Abstract
Here, a new type of PEC aptamer sensor for bisphenol A (BPA) detection was developed, in which visible-light active MoS2/Ni-Fe LDH (layered double hydroxide) heterostructure and aptamer were used as photosensitive materials and biometric elements, respectively. The combination of an appropriate amount of MoS2 and Ni-Fe LDH enhances the photocurrent response, thereby promoting the construction of the PEC sensor. Therefore, we used a simple in situ growth method to fabricate a MoS2/Ni-Fe LDH sensor to detect the BPA content. The aptasensor based on aptamer/MoS2/Ni-Fe LDH displayed a linear range toward a BPA of 0.05–10 to 50–40,000 ng L−1, and it has excellent stability, selectivity and reproducibility. In addition, the proposed aptamer sensor is effective in evaluating real water samples, indicating that it has great potential for detecting BPA in real samples.
1. Introduction
Cardiovascular disease (CVD) has been the leading cause of mortality and disability-adjusted life years worldwide. Bisphenol A (BPA) is a common endocrine disruptor that is used to manufacture polycarbonate and epoxy resins [1,2]. Manufacturing processes release BPA, which is then introduced into the environment and even food products [3,4]. Studies have revealed the relationship between BPA and the risk of several CVD outcomes and CVD risk factors [5,6]. Low concentrations of BPA could cause CVD, cytotoxicity, genotoxicity and, most severely, cancer [7]. Hence, sensitive testing for BPA is required.
Many analytical techniques have been implemented for the detection of BPA, such as amperometry [8], differential pulse voltammetry [9] and photoelectrochemical aptasensors [10]. Among these methods, some require professional operators and expensive equipment, and some even require laborious processes or severe test conditions. Electrochemical sensors are the most widely used group, due to their simple operation, high sensitivity, rapid response time, cost effective, the potential for miniaturization and the real sample analysis. The photoelectrochemical (PEC) aptasensor has attracted substantial attention through combining photoelectrode with aptamer, due to relatively low cost, miniaturization, fast response, short time and high sensitivity. The PEC aptasensor is usually used as an analytical method for monitoring various targets, including small ions, biomolecules and living cells pollutants. The PEC aptasensing process is related to the photoelectric conversion efficiency, depending on the photoinduced carrier separation and transfer of photoactive material. Due to the introduction of aptamer, the PEC platform not only possesses excellent properties of PEC technique but also obtains high selectivity for target assay. Meanwhile, PEC-based aptasensors are label-free, exempting from complex and arduous process of labeling aptamers which might affect the bio-affinity between aptamers and their target analytes. A novel photoelectrochemical (PEC) aptasensor has been employed to analyze BPA, which provided minimal background signals, high selectivity, minimal cost and wide practicability [11,12]. Thus, the PEC technique is an ideal choice to determine the low level of BPA. Here, what we should note is that selecting an appropriate photoactive material to construct the PEC sensing platform is very critical to achieve high performance for BPA detection.
Layered double hydroxide (LDH)-based materials have been applied in photocatalysis, due to their redox efficiency [13,14]. These materials show no toxicity and can be easily synthesized [15]. New LDH compounds are highly efficient for photocatalytic reactions [16]. They are arranged in the octahedral direction through oxo-bridge linkages. The unsaturated metal cations (M3+ and M2+) are also widely distributed and tunable, resulting in brucite-like layers [17,18,19]. In addition, the hydroxide ions are coordinated octahedrally. Typically, oxo-bridges promote metal-to-metal charge transfer, serving as important factors for redox reactions under visible light by slowing the recombination of electrons and holes [20].
The peculiar structure of the S-Mo-S atomic interlayer gives MoS2 excellent photocatalytic properties [21,22,23]. Recent studies have shown that MoS2 displays desirable photocatalytic activity due to its distinct electrical and optical properties and large surface area [24,25,26]. Consequently, MoS2 has been widely used to couple materials such as BiVO4 [27], WS2 [28] and TiO2 [29] to enhance electron–hole pair separation and, thus, boost PEC performance.
In this study, MoS2/NiFe-LDH nanosheets were synthesized by using a simple in situ growth approach, and a sensitive label-free PEC aptasensor was constructed on MoS2/NiFe-LDH nanosheets for the nontoxic measurement of BPA. Because of the nanoheterojunctions, Mo2S showed optimal separation of photogenerated charge carriers and also increased the visible-light absorption of NiFe-LDH. It also promoted the PEC conversion efficiency of Mo2S/NiFe-LDH. These findings demonstrated that NiFe-LDH modified by Mo2S displayed excellent PEC activity, biocompatibility and stability for efficient biomolecule detection.
2. Experimental
2.1. Reagents and Chemicals
Analytical purity reagents were employed directly. Sinopharm Chemical Reagent Co., Ltd. (Beijing, China) (www.sinoreagent.com (accessed on 2 May 2021)), provided thiourea (CH4N2S), (NH4)6Mo7O24∙4H2O, nickel nitrate hydrate (Ni(NO3)2·6H2O), ferric nitrate hydrate (Fe(NO3)3·9H2O) and ethanol, and XFNANO Materials Tech Co., Ltd. (Nanjing, China), provided glucose. BPA aptamers (5′-CCG GTG GGT GGT CAG GTG GGA TAG CGT TCC GCG TAT GGC CCA GCG CAT CAC GGG TTC GCA CCA-3′) were purchased from Shanghai Sangon Biotechnology Co. Ltd. (Shanghai, China). The 0.1 M phosphate buffer (pH = 7.0) was prepared by mixing the stock solution of Na2HPO4 and NaH2PO4.
