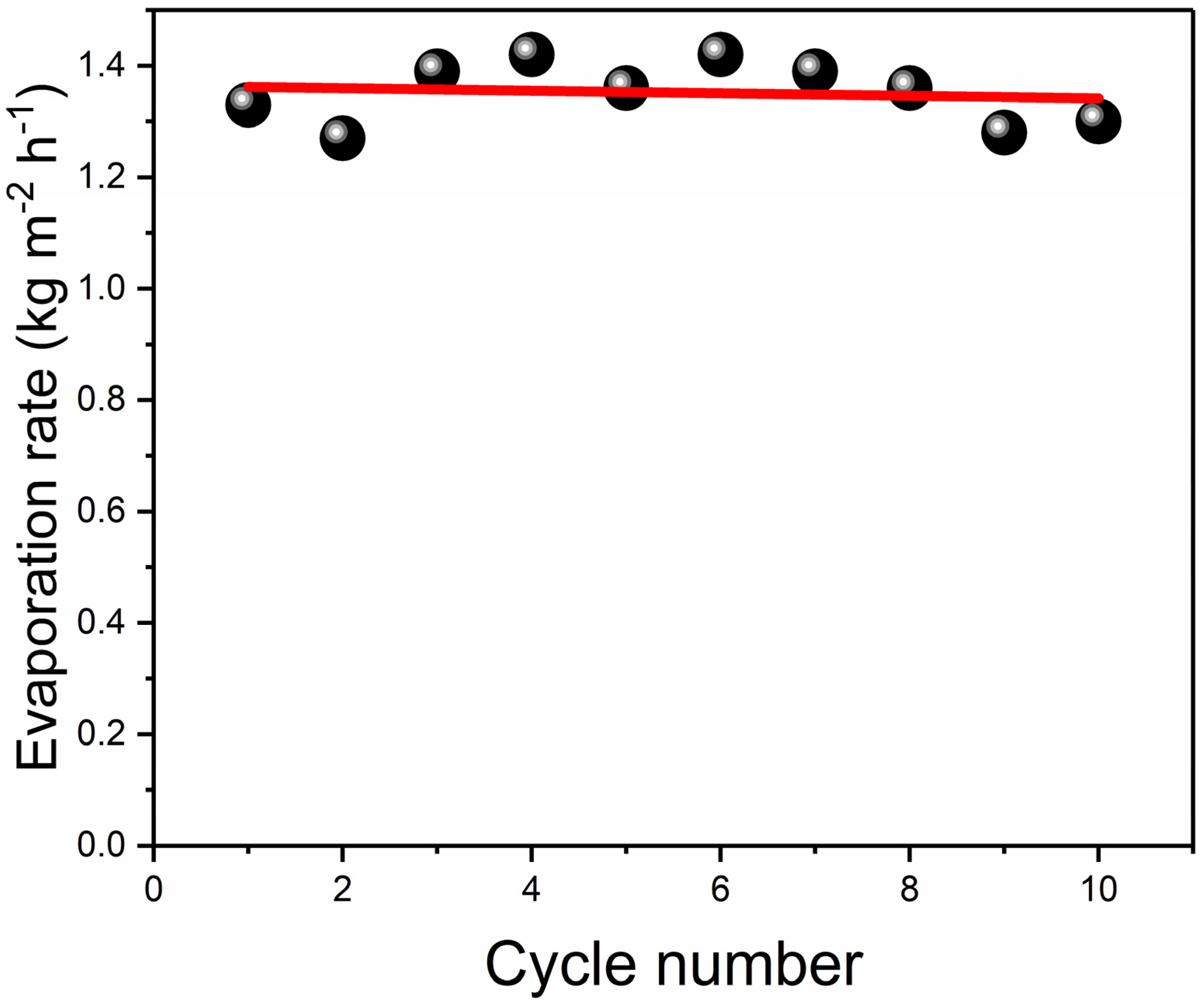
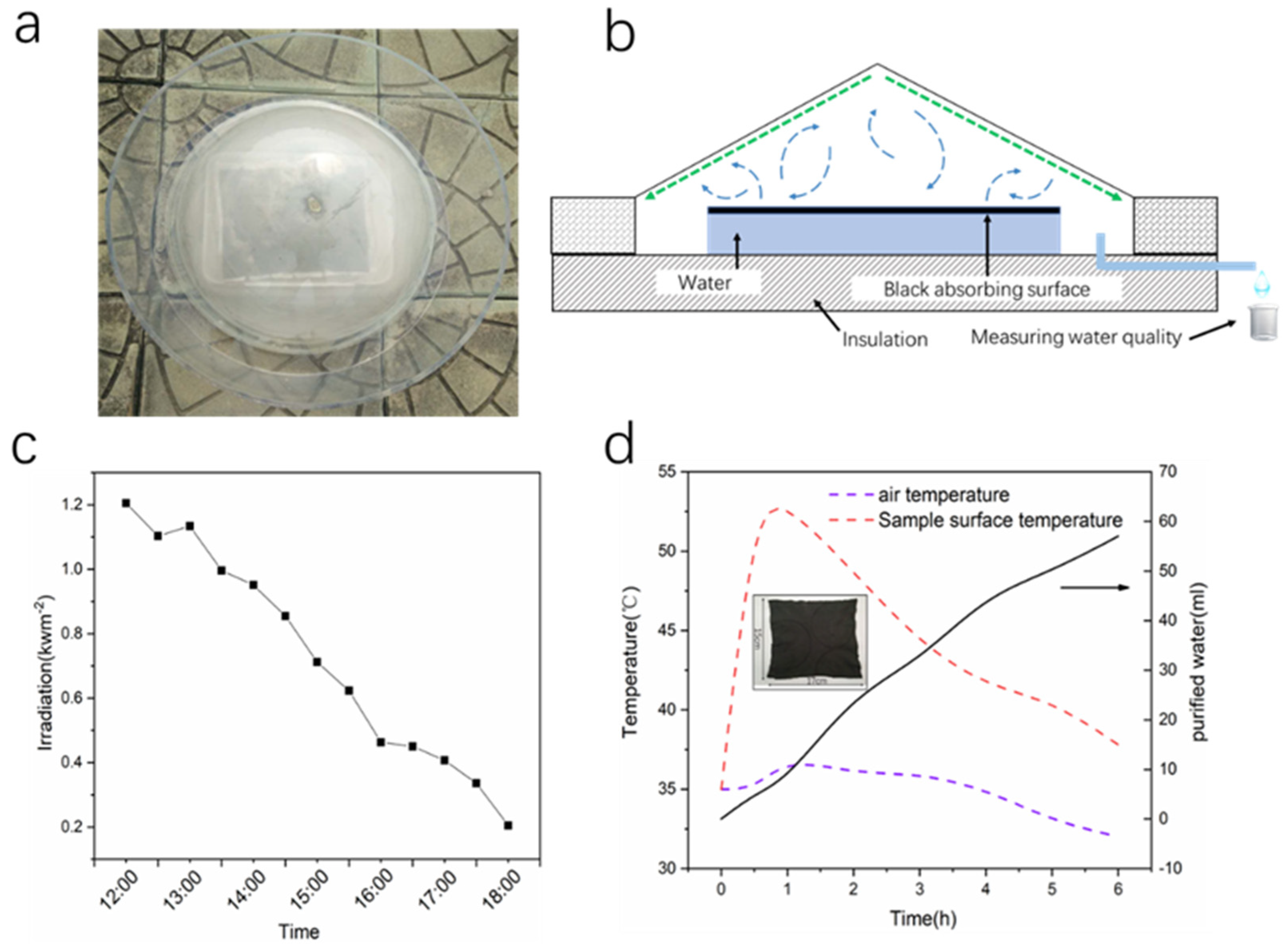
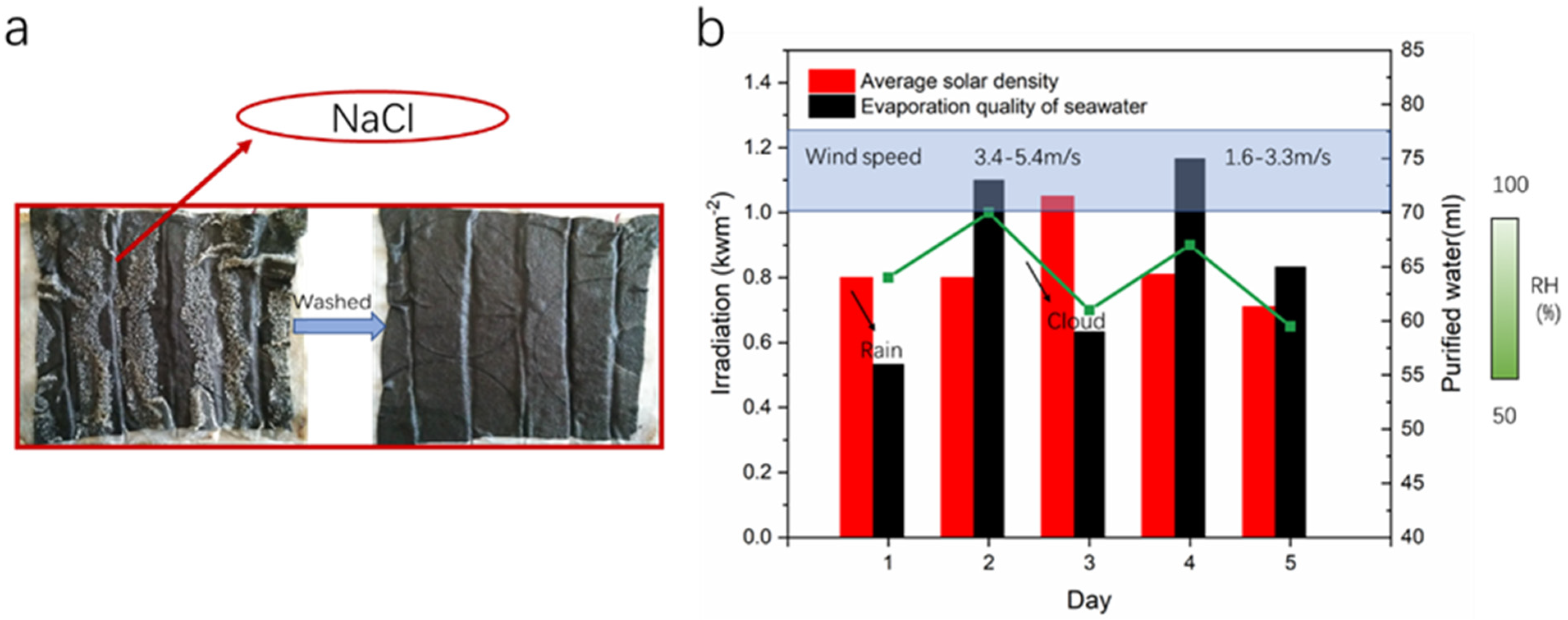
3.3. Outdoor Simplified Device Evaporation Experiment
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Elimelech, M.; Phillip, W.A. The Future of Seawater Desalination: Energy, Technology, and the Environment. Science 2011, 333, 712–717. [Google Scholar] [CrossRef] [PubMed]
- Zodrow, K.R.; Li, Q.; Buono, R.M.; Chen, W.; Daigger, G.; Dueñas-Osorio, L.; Elimelech, M.; Huang, X.; Jiang, G.; Kim, J.H.; et al. Advanced Materials, Technologies, and Complex Systems Analyses: Emerging Opportunities to Enhance Urban Water Security. Environ. Sci. Technol. 2017, 51, 10274–10281. [Google Scholar] [CrossRef] [PubMed]
- Finnerty, C.; Zhang, L.; Sedlak, D.L.; Nelson, K.L.; Mi, B. Synthetic Graphene Oxide Leaf for Solar Desalination with Zero Liquid Discharge. Environ. Sci. Technol. 2017, 51, 11701–11709. [Google Scholar] [CrossRef] [PubMed]
- Tong, T.; Elimelech, M. The Global Rise of Zero Liquid Discharge for Wastewater Management: Drivers, Technologies, and Future Directions. Environ. Sci. Technol. 2016, 50, 6846–6855. [Google Scholar] [CrossRef]
- Li, X.; Xu, W.; Tang, M.; Zhou, L.; Zhu, B.; Zhu, S.; Zhu, J. Graphene Oxide-Based Efficient and Scalable Solar Desalination under One Sun with a Confined 2d Water Path. Proc. Natl. Acad. Sci. USA 2016, 113, 13953–13958. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Lou, J.; Ni, M.; Song, C.; Wu, J.; Dasgupta, N.P.; Tao, P.; Shang, W.; Deng, T. Bioinspired Bifunctional Membrane for Efficient Clean Water Generation. ACS Appl. Mater. Interfaces 2015, 8, 772–779. [Google Scholar] [CrossRef]
- Tao, P.; Ni, G.; Song, C.; Shang, W.; Wu, J.; Zhu, J.; Chen, G.; Deng, T. Solar-driven interfacial evaporation. Nat. Energy 2018, 3, 1031–1041. [Google Scholar] [CrossRef]
- Fang, Q.; Li, T.; Chen, Z.; Lin, H.; Wang, P.; Liu, F. Full Biomass-Derived Solar Stills for Robust and Stable Evaporation To Collect Clean Water from Various Water-Bearing Media. ACS Appl. Mater. Interfaces 2019, 11, 10672–10679. [Google Scholar] [CrossRef]
- Xu, J.; Wang, Z.; Chang, C.; Fu, B.; Tao, P.; Song, C.; Shang, W.; Deng, T. Solar-driven interfacial desalination for simultaneous freshwater and salt generation. Desalination 2020, 484, 114423. [Google Scholar] [CrossRef]
- Zhou, Y.; Ding, T.; Gao, M.; Chan, K.H.; Cheng, Y.; He, J.; Ho, G.W. Controlled heterogeneous water distribution and evaporation towards enhanced photothermal water-electricity-hydrogen production. Nano Energy 2020, 77, 105102. [Google Scholar] [CrossRef]
- Gao, M.; Peh, C.K.; Zhu, L.; Yilmaz, G.; Ho, G.W. Photothermal Catalytic Gel Featuring Spectral and Thermal Management for Parallel Freshwater and Hydrogen Production. Adv. Energy Mater. 2020, 10, 2000925. [Google Scholar] [CrossRef]
- Zhu, L.; Gao, M.; Peh, C.K.N.; Ho, G.W. Recent progress in solar-driven interfacial water evaporation: Advanced designs and applications. Nano Energy 2018, 57, 507–518. [Google Scholar] [CrossRef]
- Gao, M.; Connor, P.K.N.; Ho, G.W. Plasmonic photothermic directed broadband sunlight harnessing for seawater catalysis and desalination. Energy Environ. Sci. 2016, 9, 3151–3160. [Google Scholar] [CrossRef] [Green Version]
- Zhu, M.; Li, Y.; Chen, F.; Zhu, X.; Dai, J.; Li, Y.; Yang, Z.; Yan, X.; Song, J.; Wang, Y.; et al. Plasmonic Wood for High-Efficiency Solar Steam Generation. Adv. Energy Mater. 2017, 8, 1701028. [Google Scholar] [CrossRef]
- Wang, X.; Liu, Q.; Wu, S.; Xu, B.; Xu, H. Multilayer Polypyrrole Nanosheets with Self-Organized Surface Structures for Flexible and Efficient Solar–Thermal Energy Conversion. Adv. Mater. 2019, 31, e1807716. [Google Scholar] [CrossRef]