2.2. Material Synthesis
At first, Ni (NO3)2·6H2O (1.75 g) and Fe (NO3)3·9H2O (0.81 g) were dissolved in 20 mL H2O with sonication for 0.5 h. The solution was transferred to a 50 mL Teflonlined autoclave and the heated at 150 °C, for 10 h, to obtain NiFe-LDH. Secondly, the MoS2/LDH heterojunction was prepared. An appropriate amount of (NH4)6Mo7O24∙4H2O (1.06, 2.83 and 4.24 mg) was dissolved in the mixed solution (60 mL) of ethylene glycol (EG) and H2O (EG:H2O = 1:2). Then, appropriate amounts of SC(NH2)2 (0.91, 2.43 and 3.65 mg) and the synthesized 50 mg LDH were slowly added to the suspension obtained above and continuously stirred for half an hour. The mixed solution was heated at 200 °C for 24 h in a 100 mL Teflon-lined autoclave. Finally, the product was continually washed three times with deionized water and ethanol, and then it was dried at 60 °C for 12 h.
2.3. Identification
The crystal structure of the samples was characterized by X-ray diffraction analysis (XRD, Bruker D8 Advance, Bruker AXS, Billerica, MA, USA) with Cu Kα radiation. The morphology of the samples was characterized by Scanning Electron Microscopy (SEM, TESCAN VEGA, Guanqian Technology Shanghai Co., Ltd. Shanghai, China) and Transmission Electron Microscopy (TEM, Talos F200C TEM, Thermo Fisher Scientific Electron Microscope, Beijing, China). The X-ray photoelectron spectra were obtained by X-ray photoelectron spectroscope (XPS, ESCALAB 250Xi, Thermo Fisher Scientific, Waltham, MA, USA). Diffuse reflection spectra (DRS) of the samples were texted by using a UV–Vis spectrophotometer with the reference of BaSO4 (UV1800, Shanghai Oasis, Shanghai, China). All PEC experiments were texted with CHI 660E (Beijing Huake Putian Technology Co., Ltd., Beijing, China) electrochemical workstation with a three-electrode system in 0.1 M PBS electrolyte (pH = 7). Pt wire was used as counter electrode and the reference electrode was saturated calomel electrode (SCE). Xenon lamp (PLS-SXE 300, 100 mW·cm−2, λ ≥ 420 nm Beijing Bofeilai Technology Co., Ltd. Beijing, China) was applied as the light source. The indium tin oxide (ITO) glass was used as the working electrode. Electrochemical impedance spectroscopy (EIS) was conducted over a frequency range from 1 to 1,000,000 Hz in 0.1 M PBS (pH = 7).
2.4. Fabrication of Modified Electrodes
Prior to each modification, the ITO electrodes (10 mm × 15 mm) were washed separately with water, acetone and ethanol for 5 min, respectively, and naturally dried. Typically, 5 mg catalyst powders (MoS2/LDH) were dispersed in 1 mL mixed solution (deionized water and chitosan; the water/chitosan ratio is 2/3) to form homogeneous suspension. Then ITO electrode (0.5 cm2) was coated with 20 µL suspensions. Then MoS2/LDH electrodes were coated with 20 µL BPA aptamer suspensions (1 µmol L−1) by the specific chemisorption between aptamer molecules and oxides and incubated at room temperature for 12 h. Then, they were rinsed adequately with distilled water to remove excess BPA aptamer. The fabricated aptamer/MoS2/LDH was dried and used for further studies.
3. Results and Discussion
3.1. Physical Characterization
XRD was used to investigate the phases of all synthesized materials (Figure 1). The peaks at 11.69°, 23.08°, 33.72°, 34.47°, 38.81°, 45.88°, 59.82° and 61.27° in the pattern of pure NiFe-LDH were indexed to the (003), (006), (101), (012), (015), (018), (110) and (113) NiFe-LDH lattices (JCPDS No. 40-0215). The high purity of the synthesized NiFe-LDH was confirmed by the absence of any other peaks. The maxima at 12.6°, 31.2° and 34.6° were attributed to the MoS2 lattice planes (002), (100) and (102) (JCPDS No.37-1492). The peak positions of MoS2/LDH were consistent among all composites, with both LDH and MoS2 peaks were easily distinguishable. Moreover, as the MoS2 content was increased, the (002) peak became increasingly prominent, indicating the successful synthesis of MoS2/LDH.
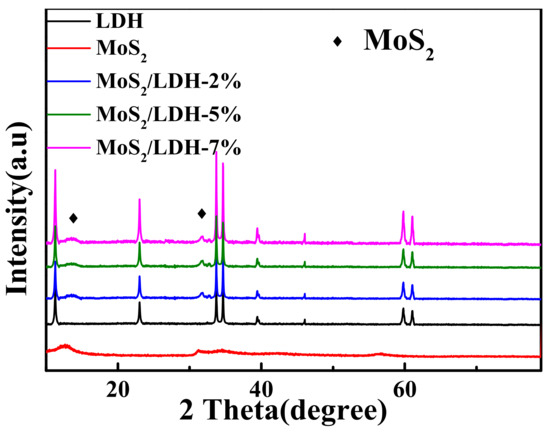
Figure 1.
XRD patterns of all materials.