- Liu, H.; Chen, C.; Wen, H.; Guo, R.; Williams, N.A.; Wang, B.; Chen, F.; Hu, L. Narrow bandgap semiconductor decorated wood membrane for high-efficiency solar-assisted water purification. J. Mater. Chem. A 2018, 6, 18839–18846. [Google Scholar] [CrossRef]
- Li, Y.; Gao, T.; Yang, Z.; Chen, C.; Luo, W.; Song, J.; Hitz, E.; Jia, C.; Zhou, Y.; Liu, B.; et al. 3D-Printed, All-in-One Evaporator for High-Efficiency Solar Steam Generation under 1 Sun Illumination. Adv. Mater. 2017, 29, 1700981. [Google Scholar] [CrossRef]
- Wu, X.; George, Y.; Chen, W.Z.; Liu, X.; Xu, H. A Plant-Transpiration-Process-Inspired Strategy for Highly Efficient Solar Evaporation. Adv. Sustain. Syst. 2017, 1, 1700046. [Google Scholar] [CrossRef]
- Chen, C.; Li, Y.; Song, J.; Yang, Z.; Kuang, Y.; Hitz, E.; Jia, C.; Gong, A.; Jiang, F.; Zhu, J.Y.; et al. Highly Flexible and Efficient Solar Steam Generation Device. Adv. Mater. 2017, 29, 1701756. [Google Scholar] [CrossRef]
- Huo, B.; Jiang, D.; Cao, X.; Liang, H.; Liu, Z.; Li, C.; Liu, J. N-Doped Graphene/Carbon Hybrid Aerogels for Efficient Solar Steam Generation. Carbon 2019, 142, 13–19. [Google Scholar] [CrossRef]
- Zhang, Q.; Xiao, X.; Wang, G.; Ming, X.; Liu, X.; Wang, H.; Yang, H.; Xu, W.; Wang, X. Silk-Based Systems for Highly Efficient Photothermal Conversion under One Sun: Portability, Flexibility, and Durability. J. Mater. Chem. A 2018, 6, 17212–17219. [Google Scholar] [CrossRef]
- Chen, T.; Wang, S.; Wu, Z.; Wang, X.; Peng, J.; Wu, B.; Cui, J.; Fang, X.; Xie, Y.; Zheng, N. A cake making strategy to prepare reduced graphene oxide wrapped plant fiber sponges for high-efficiency solar steam generation. J. Mater. Chem. A 2018, 6, 14571–14576. [Google Scholar] [CrossRef]
- Shi, L.; Wang, Y.; Zhang, L.; Wang, P. Rational design of a bi-layered reduced graphene oxide film on polystyrene foam for solar-driven interfacial water evaporation. J. Mater. Chem. A 2016, 5, 16212–16219. [Google Scholar] [CrossRef]
- Liu, K.-K.; Jiang, Q.; Tadepalli, S.; Raliya, R.; Biswas, P.; Naik, R.R.; Singamaneni, S. Wood–Graphene Oxide Composite for Highly Efficient Solar Steam Generation and Desalination. ACS Appl. Mater. Interfaces 2017, 9, 7675–7681. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, C.; Song, X.; Megarajan, S.K.; Jiang, H. A facile nanocomposite strategy to fabricate a rGO–MWCNT photothermal layer for efficient water evaporation. J. Mater. Chem. A 2017, 6, 963–971. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Zhao, D.; Yu, F.; Yang, C.; Lou, J.; Liu, Y.; Chen, Y.; Wang, Z.; Tao, P.; Shang, W.; et al. Floating Rgo-Based Black Membranes for Solar Driven Sterilization. Nanoscale 2017, 9, 19384–19389. [Google Scholar] [CrossRef] [PubMed]
- Ma, N.; Fu, Q.; Hong, Y.; Hao, X.; Wang, X.; Ju, J.; Sun, J. Processing Natural Wood into an Efficient and Durable Solar Steam Generation Device. ACS Appl. Mater Interfaces 2020, 12, 18165–18173. [Google Scholar] [CrossRef] [PubMed]
- Hummers, W.S.; Offeman, R.E. Preparation of Graphitic Oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Gao, J.; Liu, F.; Liu, Y.; Ma, N.; Wang, Z.; Zhang, X. Environment-Friendly Method To Produce Graphene That Employs Vitamin C and Amino Acid. Chem. Mater. 2010, 22, 2213–2218. [Google Scholar] [CrossRef]
- Li, K.; Chang, T.; Li, Z.; Yang, H.; Fu, F.; Li, T.; Ho, J.S.; Chen, P.Y. Biomimetic Mxene Textures with Enhanced Light-to-Heat Conversion for Solar Steam Generation and Wearable Thermal Management. Adv. Energy Mater. 2019, 9, 1901687. [Google Scholar] [CrossRef]