XPS analysis was also conducted to investigate the surface composition and chemical states of MoS2/LDH. The doublet peaks centered at 167.7 and 162.3 eV corresponded to S 2p1/2 and S 2p3/2, confirming the presence of metal sulfides (Figure 2A) [30,31]. As presented in Figure 2B, Ni2+ peaks at 855.6 and 873.7 eV were attributed to Ni 2p3/2 and Ni 2p1/2 [32]. Satellite peaks appeared at 878.9 and 861.7 eV. The peaks at 712.4 and 724.6 eV in the Fe 2p species (Figure 2C) were attributed to the Fe 2p3/2 and Fe 2p1/2 states of Fe3+, which appeared because Fe in NiFe-LDH existed in the 3+ oxidation state [33]. The peaks in the XPS spectrum of Mo 3d3/2 at 231.6 eV and Mo 3d5/2 at 228.4 eV were attributed to Mo4+ in Figure 2D [34,35]. The XPS results confirmed that MoS2/NiFe-LDH was fabricated.
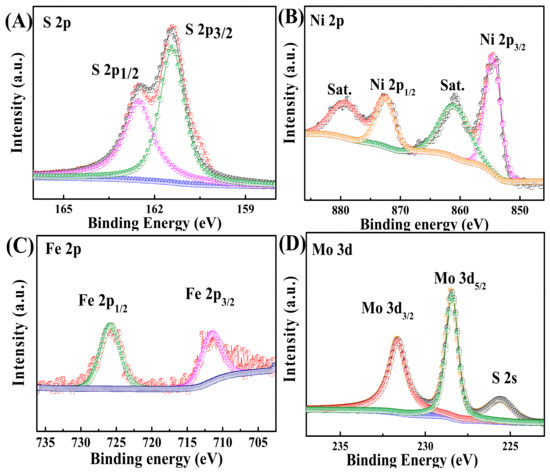
Figure 2.
XPS spectra of MoS2/LDH-5% composites: (A) S 2p, (B) Ni 2p, (C) Fe 2p and (D) Mo 3d.
SEM was used to evaluate the morphology of MoS2, LDH and MoS2/LDH-5% composites. Figure 3A shows that LDH is formed by staggered stacking of many layers. Figure 3B shows pure MoS2 as coiled and interlaced nanosheets. The microstructure of MoS2/LDH-5% is shown in Figure 3C,D. The crystallinity and composition of the as-synthesized material structure were further validated by using high-resolution transmission electron microscopy (HRTEM). Figure 3D displays the HRTEM image of the boxed region. The HRTEM data (Figure 3E) reveal two distinct lattice spacings of 0.62 and 0.252 nm, which are in close agreement with those of MoS2 (002) (0.615 nm) and LDH (012) (0.260 nm). Figure 3F–I depicts the distribution of S, Mo, Ni and Fe atoms in MoS2/LDH-5%, respectively. All elements were spread evenly across the MoS2/LDH-5%, indicating that the synthesis was successful.
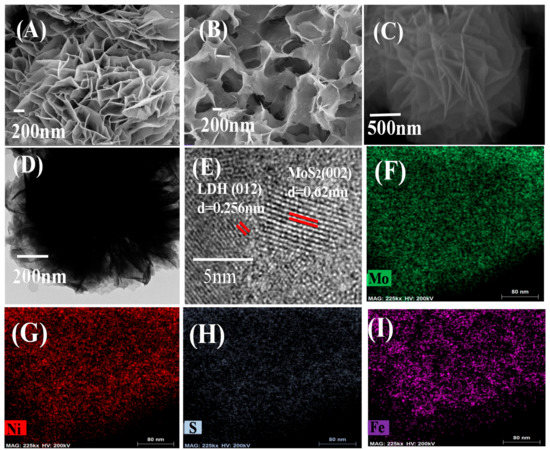
Figure 3.
SEM images of LDH (A), MoS2 (B) and MoS2/LDH-5% (C); HRTEM images (D,E); and STEM–EDX mapping images of MoS2/LDH-5% (F–I).
UV-Vis DRS spectra were measured for MoS2, LDH and MoS2/LDH-5% to learn more about their ability to handle visible light. Figure 4A shows that all catalysts displayed a high visible-light absorbance. LDH absorbed strongly at 400 nm. Due to the presence of MoS2, MoS2/LDH-5% displayed better absorption than LDH. The PL spectrum of the composite was examined to learn more about the charge separation and combination mechanisms of carriers during PEC measurements, as shown in Figure 4B. For the LDH and MoS2/LDH-5% composites, a prominent peak appeared at 450 nm, as well as a less intense peak for MoS2/LDH. As a result, the charge-carrier recombination efficiency was lowered in the composite made of MoS2 and LDH [36,37,38]. Because of these factors, MoS2/LDH-5% achieved outstanding PEC results.
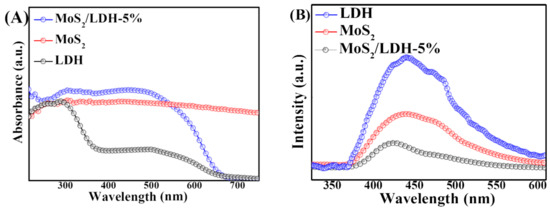
Figure 4.
UV-Vis diffuse reflectance spectra (A) and PL spectra (B) of LDH, MoS2 and MoS2/LDH-5%.