- Zhou, J.; Sun, Z.; Chen, M.; Wang, J.; Qiao, W.; Long, D.; Ling, L. Macroscopic and Mechanically Robust Hollow Carbon Spheres with Superior Oil Adsorption and Light-to-Heat Evaporation Properties. Adv. Funct. Mater. 2016, 26, 5368–5375. [Google Scholar] [CrossRef]
- Li, N.; Qiao, L.; He, J.; Wang, S.; Yu, L.; Murto, P.; Li, X.; Xu, X. Solar-Driven Interfacial Evaporation and Self-Powered Water Wave Detection Based on an All-Cellulose Monolithic Design. Adv. Funct. Mater. 2020, 31, 2008681. [Google Scholar] [CrossRef]
- Shi, M.-M.; Bao, D.; Li, S.-J.; Wulan, B.-R.; Yan, J.-M.; Jiang, Q. Anchoring PdCu Amorphous Nanocluster on Graphene for Electrochemical Reduction of N2to NH3under Ambient Conditions in Aqueous Solution. Adv. Energy Mater. 2018, 8, 1800124. [Google Scholar] [CrossRef]
- Wang, Z.; Xue, Z.; Zhang, M.; Wang, Y.; Xie, X.; Chu, P.; Zhou, P.; Di, Z.; Wang, X. Germanium-Assisted Direct Growth of Graphene on Arbitrary Dielectric Substrates for Heating Devices. Small 2017, 13, 1700929. [Google Scholar] [CrossRef]
- Hu, X.; Xu, W.; Zhou, L.; Tan, Y.; Wang, Y.; Zhu, S.; Zhu, J. Tailoring Graphene Oxide-Based Aerogels for Efficient Solar Steam Generation under One Sun. Adv. Mater. 2016, 29, 1604031. [Google Scholar] [CrossRef]
- Eda, G.; Lin, Y.-Y.; Mattevi, C.; Yamaguchi, H.; Chen, H.-A.; Chen, I.-S.; Chen, C.-W.; Chhowalla, M. Blue Photoluminescence from Chemically Derived Graphene Oxide. Adv. Mater. 2010, 22, 505–509. [Google Scholar] [CrossRef]
- Yuan, Y.; Dong, C.; Gu, J.; Liu, Q.; Xu, J.; Zhou, C.; Song, G.; Chen, W.; Yao, L.; Zhang, D. A Scalable Nickel–Cellulose Hybrid Metamaterial with Broadband Light Absorption for Efficient Solar Distillation. Adv. Mater. 2020, 32, e1907975. [Google Scholar] [CrossRef]
- Wang, Q.; Guo, Q.; Jia, F.; Li, Y.; Song, S. Facile Preparation of Three-Dimensional MoS2 Aerogels for Highly Efficient Solar Desalination. ACS Appl. Mater. Interfaces 2020, 12, 32673–32680. [Google Scholar] [CrossRef]