3.2. Photoelectrochemical Characterization
The photocurrent outputs of various electrodes were investigated at 0.1 V with visible-light stimulation in a 0.1 M phosphate-buffered solution (pH = 7.0) with ascorbic acid (AA, 0.1 M). Figure 5 shows a comparison of the photocurrents of all composites. The photocurrent of the composites increased after different quantities of MoS2 were added. In Figure 5A, MoS2/LDH-5% showed the maximum photocurrent among MoS2/LDH composites, and the photocurrent values followed the order MoS2/LDH-5% > LDH > MoS2; the photogenerated electrons of MoS2 tend to recombine with VB holes of LDH, because the VB band of LDH is higher than that of MoS2; AA is oxidized by the holes of MoS2. Then, photogenerated electron of LDH can efficiently transferred, thus reducing the charge recombination rate, which indicates that the addition of MoS2 to LDH improved the visible-light-harvesting performance, while also efficiently prevented LDH charge carrier recombination; thus, the photoelectrochemical activity was improved (Scheme 1) [39,40]. The above findings are in line with the EIS spectra (Figure S1).
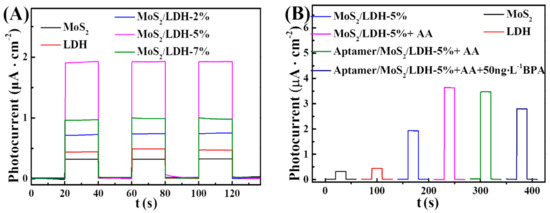
Figure 5.
(A) Photocurrent outputs of a variety of electrodes. (B) Photocurrent outputs of a variety of electrodes for BPA detection at 0.1 V vs. SCE before and after introducing AA in PBS.
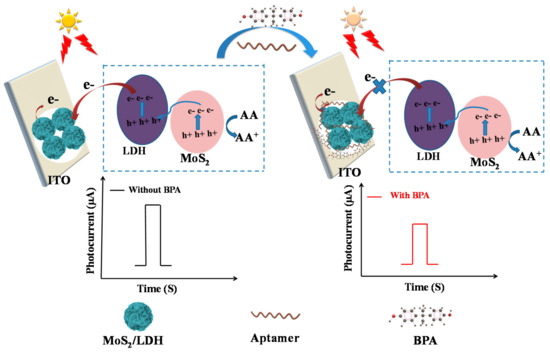
Scheme 1.
Mechanism of the PEC property based on aptamer/MoS2/LDH-5% for BPA.
Moreover, after BPA-aptamer modification, the photocurrent of aptamer/MoS2/LDH-5% decreased to 96% of the MoS2/LDH-5% because of the steric hindrance produced by the immobilized aptamer, as well as the electrostatic repulsion between the electronegative AA molecule and the negatively charged phosphate skeleton of the aptamer. Consequently, BPA solution could specifically bind to the aptamer. Since the increased steric hindrance hampered the coupling of AA and photogenerated holes and also encouraged photogenerated hole/electron interactions, the photocurrent was decreased. The above-mentioned moleculars displayed steric resistance at the electrode junction, which inhibited electron transport to the electrode surface and thereby lowered the photocurrent (Figure 5B). The electrostatic repulsion of these substances at the electrode interface prevented electron transport and enhanced electron–hole pair recombination, which lowered the photocurrent. More BPA encouraged the development of aptamer–BPA complexes, which reduced the photocurrent. The BPA concentration can be measured by monitoring the decrease of photocurrent density (Scheme 1) [37,41]. The above characteristics indicate that the MoS2/LDH-5% PEC aptasensor can be used to detect BPA.
Figure 6A shows how the applied potential was chosen. The aptamer/MoS2/LDH-5% was tested under different potentials, from −0.2 to 0.3 V in the dark and under illumination. When the potential was changed from −0.2 to 0.3 V, the current between illumination and the dark reduced abruptly. On the other hand, it declined softly when the potential was changed from −0.2 to 0.3 V; thus, 0.1 V is the most appropriate voltage for PEC. The pH of the electrolyte must be regulated during detection because it could impact PEC performance (Figure 6B). Upon increasing the pH of the electrolyte from 5 to 7, the photocurrent was increased. As the pH increased from 7 to 9, the photocurrent was decreased. The maximum photocurrent was reached at pH = 7, probably because the neutral environment was more conducive to maintaining the activity of the aptamer. The aptamer concentration (0.1–2 μmol/L) was also adjusted (Figure 6C) to obtain the maximum sensitivity. The results demonstrated that the largest photocurrent was obtained at a concentration of 1.0 μmol/L. It has been demonstrated that the optimal aptamer concentration has the capacity to trap BPA molecules’ sensitivity, while redundant immobilized aptamer exhibited a larger steric hindrance effect, which can block the BPA-capture course. Thus, 1.0 μmol/L was employed as an optimal aptamer concentration for the immobilization in the following experiments. The effect of the photocurrent on the BPA binding time, using an aptamer, was investigated. The time required for BPA to bind to the aptamer was changed from 5 to 60 s. Figure 6D shows that the photocurrent decreased over time until it stabilized, suggesting that the aptamer/BPA complex permeated the surface of the electrode.
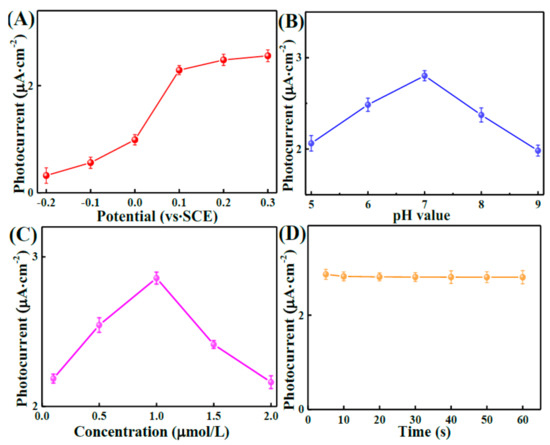
Figure 6.
Photocurrent of aptamer/MoS2/LDH-5% at different potentials (A) and PH (B). Influences of aptamer concentration (C) and binding time of BPA (D) on photocurrent of the aptamer/MoS2/LDH-5% toward BPA in PBS (pH = 7.0) containing 0. 1 mol L−1 AA, under visible-light irradiation.
In a 0.1 M phosphate buffer solution (pH = 7.0) containing ascorbic acid (AA, 0.1 M), the stable current of the aptamer/MoS2/LDH-5% electrode under dark conditions was measured. After 20 s, the visible light is turned on; the visible light is turned off after the photocurrent is increased and stabilized, the corresponding concentration of BPA is added after the dark current is stable and then the visible light is turned on to test the photocurrent at this concentration. After adding BPA, the photocurrent was decreased, as seen in Figure 7A. The linear equations were as follows: △I = 0.159 + 0.048c (R2 = 0.9946), with a linear range of 0.05–10 ng L−1; and △I = 0.696 + 6.19 × 10−5c (R2 = 0.9932), ranging from 50 to 40,000 ng L−1 (△I = I0 − I, where I0 is the photocurrent before BPA was added, and I is the photocurrent after BPA was added). Furthermore, the sensor showed a low detection limit of 0.0052 ng L−1 (S/N = 3). At low BPA levels, the local concentration at the electrode surface was rapidly depleted, as the substrate was converted into a product by the catalysis, resulting in the high sensitivity of the electrode response. At higher BPA concentrations, the nano-material is supplied with substrate for a longer period of time and the reaction proceeds over a larger time window. This, together with the possibility of fouling the electrode surface by the reaction products, results in a lower slope. It also attains the saturation level at a higher concentration. Thus, the sensor showed different linear correlations at different concentration ranges [42], as shown in Table S1. The comparison shows that the detection limit of the aptamer/MoS2/LDH-5% photoelectrode was 0.0052 ng L−1 in Table S1. The detection line of the aptamer/MoS2/LDH-5% photoelectrode was lower in area PEC. As a result, the aptasensor featured a large linear range and a low detection limit.
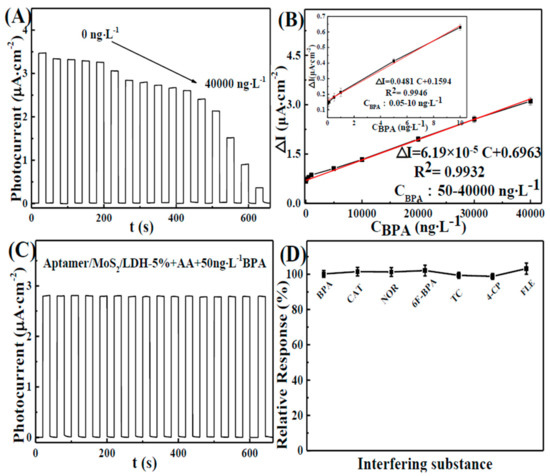
Figure 7.
(A) Photocurrent responses of aptamer/MoS2/LDH-5% electrode toward BPA at increasing concentrations (0–40,000 ng L−1). (B) Corresponding calibration plots of the BPA concentration. (C) Stable photocurrent response curve of the aptamer/MoS2/LDH-5% in the presence of 50 ng L−1 PBA. (D) The photocurrent responses of 50 ng L−1 BPA and interfering compounds, including 1000 ng L−1 hexafluorobisphenol A (6F-BPA), catechol (CAT), tetracycline (TC), 4-chlorophenol (4-CP), norfloxacin (NOR) and fleroxacin (FLE) in 0.1 M PBS at 0.1 V vs. SCE with visible-light excitation.
To evaluate the stability of the aptamer/MoS2/LDH-5% photoelectrode, it was examined for roughly 600 s (Figure 7C). The photocurrent responses of five aptamer/MoS2/LDH-5% electrodes were calculated in parallel (Figure S2A), and the stability of the aptamer/MoS2/LDH-5% photoelectrode was assessed by measuring the photocurrent sensitivity to 50 ng L−1 BPA every two days (Figure S2B). The aptamer/MoS2/LDH-5% appeared to have excellent compatibility and stability. Anti-interference studies were used to test the selectivity of aptamer/MoS2/LDH-5%. The amperometric responses of aptamer/MoS2/LDH-5% to 50 ng L−1 BPA and interfering compounds, including hexafluorobisphenol A (6F-BPA), catechol (CAT), tetracycline (TC), 4-chlorophenol (4-CP), norfloxacin (NOR), etc., are shown in Figure 7D. The interfering photocurrent was small, indicating that aptamer/MoS2/LDH-5% possesses better aptasensor BPA selectivity.
To test the dependability of the aptamer/MoS2/LDH-5% photoelectrode, Table S2, it was used to detect different concentrations of BPA in actual water samples. Shahu provided the river water (Wuhan City, Hubei Province), and boiled and filtered water samples were stored at 4 °C. The BPA sample was added to the buffer solution in the presence of ascorbic acid, and the current difference before and after adding the BPA sample was measured. The corresponding BPA concentration was calculated by bringing the current difference into the BPA calibration curve. The RSD was less than 3.24%, and the recoveries were 98.60–100.53%. These findings imply that the BPA aptasensor based on MoS2/LDH-5% can detect BPA in real-world samples.