- Song, G.; Yuan, Y.; Liu, J.; Liu, Q.; Zhang, W.; Fang, J.; Gu, J.; Ma, D.; Zhang, D. Biomimetic Superstructures Assembled from Au Nanostars and Nanospheres for Efficient Solar Evaporation. Adv. Sustain. Syst. 2019, 3, 1900003. [Google Scholar] [CrossRef]
- Zhou, L.; Tan, Y.; Wang, J.; Xu, W.; Yuan, Y.; Cai, W.; Zhu, S.; Zhu, J. 3D self-assembly of aluminium nanoparticles for plasmon-enhanced solar desalination. Nat. Photonics 2016, 10, 393–398. [Google Scholar] [CrossRef]
- Zhang, H.; Li, L.; Jiang, B.; Zhang, Q.; Ma, J.; Tang, D.; Song, Y. Highly Thermal Insulated and Super-Hydrophilic Corn Straw for Efficient Solar Vapor Generation. ACS Appl. Mater. Interfaces 2020, 12, 16503–16511. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, J.; Chu, Z.; Xing, C.; Li, W.; Liu, Z.; Chen, W. Luminescence Reduced Graphene Oxide Based Photothermal Purification of Seawater for Drinkable Purpose. Nanomaterials 2022, 12, 1622. https://doi.org/10.3390/nano12101622
Huang J, Chu Z, Xing C, Li W, Liu Z, Chen W. Luminescence Reduced Graphene Oxide Based Photothermal Purification of Seawater for Drinkable Purpose. Nanomaterials. 2022; 12(10):1622. https://doi.org/10.3390/nano12101622
Chicago/Turabian StyleHuang, Jin, Zhen Chu, Christina Xing, Wenting Li, Zhongxin Liu, and Wei Chen. 2022. "Luminescence Reduced Graphene Oxide Based Photothermal Purification of Seawater for Drinkable Purpose" Nanomaterials 12, no. 10: 1622. https://doi.org/10.3390/nano12101622
APA StyleHuang, J., Chu, Z., Xing, C., Li, W., Liu, Z., & Chen, W. (2022). Luminescence Reduced Graphene Oxide Based Photothermal Purification of Seawater for Drinkable Purpose. Nanomaterials, 12(10), 1622. https://doi.org/10.3390/nano12101622