4. Summary
For the selective detection of BPA, a new PEC method based on a MoS2/LDH-5% aptasensor was developed. The resulting PEC sensor displayed a wide linear range, low detection, acceptable stability and high selectivity, demonstrating its excellent performance for the detection of BPA in actual samples. This research provides a novel method for using PEC sensors for detecting residues in environmental samples.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/nano12010078/s1. Figure S1: EIS of all materials in PBS with Fe(CN)63+/Fe(CN)64+(5 mmol L−1). Figure S2: (A) photocurrent responses of five-paralleled aptamer/MoS2/LDH-5% photoelectrodes with 50 ng L−1 BPA; (B) stability tests of PEC base on aptamer/MoS2/LDH-5% toward 50 ng L−1 BPA. Table S1: Comparison of different ways for determination BPA. Table S2: PEC aptasensor of BPA in real river water samples. The references [43,44] were cited in Supplementary Materials.
Author Contributions
Conceptualization, H.G., Y.H. and J.L.; methodology, H.G., Y.H. and J.L.; software, H.G. and Y.H.; validation, H.G.; formal analysis, H.G. and J.L.; investigation, H.G. and Y.H.; resources, J.L.; data curation, H.G. and Y.H.; writing—original draft preparation, H.G., Y.H. and J.L.; writing—review and editing, H.G. and J.L.; visualization, Y.H.; supervision, J.L.; project administration, H.G., Y.H. and J.L.; funding acquisition, J.L.; All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by 2019 National Natural Science Foundation of China General Project (31770917).
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Peillex, C.; Kerever, A.; Lachhab, A.; Pelletier, M. Bisphenol A, bisphenol S and their glucuronidated metabolites modulate glycolysis and functional responses of human neutrophils. Environ. Res. 2021, 196, 110336. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Li, Y.Y.; Niu, Y.; Li, J.B.; Qin, Z.F. Effects of bisphenol A and its alternative bisphenol F on Notch signaling and intestinal development: A novel signaling by which bisphenols disrupt vertebrate development. Environ. Pollut. 2020, 263, 114443. [Google Scholar] [CrossRef]
- Kim, S.; Lee, I.; Lim, J.E.; Lee, A.; Moon, H.B.; Park, J.; Choi, K. Dietary contribution to body burden of bisphenol A and bisphenol S among mother-children pairs. Sci. Total Environ. 2020, 744, 140856. [Google Scholar] [CrossRef]
- Champmartin, C.; Marquet, F.; Chedik, L.; Décret, M.J.; Aubertin, M.; Ferrari, E.; Grandclaude, M.C.; Cosnier, F. Human in vitro percutaneous absorption of bisphenol S and bisphenol A: A comparative study. Chemosphere 2020, 252, 126525. [Google Scholar] [CrossRef] [PubMed]
- Kubiak, A.; Ciric, A.; Biesaga, M. Dummy molecularly imprinted polymer (DMIP) as a sorbent for bisphenol S and bisphenol F extraction from food samples. Microchem. J. 2020, 156, 104836. [Google Scholar] [CrossRef]
- Ye, Z.; Wang, Q.W.; Qiao, J.T.; Ye, B.X.; Li, G.P. Simultaneous detection of bisphenol A and bisphenol S with high sensitivity based on a new electrochemical sensor. J. Electroanaly. Chem. 2019, 854, 113541. [Google Scholar] [CrossRef]
- Jiang, S.L.; Liu, H.M.; Zhou, S.; Zhang, X.; Peng, C.; Zhou, H.; Tong, Y.Q.; Lu, Q. Association of bisphenol A and its alternatives bisphenol S and F exposure with hypertension and blood pressure: A cross-sectional study in China. Environ. Pollut. 2020, 257, 113639. [Google Scholar] [CrossRef] [PubMed]
- Cosio, M.S.; Pellicanò, A.; Brunetti, B.; Fuenmayor, C.A. A simple hydroxylated multi-walled carbon nanotubes modified glassy carbon electrode for rapid amperometric detection of bisphenol A. Sens. Actuators B Chem. 2017, 246, 673–679. [Google Scholar] [CrossRef]
- Chen, W.Y.; Mei, L.P.; Feng, J.J.; Yuan, T.; Wang, A.J.; Yu, H.Y. Electrochemical determination of bisphenol A with a glassy carbon electrode modified with gold nanodendrites. Microchim. Acta 2015, 182, 703–709. [Google Scholar] [CrossRef]
- Yan, P.C.; Mo, Z.; Xu, L.; Pang, J.Y.; Qian, J.C.; Zhao, L.; Zhang, J.M.; Chen, J.P.; Li, H.N. Plasmonic Bi microspheres doped carbon nitride heterojunction: Intensive photoelectrochemical aptasensor for bisphenol A. Electrochim. Acta 2019, 319, 10–17. [Google Scholar] [CrossRef]
- Wang, H.X.; Liang, D.; Xu, Y.; Liang, X.; Qiu, X.Q.; Lin, Z. A highly efficient photoelectrochemical sensor for detection of chlorpyrifos based on 2D/2D β-Bi2O3/g-C3N4 heterojunctions. Environ. Sci. Nano 2021, 8, 773–783. [Google Scholar] [CrossRef]
- Chen, J.X.; Zhao, G.C.; Wei, Y.; Feng, D.X.; Zhang, H.L. Construction of a novel photoelectrochemical sensor for detecting trace amount of copper (II) ion. Electrochim. Acta 2021, 370, 137736. [Google Scholar] [CrossRef]
- Fan, X.L.; Wang, T.; Gao, B.; Xie, X.X.; Zhang, S.T.; Meng, X.G.; Gong, H.; Guo, Y.X.; Huang, X.L.; He, J.P. Layered double hydroxides decorated graphic carbon nitride film as efficient photoanodes for photoelectrochemical water splitting. Catal. Today 2019, 335, 423–428. [Google Scholar] [CrossRef]
- Prasad, C.; Tang, H.; Liu, Q.Q.; Zulfiqar, S.; Shah, S.; Bahadur, I. An overview of semiconductors/layered double hydroxides composites: Properties, synthesis, photocatalytic and photoelectrochemical applications. J. Mol. Liq. 2019, 289, 111114. [Google Scholar] [CrossRef]
- Si, H.Y.; Deng, Q.X.; Yin, C.; Tavakoli, M.M.; Zhang, J.; Kong, J. Graphdiyne Coupled with g-C3N4/NiFe-Layered Double Hydroxide, a Layered Nanohybrid for Highly Efficient Photoelectrochemical Water Oxidation. Adv. Mater. Interfaces 2020, 7, 1902083. [Google Scholar] [CrossRef]
- Sayed, R.A.; Hafiz, S.E.A.E.; Gamal, N.; Hak, Y.G.; Rouby, W.M.A.E. Co-Fe layered double hydroxide decorated titanate nanowires for overall photoelectrochemical water splitting. J. Alloy. Compd. 2017, 728, 1171–1179. [Google Scholar] [CrossRef]
- Huang, J.W.; Hu, G.W.; Ding, Y.; Pang, M.C.; Ma, B.C. Mn-doping and NiFe layered double hydroxide coating: Effective approaches to enhancing the performance of α-Fe2O3 in photoelectrochemical water oxidation. J. Catal. 2016, 340, 261–269. [Google Scholar] [CrossRef]
- Iguchi, S.; Kikkawa, S.; Teramura, K.; Hosokawa, S.; Tanaka, T. Investigation of the electrochemical and photoelectrochemical properties of Ni–Al LDH photocatalysts. Phys. Chem. Chem. Phys. 2016, 18, 13811–13819. [Google Scholar] [CrossRef] [Green Version]
- Sun, L.X.; Sun, J.H.; Yang, X.J.; Bai, S.L.; Feng, Y.J.; Luo, R.X.; Li, D.Q.; Chen, A.F. An integrating photoanode consisting of BiVO4, rGO and LDH for photoelectrochemical water splitting. Dalton Trans. 2019, 48, 16091–16098. [Google Scholar] [CrossRef] [PubMed]
- Aboubakr, A.E.A.; Rouby, W.M.A.E.; Khan, M.D.; Farghali, A.A.; Revaprasadu, N. ZnCr-CO3 LDH/ruptured tubular g-C3N4 composite with increased specific surface area for enhanced photoelectrochemical water splitting. Appl. Surf. Sci. 2020, 508, 145100. [Google Scholar] [CrossRef]
- Li, Z.Z.; Meng, X.C.; Zhang, Z.S. Few-layer MoS2 nanosheets-deposited on Bi2MoO6 microspheres: A Zscheme visible-light photocatalyst with enhanced activity. Catal. Today 2018, 315, 67–78. [Google Scholar] [CrossRef]
- Trung, T.N.; Seo, D.B.; Quang, N.D.; Kim, D.J.; Kim, E.T. Enhanced photoelectrochemical activity in the heterostructure of vertically aligned few-layer MoS2 flakes on ZnO. Electrochim. Acta 2018, 260, 150. [Google Scholar] [CrossRef]
- Zhang, J.R.; Zhao, Y.Q.; Chen, L.; Yin, S.F.; Cai, M.Q. Density functional theory calculation on facet-dependent photocatalytic activity of MoS2/CdS heterostructures. Appl. Surf. Sci. 2019, 469, 27. [Google Scholar] [CrossRef]
- Fan, L.F.; Zhang, C.Y.; Liang, G.F.; Yan, W.J.; Guo, Y.J.; Bi, Y.P.; Dong, C. Highly sensitive photoelectrochemical aptasensor based on MoS2 quantum dots/TiO2 nanotubes for detection of atrazine. Sens. Actuators B Chem. 2021, 334, 129652. [Google Scholar] [CrossRef]
- Liu, X.; Wang, B.F.; Liu, M.; Liu, S.L.; Chen, W.; Gao, L.; Li, X.Y. In situ growth of vertically aligned ultrathin MoS2 on porous g-C3N4 for efficient photocatalytic hydrogen production. Appl. Surf. Sci. 2021, 554, 149617. [Google Scholar] [CrossRef]
- Hu, D.L.; Cui, H.Y.; Wang, X.Y.; Luo, F.; Qiu, B.; Cai, W.C.; Huang, H.; Wang, J.; Lin, Z.Y. Highly Sensitive and Selective Photoelectrochemical Aptasensors for Cancer Biomarkers Based on MoS2/Au/GaN Photoelectrodes. Anal. Chem. 2021, 93, 7341–7347. [Google Scholar] [CrossRef]
- Li, H.L.; Yu, K.; Lei, X.; Guo, B.J.; Fu, H.; Zhu, Z.Q. Hydrothermal Synthesis of Novel MoS2/BiVO4 Hetero-Nanoflowers with Enhanced Photocatalytic Activity and a Mechanism Investigation. J. Phys. Chem. C 2015, 119, 22681. [Google Scholar] [CrossRef]
- PesciOrcid, F.M.; Sokolikova, M.S.; Grotta, C.; Sherrell, P.C.; Reale, F.; Sharda, K.; Ni, N.; Palczynski, P.; Mattevi, C. MoS2/WS2 Heterojunction for Photoelectrochemical Water Oxidation. ACS Catal. 2017, 78, 4990. [Google Scholar]
- Liu, X.Q.; Huo, X.H.; Liu, P.P.; Tang, Y.F.; Xu, J.; Liu, X.H.; Zhou, Y.M. Assembly of MoS2 nanosheet-TiO2 nanorod heterostructure as sensor scaffold for photoelectrochemical biosensing. Electrochim. Acta 2017, 242, 327. [Google Scholar] [CrossRef]
- Zhang, H.C.; Li, Y.J.; Xu, T.H.; Wang, J.B.; Huo, Z.Y.; Wan, P.B.; Sun, X.M. Amorphous Co-doped MoS2 nanosheets coated on metallic CoS2 nanocubes as an excellent electrocatalyst for hydrogen evolution. J. Mater. Chem. A 2013, 3, 15020. [Google Scholar] [CrossRef]
- Choi, S.; Kim, C.; Lee, J.Y.; Lee, T.H.; Kwon, K.C.; Kang, S.; Choi, K.S.; Suh, J.M.; Hong, K.; Jun, S.E.; et al. Vertically aligned MoS2 thin film catalysts with Fe-Ni sulfide nanoparticles by one-step sulfurization for efficient solar water reduction. Chem. Eng. J. 2021, 418, 129369. [Google Scholar] [CrossRef]
- Que, R.H.; Liu, S.; Yang, Y.; Pan, Y.Y. High catalytic performance of core-shell structure ZnCo2O4@NiFe LDH for oxygen evolution reaction. Mater. Lett. 2021, 298, 129982. [Google Scholar] [CrossRef]
- Feng, X.T.; Jiao, Q.Z.; Chen, W.X.; Dang, Y.L.; Dai, Z.; Sui, S.L.; Zhang, J.T.; Zhao, Y.; Li, H.S.; Feng, C.H. Cactus-like NiCo2S4@NiFe LDH hollow spheres as an effective oxygen bifunctional electrocatalyst in alkaline solution. Appl. Catal. B Environ. 2021, 286, 119869. [Google Scholar] [CrossRef]
- Bu, Y.Z.; Xu, J.L.; Li, Y.W.; Liu, Q.; Zhang, Z. Enhanced photocatalytic activity of BiOI under visible light irradiation by the modification of MoS2. RSC Adv. 2017, 7, 42398. [Google Scholar] [CrossRef] [Green Version]
- Shen, J.; Wu, J.; Pei, L.; Rodrigues, M.T.F.; Zhang, Z.; Zhang, F.; Zhang, X.; Ajayan, P.M.; Ye, M. CoNi2S4-Graphene-2D-MoSe2 as an Advanced Electrode Material for Supercapacitors. Adv. Energy Mater. 2016, 6, 1600341. [Google Scholar] [CrossRef]
- Kuo, W.S.; Ho, P.H. Solar photocatalytic decolorization of dyes in solution with TiO2 film. Dye. Pigment. 2006, 71, 212–217. [Google Scholar] [CrossRef]
- Cao, J.; Xu, B.Y.; Lin, H.L. Highly improved visible light photocatalytic activity of BiPO4 through fabricating a novel p–n heterojunction BiOI/BiPO4 nanocomposite. Chem. Eng. J. 2013, 228, 482–488. [Google Scholar] [CrossRef]
- Li, M.Y.; Huang, Y.L.; Wang, S.Q. Visible light driven photoelectrochemical sensor for chromium (VI) BiOI microspheres decorated with metallic bismuth. Microchim. Acta 2019, 186, 345. [Google Scholar] [CrossRef] [PubMed]
- Yan, P.C.; Jiang, D.S.; Li, H.N. Exploitation of a photoelectrochemical sensing platform for catechol quantitative determination using BiPO4 nanocrystals/BiOI heterojunction. Anal. Chim. Acta 2018, 1042, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.H.; Zhang, C.; Ji, D.W.; Yin, G.; Wang, W.C.; Chen, Z.D. Cobalt-Doped TiO2 Nanowire Arrays Coated with NiFe Layered-Double-Hydroxide Nanoplatelets as Photoanodes for Photoelectrochemical Water Oxidation. ACS Appl. Nano Mater. 2020, 3, 6598–6608. [Google Scholar] [CrossRef]
- Chou, X.Y.; Ye, J.; He, J.H. One-Step Solvothermal Synthesis of BiPO4/Bi2MoO6 Heterostructure with Oxygen Vacancies and Z-Scheme System for Enhanced Photocatalytic Performance. ChemistrySelect 2019, 4, 8327–8333. [Google Scholar] [CrossRef]
- Dong, X.; Li, M.; Feng, N.; Sun, Y.; Yang, C.; Xu, Z.A. Nanoporous MgO based nonenzymatic electrochemical sensor for rapid screening of hydrogen peroxide in milk. RSC Adv. 2015, 5, 86485–86489. [Google Scholar] [CrossRef]
- Yang, L.Q.; Zhao, Z.J.; Hu, J.; Wang, H.B.; Dong, J.F.; Wan, X.; Cai, Z.Y.; Li, M.Y. Copper Oxide Nanoparticles with Graphitic Carbon Nitride for Ultrasensitive Photoelectrochemical Aptasensor of Bisphenol A. Electroanalysis 2020, 32, 1651–1658. [Google Scholar] [CrossRef]
- Deiminiat, B.; Gholam, H.R. A novel visible light photoelectrochemical aptasensor for determination of bisphenol A based on surface plasmon resonance of gold nanoparticles activated g-C3N4 nanosheets. J. Electroanal. Chem. 2021, 886, 115122. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).